Abstract
Background
The coronavirus disease 2019 (COVID-19) global pandemic has led to a halt in elective surgeries throughout the United States and many other countries throughout the world. Early reports suggest that COVID-19 patients undergoing surgery have an increased risk of requiring intensive care unit (ICU) admission and overall mortality.
Materials and methods
A retrospective review was performed of all COVID-19, positive with polymerase chain reaction confirmation, patients who had surgery between February 17, 2020 and April 26, 2020 at a major New York City hospital. Clinical characteristics and outcomes including ICU admission, ventilator requirement, and mortality were analyzed.
Results
Thirty-nine COVID-19 surgical patients were identified. Mean age was 53.9 y, and there were more men than women in the cohort (56.4% versus 43.6%). Twenty-two patients (56.4%) had a confirmed positive COVID-19 test preoperatively, and the remainder tested positive after their procedure. The majority (59%) of patients had an American Society of Anesthesiologists (ASA) class of 3 or higher. Postoperatively, 7 patients (17.9%) required ICU level care with a mean length of stay of 7.7 d. There were 4 deaths (10.3%) in this patient population, all of which occurred in patients who were ASA class 3 or 4.
Conclusions
This study represents the largest study to date, that objectively analyzes the outcomes of COVID-19 positive patients who underwent surgery. Overall, ICU admission rates and mortality are similar to reported rates in the literature for nonsurgical COVID-19 patients. Notably, in COVID-19 patients with ASA 1 or 2, there was a 0% mortality rate in the postoperative period.
Keywords: COVID-19, SARS-Cov-2, Surgery, Incubation period
Introduction
The 2019 novel coronavirus disease (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has evolved from an outbreak in Wuhan, China to a worldwide pandemic.1, 2, 3 In response, many countries have instituted various international travel restrictions, postexposure quarantine protocols, and community social distancing guidelines, as well as other public health measures to protect citizens and offset the pressures of overburdened health care systems.4 , 5 In the United States, the surgeon general put a pause on all elective or nonemergent surgery in an attempt to aid in the conservation of resources, minimize unnecessary exposures, and help slow the spread of disease.6
Early reports show that most patients infected with SARS-CoV-2 remain asymptomatic or have only mild symptoms. In contrast, a smaller, but striking, percentage of individuals develop severe respiratory compromise that can rapidly deteriorate to acute respiratory distress syndrome (ARDS) and even multisystem organ failure (MOF).1 Reported symptoms include fever, cough, sore throat, headache, fatigue, dyspnea, malaise, diarrhea, arthralgias, or myalgias which will typically present within 14 d of infectivity.1 , 2 , 7 However, reports of longer “incubation periods,” up to 24 d, have been cited in the literature.8
Prior studies suggest an increased risk of mortality for COVID-19 positive patients who undergo surgery. The earliest report from Iran describes three patients who had routine intra-abdominal procedures during the presumed viral incubation period, two of whom died from ARDS and MOF. The third patient had an extended hospital course but ultimately recovered from their respiratory symptoms and was discharged home.9 More recently, a study published from Wuhan, China, analyzed 34 asymptomatic patients who underwent surgery during the incubation period of COVID-19.10 The group found that 44% of the patients were admitted to the intensive care unit (ICU) with a 20.5% mortality rate.10 These findings prompted the authors to suggest that undergoing surgery during the incubation period may contribute to the high rate of ICU admission and mortality. However, they acknowledge that procedural complexity and having multiple comorbidities were associated with a worse prognosis. Theories to explain the observed trends include ventilator-associated barotrauma, hypercoagulable state from the COVID-19 disease process and/or anesthesia, a systemic cytokine response secondary to surgical insult, or even the mere additional exposures to nosocomial COVID-19 strains and other infectious organisms.5 , 8 , 11, 12, 13
As the number of confirmed cases, hospital admissions, the volume of COVID-19–associated mortality decline, and restrictions for performing elective surgeries are expected to be lifted. Understanding the virus, disease process, and complications that can arise from operating on patients with active COVID-19 or asymptomatic patients during the incubation period will help guide best practices during these uncertain times. To that end, this study was designed to describe the surgical experience at a single institution, located in a large, densely populated urban area at the height of the COVID-19 pandemic, to help determine the effect of surgery, including the use of general anesthesia, on patients with COVID-19 or asymptomatic patients who subsequently tested positive for SAR-CoV-2.
Materials and methods
Study design and participants
After obtaining institutional review board approval (i20-00644) and a waiver of informed consent given the nature of the study, a retrospective review was performed of all patients who had surgery performed between March 6, 2020 and April 26, 2020 at Bellevue Hospital Center (New York, NY). It is the oldest public hospital in the United States and serves the most affected city in the world during the COVID-19 pandemic. This time period included the time leading up to the apex of COVID-19 positive cases in New York City, the apex, and the presumed decline. Individuals were included for analysis if they had surgery and also found to be SARS-CoV-2 positive by nasopharyngeal swab polymerase chain reaction (PCR) up to 14 days preoperatively or 24 days postoperatively. For the purpose of this study, patients who had a bedside surgical procedure directly related to COVID-19 sequelae and care, including but not limited to tracheostomy tube placement, gastrostomy tube placement, and insertion of peritoneal dialysis catheters, were excluded from the study. Patients were also excluded if their surgery was performed for COVID-19–related infection or other sequelae.
