Abstract
Objectives
To clarify the meaning of elevated cardiac troponin in elite soccer athletes previously infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and screened for cardiovascular involvement in the wake of competitive sport resumption.
Methods
We designed a retrospective cohort study with the collaboration of two Italian Serie A teams. Soccer players from both rosters (58 athletes) were systematically analysed. For every SARS-CoV-2 positive athlete, the Italian Soccer Federation protocol requested full blood tests including high-sensitivity cardiac troponin I (hs-cTnI), along with a complete cardiovascular examination. We extended the analysis to SARS-CoV-2 negative athletes.
Results
A total of 13/58 players (22.4%) suffered from SARS-CoV-2infection: all had a negative cardiovascular examination and 2/13 (15%) showed increased hs-cTnI values (120.8 pg/ml and 72,6 pg/ml, respectively; upper reference level 39.2 pg/ml), which did not track with inflammatory biomarkers. Regarding the 45/58 (77.6%) non infected athletes, a slight increase in hs-cTnI was observed in 2 (4.5%) subjects (values: 61 pg/ml and 75 pg/ml respectively). All hs-cTnI positive athletes (4/58, 7%) underwent cardiac magnetic resonance (CMR), that excluded any cardiac injury.
Conclusions
In our retrospective study, SARS-CoV-2 infection in elite soccer athletes was not associated to clinical or biomarkers abnormalities. Increased hs-cTnI was rare and not significantly associated with previous SARS-COV2 infection nor with pathological findings at CMR, albeit elevated hs-cTnI was numerically more prevalent in the infected group.
Keywords: Cardiac troponin, Elite athletes, Coronavirus disease 2019 (COVID-19)
Coronavirus disease 2019 (COVID-19), the pandemic caused by severe acute respiratory syndrome coronavirus 2(SARS-CoV-2), is a respiratory illness with potential cardiovascular (CV) involvement [1,2]. Elevated cardiac troponin (cTn), a marker of cardiac injury, is detectable in a significant proportion of COVID-19, especially in the most severe cases [3]. Myocarditis is one of the possible causes of cTn elevation and a reason for concern particularly in athletes, in which it may conspire with cardiac adaptations to exercise, resulting in a pro-arrhythmic substrate [1].
Professional sport events have been placed on hold and distancing has been implemented for athletes' training. In the last months, restrictions have been progressively lifted, and recommendations dealing with COVID-19 in athletes have been published. In professional soccer, the Italian federation (FIGC, Federazione Italiana Gioco Calcio) has issued specific guidelines (http://www.sport.governo.it/it/emergenza-covid-19/lo-sport-riparte/linee-guida-per-lo-svolgimento-degli-allenamenti-per-gli-sport-di-squadra/).
We designed a cohort study implementing the FIGC protocol in the wake of professional soccer resumption. Players from two Italian Serie A teams were analysed. Both teams had a policy of extensive screening by reverse-transcriptase polymerase chain reaction (RT-PCR) assay for SARS-CoV-2 in a respiratory tract swab, which was performed weekly in the entire rosters, starting from March 1st 2020 until end June 2020. Teams' policy was to stop training for actively infected athletes, until two negative RT-PCR; then the athletes were given another two weeks rest before training resumption. All athletes at the time of the study were performing a “home training” (2 h aerobic exercise supervised through internet by team doctors), as in-person team training was still not allowed.
For every COVID-19 positive athlete, we measured hs-cTnI (ARCHITECT STAT High Sensitive Troponin, diagnostic cut-off representing the 99th percentile of 34.2 pg/ml in men [4]). The protocol included: complete blood count, alanine transaminase (ALT), aspartate transaminase (AST), gamma-glutamyl-transferase (GGT), creatine kinase (CPK), CPK myocardial band (CPK-MB), lactate dehydrogenase (LDH), partial thromboplastin time (PTT), international normalized ratio (INR), serum protein electrophoresis, ferritin, interleukin-6, C-reactive protein, D-dimer and urine test. The CV examination included clinical visit, electrocardiogram (ECG), echocardiography, cardiopulmonary exercise testing, and 24 h ECG Holter monitoring.
