Abstract
Background
Despite therapeutic advances, the management of chronic obstructive pulmonary disease (COPD) remains complex. There is growing interest in multidimensional, mind-body exercises to improve both physical and psychosocial aspects of COPD burden. Few US data are available in this population on tai chi (TC) a mind-body exercise incorporating physical activity, breathing and mindful awareness. We explored feasibility and preliminary efficacy of TC in COPD in an US academic medical setting.
Methods
Patients with COPD Global Obstructive Lung Disease (GOLD) stages 2–4 were randomised to a 12-week TC programme or education control. At 12 weeks, those in TC were randomised again to continue in maintenance classes or not to further explore optimal duration. All groups were followed to 24 weeks. Feasibility/safety parameters were analysed descriptively. Preliminary between-group differences were estimated in symptoms (dyspnoea, fatigue), health-related quality-of-life (Chronic Respiratory Questionnaire CRQ), cognitive-emotional measures (mood, COPD self-efficacy) and functional status (6 min walk test, lower body strength, flexibility, physical activity).
Results
Ninety-two subjects were randomised (N=61 TC, N=31 education). Mean age was 68±8 years, 66% male, mean forced expiratory volume in 1 s % predicted 57±13, 28% were GOLD stage 3–4. Overall retention was 85%. Nineteen adverse events occurred, most being study-unrelated COPD exacerbations. From baseline to 12 weeks, there were between-group improvements favouring TC, in CRQ-total (Cohen’s d effect size (ES)=0.46; adj mean diff (AMD)=0.31), CRQ-emotion (ES=0.54; AMD=0.49), Centre for Epidemiologic Studies Depression (ES=−0.37; AMD=2.39) and Patient-Reported Outcome Measurement Information System (PROMIS)-fatigue (ES=−0.34; AMD=−0.17). From baseline to 24 weeks, there was an improvement favouring TC in CRQ-dyspnoea (ES=0.41; AMD=0.46). Among TC participants, there was a positive effect of maintenance classes on self-efficacy (ES=−0.69; AMD=−0.40), 6 min walk (ES=0.56; AMD=49.26 feet), PROMIS-fatigue (ES=−0.41; AMD=−0.28) and chair stand (0.43; AMD=0.56).
Conclusion
TC in patients with COPD is feasible and safe. Preliminary analyses support a potential modest role in improving quality-of-life, cognitive-emotional health and function that should be further studied.
Trial registration number
IRB reference
BIDMC 2010P-000412; VA 2540.
Keywords: exercise, complementary medicine
Key messages.
Few studies are available in the USA to guide recommendations for tai chi exercise for patients with chronic obstructive pulmonary disease (COPD). We sought to examine the safety and feasibility of tai chi as a potential therapeutic intervention that uniquely addresses the biopsychosocial issues of COPD.
Tai chi, as a mind-body exercise for patients in the USA with moderate to severe COPD, is feasible and safe.
This study provides valuable information regarding practical issues of study implementation and engagement as well as discussion of the potential role of tai chi in supporting health-related quality of life, cognitive-emotional measures and functional status in patients with COPD.
Background
While the physical limitations are well known, there has been increasing appreciation for the multidimensionality of chronic obstructive pulmonary disease (COPD).1 2 Patients with COPD suffer from dyspnoea and exercise intolerance, and the resulting decrease in physical activity is associated with increased risk of hospitalisations, acute exacerbations and death.3–8 However, the patient experience and disease burden are a consequence of not only physical and functional problems, but overlaid with psychosocial, emotional, cognitive and behavioural considerations. Anxiety and depression, for example, are prevalent in COPD,9 independently associated with poor outcomes, and serve as additional barriers for patients towards self-care and overall self-efficacy.10
Tai chi (TC) is a low-impact, multimodal mind-body exercise that integrates dynamic postural control, flexibility, strength and breath training, along with mindful, internal awareness.11 12 TC has been shown to beneficially affect multiple physical and psychological processes in both healthy and chronic disease populations, such as cardiovascular, neurological and chronic pain conditions.13–15 Studies have reported benefits including musculoskeletal strength, flexibility, balance, fall prevention, as well as improved cardiovascular indices, mood, stress and cognitive function, supporting TCs safety and promise for prevention and rehabilitation.16–18
A number of components of TC may be relevant to the treatment of deconditioned, pulmonary patients with COPD (eg, physical exercise, breathing training, mind-body awareness, stress management),19 20 however, there have been few large-scale studies of TC in COPD. One recent, randomised controlled trial (RCT), conducted in China, reported TC was equivalent to pulmonary rehabilitation for improving health-related quality of life (HRQL) at 12 weeks.21 Recent meta-analyses suggest benefits for outcomes including 6 min walk test (6MWT) distance, symptom of dyspnoea, spirometric indices and HRQL.22–24 However, the evidence to date has been inconclusive due to methodological limitations,23 25–27 and importantly, the majority of these studies have been conducted in Asia. TC’s role in the management of chronic disease in the USA is still poorly understood. In addition, the duration of most studies evaluating TC interventions has been 12 weeks; however, the optimal duration/dose of TC for COPD is unknown. Studies in other populations have suggested that longer durations, booster or maintenance sessions, similar to approaches used in pulmonary rehabilitation, may be beneficial.28 29 As TC is an increasingly available modality in the USA, rigorous data are needed to guide clinical recommendations in this country.
In this context, we sought to examine TC as a potential therapeutic intervention that uniquely addresses the biopsychosocial issues of COPD. Our primary aim was to examine safety and feasibility of conducting a TC trial in patients with COPD within a US academic medical setting to inform design features for a future fully powered RCT. Consistent with this aim, we assessed preliminary estimates of effect of TC in patients with COPD on HRQL, cognitive-emotional and functional outcomes, and explored effects of longitudinal intervention dose.
Methods
Participants were randomised (2:1:1) to a 12-week TC programme, a time/attention-matched education control, or a third exploratory arm of mind-body breathing exercise. The analysis of this paper describes the primary comparison of participants in the first two arms (TC vs education). At the end of the first 12 weeks, participants in TC were randomised a second time to either continue TC in a ‘maintenance’ class, or to resume usual care. All groups were followed to 24 weeks. Questionnaires and functional tests were assessed at baseline, 12 and 24 weeks. Staff performing testing were blinded to participants’ group allocation. Our analysis follows the Consolidated Standards of Reporting Trials (CONSORT) guidelines for pilot studies30 primarily evaluating feasibility. Further analyses were performed for hypothesis generation to inform future trial planning.
Study population recruitment and randomisation
Participants were identified (August 2011–June 2015) through hospital databases from primary care and pulmonary clinics at three large academic medical centres in Boston and their affiliated sites: Beth Israel Deaconess Medical Center, VABoston Healthcare System and Boston Medical Center. Potentially eligible patients were sent recruitment mailings and telephoned. All subjects provided written informed consent. Initial randomisation by study staff occurred after baseline testing. The second randomisation in the TC group occurred after 12-week testing. Group assignments were generated by the study biostatistician using a permuted blocks method with randomly varying block size.
We included participants with moderate to severe COPD defined by (1) Global Obstructive Lung Disease (GOLD) stages 2,3 or 4 with symptoms of dyspnoea (forced expiratory volume in 1 s (FEV1) ≤80% and FEV1/forced vital capacity <0.70 or CT evidence of emphysema) and (2) age ≥40 years. We excluded those with: (1) respiratory failure or GOLD stage 4 who were unable to perform a 6 MWT; (2) COPD exacerbation requiring steroids, antibiotics, ED visit or hospitalisation within the past 2 weeks; (3) planned thoracic surgery within the next 3 months; (4) hypoxaemia on 6 MWT or cardiopulmonary exercise test (oxygen saturation <88% on supplemental oxygen); (5) inability to ambulate due to vascular or neuromuscular conditions preclude a walk test; (6) clinical signs of unstable cardiovascular disease (eg, chest pain on 6MWT); (7) severe cognitive dysfunction (Mini-Mental Status Examination ≤24); (8) non-English speaking; (9) current participation in pulmonary rehabilitation or regular practice of TC and (10) unstable/untreated clinical depression.