Data collection
All patients required a documented positive COVID-19 test. Testing related to this study was performed by PCR confirmation through the Bellevue Hospital laboratory and approved outside vendors using nasopharyngeal swab samples. The two primary tests used were the COVID-19 reverse transcription-PCR assay developed by Bio-Reference Laboratories (Bio-Reference Laboratories, Inc, Elmwood Park, NJ) for which information related to sensitivity and specificity is not yet available, and the Cepheid Xpert Xpress SARS-CoV-2rapid test (Cepheid Inc, Sunnyvale, CA) reported to have a sensitivity of 95% and a specificity of 100%.14
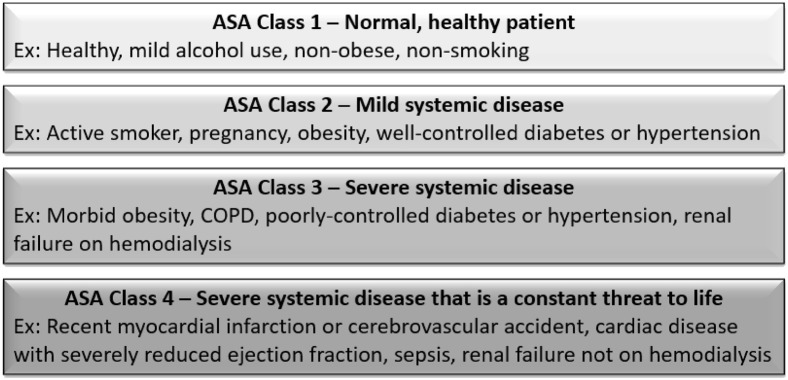
Demographic data, patient comorbidities, and procedural information were collected. Surgical outcomes including operative details, escalation to ICU level care, or need for prolonged postoperative ventilation were recorded. Dates of symptoms onset, emergency room visits, surgery, and hospital admission and discharge were systematically captured. Patients were classified by the American Society of Anesthesiologists (ASA) physical status presurgical classification15 (Fig. 1 ).
Fig. 1.
American Society of Anesthesiologists (ASA) physical status classification system. (Color version of figure is available online.)
Statistical analysis
Statistical analysis was performed using SPSS statistical software package, version 25 (IBM, Armonk, NY). Descriptive statistics and frequencies were calculated for the entire patient population and each subgroup analyzed. For comparisons between groups, the appropriate parametric or nonparametric tests were conducted based on the nature of variable and included the Student's t-test, the Mann-Whitney U test, and the chi-squared or Fischer's exact test. For the purpose of two-tailed univariate analysis, the cutoff for statistical significance was set at an alpha (α) of 0.05.
Results
Demographics of patient population
A total of 39 PCR-confirmed COVID-19 symptomatic patients underwent a surgical procedure at Bellevue Hospital Center from March 6, 2020 to April 26, 2020. The mean age was 53.9 y (range 9-91 y), and there was a predominance of male patients in the cohort, with 22 men (56.4%) compared with 17 women (43.6%). Many racial and ethnic backgrounds were represented, however, nearly half were identified as having Hispanic or Latin ethnicity (46.2%). The majority (71.8%) were domiciled in a house or an apartment; the remainder were either homeless or shelter-domiciled (15.4%), resided in a long-term care facility (7.7%), or were incarcerated in the New York City correctional system (5.1%) (Table 1 ).
Table 1.
Patient demographics, comorbidities, and preoperative COVID-19 course.
| Characteristics | n (%) | Symptoms/Comorbidities | n (%) |
|---|---|---|---|
| Patients | 39 | Preoperative symptoms | |
| Gender | Any preoperative COVID-19 symptoms | 20 (51.3%) | |
| Male | 22 (56.4%) | Cough | 10 (25.6%) |
| Female | 17 (43.6%) | Objective fever | 10 (25.6%) |
| Age (years)∗ | 53.92 ± 20.30 | Fatigue/malaise | 4 (10.3%) |
| BMI (kg/m2)∗ | 26.89 ± 8.19 | Shortness of breath | 5 (12.8%) |
| Race/ethnicity | Myalgia or arthralgia | 1 (2.6%) | |
| Caucasian | 6 (15.4%) | Anosmia or abnormal smell | 0 |
| African American | 5 (12.8%) | Ageusia or abnormal taste | 1 (2.6%) |
| Hispanic/Latinx | 18 (46.2%) | ||
| Asian | 0 | ||
| Other/not reported | 10 (25.6%) | Preoperative testing/workup | |
| Living situation | Preoperative positive COVID-19 test | 22 (56.4%) | |
| House/apartment | 28 (71.8%) | Preoperative admission for COVID-19 | 3 (7.7%) |
| Shelter-domiciled/homeless | 6 (15.4%) | Preoperative ICU for COVID-19 | 1 (2.6%) |
| Long-term facility | 3 (7.7%) | Radiologic evidence of pneumonia | |
| Incarcerated | 2 (5.1%) | X-ray | 15 (38.5%) |
| Tobacco use | CT scan | 6 (15.4%) | |
| Active | 3 (8.8%) | Time from symptom onset to surgery (days)∗ | 14 ± 6 (range: 1-22) |
| Former | 5 (14.7%) | Time from positive COVID-19 test to surgery (days)∗ | 2.5 ± 12 (range: –33-24)† |
| Hypertension | 18 (46.2%) | ||
| Congestive heart failure | 3 (7.7%) | ||
| Coronary artery disease | 5 (12.8%) | Pharmacologic management | |
| Cerebrovascular accident | 2 (5.1%) | Azithromycin | 13 (33.3%) |
| COPD | 5 (12.8%) | Hydroxychloroquine | 17 (43.6%) |
| Diabetes mellitus | 4 (10.3%) | Other antibiotics (excluding peri-operative) | 28 (71.8%) |
| Chronic kidney disease | 5 (12.8%) | Lopinavir-ritonavir | 3 (7.7%) |
| Hemodialysis | 4 (10.3%) | Remdesivir | 0 |
| Active malignancy | 1 (2.6%) | Tocilizumab | 0 |
| History of malignancy | 2 (5.1%) | Steroids | 1 (2.6%) |
| Autoimmune disorder | 1 (2.6%) | Immunoglobulin | 0 |
BMI = body mass index; COPD = chronic obstructive pulmonary disease.