In COVID-19 negative athletes, we also measured hs-cTnI, performed first level clinical examination and ECG (with second-level cardiac tests planned only in case of abnormalities). Our study protocol also called for cardiac magnetic resonance (CMR) in athletes with elevated hs-cTnI, as well as for repeated measurements of biomarkers. CMR protocol included multiplane cine imaging, short-tau inversion recovery (STIR) and late gadolinium enhancement (LGE) (see Supplementary Methods). We collected anonymized data for the present study from electronic and paper medical records, including demographics, cardiovascular risk factors and co-existing conditions.
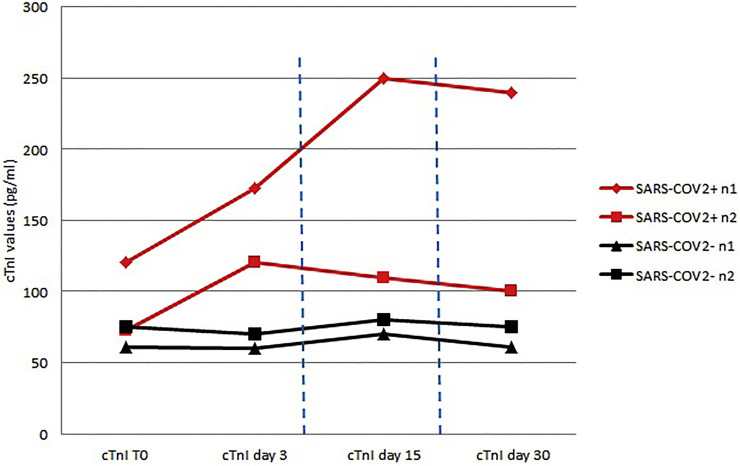
Fifty-eight players (31 from Team-A, and 27 from Team-B) were included, of whom 13/58 (22.4%) had suffered a previous infection. Results are reported in Table 1 . Among the 13 COVID-19 positive athletes, CV examination did not demonstrate any abnormality. Two/13 (15%) had increased hs-cTnI (peak 120.8 pg/ml and 72.6 pg/ml), which did not track with inflammatory biomarkers (see Table 1); hs-cTnI was re-checked at 48 and 72 h, up to 30 days (Fig. 1 ) documenting stable values. All the 45 COVID-19 negative players had a negative CV examination. In 2/45 (4.5%) athletes we documented a slight increase in hs-cTnI (61 pg/ml and 75 pg/ml, respectively), with stable values at 48 and 72 h, up to 30 days (Fig. 1). Chi-square statistic (see Supplementary Table) shows no relationship between COVID-19 positive status and hs-cTnI elevation (p-value: 0.17).
Table 1.
Athletes undergoing advanced cardiovascular examination. Number 1 to 13: SARS-CoV-2 positive athletes. Number 14 to 15: SARS-CoV-2 negative athletes. SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
| Case No. | SARS-CoV-2 | ECG | Echo | 24 h HM | Stress Test | Hs-cTnI first value (NV: 39,2 pg/mL) | Blood exams/inflammatory biomarkers /coagulation markers* | CMR | Remarks |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Positive | Negative T wave in DIII | Normal | Normal | Normal, maximal |
**120.8 pg/ml | No abnormal values | Negative | Mild respiratory symptoms during infection |
| 2 | Positive | Normal | Normal | Normal | Normal, maximal |
<39.2 pg/ml | No abnormal values | NP | Nothing |
| 3 | Positive | Normal | Pericardial effusion (3 mm) | Normal | Normal, maximal |
<39.2 pg/ml | No abnormal values | NP | Echo follow-up suggested for minimal pericardial effusion |
| 4 | Positive | Normal | Normal | Normal | Normal, maximal |
**72.6 pg/ml | High-normal CK values (298 U/l, ULN 304 U/l) |
Negative | Mild respiratory symptoms during infection |
| 5 | Positive | Negative T wave in DIII, aVF |
Normal | Normal | Normal, maximal |
<39.2 pg/ml | No abnormal values | NP | Previously documented negative T wave in DIII, aVF |
| 6 | Positive | Normal | Normal | monomorphic PVCs (LVOT origin) |
Normal maximal |
<39.2 pg/ml | No abnormal values | NP | PVCs suppression during exercise test |
| 7 | Positive | Normal | Normal | NSVT (5 beats) |
Normal maximal |
<39.2 pg/ml | No abnormal values | Negative | Negative repeated 24 h Holter |
| 8 | Positive | Normal | Normal | Normal | Normal maximal |
<39.2 pg/ml | No abnormal values | NP | Nothing to declare |
| 9 | Positive | Normal | Normal | Normal | Normal maximal |
<39.2 pg/ml | No abnormal values | NP | Nothing to declare |
| 10 | Positive | Normal | Normal | Normal | Normal maximal |
<39.2 pg/ml | No abnormal values | NP | Nothing to declare |
| 11 | Positive | Normal | Normal | Normal | Normal maximal |
<39.2 pg/ml | No abnormal values | NP | Nothing to declare |
| 12 | Positive | Normal | Normal | Normal | Normal maximal |
<39.2 pg/ml | No abnormal values | NP | Nothing to declare |
| 13 | Positive | Normal | Normal | Notmal | Normal maximal |
<39.2 pg/ml | No abnormal values | NP | Nothing to declare |