Intervention/education control
TC and education classes were conducted twice weekly for 1 hour for the first 12 weeks. Those randomised to continue TC class after 12 weeks were offered classes once weekly for another 12 weeks. A limited number of make-up classes (3–5 per cohort) were offered. Three experienced instructors were certified graduates of a 2.5-year instructor training programme (average 19 years teaching experience, including working with medical populations in the context of clinical trials). Following dual intervention and control protocol training, the same instructors administered both TC and education classes to minimise confounding of instructor personality and teaching style within groups. All groups received standard care, including medications according to American Thoracic Society guidelines for managing COPD and regularly scheduled provider visits.31
TC intervention
The TC intervention was designed for an older, physically limited population with COPD. Details of the intervention are published elsewhere.19 In brief, the classes emphasised essential TC movements that could be done repetitively in a flowing manner. The five core TC movements—‘raising the power’, ‘withdraw and push’, 'grasp the sparrow’s tail', ‘brush knee twist step’ and ‘cloud hands’—were based on the traditional Cheng Man-Ch’ing’s Yang-style short form.11 Classes also included mindful breathing techniques and a complementary set of traditional TC warm-up exercises focused on loosening the physical body, providing low to moderate aerobic activity, incorporating mindfulness and imagery into movement, and generating body and breath awareness. Each session concluded with a cool-down exercise of seated self-massage of the face, abdomen, flanks and mid-back.
Subjects in TC received a 45 min digital video disc and an audio file to facilitate home practice and encouraged to practise three times a week for 30 min. There was no new content for those randomised to the TC ‘maintenance’ class, which served to review and practice material from the first 12 weeks.
Education control
Content was based on information from the American Thoracic Society, American College Chest Physicians and the Global Obstructive Lung Disease Patient Guide.32–37 Educational modules included: anatomy of the lungs, managing COPD symptoms, smoking cessation, diagnostic tests, understanding COPD meds, managing acute exacerbations, managing stress, exercise, nutrition, sleep, mental health, oxygen therapy, surgical options, pulmonary rehabilitation and advance care planning. Time was spent with both didactic as well as informal group discussion of the educational material moderated by the instructor.
Outcome measures
Feasibility and adherence
A priori defined benchmarks for study feasibility included: proportion of eligible patients willing to participate >10%, reasonable rate of participant recruitment of ≥5 patients per month, a majority of participants with ≥70% class attendance,<20% lost to follow-up and >80% completion of outcome testing. Intervention feasibility was assessed by class attendance and home practice. During the first 12 weeks, home TC practice was tracked through logs at each class. During the second 12 weeks, those randomised to continue TC classes were similarly tracked with logs at each class; those not randomised to continue TC classes were queried about home practice once a month by phone.
Safety and adverse event monitoring
During the first 12 weeks, detailed changes in symptoms and new events were queried at each class. At 12 and 24 weeks testing visits, we asked about emergency room visits and hospitalisations, as well as medical symptoms/events in the past 12 weeks including muscle strain, dizziness, shortness of breath or COPD exacerbation, fatigue, falls, palpitations and psychological stress. For any reportable adverse event, we ascertained study relatedness, severity, any change in medications, medical care or hospital visits. Medical record review was conducted for further details of serious adverse events, defined by our institutional review boards to be events that require hospitalisation, are life-threatening, or result in persistent or significant disability/incapacity.
Symptoms and HRQL
Disease-specific HRQL was measured with the 20-item self-administered, standardised version of the Chronic Respiratory Disease Questionnaire (CRQ). We analysed the total score and subscales in the four domains—dyspnoea, fatigue, emotional function and mastery, with minimal clinically important difference (MCID) of 0.4.38 Dyspnoea was measured with the University of California, San Diego Shortness of Breath Questionnaire (UCSD SOB), a 24-item self-administered instrument that assesses the degree to which patients feel short of breath while performing different activities of daily living, with MCID of 5.39 40
Cognitive-Emotional Measures
We used the COPD self-efficacy scale (CSES) to measure level of confidence to manage or avoid breathing difficulties in different situations, including times of negative affect, intense emotions, physical exertion, at-risk behaviours or adverse weather/environment conditions. Higher scores correspond to lower confidence in managing dyspnoea.41 Depression was measured using the Centre for Epidemiology Studies-Depression Scale (CES-D),42 a validated 20-item instrument, measuring psychological impairment including feelings of depression, worthlessness, loneliness, energy level and fear. A score of <15 indicates no depression.43 44 We used the Multidimensional Scale of Perceived Social Support (MSPSS), a validated 12-item instrument to assess the degree of perceived social support provided in subscale areas of the subject’s existing social network (family, friends and significant others).45
Functional status
The 6 MWT, a standardised assessment that measures the maximum distance walked in 6 min, was used as a measure of exercise capacity.46 Patients were allowed to stop as often as needed and to use supplemental oxygen if usually prescribed for activity. The 6 MWT distance has been shown to be an independent correlate of COPD prognosis and survival. Published MCID is 26 m in those with severe COPD.47 48 The Chair Stand test was used to assess lower body strength and endurance. This validated test instructs subjects with arms folded to rise to a standing position and then return to a seated position as many times as possible within 30 s.49 The Chair Sit and Reach was used to assess lower body flexibility, primarily the hamstring, for use in older or deconditioned populations.50
We used the Patient-Reported Outcome Measurement Information System (PROMIS) Fatigue Short Form 7a to measure functional fatigue.51 To track patients self-reported level of physical activity outside of exercise classes, we used the Community Health Activities Model Programme for Seniors (CHAMPS) Physical Activity Questionnaire for Older Adults,52 a 41-item validated instrument in the elderly, which assesses physical activity from several domains, including leisure, household and occupational. Weekly frequency and total time spent allow estimation of caloric expenditure, presented in kilocalories per week.
Other data collection
We collected baseline information on sociodemographics and baseline clinical factors, including prior pulmonary rehabilitation, supplemental O2 use, Body Mass Index, Obstruction, Dyspnoea and Exercise (BODE) index (which includes FEV1% predicted, 6 MWT distance, Modified Medical Research Council dyspnoea score, body mass index53) and Charlson Comorbidity Index.54
Statistical analysis
Feasibility and safety
We used descriptive statistics to report study and group feasibility according to prespecified measures: (1) recruitment (proportion of eligible patients willing to participate, monthly recruitment rate), (2) group adherence (class attendance) and (3) study protocol completion (proportion lost to follow-up, proportion completing outcome testing). TC home practice hours across 24 weeks are reported descriptively.
Safety was evaluated through number of adverse events reported per type according to expectedness and severity per study arm. Safety was defined as no occurrence of serious adverse events related to either the intervention or education group assignment.
Preliminary estimates of effect
Since the publication of our initial protocol paper,19 there has been increased awareness of the limitations and appropriate reporting of results in pilot studies.30 While one of the study’s initial primary aims was feasibility, the study was also powered on pre-specified outcomes of overall CRQ and 6MWT distance and proposed preliminary between-group comparisons using adjusted analysis of covariance. We recognise that sample size estimates, largely based on a small study,55 are likely to be inaccurate. Thus, while we have conducted exploratory efficacy analyses, we emphasise the primary purpose of this pilot to be feasibility and safety.
