Abstract
Noonan syndrome is a pleomorphic genetic disorder, in which a high percentage of affected individuals have cardiovascular involvement, most prevalently various forms of congenital heart disease (i.e., pulmonary valve stenosis, septal defects, left-sided lesions, and complex forms with multiple anomalies). Care includes attentiveness to several comorbidities, some directly impacting cardiac management (bleeding diatheses and lymphatic anomalies). More than 50% of patients with Noonan syndrome harbor PTPN11 pathogenic variation, which results in hyperactivation of RAS/mitogen-activated protein kinase signaling. Several other disease genes with similar biological effects have been uncovered for NS and phenotypically related disorders, collectively called the RASopathies. Molecular diagnosis with gene resequencing panels is now widely available, but phenotype variability and in some cases, subtlety, continues to make identification of Noonan syndrome difficult. Until genetic testing becomes universal for patients with congenital heart disease, alertness to Noonan syndrome’s broad clinical presentations remains crucial. Genotype-phenotype associations for Noonan syndrome enable better prognostication for affected patients when a molecular diagnosis is established. We still lack Noonan syndrome-specific treatment; however, newly developed anticancer RAS pathway inhibitors could fill that gap if safety and efficacy can be established for indications such as pulmonary valve stenosis.
Keywords: congenital heart disease, Noonan syndrome, RASopathy
1 |. NOONAN SYNDROME AND THE RASOPATHIES
In 1968, Dr. Jacqueline Noonan described the eponymous syndrome, grouping nine patients displaying a similar array of clinical characteristics: “remarkably similar facies with a previously unrecognized syndrome of valvular pulmonary stenosis and multiple extracardiac anomalies” (Noonan, 1968). These features, which may be more or less subtle, include facial characteristics (typically, hypertelorism, ptosis, and low-set ears), short stature, and chest abnormalities (pectus excavatum). Additional extracardiac features include neurodevelopmental disabilities, cryptorchidism, delayed puberty, lymphedema, bleeding disorders, and a slightly increased predisposition to hematologic and solid cancers (Roberts, Allanson, Tartaglia, & Gelb, 2013). Transmission was established to be autosomal dominant, although an autosomal recessive form has recently been identified (Johnston et al., 2018). The phenotype is variable, but penetrance is believed to be complete.
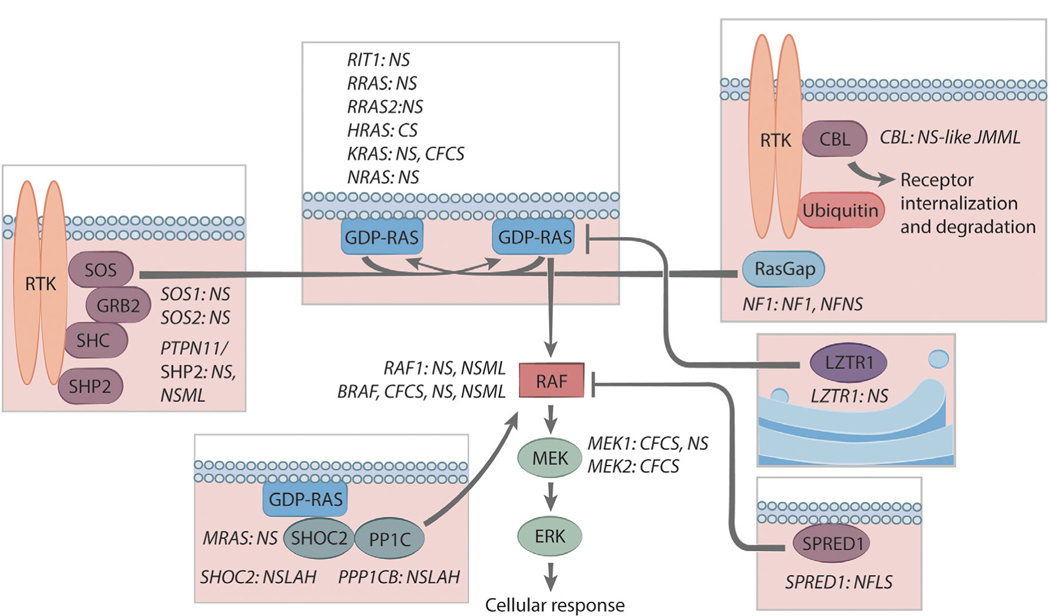
After the initial description of Noonan syndrome (NS), several other traits with overlapping features were described: Costello syndrome, cardiofaciocutaneous syndrome, NS with multiple lentigines (previously known as LEOPARD syndrome) (Tartaglia, Gelb, & Zenker, 2011), and NS-like with loose anagen hair. With the elucidation of their molecular genetics, NS and these related disorders are now known as the RASopathies because they share a common pathophysiology, that is, perturbation of the RAS/mitogen-activated protein kinase (MAPK) signal transduction pathway (Figure 1). Since 2001, when PTPN11, the first disease gene for NS, was discovered (Tartaglia et al., 2001), the degree of molecular heterogeneity underlying the RASopathies has become evident (Table 1).
FIGURE 1.