| 14 | Negative | Normal | Normal | Normal | Normal maximal |
**61 pg/ml | High IL-6 (25.6 pg/ml, ULN 16.4 pg/ml | Negative | Nothing to declare |
| 15 | Negative | Normal | Normal | Normal | Normal maximal |
**75 pg/ml | Normal values | Negative | Documented athlete's heart |
Abbreviations: No = number; NV = normal values; hs-cTnI = high sensitivity ncardiac troponin I; ECG = Electrocardiogram; Echo = Echocardiography; HM = Holter monitoring; CMR = Cardiac magnetic resonance; FU = follow-up; NP = not performed; AV = atrioventricular; PVC = premature ventricular contraction; LVOT = left ventricular outflow tract; NSVT = non-sustained ventricular tachycardia; ULN = upper level of normality).
* Blood exams included: complete blood count, alanine transaminase (ALT), aspartate transaminase (AST), gamma-glutamyl transferase (GGT), creatine kinase (CPK), CPK myocardial band (CPK-MB), lactate dehydrogenase (LDH); inflammation biomarkers included: interleukin −6 (IL-6), C-reactive protein; coagulation markers included: D-dimer, fibrinogen, international normalized ratio (INR) ** = abnormal hs-cTnI values.
Fig. 1.
Time course of high sensitive cardiac troponin in 4 athletes (2 with and 2 without previous SARS-CoV-2 infection) over 30 days. SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
CMR in the 4 hs-cTnI positive athletes, performed at 27–41 days after COVID-19 diagnosis, ruled out any cardiac disease or injury. Cine images revealed normal left and right ventricle volumes and systolic function, with no segmental wall motions abnormalities; moreover, no myocardial edema, no increased myocardial-to-skeletal muscle intensity ratio on STIR images, and no pericardial effusion were documented. Finally, LGE images were negative (see Supplementary Figs. 1 and 2).
Raised cardiac markers in COVID-19 infection during the acute phase are associated with poor prognosis [3]. This elevation has several potential mechanisms, including direct cardiac myocyte infection, cytokine storm, hypoxia-mediated damage, or microvascular endothelitis [1]. The main concern for athletes with documented SARS-CoV2 infection is the risk of active or chronic myocarditis [5]. Indeed, CMR signs consistent with myocardial inflammation have been reported in up to 60% of patients recently recovered from COVID-19 [6]. Moreover, among 26 SARS-CoV-2-positive athletes, 12/26 (46%) demonstrated LGE and 4/26 (15%) had both abnormal T2 values and LGE by CMR [2]. It remains unclear whether hs-cTn assay can be considered a reliable “gatekeeper” for advanced investigations in athletes, as well as the role of CMR.
Our data may provide insights to physicians and politicians involved in these difficult decisions, since with our systematic screening protocol, we found no clinical or CMR evidence for myocarditis in 13 athletes with a previous, mostly asymptomatic, SARS-CoV2 infection. Increased hs-cTnI was rare and not significantly associated with previous SARS-COV2 infection nor with pathological findings at CMR, albeit elevated hs-cTnI was numerically more prevalent in the infected group. Conversely, we found persistently elevated hs-cTnI also in non-infected athletes, raising concerns on the policy of measuring this very sensitive myocardial injury biomarker in all SARS-CoV2 infected, irrespective of symptoms or signs. It should be emphasized that SARS-CoV-2 does not seem to be a cardiotropic virus. Indeed, a less than 2% prevalence of active myocyte infection was found in 277 individuals across 22 autopsy studies [7]. Additionally in the acute phase, troponins, when elevated, do track with inflammatory and coagulation markers, suggesting non-specific cytokine-mediated cardiotoxicity [8]. On the other hand, in athletes, hs-troponins can be elevated even in absence of overt myocardial disease or injury, due to repeated exercise, especially in the young subjects [9,10].