Effect size (ES) estimates are presented as standardised differences between groups, comparing change scores between groups (baseline to 12 weeks, and baseline to 24 weeks) and calculation of Cohen’s d. Hedges and Olkin’s56 formula for CIs for standardised differences was used to calculate 95% CIs for Cohen’s d. The magnitude of ES of 0.2, 0.5 and 0.8 are used to represent small, medium and large ES, respectively.57 We also report effects sizes estimated by comparing change scores in the second 12 weeks (24 minus 12 weeks) between those in TC who received additional maintenance classes and those who did not. We fit linear regression models using generalised estimating equations methods to account for the longitudinal repeated measures. We used contrasts from these models to estimate the difference in mean changes in outcomes between groups between baseline and 12 weeks and between baseline and 24 weeks, with models adjusted for baseline values that were imbalanced at baseline: Charlson Comorbidity Index, CES-D score and BODE index. Treatment group assignment, time point and their interaction were incorporated into each model as categorical variables. Analysis of covariance using change score between 12 weeks and 24 weeks as the outcome was used to estimate the difference in mean changes between the TC subgroups. All analyses were performed using SAS statistical software, V.9.4 (SAS Institute). According to the CONSORT figure, analyses were conducted in the subjects with complete data at each of the 12 and 24-week time points. We did not impute missing data.
Patient and public involvement
Development of the research question and outcome measures, as well as assessment of the burden of intervention were informed by patients’ priorities, experience and preferences as expressed in our prior studies of TC in chronic cardiopulmonary disease, including an initial pilot in patients with COPD.55 Patients were not directly involved in the design or conduct of this study. Results are disseminated to patients and the public through scientific publication.
Results
Recruitment
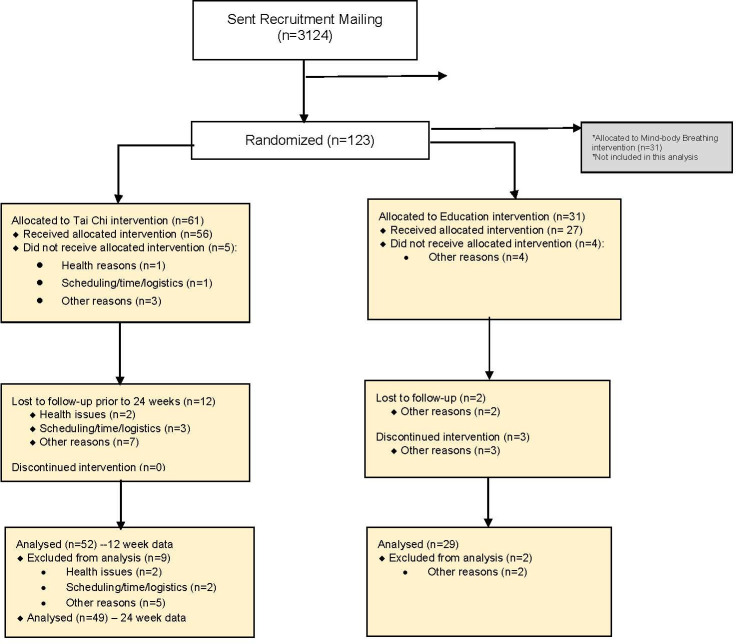
A total of 92 subjects were randomised (N=61 TC, n=31 education control). Figure 1 shows the CONSORT diagram focused on these two primary arms within the overall randomisation scheme of 123 subjects (N=31 in a third exploratory mind-body breathing arm not included in this analysis). Of the 2828 participants contacted, 4.4% were willing to participate. The rate of recruitment was on average three patients per month. The main reasons for refusal to participate included transportation/distance (25%), lack of interest (16%), too busy/no time (14%) and health issues (10%).
Figure 1.
CONSORT flow diagram. CONSORT, Consolidated Standards of Reporting Trials.
Study population
Table 1 details the baseline characteristics. The mean±SD age was 68 years±8.5, 66% were male, mean FEV1% predicted 57.3±13.8, 28.3% were GOLD stage 3–4. Mean pack years was 55±35; 16% were on supplemental O2. A large proportion of subjects reported significant limitations and comorbidities—23% with coronary disease or heart failure, 21% with cancer, 37% with chronic musculoskeletal issues or back pain and 21% with significant limitation of an extremity (missing limb, paralysed, weakness). Most characteristics were sufficiently balanced between groups, however, subjects in TC at baseline were more likely to have cardiovascular disease, score higher on the depression scale and have a less favourable COPD prognostic index (higher BODE).
Table 1.
Baseline sociodemographic and clinical characteristics
| Characteristic* | Tai chi (N=61) | Education (N=31) |
| Age (mean, SD) | 68.6 (9.2) | 68.1 (6.7) |
| Male sex | 43 (71) | 18 (58) |
| Race | ||
| White | 48 (79) | 26 (84) |
| Black | 7 (12) | 4 (13) |
| Other | 6 (10) | 1 (3) |
| Annual income <US$35K | 37 (61) | 15 (48) |
| Married/living with partner | 20 (33) | 14 (45) |
| Unemployed/retired/disabled | 56 (92) | 27 (87) |
| FEV1 % predicted NHANES (mean±SD) | 57.8 (14.3) | 56.4 (13.0) |
| Charlson Comorbidity Index (mean±SD) | 6.8 (2.5) | 6.4 (2.4) |
| GOLD stage (mean, SD) | 2.3 (0.6) | 2.4 (0.6) |
| Stage I–II | 45 (74) | 21 (68) |
| Stage III–IV | 16 (26) | 10 (32) |
| BODE index (mean, SD) | 2.8 (1.8) | 2.4 (1.1) |
| 0–2 | 27 (44) | 20 (65) |
| 3–4 | 23 (38) | 11 (36) |
| 5 or greater | 11 (18.0) | 0 (0) |
| Regular, daily oxygen use | 11 (18) | 4 (13) |
| Smoking pack-years (mean, SD) | 57.6 (38.2) | 50.4 (30.0) |
| Completed pulmonary rehabilitation | 12 (20) | 11 (36) |
| Comorbidities | ||
| CVD (CAD, angina, angioplasty, MI) | 23 (38) | 7 (23) |
| Heart failure | 7 (12) | 2 (7) |
| Cancer | 14 (23) | 5 (16) |
| Hypertension | 42 (69) | 18 (58) |
| Limitation of limb | 14 (23) | 5 (16) |
| OA, sciatica, chronic back pain | 31 (51) | 16 (52) |
| Peripheral vascular disease | 4 (7) | 0 (0) |
| Stroke or cerebrovascular disease | 7 (12) | 2 (7) |
| Connective tissue disease | 9 (15) | 3 (10) |
| Obstructive sleep apnea | 14 (23) | 6 (20) |
| CES-D (mean±SD) | 14.9 (11.7) | 8.7 (7.8) |
*N (%) unless otherwise noted.
BODE, Body Mass Index, Obstruction, Dyspnoea and Exercise; CAD, coronary artery disease; CES-D, Centre for Epidemiologic Studies Depression; CVD, cardiovascular disease; FEV, forced expiratory volume; GOLD, Global Obstructive Lung Disease; MI, myocardial infarction; NHANES, National Health and Nutrition Examination Survey; OA, osteoarthritis.;
Adherence
The overall rate of group adherence (class attendance) was 65% (62% in TC, 68% in education). The majority (59% in TC, 74% in education) attended ≥70% of classes. Table 2 provides details of frequency and duration of TC practice both in class and home practice among subjects with available practice data (82% of total; N=50/61 in TC). In the first 12 weeks, TC participants logged 87 (±35) min/week of class time, and 94 (±76) min/week of home practice. During the second 12 weeks, those randomised to continue weekly TC reported 88 (±66) min/week of class plus home practice, while those without maintenance classes logged 69 (±59) min/week of home practice.
Table 2.