Spectrum of RAS-MAPK pathway disease genes and associated RASopathies. Proteins having roles in the RAS-MAPK are shown, and the ones for which pathogenic variation in the encoding genes results in one or more RASopathies are indicated. The proteins depicted within the boxes largely reside at the cell membrane (indicated with the schematic of lipid bilayers, except LZTR1, which is associated with the Golgi apparatus). The proteins shown outside of the boxes principally reside in the cytosol. Abbreviations: CFCS, cardiofaciocutaneous syndrome; CS, Costello syndrome; NF1, neurofibromatosis type 1; NFNS, neurofibromatosis-Noonan syndrome; NS, Noonan syndrome; NSLAH, Noonan syndrome with loose anlagen hair; NSML, Noonan syndrome with multiple lentigines; NS-like JMML, Noonan syndrome-like disorder with juvenile myelomonocytic leukemia; RTK, Receptor tyrosine kinases. Copyright, Ni-ka Ford, Icahn School of Medicine at Mount Sinai
TABLE 1.
RASopathy disorders and their known disease genes
| RASopathy | Disease genes | Cases accounted for by known disease genes (%) |
|---|---|---|
| Noonan syndrome | PTPN11, SOS1, RAF1, KRAS, MRAS, NRAS, RRAS, BRAF, RIT1, SOS2, LZTR1, RASA2, RRAS, RRAS2 MAP2K1 | 85 |
| NS with multiple lentigines | PTPN11, RAF1, BRAF | >90 |
| NS-like with loose anagen hair | SHOC2, PPP1CB | 100 |
| Costello syndrome | HRAS | 100 |
| Cardiofaciocutaneous syndrome | BRAF, MAP2K1, MAP2K2, KRAS | 70 |
| Noonan-like with juvenile myelomonocytic leukemia | CBL | 100 |
Citations for Table 1: Tidyman and Rauen (2016); Aoki et al. (2013); Yamamoto et al. (2015); Tajan, Paccoud, Branka, Edouard, and Yart (2018); Yu et al. (2019).
Bold indicates definitive or strong; non-bold indicates moderate or limited.
2 |. CONGENITAL HEART DISEASE IN NS
One of the principal features of NS, identified from the very first description, is cardiac involvement (Table 2). Indeed, affected patients present a wide spectrum of cardiac-related disease, which fall into two main categories: congenital heart disease (CHD), present in ~80% of patients, and hypertrophic cardiomyopathy (HCM), found in ~20% of patients (Marino et al., 1999). NS-associated CHD, which is the topic of this review, most often manifests as pulmonary valve stenosis (PVS), with an estimated prevalence of ~40%, but other types have been described, notably atrial (8%) or ventricular septal defects (ASD/VSD), as well as atrioventricular canal defects (AVCDs) (15%) (Calcagni et al., 2017). Less frequently, patients present with left-sided forms of CHD including mitral valve stenosis (6%), aortic valve stenosis (AS), and aortic coarctation (9%) (Lam, Corno, Oorthuys, & Marcelletti, 1982). Rarely, tetralogy of Fallot or patent ductus arteriosus are observed in NS. Often, patients will display complex cardiac phenotypes with multiple defects such as PVS and AS or CHD and HCM (Prendiville et al., 2014).
TABLE 2.
Spectrum of cardiac abnormalities in Noonan syndrome
| No cardiovascular disease | 10–16% | |
| Congenital heart disease | Pulmonary valve stenosis | 25–71% |
| Atrial septal defect | 4–57% | |
| Ventricular septal defect | 1–14% | |
| Atrioventricular canal defect | 1–13% | |
| Mitral abnormalities | 2–17% | |
| Aortic coarctation | 2–9% | |
| Patent ductus arteriosus | 1–6% | |
| Tetralogy of Fallot | 1–4% | |
| Other cardiovascular | Hypertrophic cardiomyopathy | 10–29% |
| Arterial aneurysms | <1% |
Data obtained from 12 cohorts of patients with a clinically established NS diagnosis, with n ≥ 20: (Burch et al., 1993; Ishizawa, Oho, Dodo, Katori, & Homma, 1996; Marino, Digilio, Toscano, Giannotti, & Dallapiccola, 1999; Bertola et al., 2000; Tartaglia et al., 2002; Sarkozy et al., 2003; Shaw, Kalidas, Crosby, Jeffery, & Patton, 2007; Sznajer et al., 2007; Smpokou, Tworog-Dube, Kucherlapati, & Roberts, 2012; Colquitt & Noonan, 2014; Li, X et al., 2019).
After the elucidation of the underlying genetic variants causing NS, it became possible to look for genotype-phenotype associations with respect to NS-associated CHD. Several have emerged; others may exist but not have been recognized due to small patient numbers. PTPN11 pathogenic variants, for example, constitute nearly 80% of the identified causes of NS among patients presenting with PVS or ASD (Prendiville et al., 2014); conversely, PTPN11-mutant NS is negatively associated with HCM (6% vs. 26% of non-PTPN11 NS patients) (Tartaglia et al., 2002). Similarly, patients with NS and AVCDs predominantly have pathogenic variants in PTPN11 (Digilio et al., 2013). RAF1 pathogenic variants, which are strongly associated with HCM, are negatively associated with PVS (Pandit et al., 2007). Pathogenic variation in RIT1 predisposes patients to both HCM and valve abnormalities (Gelb, Roberts, & Tartaglia, 2015). NS-causing SOS1 variants most often result in PVS, much like PTPN11 hits (Lepri et al., 2011).