Mean ± Standard Deviation.
Only 1 patient in deceased group placed on ventilator postoperatively.
Baseline health and medical comorbidities
With respect to the general health of this patient cohort before COVID-19 infection, the mean body mass index (BMI) was 26.89. Nearly a quarter had a history of current or prior tobacco use (8.8% active, 14.7% former). Hypertension was the most common comorbidity, present in 18 patients (46.2%), followed by coronary artery disease, chronic obstructive pulmonary disease (COPD), and chronic kidney disease in 5 patients (12.8%) each. 4 individuals (10.3%) had renal failure at baseline and were on hemodialysis before the COVID-19 pandemic (Table 1).
COVID-19 infection in preoperative and perioperative periods
Among these 39 patients, 22 (56.4%) had a confirmed positive COVID-19 test before surgery. Three individuals required hospital admission for treatment of their COVID-19 infection preoperatively with 1 needing escalation to the ICU. For those patients with an unknown viral status preoperatively, the mean number of days between a SARS-CoV-2 positive test result and surgery was 5.6 (range 0-33). It must be noted that the individual who initially tested positive more than 30 d out from surgery had a repeat positive test 2 d before being taken to the operating room. For patients whose viral testing was performed subsequently to surgery, testing occurred at an average of 12.88 d (range 0-24) after their procedure (Table 1).
Twenty patients (51.3%) had documented evidence of at least one COVID-19 symptom in the preoperative period. Cough and objective fever were the most commonly cited (n = 10, 25.6%) followed by shortness of breath in 5 individuals (12.8%). Symptoms began an average of 14 d (range 1-22) before surgery (Table 1).
Surgical interventions and outcomes in the setting of COVID-19 infection
These 39 patients underwent a total of 42 procedures in the operating room over the duration of this study. For the purposes of statistical analysis, only the primary procedure to address their underlying problem was included (e.g., an initial amputation was selected over subsequent revision or debridement). Nearly, all cases were performed for emergent or urgent indications. General surgery and orthopedic/hand surgery were responsible for treating the largest percentages of these patients (n = 8, 20.5%), followed by neurosurgery, vascular surgery, and cardiothoracic surgery/interventional cardiology (n = 4, 10.7%) (Table 2 ).
Table 2.
Operative details, outcomes, and complications.
| n (%) | n (%) | ||
|---|---|---|---|
| Operative characteristics | Operative outcomes | ||
| Surgical service | COVID-related inability to extubate in OR | 3 (7.7%) | |
| Trauma | 3 (7.7%) | New postoperative COVID-related ICU requirement | 7 (17.9%) |
| General | 8 (20.5%) | Postoperative ICU length of stay (days)∗ | 7.7 ± 4.1 |
| Cardiothoracic/interventional cardiology | 4 (10.3%) | Respiratory support | |
| Vascular | 4 (10.3%) | Supplemental oxygen | 9 (23.1%) |
| Orthopedic/hand | 8 (20.5%) | CPAP/BiPAP | 1 (2.6%) |
| Neurosurgery | 4 (10.3%) | Intubation | 5 (13.2%) |
| Plastic/OMFS | 3 (7.7%) | Extubated | 3 (60%) |
| Pediatric | 2 (5.1%) | Days on ventilator∗ | 10.2 ± 3.3 |
| OB-Gyn | 1 (2.6%) | COVID-related severe complications | |
| Podiatry | 2 (5.1%) | ARDS | 5 (12.8%) |
| ASA classification | Shock requiring pressors | 6 (15.4%) | |
| 1 | 4 (10.3%) | Acute kidney injury | 6 (15.4%) |
| 2 | 12 (30.8%) | New dialysis requirement | 0 |
| 3 | 15 (38.5%) | ECMO | 0 |
| 4 | 8 (20.5%) | Pulmonary embolism | 0 |
| Procedure length (mins)∗ | 177.3 ± 115.1 | Deep venous thrombosis | 0 |
| Type of anesthesia | Other ischemic or thrombotic complication | 1 (2.6%) | |
| General | 35 (89.7%) | Secondary infection | 5 (13.2%) |
| IV sedation/local | 4 (10.3%) | Disposition | |
| Airway management | Hospital length of stay (days)∗ | 20.9 ± 29.1 | |
| Endotracheal tube | 34 (87.2%) | Discharged from hospital | 26 (66.7%) |
| Laryngeal mask airway | 2 (5.1%) | Remain admitted to hospital | 9 (23.1%) |
| Nasal cannula oxygen or N/A | 3 (7.7%) | Deceased | 4 (10.3%) |
ASA = American Society of Anesthesiologists.