Tai chi intervention adherence
| Phase 1 (baseline to 12 weeks) |
Phase 2 maintenance (12–24 weeks) |
||
| Mean (SD) | |||
| Tai chi class 2x/week (n=50) | Tai chi class 1 x/week (n=25) | No additional classes (n=25) | |
| Classes attended (N) | 17.3 (6.9) | 7.1 (4.6) | – |
| Class time (min/week) | 86.7 (34.7) | 35.6 (22.8) | – |
| Home practice time (min/week) | 94.4 (75.8) | 52.1 (50.4) | 68.8 (58.6) |
| Home practice frequency (sessions/week) | 2.9 (1.6) | 1.8 (1.5) | 0.6 (0.4) |
| Total minutes of tai chi (min/week) | 181.1 (98.3) | 87.7 (66.1) | 68.8 (58.6) |
In total, 78 subjects (85%) completed the 24-week protocol and 6-month outcome testing; 15% (14/92) were lost to follow-up (12 in TC, 2 in education). Outcome data were available at 12 weeks in N=81 and at 24 weeks in N=78 subjects.
Safety
A total of 19 reportable adverse events occurred during the 12-week intervention period (16 in TC, 3 in education). These included 9 COPD exacerbations (seven in TC, two in education); 15/19 (79%) events were deemed unrelated to the study. Of the four that were possibly related, all were musculoskeletal flares in patients with prior histories of chronic pain and/or osteoarthritis (two back pain, one chest muscle pain, one knee pain). In the second 12 weeks, there were no events in the TC group deemed related to the study. Outside of reportable adverse events, we documented 280 individual mentions of various symptom occurrences over the course of the study, reported by 55/61 (90%) subjects in TC and 29/31 (94%) in education. Table 3 reports the common symptoms that did not meet criteria for a reportable adverse event among the patients in each group, including muscle soreness, dizziness/fainting, shortness of breath, fatigue, falls, palpitations and psychological stress.
Table 3.
Common symptoms (not meeting criteria for reportable adverse event)
| Symptom | N (%) Tai chi (N=61) |
N (%) education (N=31) |
| Muscle soreness | 24 (39) | 14 (45) |
| Dizziness/fainting | 17 (28) | 8 (26) |
| Shortness of breath | 28 (46) | 10 (32) |
| Fatigue | 17 (28) | 8 (26) |
| Falls | 5 (8) | 3 (10) |
| Heart palpitations | 7 (11) | 7 (23) |
| Psychological stress | 15 (25) | 6 (19) |
Preliminary effect estimates
Table 4 presents the effect of TC and education on HRQL, cognitive-emotional measures and functional status, including (1) unadjusted mean changes (SD) within the TC and education groups, (2) between-group ES/standardised differences (Cohen’s d) and (3) between-group mean differences (95% CI) adjusted for CES-D, BODE and Charlson Comorbidity Score. Similar models that additionally adjusted for age and gender (not shown) did not significantly change results.
Table 4.
Within-group and between-group changes in quality of life, cognitive-emotional measures and functional status
| Baseline to 12 weeks | Baseline to 24 weeks | |||||||
| Outcome measure | Tai chi (N=52) Mean change (SD) |
Education (N=29) Mean change (SD) |
Between-group effect size (95% CI) | Adjusted between- group Mean difference (95% CI)* | Tai chi (N=49) Mean change (SD) |
Education (N=29) Mean change (SD) |
Between- group effect size (95% CI) |
Adjusted between- group mean difference (95% CI)* |
| Health-related quality of life and symptoms | ||||||||
| CRQ dyspnoea* | 0.29 (1.31) |
0 (0.83) |
0.26 (−0.20 to 0.71) |
0.29 (−0.16 to 0.75) |
0.23 (1.31) |
−0.25 (1.05) |
0.41 (−0.06 to 0.87) |
0.46 (−0.05 to 0.97) |
| CRQ emotion* | 0.25 (0.75) |
−0.18 (0.84) |
0.54 (0.08 to 1.00) |
0.49 (0.13 to 0.85) |
0.09 (1) |
0.16 (0.79) |
−0.08 (−0.54 to 0.38) |
−0.01 (−0.4 to 0.38) |
| CRQ fatigue* | 0.2 (1.23) |
0.15 (0.74) |
0.05 (−0.40 to 0.51) |
0.08 (−0.34 to 0.50) |
−0.06 (1.04) |
0.03 (0.9) |
−0.09 (−0.55 to 0.37) |
−0.05 (−0.47 to 0.38) |
| CRQ mastery* | 0.27 (0.94) |
0.02 (0.97) |
0.27 (−0.19 to 0.72) |
0.26 (−0.16 to 0.69) |
0.41 (0.89) |
0.25 (0.91) |
0.18 (−0.28 to 0.64) |
0.15 (−0.25 to 0.55) |
| CRQ total* | 0.25 (0.74) |
−0.03 (0.47) |
0.46 (0 to 0.92) |
0.31 (0.05 to 0.57) |
0.16 (0.77) |
0.05 (0.57) |
0.16 (−0.30 to 0.62) |
0.13 (−0.17 to 0.42) |
| UCSD shortness of breath | −1.7 (11.73) |
0.85 (7.23) |
−0.26 (−0.72 to 0.19) |
−2.58 (−6.63 to 1.47) |
−0.09 (14.81) |
3.06 (9.95) |
−0.25 (−0.71 to 0.21) |
−3.28 (−8.66 to 2.11) |
| PROMIS fatigue* | −0.13 (0.4) |
0.02 (0.5) |
−0.34 (−0.80 to 0.12) |
−0.17 (−0.38 to 0.03) |
−0.04 (0.67) |
−0.01 (0.51) |
−0.05 (−0.51 to 0.41) |
−0.05 (−0.30 to 0.21) |
| Cognitive-emotional measures | ||||||||
| COPD self-efficacy | 0.13 (0.58) |
0.15 (0.43) |
−0.04 (−0.49 to 0.41) |
0 (−0.22 to 0.22) |
0.16 (0.62) |
0.13 (0.53) | 0.06 (−0.40 to 0.52) |
0.07 (−0.19 to 0.33) |
| CES-D score* | −2.57 (7.73) |
−0.07 (5.78) |
−0.37 (−0.82 to 0.09) |
−2.39 (−5.28 to 0.49) |
−1.41 (9.44) |
0.21 (8.17) |
−0.18 (−0.64 to 0.28) |
−1.42 (−5.28 to 2.43) |
| MSPSS* | 0.14 (1) |
−0.03 (0.63) |
0.20 (−0.26 to 0.65) |
0.13 (−0.21 to 0.48) |
0.27 (1.28) |
−0.04 (0.61) |
0.30 (−0.16 to 0.76) |
0.26 (−0.15 to 0.66) |
| Functional status | ||||||||
| 6 min walk test | 35.3 (118.6) |
30.5 (121.6) |
0.04 (−0.41 to 0.49) |
1.4 (−52.9 to 55.7) |
12.8 (138.3) |
−12.3 (158.1) |
0.17 (−0.30 to 0.64) |
27.7 (−40.7 to 96.0) |
| Chair stand | 0.63 (1.74) |
1.07 (2.31) |
−0.21 (−0.67 to 0.24) |
−0.43 (−1.39 to 0.53) |
0.57 (2.25) |
0.9 (1.99) |
−0.16 (−0.62 to 0.31) |
−0.30 (−1.25 to 0.66) |
| Chair sit and reach | 0.28 (3.18) |
−0.43 (2.91) |
0.23 (−0.22 to 0.69) |
0.67 (−0.68 to 2.03) |
0.36 (3.08) |
−0.29 (2.62) |
0.23 (−0.24 to 0.69) |
0.67 (−0.61 to 1.96) |
| Calories-Mod Intensity Exercise† | −216 (2311) |
−177 (1765) |
−0.02 (−0.47 to 0.44) |
−36 (−905 to 834) |
−356 (1976) |
591 (2798) |
−0.39 (−0.86 to 0.07) |
−874 (−2003 to 254) |
| Freq-Mod Intensity Exercise | −1.26 (6.52) |
−0.38 (5.92) |
−0.14 (−0.59 to 0.31) |
−0.92 (−3.56 to 1.72) |
1.93 (18.89) |
−0.74 (7.24) |
0.19 (−0.27 to 0.64) |
2.81 (−2.85 to 8.47) |
*Adjusted for 12 weeks values, and baseline CESD, BODE (Body Mass Index, Obstruction, Dyspnoea, Exercise) and Charlson Comorbidity Score.