Ideally, providers would be able to use variant-specific genotype-phenotype associations to guide families affected with NS more accurately, but efforts to achieve that have been hampered by the degree of molecular heterogeneity underlying NS. For PTPN11, the commonest NS gene (~50% of cases), attempts have been made to associate CHD with the protein domain altered (Sznajer et al., 2007), but no clear genotype-phenotype association has been established for patients with NS as of yet.
The pathogenesis of CHD in NS, particularly with respect to lesion diversity, is only partially understood. For PTPN11’s protein product, the protein tyrosine phosphatase SHP2, a clear role in valvular morphogenesis has been established. Specifically, activation of the MAPK pathway through epidermal growth factor receptors is a principal mechanism driving epithelial-mesenchymal transition (EMT) in semilunar valve formation (Krenz, Yutzey, & Robbins, 2005). Modeling of PTPN11-related NS in mice similarly showed that the heart defects arose from mutant SHP2’s activity in the developing endocardium (the source of valve-related EMT), not in the myocardium or neural crest cells. EMT was perturbed, and signaling downstream to the EGFR-related receptor, ErbB, was implicated. Mouse studies have also shown that genetic background influences the severity and type of CHD, implicating genetic modifiers (Araki et al., 2009). Overall, more research is needed in order to understand (or, more importantly, predict) what form of CHD will arise in individual patients affected by NS as well as the severity of that CHD.
3 |. SPECIFIC MANAGEMENT OF CHD IN PATIENTS DIAGNOSED WITH NS
Overall, CHD arising in the context of NS is dictated by the nature of the lesion(s) and the hemodynamics, not by the fact that the patient has NS. However, there is some information about the commonest form of CHD observed in NS, PVS, which is informed by the trait per se. In addition, some NS comorbidities are relevant for planning periinterventional care for affected patients.
3.1 |. Pulmonary valve stenosis
In NS, the severity of PVS is observed to be mild in ~60% of cases, moderate in ~10%, and severe in ~30% (Colquitt & Noonan, 2014; Shaw et al., 2007). For mild PVS in NS, the natural history is similar to patients with PVS who do not have NS; stenosis tends to be nonprogressive and unlikely to require intervention. Of note, approximately one-half of such cases will have an additional cardiac lesion that will require intervention, most commonly a secundum ASD.
Among patients with NS and PVS with moderate or severe obstruction, the rates of intervention are higher (~50% and 100%, respectively). The pulmonary valve leaflets are frequently dysplastic with commissural fusion and thickened leaflets. As a result, the standard intervention using percutaneous balloon valvuloplasty is often not as successful as is typical for PVS (McCrindle, 1994). Indeed, after initial balloon valvuloplasty, pulmonary gradient remained unfavorably elevated in 80% of patients with NS (Holzmann et al., 2018), and reintervention was necessary in up to 65% of cases (Holzmann et al., 2018; Prendiville et al., 2014).
When catheterization-based intervention fails or is deemed not feasible in patients with NS, surgical valvotomy appears to be highly successful. Although cardiac surgical outcomes data for NS are limited, an examination of a small cohort of affected individuals (mean age 23 years) found hospital lengths of stay that were deemed comparable to those for patients without genetic syndromes (Hemmati et al., 2019).
3.2 |. Atrioventricular canal defects
AVCDs are observed in up to 15% of individuals with NS. Partial AVCD is the commonest form of this lesion. These defects may be accompanied by subaortic stenosis, related to abnormalities in the mitral valve apparatus or HCM. Unusual mitral valve involvement (parachute mitral valve and double mitral valve) has also been described. Complete AVCDs, while rare, do occur (Digilio et al., 2013; Pradhan, Pandey, Usman, Kumar, & Mishra, 2013). As yet, there is insufficient information to know whether there is a tendency for the anatomy of specific Rastelli classification(s) or about surgical outcomes.
3.3 |. Relevant comorbidities
There are two comorbidities in NS that are relevant for periprocedural care, particularly for surgical interventions: chylothorax and bleeding diatheses.
The development of the lymphovascular system appears to be frequently perturbed in NS. During fetal life, this manifests as increased nuchal translucency or cystic hygroma. Those features generally resolve later during fetal life but are believed to underlie the pterygium colli (webbed neck) that is frequently observed as a feature of NS. Moreover, 5–10% of patients with NS will develop lymphatic defects, most frequently peripheral lymphedema, in adolescence or young adulthood. As with other genetic disorders for which perturbed lymphovascular defects are observed such as Down syndrome, open-heart surgery unmasks these abnormalities, resulting in postoperative chylothoraces. In patients with NS undergoing cardiac surgery, the reported prevalence of chylothorax is 10% (Hemmati et al., 2019)n-this comorbidity, however, does not seem to negatively prolong postoperative length of stay in NS patient series, as mentioned above. Chylous pericardial effusions, although considerably rarer, have also been described. Recently, a study of lymphatic imaging in patients with NS, nearly all with CHD and chylothorax, showed that thoracic duct abnormalities, retrograde intercostal flow, and pulmonary lymphatic perfusion were highly prevalent (Biko et al., 2019). Thus, it is unlikely that the postoperative chylothorax observed in patients with NS results from direct surgical injury to the thoracic duct in nearly all instances. Management of NS-associated postoperative chylothorax is, at present, not different from when it arises in other patients. However, there is an emerging role for catheter-based interventions depending on the results of lymphangiography (Savla, Itkin, Rossano, & Dori, 2017). As discussed below, medical therapy may also become possible for some of these patients.