Mean ± Standard Deviation.
Mean procedure length was approximately 177.3 min, and nearly, 90% of patients received general anesthesia (n = 35) with endotracheal intubation (n = 34). The ASA class was 3 or higher in 59% of the population, including 8 (20.5%) patients meeting criteria for class 4 (Table 2).
The anesthesiologists were unable to extubate 3 patients (7.7%) at the immediate conclusion of the procedure, secondary to difficulty weaning oxygen and ventilatory support requirements in 2 individuals and the presence of a hemopneumothorax in another, while 7 (17.9%) patients were found to have COVID-related ICU needs that had not been present preoperatively, with an average postoperative ICU length of stay of 7.7 d (range 2-15 d [The patient admitted for 15 d remains in the ICU as of the time of manuscript submission]). Preoperatively, no patient was noted to have supplemental oxygen requirements nor were any patients on ventilators. Twenty-six (66.7%) patients remained on room air postoperatively and did not require any respiratory intervention, whereas approximately one quarter (n = 9) were unable to maintain adequate oxygen saturation requirements that improved with supplemental oxygen. Overall, 5 patients (13.2%) were intubated at some point in their postoperative course, with a mean duration of time on the ventilator of 10.2 d. Three of these patients have successfully been extubated at the time of manuscript submission, another remains intubated, and the last expired while on the ventilator (Table 2).
Severe complications of COVID-19 developed in 10 patients (25.6%). Shock requiring pharmacologic blood pressure support and acute kidney injury was the most common, occurring in 6 individuals each (15.4%). ARDS and secondary infection were also seen in 5 patients each. One patient suffered from a vaso-occlusive complication in the form of acute lower extremity ischemia secondary to an undetermined but likely embolic or in situ thrombotic etiology (Table 2).
At the time of manuscript submission, 9 patients (23.1%) remain admitted to the hospital, whereas 26 (66.7%) have been discharged home or to rehabilitation facilities (Table 2). All patients who had been discharged had confirmed proof of life with documented follow-up within the Bellevue Hospital Center system within 10 d of the final review of the clinical chart. There were 4 deaths (10.3%) among this cohort, all of which occurred in patients who were ASA class 3 or 4 (Table 3 ). Each death occurred at least a week after surgery, and 75% were in patients who had been admitted to the ICU, with the date of ICU admission coming a minimum of 12 d postoperatively. The causes of death have only been determined for 2 of these individuals (multiorgan system failure and secondary to cardiac arrest) (Table 3).
Table 3.
Characteristics of deceased patients.
| Age, Gender | Comorbidities | Preopp COVID-19 symptoms or findings | COVID-19 test date | Surgery date | Procedure (length) | ASA class | Date of death | COVID-related complications | Cause of death |
|---|---|---|---|---|---|---|---|---|---|
| 57, F | Active smoker, HTN, COPD, CKD on dialysis, HIV, hepatitis B, hepatitis C | Pneumonia on X-ray | 3/30/2020 | November 3, 2020 | Arteriovenous fistula revision, graft placement (178) | 4 | May 4, 2020 | Secondary infection | Unknown, found unresponsive |
| 86, M | Former smoker, HTN, CHF, CAD, COPD, Parkinson's disease | Pneumonia on X-ray | 3/29/2020 | 3/13/2020 | Open reduction and internal fixation of hip (130) | 3 | 3/31/2020 | ARDS, shock requiring pressors, acute kidney injury | Multiorgan failure |
| 54, F | HTN, CKD on dialysis, mitral valve endocarditis, hyperparathyroidism | Fever, pneumonia on X-ray | August 4, 2020 | January 4, 2020 | Mitral valve replacement (330) | 4 | 4/22/2020 | ARDS, shock requiring pressors | Bradycardiac arrest |
| 65, M | Former smoker, COPD, CKD, HIV, hepatitis B, hepatitis C, IV drug use, cirrhosis with hepatopulmonary syndrome | None | 4/16/2020 | December 4, 2020 | Arthroscopic irrigation and debridement for pyogenic arthritis of knee (93) | 3 | 4/19/2020 | Secondary infection | Unknown, found unresponsive |
Differences in baseline characteristics and surgical outcomes among different ASA classes
Patients were grouped as per low (1 or 2) or high (3 or 4) ASA class and compared on the basis of demographics, health history, COVID-19 infection course, and surgical outcomes (Table 4 ). There were 16 patients in the low ASA class group and 23 in the high ASA class group. Those in the low ASA class group tended to be younger (mean age 46.5 versus 59.1 y, P = 0.056) and have lower mean BMI 24.75 versus 28.35, P = 0.193), although neither comparison yielded statistical significance. Patients in the high ASA class group had significantly higher rates of hypertension (60.9% versus 25%, P = 0.049), whereas the rates of coronary artery disease, COPD, and chronic kidney disease were not significant. There was no discernible difference in the rates patients experienced preoperative COVID-19 symptoms (low ASA = 50% versus high ASA = 52.2%, P = 0.894). Patients in the low ASA class group more frequently had a known positive COVID-19 test before surgery (75% versus 43.5%, P = 0.099), but this difference was not statistically significant.
Table 4.