†Kcal/week.
CES-D, Centre for Epidemiologic Studies Depression; COPD, chronic obstructive pulmonary disease; CRQ, Chronic Respiratory Questionnaire; MSPSS, Multidimensional Scale of Perceived Social Support; PROMIS, Patient-Reported Outcomes Measurement Information System; UCSD, University of California San Diego.
From baseline to 12 weeks, there was a moderate size between-group improvement in CRQ-total score (ES=0.46; adj mean diff 0.31) and CRQ emotion subscale (ES=0.54; adj mean diff 0.49), and a small-moderate size improvement in CES-D depression (ES=−0.37; adj mean diff −2.39) and PROMIS-fatigue score (ES=−0.34; adj mean diff −0.17) favouring TC. Likewise, smaller, yet positive, between-group changes were seen in UCSD SOB score (ES=−0.26; adj mean diff −2.58), MSPSS (ES=0.2; adj mean diff 0.13) and the chair sit and reach (ES=0.23; adj mean diff 0.67) at 12 weeks which were sustained at 24 weeks.
Additionally, from baseline to 24 weeks, there was a small-moderate size improvement with TC in CRQ-dyspnoea (ES=0.41; adj mean diff=0.46). Changes in CRQ-emotion at 12 weeks and CRQ-dyspnoea at 24 weeks were clinically significant. There was also an apparent small-size effect on caloric expenditure in moderate to intensity activities (not including TC) favouring education (ES=−0.39; adj mean diff −874.5).
Table 5 presents within-group change and between-group differences and ES in the two TC groups (continued TC vs no further TC) in the second 12 weeks in an exploration of TC dose/duration. Favouring those who received additional ‘maintenance’ classes compared with those who completed classes at 12 weeks, there was a moderate-large improvement in COPD self-efficacy (ES=−0.69; adj mean diff −0.40), 6MWT distance (ES=0.56; adj mean diff 49.26 feet), fatigue (ES=−0.41; adj mean diff −0.28), and chair stand (0.43; adj mean diff 0.56). There was an increase in frequency of moderate to intensity exercise as assessed by the CHAMPS questionnaire (ES=−0.38; adj mean diff −8.62) and in perceived social support (ES=−0.52; adj mean diff −0.51) in those TC participants who did not receive additional classes.
Table 5.
Within-group and between-group changes in quality of life, cognitive-emotional measures and functional status in the tai chi subgroup from 12 to 24 weeks
| Outcome measure | Phase 2 maintenance (12–24 weeks) | |||
| Weekly tai chi class (n=23) Mean change (SD) |
No additional tai chi class (n=25) Mean change (SD) |
Between-group effect size (95% CI) | Adjusted between-group difference at week 24 (95% CI)* | |
| Health-related quality of life and symptoms | ||||
| *CRQ dyspnoea | −0.05 (1.28) |
−0.24 (1.33) |
0.15 (−0.42 to 0.71) |
0.28 (−0.48 to 1.05) |
| *CRQ emotion | −0.13 (0.85) |
−0.17 (1.03) |
0.04 (−0.52 to 0.61) |
0.05 (−0.49 to 0.58) |
| *CRQ fatigue | −0.12 (1.48) |
−0.4 (1.17) |
0.21 (−0.36 to 0.78) |
0.22 (−0.56 to 0.99) |
| *CRQ mastery | 0.02 (0.49) |
0.16 (0.9) |
−0.19 (−0.76 to 0.38) |
−0.13 (−0.55 to 0.30) |
| *CRQ total | −0.08 (0.74) |
−0.17 (0.94) |
0.11 (−0.46 to 0.67) |
0.11 (−0.39 to 0.60) |
| UCSD shortness of breath | 0.74 (9.61) |
2.72 (11.94) |
−0.19 (−0.76 to 0.38) |
−2.18 (−8.61 to 4.25) |
| *PROMIS fatigue | −0.06 (0.75) |
0.24 (0.71) |
−0.41 (−0.98 to 0.16) |
−0.28 (−0.71 to 0.16) |
| Cognitive-emotional measures | ||||
| *COPD self-efficacy | −0.13 (0.48) |
0.27 (0.68) |
−0.69 (−1.28 to to 0.11) |
−0.40 (−0.75 to to 0.06) |
| *CES-D score | 1.91 (7.38) |
0.47 (9.69) |
0.17 (−0.40 to 0.73) |
1.22 (−3.64 to 6.08) |
| *MSPSS | −0.19 (1.24) |
0.37 (0.86) |
−0.52 (−1.10 to 0.05) |
−0.51 (−1.15 to 0.12) |
| Functional status | ||||
| 6 min walk test | 0.3 (104.2) |
−50.6 (77.5) |
0.56 (−0.05 to 1.16) |
49.3 (−9.9 to 108.5) |
| Chair stand | 0.18 (1.26) |
−0.52 (1.97) |
0.42 (−0.17 to 1.02) |
0.56 (−0.48 to 1.60) |
| Chair sit and reach | −0.32 (3.14) |
0.57 (2.71) |
−0.30 (−0.89 to 0.29) |
−0.92 (−2.79 to 0.96) |
| Calories-mod intensity exercise† | −105 (2102) |
−39 (2309) |
−0.03 (−0.60 to 0.54) |
−202 (−1509 to 1106) |
| Freq-mod intensity exercise | −0.63 (4.74) |
7.34 (27.99) |
−0.38 (−0.95 to 0.20) |
−8.62 (−19.72 to 2.48) |
*Adjusted for 12 weeks values, and baseline CESD, BODE (Body Mass Index, Obstruction, Dyspnoea, Exercise) and Charlson Comorbidity Score.
†Kcal/week.
CES-D, Centre for Epidemiologic Studies Depression; COPD, chronic obstructive pulmonary disease; CRQ, Chronic Respiratory Questionnaire; MSPSS, Multidimensional Scale of Perceived Social Support; PROMIS, Patient-Reported Outcomes Measurement Information System; UCSD, University of California San Diego.
From baseline to 24 weeks, in those who received additional maintenance TC classes compared with those in the education control, there were large improvements in CRQ total (ES=0.57; adj mean diff 0.32 (95% CI 0.02 to 0.61)) as well as in the dyspnoea subscale (ES=0.59; adj mean diff 0.70 (95% CI 0.08 to 1.32)).
Discussion
A 12–24 weeks TC programme is feasible and safe in patients with moderate to severe COPD with multiple comorbidities. Despite suboptimal recruitment rates, our study demonstrated reasonable retention and adherence. Similar to prior exercise studies in COPD, patients reported time conflicts, travel issues and medical comorbidities as commonly encountered barriers to enrolment. Per our CONSORT diagram, our two largest reasons for non-enrolment were ‘passive decline’ and ‘transportation/distance’. We believe these reflect inefficiencies in our recruitment process (eg, mass mailings based on a hospital database), as well as our large catchment area as a tertiary hospital which may have amplified transportation barriers for twice weekly in-person classes. Once enrolled, medically complex and deconditioned patients with COPD are well recognised as a difficult group to engage. Overall, rates of participation in our study were similar or better than other clinical behavioural trials in this population.58 59 Attendance at TC classes was better than general rates of adherence to conventional pulmonary rehabilitation, which is often underused with rates of attrition up to 60%.58–61 Like prior literature, COPD exacerbations and health-related issues were common barriers to engagement.62 We also demonstrated adequate adherence to our education class, suggesting perceived value in this interaction. Further studies will need expanded, targeted recruitment efforts and additional make-up classes to further optimise adherence. Rates of TC home practice, on the other hand, were excellent, with mean practice meeting our recommended 30 min three times per week. There was variability across individuals, but some reported considerably more home practice (up to 165 min per week).