A bleeding diathesis is present in 65% of NS patients (Artoni et al., 2014; Briggs & Dickerman, 2012; Morice, Harroche, Cairet, & Khonsari, 2018), necessitating hematological evaluation prior to major surgical procedures. The underlying issues leading to the bleeding tendencies are diverse; they include single or multiple coagulation factor deficiencies (most commonly Factors XI, XII, and VIII), von-Willebrand disease, and/or platelet-related disorders. In general, evaluation should be undertaken in consultation with a hematologist as a typical pre-cardiac surgical workup (CBC with differential, PT, aPTT) is not sufficiently sensitive for detecting clinically relevant bleeding diatheses in NS. When conducting basic coagulation screening, 39% of NS patients will present abnormalities, and a negative screen does not eliminate the possibility of perioperative bleeding events (Perez Botero et al., 2017). While no understanding of the associations with coagulation factor deficiencies or von-Willebrand disease has yet emerged, a recent elegant study using an NS mouse model and patient-derived platelets provided the first insights into the platelet-related disorders for PTPN11-related NS (Bellio et al., 2019). In brief, SHP2 has a role in several signaling events related to platelet aggregation as well as thrombus formation and growth that are perturbed due to the gain-of-function changes present in NS-mutant SHP2 proteins.
3.4 |. Initial and follow-up cardiac evaluations
At the time of diagnosis, a baseline cardiac evaluation including EKG and echocardiogram is recommended for patients with NS. If CHD or HCM is detected at the initial study, then follow-up is undertaken as per the typical protocol used by individual care providers. Particularly if the initial study is done in early infancy, repeat echocardiography is recommended through age 3 and again in later childhood to surveil for HCM. At present, it is unclear what the upper age limit is for the onset of HCM due to NS.
Arrhythmias are a possible, albeit infrequent, complication of NS. While multifocal atrial tachycardia and atrial ectopic tachycardia have been primarily associated with a different RASopathy, Costello syndrome, those arrhythmias have been observed in young infants with NS as well (Levin et al., 2018).
Arterial aneurysms are also associated with NS. There are several case reports describing aneurysms of coronary and carotid arteries as well as of the aorta (Mauro, Flors, Hoyer, Norton, & Hagspiel, 2016; Tahir et al., 2017). Similarly, CBL pathogenic variants have been associated with the development of moyamoya disease, underlining a potential role in cerebrovascular proliferation (Hyakuna et al., 2015). The prevalence of aneurysms of other arteries is not known but, at present, seems too low to recommend routine surveillance. Relatedly, a review of aortic root diameters from routine echocardiograms performed on patients with NS showed that aneurysms (defined as Z score ≥2) were prevalent (~20%) (Cornwall, Green, Nielsen, & Gelb, 2014). To date, aortic dissection has not been associated with NS so the clinical relevance of these aortic root aneurysms remains uncertain.
4 |. DIAGNOSING NS IN PATIENTS WITH CHD
In order for the information about NS that is relevant for the care of patients with the disorder to be applied, it is, of course, necessary for the diagnosis to have been established. For the time being, clinical genetics examination and genetic testing are not routine for all patients with CHD. If in the future, as can be reasonably expected, all CHD patients get at least gene panel resequencing, then the RASopathy genes will be routinely assessed so the issue of NS detection will subside.
For the time being, detection of NS depends on the clinical detection of one or more suggestive features, prompting an evaluation. Sometimes, the indications are obvious—for instance, a child with an affected parent or a baby with typical facial dysmorphia and PVS. With the more widespread use of RASopathy gene panel testing, it has become clear that NS can be subtle in its presentation, even as it is still believed to be completely penetrant. Thus, care providers need to be attentive to this possible diagnosis in order to avoid missing it.
In the context of CHD, the focus has been on patients with PVS as that is the commonest lesion. A study of a cohort of 204 patients with PVS found that 6% had NS (Anderson et al., 2019); of note, the cases with non-syndromic PVS were not routinely tested for NS. Compared to non-syndromic cases of PVS, those with PVS due to NS were more likely to have features associated with NS: short stature, pectus deformity, neurodevelopmental delays or other, unexpected cardiac issues such as HCM or AS. While there are no data about this for other forms of CHD associated with NS, it seems reasonable to extrapolate: NS can be considered for any child with a form of CHD associated with NS and exhibiting other features of NS.