Comparison of patient characteristics and surgical outcomes among different ASA classes.
| Characteristics | ASA class 1/2 | ASA class 3/4 | P-value |
|---|---|---|---|
| Patients | 16 | 23 | - |
| Demographics | |||
| Age (years)∗ | 46.5 ± 22.3 | 59.1 ± 17.5 | 0.056 |
| BMI (kg/m2)∗ | 24.75 ± 5.5 | 28.35 ± 9.46 | 0.193 |
| Race/ethnicity | 0.420 | ||
| Caucasian | 3 (18.8%) | 3 (13%) | |
| African American | 1 (6.3%) | 4 (17.4%) | |
| Hispanic/Latinx | 10 (62.5%) | 8 (34.8%) | |
| Asian | 0 | 0 | |
| Other/not reported | 2 (12.5%) | 8 (34.8%) | |
| Comorbidities | |||
| Tobacco use | |||
| Active | 2 (14.3%) | 1 (5%) | 0.555 |
| Former | 0 | 5 (25%) | 0.063 |
| Hypertension | 4 (25%) | 14 (60.9%) | 0.049 |
| Congestive heart failure | 0 | 3 (13%) | 0.255 |
| Coronary artery disease | 0 | 5 (21.7%) | 0.066 |
| Cerebrovascular accident | 0 | 2 (8.7%) | 0.503 |
| COPD | 0 | 5 (21.7%) | 0.066 |
| Diabetes mellitus | 1 (6.3%) | 3 (13%) | 0.631 |
| Chronic kidney disease | 0 | 5 (21.7%) | 0.066 |
| Hemodialysis | 0 | 4 (17.4%) | 0.130 |
| Preoperative clinical course | |||
| Any preoperative COVID-19 symptoms | 8 (50%) | 12 (52.2%) | 0.894 |
| Preoperative positive COVID-19 test | 12 (75%) | 10 (43.5%) | 0.099 |
| Preoperative admission for COVID-19 | 0 | 3 (13%) | 0.255 |
| Time from symptom onset to surgery (days)∗ | 15.3 ± 4.5 | 17.7 ± 6.4 | 0.666 |
| Time from positive COVID-19 test to surgery (days)∗ | 0.3 ± 8.5 | 4 ± 14 | 0.358 |
| Surgical outcomes | |||
| Procedure length (mins)∗ | 171.3 ± 99 | 181.5 ± 127.1 | 0.789 |
| COVID-related inability to extubate in OR | 1 (6.3%) | 2 (8.7%) | 1.000 |
| New postoperative COVID-related ICU requirement | 2 (12.5%) | 5 (21.7%) | 0.678 |
| Postoperative ICU length of stay (days)∗ | 6 ± 5.7 | 8.1 ± 4.0 | 0.667 |
| Intubation | 1 (6.3%) | 4 (18.2%) | 0.374 |
| Days on ventilator∗ | 10† | 10.3 ± 3.8 | 1.000 |
| Hospital length of stay (days)∗ | 6.6 ± 6.7 | 30.8 ± 34.3 | 0.008 |
| Remain admitted to hospital | 2 (12.5%) | 7 (30.4%) | 0.262 |
| Deceased | 0 | 4 (17.4%) | 0.130 |
Bold indicates P < 0.05.
ASA = American Society of Anesthesiologists.
Mean ± Standard Deviation.
Only 1 patient in ASA class 1/2 placed on ventilator postoperatively.
Regarding postoperative outcomes, no statistically significant differences were detected with respect to a new COVID-related need for ICU management (low ASA = 12.5% versus high ASA = 21.7%, P = 0.678) or ICU length of stay (low ASA = 6 days versus high ASA = 8.1 d, P = 0.667). However, patients in the low ASA class group had significantly shorter durations of their overall hospital length of stay, with a mean of 6.6 d compared with 30.8 d (P = 0.008). Finally, there was a 17.4% mortality rate (n = 4) in the high ASA class group, compared with zero deaths in the low ASA class group (P = 0.130).
Comparison of baseline characteristics between living and deceased patients
To better understand mortality in the postoperative patient with COVID-19, the patient population was divided on the basis of whether or not they remained alive at the time of manuscript submission (n = 35) or were deceased (n = 4). Patients who ultimately died were on average older (65.5 versus 52.6 y, P = 0.195) and had lower BMIs (21.3 versus 27.6, P = 0.103) although neither of these comparisons reached statistical significance. They had higher rates of every comorbidity with the exception of diabetes mellitus and a history of cerebrovascular accident, and these rates were statistically significantly different with respect to COPD (75% versus 5.7%, P = 0.004), chronic kidney disease (75% versus 5.7%, P = 0.004), and being hemodialysis-dependent (50% versus 5.7%, P = 0.045). It bears noting that none of the deceased patients were known to be COVID-19 positive preoperatively, compared with 62.9% of those who remain alive (P = 0.029) (Table 5 ).
Table 5.