Through rigorous systematic symptom tracking, we were able to capture a comprehensive profile of adverse events. The similar prevalence of common complaints in both groups likely reflect expected symptomatology in a chronic, comorbid pulmonary population. Only a small percentage of symptoms resulted in reportable adverse events, with half of events being COPD exacerbations. There were no significant study-related adverse events supporting the safety of TC in this population.
Preliminary effect estimates should be interpreted with caution, but offer information on (1) outcomes that may be sensitive to change with TC compared with education and (2) over what time period. These estimates inform design and choice of measures for a future, fully powered trial in COPD in the USA. Overall, data suggest that psychological/emotional outcomes may be sensitive to change with TC practice early on, while certain functional/physical effects may be detected after several months. In particular, at 12 weeks, we report trends of modest improvements in depression, HRQL (particularly the emotion subscale) and fatigue. Changes seen in CRQ-emotion in the first 12 weeks were clinically meaningful differences. In those who continue TC classes until 24 weeks, maintenance of exercise capacity (6MWT) and changes in lower body strength/endurance (chair stand) may become more evident, and this may be coupled with increasing COPD self-efficacy.63 Baseline indices of COPD severity and functional status, depressive symptoms, and degree of comorbidity will be important factors to consider in future analyses.
Our results support prior literature, largely from China,23 26 reporting improvements in HRQL and 6MWT distance in studies comparing TC with usual care, although our ES are notably smaller. This disparity might be due to multiple factors, including differences in study population, intervention style and implementation, dose (eg, frequency of classes) and medical environment (eg, degree of optimised medical management). Our study included a more ethnically diverse Western population and used a relatively low-intensity TC protocol with more practical, yet less frequent instruction than typically practiced in China. Participants in our study were all receiving standard medical therapy, in contrast to the investigation by Polkey et al21 where participants were bronchodilator-naive. We also used an attention-matched education control, which may have further diminished differences.
In the context of the biopsychosocial model,64 65 our data suggest that in a COPD population with multiple comorbidities, emotional health, HRQL and self-efficacy may be important areas of future focus. The role of psychoemotional factors in COPD disease management and healthcare engagement is well recognised.65 66 and there is increasing interest in behavioural medicine approaches.67 For example, psychological distress, including depression and anxiety is prevalent in COPD and increases risk of exacerbation. Cognitive behavioural pathways linking psychological symptoms to low self-efficacy, poor coping and poor self-care behaviours are postulated to play a role.68 Models of physical activity promotion in COPD call for addressing both health related (eg, fatigue), as well as psychological and attitudinal barriers (eg, low self-efficacy, locus-of-control, emotion regulation, motivation).69 Indeed, more favourable COPD self-efficacy in both physical and affective subdomains have been associated with better HRQL and exercise capacity.70Self-regulation through mind-body therapies may be a mediator for physical and psychological improvements and catalysing positive behavioural change, like smoking cessation or physical activity, in other populations.71 72 A multimodal exercise, like TC, that incorporates mind-body awareness, stress management, as well as physical activity may target many biopsychosocial processes in COPD beyond physical exercise alone.73
In this preliminary investigation, we also explored dosing with a 3-month TC maintenance class. Data show decreased home practice in the second 12 weeks, even in those in maintenance classes. This may reflect natural history which is well described in exercise studies60 or might relate to the cessation of classes or decrease in class frequency. Classes for those randomised to continue were offered only once weekly, and attendance decreased substantially. Nonetheless, possible emerging differences between the TC subgroups suggest that some amount of continued support after 12 weeks might be beneficial, although overall differences between TC and education after 24 weeks were small. In a relatively sick population, a more gradual cultivation of self-efficacy, with small successes that motivate towards continued positive self-care behaviours may contribute to physical benefits over a longer period of time.74
We acknowledge that our education control may have been active. There was an apparent decrease in caloric expenditure in moderate to intensity activities in TC and increase in education at 24 weeks, although CIs are wide. One caveat is that these data did not account for TC (classes or practice) and thus caloric expenditure may be underestimated. Provision of relevant health education with group discussion could have affected motivation towards physical activity. Additionally, education classes were taught by the same TC teachers. Together with an in-person peer support group, these classes offered a parallel, socially supportive environment. We did not, however, detect an improvement in social support in the education group with the MPSS instrument we used.
To date, there is no consensus regarding optimal forms of exercise (eg, type, intensity, frequency, duration) for patients with COPD.75 Older adults spend a major proportion of time in activities of lesser intensity. Recent trends have recognised that these activities may still confer benefits of physical exercise.76 77 Low to moderate intensity exercises like TC may be further studied as viable options. While preliminary effects are modest, TC’s potential to impact multiple neurocognitive and psychoemotional processes, decrease psychological distress and support self-efficacy towards positive self-care behaviours and physical activity may fit within a contemporary integrated care to decrease morbidity in deconditioned patients with COPD.
The main limitation of this study was the small sample. Our primary purpose was to assess feasibility. Thus, the study was likely underpowered to detect significant differences in multiple outcomes. Sample size was informed by a small pilot55 where estimates may be unstable and CIs wide. Nonetheless, this study provides rigorous preliminary data in an outpatient COPD population at tertiary centres in the USA regarding safety and feasibility and informs the design and conduct of a larger, more definitive trial.
Footnotes
Contributors: GY: conceptualisation/design, analysis and interpretation of data, implementation, manuscript writing. DL: implementation, acquisition of data. PW: design, analysis and interpretation of data, manuscript writing and review. DB and EK: interpretation of data, implementation, manuscript review. HRN and AP: analysis and interpretation of data. RD: analysis and interpretation of data, manuscript review. MM: design, analysis and interpretation of data, implementation, manuscript writing and review.
Funding: This study was supported by an award from the National Center for Complementary and Integrative Health (NCCIH) at the National Institutes of Health (NIH) (R01AT005436). GY was supported by K24AT009465. PW was supported by K24AT009282.
Disclaimer: Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCCIH or the NIH.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication: Not required.
Ethics approval: All study procedures followed HIPAA guidelines and were approved by each institution’s human subjects review board.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available on reasonable request.