For patients with CHD but without any other feature of NS (i.e., isolated CHD), the available evidence suggests that NS prevalence will be extremely low. One study looked at PTPN11 variation among cohorts of patients with isolated aortic coarctation (n = 157) and AVSD (n = 24), finding a single patient with isolated AVSD who harbored a novel PTPN11 variant that might cause NS (Weismann et al., 2005). This issue has not been examined for isolated PVS, but it would be surprising if it turned out otherwise. Thus, NS evaluation is unlikely to have a worthwhile yield for patients with isolated PVS or other NS-associated forms of CHD. A possible exception might be a young infant with PVS, particularly with a dysplastic valve, in whom other features of NS such as growth, dysmorphia, pectus deformity, and neurodevelopment might not have emerged yet.
When NS or other RASopathy is suspected (differentiating the two in newborns and young infants can be challenging), evaluation by a care provider with expertise in making the diagnosis, generally a clinical geneticist, is appropriate. That evaluation will typically entail a careful medical history, including family history, physical examination to elicit features, even subtle ones, of these traits, and genetic testing. For the genetic testing, the current standard is to undertake resequencing of the RASopathy genes through a panel. Thanks to the capacity of next-generation sequencing machines, such gene sequencing panels now may include genes such as NF1 that can result in overlapping phenotypes. Compared to many other traits, the interpretation of variants in genes underlying the RASopathies is relatively straightforward. When there is uncertainty about a particular variant, demonstrating that it arose de novo in an affected child with a sporadic RASopathy can help resolve the interpretation. Of note, the currently known NS genes account for ~85% of cases in aggregate. Thus, a child diagnosed confidently with NS who has negative genetic testing can still be assumed to have the disorder.
Gene resequencing panels will become obsolete at some point. The cost of sequencing human exomes or genomes has declined spectacularly over the past several years (Sboner, Mu, Greenbaum, Auerbach, & Gerstein, 2011). At some point, exome or genome sequencing will become the primary test, providing several possible advantages-discovery of tangentially related traits that were not on the differential diagnosis list, ability to revisit the data as new RASopathy genes are discovered, and simultaneous detection of actionable variation in genes unrelated to NS (e.g., cancer predisposition variants).
5 |. FUTURE PROSPECTS FOR TREATING NS-RELATED CHD
The pathogenesis of NS appears to result primarily from gain-of-function changes in RAS/MAPK signal transduction. This is encouraging with respect to devising novel therapeutic strategies for NS in two respects. First, the RAS/MAPK pathway is reasonably well understood. It comprises a series of protein phosphorylation events mediated by protein kinases, a class of proteins that is generally amenable to small-molecule inhibition. Second, acquired gene alterations resulting in RAS/MAPK gain of function are prevalent in cancer. As such, there has been a strong impetus to develop drugs that inhibit RAS/MAPK pathway proteins. To date, the most advanced ones are MEK inhibitors-trametinib, cobimetinib, and binimetinib are FDA approved for use in BRAF-mutant cancers, particularly melanoma (Grimaldi et al., 2017). Other pathway inhibitors are in various stages of drug development.
Can drugs inhibiting the RAS/MAPK pathway, which is needed for essential biological processes such as hematopoiesis, be given safely to children with NS? And, if so, would there be efficacy with respect to CHD? For MEK inhibitors, there are side effects, particularly involving the skin, which are well described. Those, of course, are related to the use of the medication for relatively short periods of time as is common for anticancer therapies. It is unclear whether the side-effect profile for young children with NS will be similar or not, particularly as therapy might be needed for longer durations. Of note, there is a major biological difference as well: patients with cancer have RAS/MAPK gain-of-function variation only in their tumor cells whereas individuals with NS have their genetic variant, which tends to engender milder gain of function, in all of their cells. Taken as a whole, it is difficult to predict how children with NS will respond to a MEK inhibitor or other drugs targeting the RAS/MAPK pathway.
To date, there is only one publication describing the use of RAS/MAPK pathway targeted therapy for NS (Andelfinger et al., 2019). Trametinib was given to two infants with the severe, rapidly progressive form of HCM, due, in both cases, to RIT1 pathogenic variants. In both instances, the HCM improved with regression of left ventricular mass, outflow tract obstruction, and heart failure. While this was an uncontrolled experience with giving trametinib on a compassionate-usage basis, which limits the conclusions one can draw, it is noteworthy that the outcomes for young infants with NS-related HCM with early-onset heart failure is ordinarily dismal (Wilkinson et al., 2012). The treatment was well tolerated with only a self-limited rash. Of note, the patients’ trametinib therapy is ongoing as treatment cessation appeared to be associated with HCM recurrence; neither patient has experienced additional adverse effects as yet (Gregor Andelfinger, personal communication).
Both of the two infants with NS treated with trametinib for severe HCM also had PVS with dysplastic valve leaflets (Andelfinger et al., 2019 and personal communications from Gregor Andelfinger and Michael Hofbeck). For one, balloon valvuloplasty was attempted but was unsuccessful. With trametinib treatment, both patients’ obstruction improved (pretreatment gradients, 62 and 45 mmHg; posttreatment gradient, 8 and 21 mm Hg, respectively) (Andelfinger et al., 2019) and their dysplastic valve morphology resolved (personal communications from Gregor Andelfinger and Michael Hofbeck). While two uncontrolled experiences cannot prove anything, it is intriguing to consider that inhibition of the RAS/MAPK hyperactivation may allow remodeling of an abnormal valve, resulting in a nearly normal outcome. If further experience with trametinib or other pathway inhibitors in NS strengthens our confidence in the safety profile, then it might be feasible to undertake a systematic study of the effects of such drugs for PVS in NS.