Comparison of living and deceased patients across all ASA classes and only ASA class 3/4.
| Characteristics | Deceased | All alive | P-value† | ASA class 3/4 alive | P-value‡ |
|---|---|---|---|---|---|
| Patients | 4 | 35 | - | 19 | - |
| Demographics | |||||
| Age (years)∗ | 65.5 ± 14.4 | 52.6 ± 20.6 | 0.195 | 57.7 ± 18.1 | 0.409 |
| BMI (kg/m2)∗ | 21.3 ± 3.2 | 27.57 ± 8.38 | 0.103 | 29.9 ± 9.7 | 0.042 |
| Race/ethnicity | 0.876 | 0.671 | |||
| Caucasian | 2 (50%) | 4 (11.4%) | 1 (5.3%) | ||
| African American | 0 | 5 (14.3%) | 4 (21.1%) | ||
| Hispanic/Latinx | 0 | 18 (51.4%) | 8 (42.1%) | ||
| Asian | 0 | 0 | 0 | ||
| Other/not reported | 2 (50%) | 8 (22.9%) | 6 (31.6%) | ||
| Comorbidities | |||||
| Tobacco use | |||||
| Active | 1 (25%) | 2 (6.7%) | 0.322 | 0 | 0.200 |
| Former | 2 (50%) | 3 (10%) | 0.094 | 3 (18.8%) | 0.249 |
| Hypertension | 3 (75%) | 15 (42.9%) | 0.318 | 11 (57.9%) | 1.000 |
| Congestive heart failure | 1 (25%) | 2 (5.7%) | 0.284 | 2 (10.5%) | 0.453 |
| Coronary artery disease | 1 (25%) | 4 (11.4%) | 0.436 | 4 (21.1%) | 1.000 |
| Cerebrovascular accident | 0 | 2 (5.7%) | 1.000 | 2 (10.5%) | 1.000 |
| COPD | 3 (75%) | 2 (5.7%) | 0.004 | 2 (10.5%) | 0.021 |
| Diabetes mellitus | 0 | 4 (11.4%) | 1.000 | 3 (15.8%) | 1.000 |
| Chronic kidney disease | 3 (75%) | 2 (5.7%) | 0.004 | 2 (10.5%) | 0.021 |
| Hemodialysis | 2 (50%) | 2 (5.7%) | 0.045 | 2 (10.5%) | 0.125 |
| Preoperative clinical course | |||||
| Any preoperative COVID-19 symptoms | 1 (25%) | 19 (54.3%) | 0.342 | 11 (57.9%) | 0.317 |
| Preoperative positive COVID-19 test | 0 | 22 (62.9%) | 0.029 | 10 (52.6%) | 0.104 |
| Preoperative admission for COVID-19 | 0 | 3 (8.6%) | 1.000 | 3 (15.8%) | 1.000 |
| Time from symptom onset to surgery (days)∗ | 12.7 ± 6.7 | 14.3 ± 6.1 | 0.712 | 14 ± 6.6 | 0.840 |
| Time from positive COVID-19 test to surgery (days)∗ | 11.5 ± 7.1 | 1.4 ± 12.1 | 0.066 | 2.4 ± 14.6 | 0.218 |
| Surgical outcomes | |||||
| Procedure length (mins)∗ | 182.8 ± 104.1 | 176.7 ± 117.7 | 0.806 | 181.2 ± 133.9 | 0.845 |
| COVID-related inability to extubate in OR | 0 | 3 (8.6%) | 1.000 | 2 (10.5%) | 1.000 |
| New postoperative COVID-related ICU requirement | 2 (50%) | 5 (14.3%) | 0.141 | 3 (15.8%) | 0.194 |
| Postoperative ICU length of stay (days)∗ | 5.7 ± 3.1 | 8.7 ± 4.4 | 0.381 | 10 ± 3.8 | 0.229 |
| Intubation | 1 (25%) | 4 (11.8%) | 0.446 | 3 (16.7%) | 1.000 |
| Days on ventilator∗ | 9 | 10.5 ± 3.7 | 0.800 | 10.7 ± 4.5 | 1.000 |
| Hospital length of stay (days)∗ | 13.3 ± 15.3 | 21.7 ± 30.3 | 0.639 | 34.5 ± 36.2 | 0.054 |
Bold indicates P < 0.05.
ASA = American Society of Anesthesiologists.
Mean ± Standard Deviation.
P-values for comparison of deceased and all patients who remain alive.
P-values for comparison of deceased and patients with ASA class 3 or 4 who remain alive.
The cohort of survivors was then further stratified to include only those with ASA class 3 or 4 status. A significant difference in BMI was identified, as those who remained alive were had a mean BMI 8 points higher than the deceased (29.9 versus 21.3, P = 0.042). In addition, patients who died had significantly higher rates of COPD (75% versus 10.5%, P = 0.021) and chronic kidney disease (75% versus 10.5%, P = 0.021) (Table 5).
Discussion
As the number of confirmed COVID-19 cases decreases, surgeons, anesthesiologists, infectious disease experts, and hospital administrators will have the challenging task of determining when elective surgery can safely recommence. Here, we present clinical data on the potential additional surgical risk of operating on COVID-19 patients or asymptomatic patients who are hypothetically in the incubation period of the disease.