References
- 1.Soriano JB, Rodríguez-Roisin R. Chronic obstructive pulmonary disease overview: epidemiology, risk factors, and clinical presentation. Proc Am Thorac Soc 2011;8:363–7. 10.1513/pats.201102-017RM [DOI] [PubMed] [Google Scholar]
- 2.Mannino DM, Braman S. The epidemiology and economics of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2007;4:502–6. 10.1513/pats.200701-001FM [DOI] [PubMed] [Google Scholar]
- 3.Moy ML, Teylan M, Weston NA, et al. Daily step count predicts acute exacerbations in a US cohort with COPD. PLoS One 2013;8:e60400. 10.1371/journal.pone.0060400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen HQ, Chu L, Amy Liu I-L, et al. Associations between physical activity and 30-day readmission risk in chronic obstructive pulmonary disease. Ann Am Thorac Soc 2014;11:695–705. 10.1513/AnnalsATS.201401-017OC [DOI] [PubMed] [Google Scholar]
- 5.Moy ML, Teylan M, Danilack VA, et al. An index of daily step count and systemic inflammation predicts clinical outcomes in chronic obstructive pulmonary disease. Ann Am Thorac Soc 2014;11:149–57. 10.1513/AnnalsATS.201307-243OC [DOI] [PubMed] [Google Scholar]
- 6.Wan ES, Kantorowski A, Polak M, et al. Long-Term effects of web-based pedometer-mediated intervention on COPD exacerbations. Respir Med 2020;162:105878. 10.1016/j.rmed.2020.105878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moy ML, Gould MK, Liu I-LA, et al. Physical activity assessed in routine care predicts mortality after a COPD hospitalisation. ERJ Open Res 2016;2. 10.1183/23120541.00062-2015. [Epub ahead of print: 17 03 2016]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waschki B, Kirsten A, Holz O, et al. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort study. Chest 2011;140:331–42. 10.1378/chest.10-2521 [DOI] [PubMed] [Google Scholar]
- 9.Yohannes AM, Alexopoulos GS. Depression and anxiety in patients with COPD. Eur Respir Rev 2014;23:345–9. 10.1183/09059180.00007813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panagioti M, Scott C, Blakemore A, et al. Overview of the prevalence, impact, and management of depression and anxiety in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2014;9:1289–306. 10.2147/COPD.S72073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng M. Master Cheng’s thirteen chapters on tʼai-chi chʻüan = [Zhengzi tai ji quan shi san pianp]. Brooklyn, NY: Sweet Chʼi Press, 1983. [Google Scholar]
- 12.Helm B. Gateways to health: Taijiquan and traditional Chinese medicine. Taijiquan J 2002:8–12. [Google Scholar]
- 13.Liu T, Chan AW, Liu YH, et al. Effects of Tai Chi-based cardiac rehabilitation on aerobic endurance, psychosocial well-being, and cardiovascular risk reduction among patients with coronary heart disease: a systematic review and meta-analysis. Eur J Cardiovasc Nurs 2018;17:368–83. 10.1177/1474515117749592 [DOI] [PubMed] [Google Scholar]
- 14.Chen Y-W, Hunt MA, Campbell KL, et al. The effect of Tai Chi on four chronic conditions-cancer, osteoarthritis, heart failure and chronic obstructive pulmonary disease: a systematic review and meta-analyses. Br J Sports Med 2016;50:397–407. 10.1136/bjsports-2014-094388 [DOI] [PubMed] [Google Scholar]
- 15.Kong LJ, Lauche R, Klose P, et al. Tai Chi for chronic pain conditions: a systematic review and meta-analysis of randomized controlled trials. Sci Rep 2016;6:25325. 10.1038/srep25325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Easwaran K, Gopalasingam Y, Green DD, et al. Effectiveness of Tai Chi for health promotion for adults with health conditions: a scoping review of meta-analyses. Disabil Rehabil 2020:1–12. 10.1080/09638288.2020.1725916 [DOI] [PubMed] [Google Scholar]
- 17.Wang C, Collet JP, Lau J. The effect of Tai Chi on health outcomes in patients with chronic conditions: a systematic review. Arch Intern Med 2004;164:493–501. 10.1001/archinte.164.5.493 [DOI] [PubMed] [Google Scholar]
- 18.Wayne P, Fuerst M. The Harvard medical school guide to tai chi: 12 weeks to a healthy body, strong heart, and sharp mind. 1st ed Boston: Shambhala, 2013: 336 p. [Google Scholar]
- 19.Yeh GY, Wayne PM, Litrownik D, et al. Tai chi Mind-body exercise in patients with COPD: study protocol for a randomized controlled trial. Trials 2014;15:337. 10.1186/1745-6215-15-337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wayne PM, Kaptchuk TJ. Challenges inherent to t'ai chi research: part I--t'ai chi as a complex multicomponent intervention. J Altern Complement Med 2008;14:95–102. 10.1089/acm.2007.7170A [DOI] [PubMed] [Google Scholar]
- 21.Polkey MI, Qiu Z-H, Zhou L, et al. Tai Chi and pulmonary rehabilitation compared for treatment-naive patients with COPD: a randomized controlled trial. Chest 2018;153:1116–24. 10.1016/j.chest.2018.01.053 [DOI] [PubMed] [Google Scholar]
- 22.Wu W, Liu X, Wang L, et al. Effects of tai chi on exercise capacity and health-related quality of life in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis 2014;9:1253–63. 10.2147/COPD.S70862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ngai SP, Jones AY, WWS T. Tai chi for chronic obstructive pulmonary disease (COPD). Cochrane airways group, editor. Cochrane Database Syst Rev 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu L-L, Lin Z-K, Weng H-D, et al. Effectiveness of meditative movement on COPD: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis 2018;13:1239–50. 10.2147/COPD.S159042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan J-H, Guo Y-Z, Yao H-M, et al. Effects of tai chi in patients with chronic obstructive pulmonary disease: preliminary evidence. PLoS One 2013;8:e61806. 10.1371/journal.pone.0061806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ding M, Zhang W, Li K, et al. Effectiveness of T’aiChi and qigong on chronic obstructive pulmonary disease: a systematic review and meta-analysis. J Altern Complement Med 2014;20:79–86. 10.1089/acm.2013.0087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gendron LM, Nyberg A, Saey D, et al. Active Mind-body movement therapies as an adjunct to or in comparison with pulmonary rehabilitation for people with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2018;10:CD012290. 10.1002/14651858.CD012290.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salmoirago-Blotcher E, Wayne PM, Dunsiger S, et al. Tai chi is a promising exercise option for patients with coronary heart disease declining cardiac rehabilitation. J Am Heart Assoc 2017;6. 10.1161/JAHA.117.006603. [Epub ahead of print: 11 Oct 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee AC, Harvey WF, Price LL, et al. Dose-Response effects of tai chi and physical therapy exercise interventions in symptomatic knee osteoarthritis. PM&R 2018;10:712–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eldridge SM, Chan CL, Campbell MJ, et al. Consort 2010 statement: extension to randomised pilot and feasibility trials. BMJ 2016:i5239 10.1136/bmj.i5239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of physicians, American College of chest physicians, American thoracic Society, and European respiratory Society. Ann Intern Med 2011;155:179. 10.7326/0003-4819-155-3-201108020-00008 [DOI] [PubMed] [Google Scholar]
- 32.LWWCOPD Living well with COPD, 2020. Available: https://www.livingwellwithcopd.com/en/about.html
- 33.American Thoracic Society and European Respiratory Society Standards for diagnosis and management of patients with COPD. Available: https://www.thoracic.org/professionals/clinical-resources/
- 34.Global Initiative for Chronic Obstructive Lung Disease What you can do about a lung disease called COPD. Available: http://www.goldcopd.org
- 35.American Thoracic Society Patient Information Series & Patient Health Series. Available: https://www.thoracic.org/patients/patient-resources/
- 36.COPD Foundation What is COPD. Available: https://www.copdfoundation.org/What-is-COPD/Understanding-COPD/What-is-COPD.aspx
- 37.Patient Education Resources [Internet]. CHEST American College of CHEST Physician. Available from. Available: http://www.chestnet.org/Publications/Other-Publications/Patient-Education-Guides
- 38.Schünemann HJ, Puhan M, Goldstein R, et al. Measurement properties and interpretability of the chronic respiratory disease questionnaire (CRQ). COPD 2005;2:81–9. 10.1081/COPD-200050651 [DOI] [PubMed] [Google Scholar]