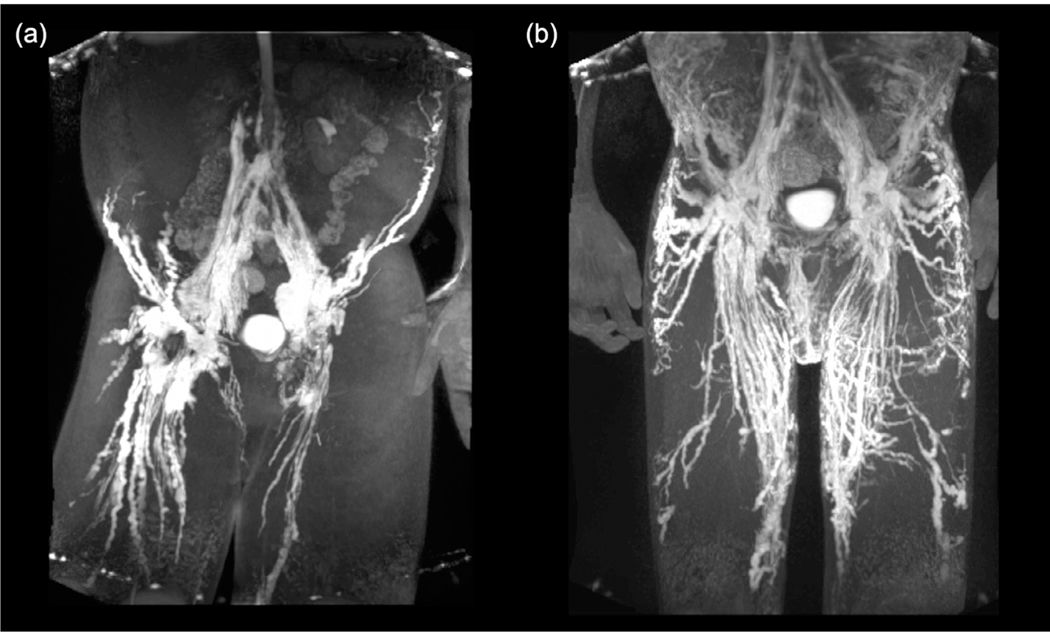
Recent information also indirectly suggests that RAS pathway inhibition might be useful for the treatment of lymphovascular abnormalities in NS. Two patients with central conducting lymphatic anomaly (CCLA) were observed to have the same gain-of-function de novo Ser214Pro allele in ARAF (Li, D et al., 2019). This allele altered the same amino acid residue as observed in pathogenic RAF1 variants underlying NS. Modeling in zebrafish revealed lymphatic defects, which were rescued by treatment with the MEK inhibitor cobimetinib. One of the two patients with CCLA was subsequently treated with trametinib. This therapy resulted in a dramatic improvement in his lymphatic system-related symptoms and, most impressively, pre-treatment and posttreatment lymphangiography revealed remodeling of his lymphovascular system (Figure 2). Current efforts are underway to initiate a MEK inhibitor treatment trial of patients with severe lymphatic disease with RASopathy pathogenic variation, with individuals with NS expected to constitute a significant proportion.
FIGURE 2.
Effect of MEK inhibitor treatment on lymphatic abnormalities in a patient with a gain-of-function pathogenic ARAF variant. Coronal maximal intensity projections of contrast lymphangiograms of the pelvis and thighs before (left) and after (right) MEK-inhibitor treatment of proband. Pretreatment, the patient presents distinct dilation and beading of the lymphatic system; after a 12-month treatment, the patient displays resorption of the dilated ducts and formation of new and symmetrical lymphatic networks. Figure reprinted with permission from Springer Nature (Li, D et al., 2019)
6 |. CONCLUSION AND PERSPECTIVES
In more than 50 years since Dr. Noonan described children with PVS and a stereotypic appearance, our understanding of NS has increased substantially. While PVS is the commonest form of CHD associated with this trait, we now know that a broader array of lesions exists, necessitating a higher index of suspicion for care providers. Moreover, while PVS with dysplastic pulmonary valve leaflets has been considered a hallmark of disease and led to the belief that balloon valvuloplasty is fruitless for patients with NS, epidemiologic studies have shown that NS-associated PVS is often mild and nonprogressive as well as amenable to catheter-based interventions in select cases when more severe. We also have a clearer sense of NS-associated bleeding diatheses and lymphatic abnormalities, which require attentiveness when cardiac surgical interventions are undertaken.