The suggested notion that a patient undergoing surgery who is either asymptomatic or subsequently infected by a coronavirus is not novel. In 2015, the Middle East region experienced a similar, albeit much smaller scale, coronavirus outbreak.2 , 8 Coined Middle East respiratory syndrome coronavirus, this similar respiratory virus exhibited high fatality and transmissibility characteristics. In one hospital where a Middle East respiratory syndrome coronavirus minioutbreak occurred, the cardiac ward was preferentially susceptible, and ultimately, 5 of the 6 patients who had undergone recent cardiac surgery died secondary to respiratory failure.16
Currently, studies in the literature associating COVID-19 and increased surgical risk with poorer outcomes are limited to small case reports or make generalized conclusions that are not supported by the data. Recently, a multihospital study from Wuhan China reported 20% mortality rate in 34 patients.10 However, it lacks a comparison group and most of the patients who died had multiple comorbidities or underwent index procedures that involved thoracic surgery, suggesting that many of these patients had some degree of respiratory compromise at baseline. Publishing these data during these challenging times on a novel virus is quite valuable. However, generalized conclusions regarding COVID-19 and its associated perioperative risk for otherwise healthy patients that fall in the ASA 1 or 2 category should not be made based off such studies alone. In our cohort of patients who underwent surgery, all patients in the ASA 1 or 2 class have survived. In a recent meta-analysis of over 650 patients, the authors found that 20% of hospitalized, infected COVID-19 patients required an ICU admission and 14% of the hospital patients had fatal outcomes.7 Our data, composed of surgical patients (18% ICU admission rate and 10% mortality), aligns with the results these authors present, suggesting that patients undergoing surgery do not appear to have a significantly increased risk of mortality.
Each surgical subspecialty has a cohort of medically indicated, high priority procedures that are not considered emergent. For the months during which the COVID-19 was at its peak in New York City, these procedures were either discouraged or prohibited by hospital institutions. With limited understanding of the surgical and/or anesthetic impact on the physiology of those infected with SARS-CoV-2, this can be challenging for surgeons, patients, and their families.17 During these times of uncertainty, there is a moral and ethical obligation for surgeons to remain current on the evolving literature and best practices. It is imperative health care providers clearly convey the risks associated with each procedure while acknowledging the potential for additional contributing factors such as COVID-19 that may alter the risk profile for that procedure or the subsequent postoperative care. Conversely, although the timeline of societal recovery from this pandemic is uncertain, and many anticipate that the slow return to “normal” may take several more months. During this time period, the potential repercussions from improper, generalized cancellation or delay of all nonemergent surgeries may yield significant pain, distress, and morbidity for patients and society.6 Therefore, careful consideration, risk stratification, and preoperative patient counseling are imperative as bans lift on elective surgery.
As with all studies, this study has its limitations. First, it is a retrospective review from a single hospital. However, given the severity of this pandemic and the rapidly evolving health care regulations regarding elective surgery, the authors believe that publishing these results adds value to the medical community as we begin to evaluate the future of elective surgery in the near term. While many patients in our cohort were confirmed SARS-CoV-2 positive before surgery, there were several patients in the early part of this study, when testing kits were unavailable or their use was not widespread, that were presumed to be in the incubation period based on the estimated time from the day they subsequently tested positive for the virus on PCR-based testing. We included all patients who tested positive up to 14 d before their surgery or up to 24 d after their surgery, based on the longest incubation time that has been reported to date in an effort to ensure that we captured all infected patients who underwent surgery at our institution.8 As a tertiary care center in New York City, the epicenter of the COVID-19 pandemic in the United States, our institution is in a unique position to perform this study. Despite the intermission on elective cases, longstanding referral and transfer patterns from other city hospitals contributed to the constant but low volume of emergent operative cases at our institution. The overwhelming majority, 80% of our reported cases, had surgery dates after the New York State mandate to cancel of elective surgery in New York City. Therefore, elective procedures are inherently under-represented, however, it remains the largest reported series published to date on this topic. While a majority of cases included were for urgent or emergent indications, they represent a wide variety of surgical procedures commonly performed as part of care in a level 1 trauma center (e.g., open reduction internal fixation of hip fractures, coronary artery bypass grafting, laparoscopic cholecystectomy, appendectomy, etc.). Nonetheless, it must be acknowledged that with the constraints of the cancellation of elective cases as well as a hesitancy to operate on patients with confirmed or suspected COVID-19 infection, it was necessary for the purposes of this study to group procedures of varying complexity from all surgical specialties, stratifying patients only by ASA class. Ideally, as the COVID-19 pandemic evolves and more data become available both within New York City as well as the country and the world at large, coordinated efforts can be made between centers to pool outcomes and further refine our understanding of the interplay between COVID-19 and postoperative outcomes.
Finally, our intention is to determine the impact of COVID-19 with postoperative complication and mortality. Unfortunately, a recently implemented New York City moratorium limits the performance of autopsies during this COVID-19 pandemic. The cause of death for 3 of the 4 patients is presumed to be attributed to COVID-19. One patient, a 65-year-old SARS-Cov-2 positive, drug dependent, active smoker was diagnosed with pyogenic arthritis, underwent knee wound irrigation and debridement and expired unexpectantly 8 d after surgery while recovering on the floor. Patients with severe COVID-19 illness have a propensity for a hypercoagulable state, however, an autopsy could not be performed. Although he had tested positive for SARS-CoV-2, he remained asymptomatic during his hospitalization and was only tested because his roommate had been found to be positive for COVID-19. Further, this patient received none of the treatments being used at that time to combat the virus, including hydroxychloroquine, tocilizumab, or corticosteroids, and thus the true cause of death remains unknown. We included this patient in the analysis, despite significant contemplation that the cause of death was unrelated to COVID-19, which would only strengthen our findings and conclusions. While this study would indeed be strengthened by comparing this COVID positive cohort to a matched control of patients undergoing surgery who were COVID negative, we are limited by the study period (a <2 mo duration) and single-institution setting. This prevented our ability to obtain a properly matched cohort of control patients with similar patient and operative characteristics. Nevertheless, our case series is valuable in its size and timeliness as we continue to battle the COVID-19 pandemic today.