- 39.Verrill D, Barton C, Beasley W, et al. The effects of short-term and long-term pulmonary rehabilitation on functional capacity, perceived dyspnea, and quality of life. Chest 2005;128:673–83. 10.1378/chest.128.2.673 [DOI] [PubMed] [Google Scholar]
- 40.Jones PW, Beeh KM, Chapman KR, et al. Minimal clinically important differences in pharmacological trials. Am J Respir Crit Care Med 2014;189:250–5. 10.1164/rccm.201310-1863PP [DOI] [PubMed] [Google Scholar]
- 41.Wigal JK, Creer TL, Kotses H. The COPD self-efficacy scale. Chest 1991;99:1193–6. 10.1378/chest.99.5.1193 [DOI] [PubMed] [Google Scholar]
- 42.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401. [Google Scholar]
- 43.van Manen JG. Risk of depression in patients with chronic obstructive pulmonary disease and its determinants. Thorax 2002;57:412–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Himmelfarb S, Murrell SA. Reliability and validity of five mental health scales in older persons. J Gerontol 1983;38:333–9. 10.1093/geronj/38.3.333 [DOI] [PubMed] [Google Scholar]
- 45.Zimet GD, Powell SS, Farley GK, et al. Psychometric characteristics of the multidimensional scale of perceived social support. J Pers Assess 1990;55:610–7. [DOI] [PubMed] [Google Scholar]
- 46.Guyatt GH, Sullivan MJ, Thompson PJ, et al. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J 1985;132:919–23. [PMC free article] [PubMed] [Google Scholar]
- 47.Gerardi DA, Lovett L, Benoit-Connors ML, et al. Variables related to increased mortality following out-patient pulmonary rehabilitation. Eur Respir J 1996;9:431–5. 10.1183/09031936.96.09030431 [DOI] [PubMed] [Google Scholar]
- 48.Polkey MI, Spruit MA, Edwards LD, et al. Six-minute-walk test in chronic obstructive pulmonary disease: minimal clinically important difference for death or hospitalization. Am J Respir Crit Care Med 2013;187:382–6. 10.1164/rccm.201209-1596OC [DOI] [PubMed] [Google Scholar]
- 49.Jones CJ, Rikli RE, Beam WC. A 30-S chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exerc Sport 1999;70:113–9. 10.1080/02701367.1999.10608028 [DOI] [PubMed] [Google Scholar]
- 50.Jones CJ, Rikli RE, Max J, et al. The reliability and validity of a chair Sit-and-Reach test as a measure of hamstring flexibility in older adults. Res Q Exerc Sport 1998;69:338–43. 10.1080/02701367.1998.10607708 [DOI] [PubMed] [Google Scholar]
- 51.Rose M, Bjorner JB, Becker J, et al. Evaluation of a preliminary physical function item bank supported the expected advantages of the patient-reported outcomes measurement information system (PROMIS). J Clin Epidemiol 2008;61:17–33. 10.1016/j.jclinepi.2006.06.025 [DOI] [PubMed] [Google Scholar]
- 52.Stewart AL, Mills KM, King AC, et al. Champs physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc 2001;33:1126–41. 10.1097/00005768-200107000-00010 [DOI] [PubMed] [Google Scholar]
- 53.Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004;350:1005–12. 10.1056/NEJMoa021322 [DOI] [PubMed] [Google Scholar]
- 54.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 55.Yeh GY, Roberts DH, Wayne PM, et al. Tai chi exercise for patients with chronic obstructive pulmonary disease: a pilot study. Respir Care 2010;55:1475–82. [PMC free article] [PubMed] [Google Scholar]
- 56.Hedges LV, Olkin I. Statistical methods for meta-analysis. Orlando: Academic Press, 1985: 369 p. [Google Scholar]
- 57.Cohen J. Statistical power analysis for the behavioral sciences. New York: Academic Press, 1977: 474 p. [Google Scholar]
- 58.Li Y, Qian H, Yu K, et al. Nonadherence in home-based pulmonary rehabilitation program for COPD patients. Can Respir J 2020;2020:1–7. 10.1155/2020/5146765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Butts JF, Belfer MH, Gebke KB. Exercise for patients with COPD: an integral yet underutilized intervention. Phys Sportsmed 2013;41:49–57. 10.3810/psm.2013.02.1999 [DOI] [PubMed] [Google Scholar]
- 60.Hayton C, Clark A, Olive S, et al. Barriers to pulmonary rehabilitation: characteristics that predict patient attendance and adherence. Respir Med 2013;107:401–7. 10.1016/j.rmed.2012.11.016 [DOI] [PubMed] [Google Scholar]
- 61.Boutou AK, Tanner RJ, Lord VM, et al. An evaluation of factors associated with completion and benefit from pulmonary rehabilitation in COPD. BMJ Open Respir Res 2014;1:e000051. 10.1136/bmjresp-2014-000051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oates GR, Niranjan SJ, Ott C, et al. Adherence to pulmonary rehabilitation in COPD: a qualitative exploration of patient perspectives on barriers and facilitators. J Cardiopulm Rehabil Prev 2019;39:344–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Robinson SA, Shimada SL, Quigley KS, et al. A web-based physical activity intervention benefits persons with low self-efficacy in COPD: results from a randomized controlled trial. J Behav Med 2019;42:1082–90. 10.1007/s10865-019-00042-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Borrell-Carrió F, Suchman AL, Epstein RM. The biopsychosocial model 25 years later: principles, practice, and scientific inquiry. Ann Fam Med 2004;2:576–82. 10.1370/afm.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yeh GY, Horwitz R. Integrative medicine for respiratory conditions. Med Clin North Am 2017;101:925–41. 10.1016/j.mcna.2017.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Franssen FME, Smid DE, Deeg DJH, et al. The physical, mental, and social impact of COPD in a population-based sample: results from the longitudinal aging study Amsterdam. NPJ Prim Care Respir Med 2018;28:30. 10.1038/s41533-018-0097-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.von Leupoldt A, Fritzsche A, Trueba AF, et al. Behavioral medicine approaches to chronic obstructive pulmonary disease. ann. behav. med. 2012;44:52–65. 10.1007/s12160-012-9348-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Laurin C, Moullec G, Bacon SL, et al. Impact of anxiety and depression on chronic obstructive pulmonary disease exacerbation risk. Am J Respir Crit Care Med 2012;185:918–23. 10.1164/rccm.201105-0939PP [DOI] [PubMed] [Google Scholar]
- 69.Kosteli M-C, Heneghan NR, Roskell C, et al. Barriers and enablers of physical activity engagement for patients with COPD in primary care. Int J Chron Obstruct Pulmon Dis 2017;12:1019–31. 10.2147/COPD.S119806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jackson BE, Coultas DB, Ashmore J, et al. Domain-Specific self-efficacy is associated with measures of functional capacity and quality of life among patients with moderate to severe chronic obstructive pulmonary disease. Ann Am Thorac Soc 2014;11:310–5. 10.1513/AnnalsATS.201308-273BC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gawande R, To MN, Pine E, et al. Mindfulness training enhances self-regulation and facilitates health behavior change for primary care patients: a randomized controlled trial. J Gen Intern Med 2019;34:293–302. 10.1007/s11606-018-4739-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Salmoirago-Blotcher E, Carey MP. Can mindfulness training improve medication adherence? integrative review of the current evidence and proposed conceptual model. Explore 2018;14:59–65. 10.1016/j.explore.2017.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nici L, ZuWallack R, American Thoracic Society Subcommittee on Integrated Care of the COPD Patient . An official American thoracic Society workshop report: the integrated care of the COPD patient. Proc Am Thorac Soc 2012;9:9–18. 10.1513/pats.201201-014ST [DOI] [PubMed] [Google Scholar]
- 74.Bandura A. Self-Efficacy: toward a unifying theory of behavioral change. Psychol Rev 1977;84:191–215. 10.1037/0033-295X.84.2.191 [DOI] [PubMed] [Google Scholar]
- 75.Spruit MA, Burtin C, De Boever P, et al. Copd and exercise: does it make a difference? Breathe 2016;12:e38–49. 10.1183/20734735.003916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hekler EB, Buman MP, Haskell WL, et al. Reliability and validity of CHAMPS self-reported sedentary-to-vigorous intensity physical activity in older adults. J Phys Act Health 2012;9:225–36. 10.1123/jpah.9.2.225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zainuldin R, Mackey MG, Alison JA. Optimal intensity and type of leg exercise training for people with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2011:CD008008. 10.1002/14651858.CD008008.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]