Over the past 18 years, the genetic basis of NS and its phenotypically related disorders has been elucidated. Although that project remains incomplete, the set of known RASopathy genes has enabled relatively robust genetic testing. Paired with the several genotype-phenotype associations that have been established for NS, molecular diagnoses are enabling earlier and more precise identification of affected individuals, particularly for patients who have a subtle or atypical presentation, as well as a better prognostication of CHD, HCM, and extracardiac manifestations of the trait. Ongoing research regarding NS pathogenesis is informing efforts to devise NS-specific therapeutics with some tantalizing possibilities on the horizon.
ACKNOWLEDGMENTS
This work was supported by NIH National Heart, Lung and Blood Institute (R35HL135742) to B.D.G. and by the Liliane Bettencourt INSERM Foundation (L.L.).
Funding information
Fondation Bettencourt Schueller; NIH/NHLBI, Grant/Award Number: R35HL135742
Footnotes
CONFLICT OF INTEREST
The Icahn School of Medicine at Mount Sinai receives royalties for genetic testing of Noonan syndrome from GeneDx, Prevention Genetics, LabCorp, and Correlegan, of which B.D.G. receives a portion.
REFERENCES
- Andelfinger G, Marquis C, Raboisson M-J, Théoret Y, Waldmüller S, Wiegand G, … Hofbeck M. (2019). Hypertrophic cardiomyopathy in Noonan syndrome treated by MEK-inhibition. Journal of the American College of Cardiology, 73(17), 2237–2239. 10.1016/j.jacc.2019.01.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson K, Cnota J, James J, Miller EM, Parrott A, Pilipenko V, … Shikany A. (2019). Prevalence of Noonan spectrum disorders in a pediatric population with valvar pulmonary stenosis. Congenital Heart Disease, 14(2), 264–273. 10.1111/chd.12721 [DOI] [PubMed] [Google Scholar]
- Aoki Y, Niihori T, Banjo T, Okamoto N, Mizuno S, Kurosawa K, … Matsubara Y. (2013). Gain-of-function mutations in RIT1 cause Noonan syndrome, a RAS/MAPK pathway syndrome. American Journal of Human Genetics, 93(1), 173–180. 10.1016/j.ajhg.2013.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki T, Chan G, Newbigging S, Morikawa L, Bronson RT, & Neel BG (2009). Noonan syndrome cardiac defects are caused by PTPN11 acting in endocardium to enhance endocardial-mesenchymal transformation. Proceedings of the National Academy of Sciences of the United States of America, 106(12), 4736–4741. 10.1073/pnas.0810053106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artoni A, Selicorni A, Passamonti SM, Lecchi A, Bucciarelli P, Cerutti M, … Martinelli I. (2014). Hemostatic abnormalities in Noonan syndrome. Pediatrics, 133(5), e1299–e1304. 10.1542/peds.2013-3251 [DOI] [PubMed] [Google Scholar]
- Bellio M, Garcia C, Edouard T, Voisin S, Neel BG, Cabou C, … Severin S. (2019). Catalytic dysregulation of SHP2 leading to Noonan syndromes impacts on platelet signaling and functions. Blood, 134, 2304–2317. 10.1182/blood.2019001543 [DOI] [PubMed] [Google Scholar]
- Bertola DR, Kim CA, Sugayama SM, Albano LM, Wagenführ J, Moysés RL, & Gonzalez CH (2000). Cardiac findings in 31 patients with Noonan’s syndrome. Arquivos Brasileiros de Cardiologia, 75(5), 409–412. [DOI] [PubMed] [Google Scholar]
- Biko DM, Reisen B, Otero HJ, Ravishankar C, Victoria T, Glatz AC, … Dori Y. (2019). Imaging of central lymphatic abnormalities in Noonan syndrome. Pediatric Radiology, 49(5), 586–592. 10.1007/s00247-018-04337-6 [DOI] [PubMed] [Google Scholar]
- Briggs BJ, & Dickerman JD (2012). Bleeding disorders in Noonan syndrome. Pediatric Blood & Cancer, 58(2), 167–172. 10.1002/pbc.23358 [DOI] [PubMed] [Google Scholar]
- Burch M, Sharland M, Shinebourne E, Smith G, Patton M, & McKenna W. (1993). Cardiologic abnormalities in Noonan syndrome: Phenotypic diagnosis and echocardiographic assessment of 118 patients. Journal of the American College of Cardiology, 22(4), 1189–1192. 10.1016/0735-1097(93)90436-5 [DOI] [PubMed] [Google Scholar]
- Calcagni G, Limongelli G, D’Ambrosio A, Gesualdo F, Digilio MC, Baban A, … Marino B. (2017). Cardiac defects, morbidity and mortality in patients affected by RASopathies. CARNET study results. International Journal of Cardiology, 245, 92–98. 10.1016/j.ijcard.2017.07.068 [DOI] [PubMed] [Google Scholar]
- Colquitt JL, & Noonan JA (2014). Cardiac findings in Noonan syndrome on long-term follow-up. Congenital Heart Disease, 9(2), 144–150. 10.1111/chd.12102 [DOI] [PubMed] [Google Scholar]