Acknowledgement of grant support
No sources of any support for all authors in the form of grants, equipment, drugs, or any combination of these.
Declaration of Competing Interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Footnotes
All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcard.2020.11.039.
Appendix A. Supplementary data
Supplementary Methods and Supplemtary Figure 1 & 2
Supplementary Table
References
- 1.Guzik T.J., Mohiddin S.A., Dimarco A., Patel V., Savvatis K., Marelli-Berg F.M., Madhur M.S., Tomaszewski M., Maffia P., D’Acquisto F., Nicklin S.A., Marian A.J., Nosalski R., Murray E.C., Guzik B., Berry C., Touyz R.M., Kreutz R., Wang D.W., Bhella D., Sagliocco O., Crea F., Thomson E.C., McInnes I.B. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc. Res. 2020 Aug 1;116(10):1666–1687. doi: 10.1093/cvr/cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rajpal S., Tong M.S., Borchers J., Zareba K.M., Obarski T.P., Simonetti O.P., Daniels C.J. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol. 2020 Sep;11 doi: 10.1001/jamacardio.2020.4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nie S.F., Yu M., Xie T., Yang F., Wang H.B., Wang Z.H., Li M., Gao X.L., Lv B.J., Wang S.J., Zhang X.B., He S.L., Qiu Z.H., Liao Y.H., Zhou Z.H., Cheng X. Cardiac troponin I is an independent predictor for mortality in hospitalized patients with COVID-19. Circulation. 2020 Aug 11;142(6):608–610. doi: 10.1161/CIRCULATIONAHA.120.048789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Apple F.S., Ler R., Murakami M.M. Determination of 19 cardiac troponin I and T assay 99th percentile values from a common presumably healthy population. Clin. Chem. 2012 Nov;58(11):1574–1581. doi: 10.1373/clinchem.2012.192716. [DOI] [PubMed] [Google Scholar]
- 5.Eichhorn C., Bière L., Schnell F., Schmied C., Wilhelm M., Kwong R.Y., Gräni C. Myocarditis in athletes is a challenge: diagnosis, risk stratification, and uncertainties. JACC Cardiovasc. Imaging. 2020 Feb;13(2 Pt 1):494–507. doi: 10.1016/j.jcmg.2019.01.039. [DOI] [PubMed] [Google Scholar]
- 6.Puntmann V.O., Carerj M.L., Wieters I., Fahim M., Arendt C., Hoffmann J., Shchendrygina A., Escher F., Vasa-Nicotera M., Zeiher A.M., Vehreschild M., Nagel E. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 Jul;27 doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halushka M.K., Van der Heide M.S. Myocarditis is rare in COVID-19 autopsies:cardiovascular findings across 277 postmortem examinations. Cardiovasc. Pathol. 2021;50:107300. doi: 10.1016/j.carpath.2020.107300. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li C., Jiang J., Wang F., Zhou N., Veronese G., Moslehi J.J., Ammirati E., Wang D.W. Longitudinal correlation of biomarkers of cardiac injury, inflammation, and coagulation to outcome in hospitalized COVID-19 patients. J. Mol. Cell. Cardiol. 2020 Oct;147:74–87. doi: 10.1016/j.yjmcc.2020.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cirer-Sastre R., Legaz-Arrese A., Corbi F., George K., Nie J., Carranza-García L.E., Reverter-Masià J. Cardiac biomarker release after exercise in healthy children and adolescents: a systematic review and meta-analysis. Pediatr. Exerc. Sci. 2019 Feb 1;31(1):28–36. doi: 10.1123/pes.2018-0058. [DOI] [PubMed] [Google Scholar]
- 10.Cirer-Sastre R., Legaz-Arrese A., Corbi F., López-Laval I., Puente-Lanzarote J., Hernández-González V., Reverter-Masià J. Effect of training load on post-exercise cardiac troponin T elevations in young soccer players. Int. J. Environ. Res. Public Health. 2019 Dec 2;16(23):4853. doi: 10.3390/ijerph16234853. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Methods and Supplemtary Figure 1 & 2
Supplementary Table