Conclusions
We have critically analyzed the hospitalizations and postoperative outcomes for 39 PCR-confirmed SARS-Cov-2 patients who underwent surgery during the apex of the COVID-19 epidemic in a large volume New York City hospital. The rate of ICU admission and mortality of these patients appears to be similar to nonsurgical COVID-19 patients who have been reported in the literature. It was encouraging to find that none of the patients with ASA 1 or 2 scores died in the immediate postoperative period. These findings support the risk stratification of elective surgery algorithms that Dr Stahel presents in his recent editorial.6 We encourage all researchers to work together and continue accruing objective data, so that health care professionals and hospitals are better able to risk stratify COVID-19 positive surgical candidate patients to achieve safe clinical outcomes.
Disclosure
The authors have no financial interest to declare in relation to the content of this work.
Author’s contributions
The project idea and study design were conceptualized and put forth by N.M.V., J.M.B., and S.C.W. Refinement of this study design was performed by S.C.W., D.A.D., and Chiu. Data collection was performed by N.M.V., J.M.B., S.C.W., with data analysis performed by J.M.B. Manuscript writing was performed by N.M.V., J.M.B., D.A.D., and S.C.W., with manuscript editing being performed by all members of the team. Figures were generated by D.A.D. and J.M.B. Chiu served as PI for the study and oversaw the project.
References
- 1.Burhan E., Prasenohadi P., Rogayah R., Isbaniyah F., Reisa T., Dharmawan I. Clinical Progression of COVID-19 patient with extended incubation period, delayed RT-PCR time-to-positivity, and potential Role of chest CT-scan. Acta Med Indones. 2020;52:80–83. [PubMed] [Google Scholar]
- 2.Chakraborty C., Sharma A.R., Sharma G., Bhattacharya M., Lee S.S. SARS-CoV-2 causing pneumonia-associated respiratory disorder (COVID-19): diagnostic and proposed therapeutic options. Eur Rev Med Pharmacol Sci. 2020;24:4016–4026. doi: 10.26355/eurrev_202004_20871. [DOI] [PubMed] [Google Scholar]
- 3.Coronavirus live Updates: W.H.O. Declares pandemic as number of infected countries Grows. The New York Times; New York, NY: 2020. [Google Scholar]
- 4.Doussot A., Heyd B., Lakkis Z. We asked the experts: How do we maintain surgical quality standards for enhanced recovery programs after cancer surgery during the COVID-19 outbreak? World J Surg. 2020;44:2051–2052. doi: 10.1007/s00268-020-05546-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lepre L., Costa G., Virno V.A., et al. Acute care surgery and postoperative COVID-19 pneumonia: a surgical and environmental challenge. ANZ J Surg. 2020;90:1160–1161. doi: 10.1111/ans.15962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stahel P.F. How to risk-stratify elective surgery during the COVID-19 pandemic? Patient Saf Surg. 2020;14:8. doi: 10.1186/s13037-020-00235-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodriguez-Morales A.J., Cardona-Ospina J.A., Gutierrez-Ocampo E., et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Trav Med Infect Dis. 2020:101623. doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y., Wang Y., Chen Y., Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J Med Virol. 2020;92:568–576. doi: 10.1002/jmv.25748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aminian A., Safari S., Razeghian-Jahromi A., Ghorbani M., Delaney C.P. COVID-19 outbreak and surgical practice: Unexpected fatality in perioperative period. Ann Surg. 2020;272:e27–e29. doi: 10.1097/SLA.0000000000003925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lei S., Jiang F., Su W., et al. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. EClinicalMedicine. 2020:100331. doi: 10.1016/j.eclinm.2020.100331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ben Abdallah I., La Collegiale A., Coscas R., et al. Early experience in Paris with the impact of the Covid-19 pandemic on Vascular surgery. J Vasc Surg. 2020;72:373. doi: 10.1016/j.jvs.2020.04.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Connors J.M., Levy J.H. Thromboinflammation and the hypercoagulability of COVID-19. J Thromb Haemost. 2020;18:1559–1561. doi: 10.1111/jth.14849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spiezia L., Boscolo A., Poletto F., et al. COVID-19-Related severe hypercoagulability in patients admitted to intensive care Unit for acute respiratory failure. Thromb Haemost. 2020;120:998–1000. doi: 10.1055/s-0040-1710018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loeffelholz M.J., Alland D., Butler-Wu S.M., et al. Multicenter evaluation of the Cepheid Xpert Xpress SARS-CoV-2 test. J Clin Microbiol. 2020;58:e00926-20. doi: 10.1128/JCM.00926-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ASA Physical Status Classification System. 2019. https://www.asahq.org/standards-and-guidelines/asa-physical-status-classification-system Available at: [Google Scholar]
- 16.Nazer R.I. Outbreak of Middle East respiratory syndrome-coronavirus causes high fatality after cardiac Operations. Ann Thorac Surg. 2017;104:e127–e129. doi: 10.1016/j.athoracsur.2017.02.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Milner R., Roggin K.K., Angelos P., J.B M. Unknown unknowns: surgical consent during the COVID-19 pandemic. Ann Surg. 2020:229–233. doi: 10.1097/SLA.0000000000003995. [DOI] [PMC free article] [PubMed] [Google Scholar]