- Cornwall JW, Green RS, Nielsen JC, & Gelb BD (2014). Frequency of aortic dilation in Noonan syndrome. The American Journal of Cardiology, 113(2), 368–371. 10.1016/j.amjcard.2013.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digilio MC, Romana Lepri F, Lisa Dentici M, Henderson A, Baban A, Cristina Roberti M, … Dallapiccola B. (2013). Atrioventricular canal defect in patients with RASopathies. European Journal of Human Genetics, 21(2), 200–204. 10.1038/ejhg.2012.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelb BD, Roberts AE, & Tartaglia M. (2015). Cardiomyopathies in Noonan syndrome and the other RASopathies. Progress in Pediatric Cardiology, 39(1), 13–19. 10.1016/j.ppedcard.2015.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi AM, Simeone E, Festino L, Vanella V, Strudel M, & Ascierto PA (2017). MEK inhibitors in the treatment of metastatic melanoma and solid tumors. American Journal of Clinical Dermatology, 18(6), 745–754. 10.1007/s40257-017-0292-y [DOI] [PubMed] [Google Scholar]
- Hemmati P, Dearani JA, Daly RC, King KS, Ammash NM, Cetta F, & Schaff HV (2019). Early outcomes of cardiac surgery in patients with Noonan syndrome. Seminars in Thoracic and Cardiovascular Surgery, 31(3), 507–513. 10.1053/j.semtcvs.2018.12.004 [DOI] [PubMed] [Google Scholar]
- Holzmann J, Tibby SM, Rosenthal E, Qureshi S, Morgan G, & Krasemann T. (2018). Results of balloon pulmonary valvoplasty in children with Noonan’s syndrome. Cardiology in the Young, 28(5), 647–652. 10.1017/S1047951117002827 [DOI] [PubMed] [Google Scholar]
- Hyakuna N, Muramatsu H, Higa T, Chinen Y, Wang X, & Kojima S. (2015). Germline mutation of CBL is associated with moyamoya disease in a child with juvenile myelomonocytic leukemia and Noonan syndrome-like disorder. Pediatric Blood & Cancer, 62(3), 542–544. 10.1002/pbc.25271 [DOI] [PubMed] [Google Scholar]
- Ishizawa A, Oho S, Dodo H, Katori T, & Homma SI (1996). Cardiovascular abnormalities in Noonan syndrome: The clinical findings and treatments. Acta Paediatrica Japonica: Overseas Edition, 38(1), 84–90. [DOI] [PubMed] [Google Scholar]
- Johnston JJ, van der Smagt JJ, Rosenfeld JA, Pagnamenta AT, Alswaid A, Baker EH, … Biesecker LG (2018). Autosomal recessive Noonan syndrome associated with biallelic LZTR1 variants. Genetics in Medicine, 20(10), 1175–1185. 10.1038/gim.2017.249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenz M, Yutzey KE, & Robbins J. (2005). Noonan syndrome mutation Q79R in Shp2 increases proliferation of valve primordia mesenchymal cells via extracellular signal-regulated kinase 1/2 signaling. Circulation Research, 97(8), 813–820. 10.1161/01.RES.0000186194.06514.b0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam J, Corno A, Oorthuys HWE, & Marcelletti C. (1982). Unusual combination of congenital heart lesions in a child with Noonan’s syndrome. Pediatric Cardiology, 3(1), 23–26. 10.1007/BF02082326 [DOI] [PubMed] [Google Scholar]
- Lepri F, De Luca A, Stella L, Rossi C, Baldassarre G, Pantaleoni F, … Tartaglia M. (2011). SOS1 mutations in Noonan syndrome: Molecular spectrum, structural insights on pathogenic effects, and genotype-phenotype correlations. Human Mutation, 32(7), 760–772. 10.1002/humu.21492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin MD, Saitta SC, Gripp KW, Wenger TL, Ganesh J, Kalish JM, … Lin AE (2018). Nonreentrant atrial tachycardia occurs independently of hypertrophic cardiomyopathy in RASopathy patients. American Journal of Medical Genetics, Part A, 176(8), 1711–1722. 10.1002/ajmg.a.38854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, March ME, Gutierrez-Uzquiza A, Kao C, Seiler C, Pinto E, … Hakonarson H. (2019). ARAF recurrent mutation causes central conducting lymphatic anomaly treatable with a MEK inhibitor. Nature Medicine, 25(7), 1116–1122. 10.1038/s41591-0190479-2 [DOI] [PubMed] [Google Scholar]
- Li X, Yao R, Tan X, Li N, Ding Y, Li J, … Wang X. (2019). Molecular and phenotypic spectrum of Noonan syndrome in Chinese patients. Clinical Genetics, 96(4), 290–299. 10.1111/cge.13588 [DOI] [PubMed] [Google Scholar]
- Marino B, Digilio MC, Toscano A, Giannotti A, & Dallapiccola B. (1999). Congenital heart diseases in children with Noonan syndrome: An expanded cardiac spectrum with high prevalence of atrioventricular canal. The Journal of Pediatrics, 135(6), 703–706. 10.1016/s0022-3476(99)70088-0 [DOI] [PubMed] [Google Scholar]
- Mauro DM, Flors L, Hoyer AW, Norton PT, & Hagspiel KD (2016). Development of bilateral coronary artery aneurysms in a child with Noonan syndrome. Pediatric Radiology, 46(3), 422–425. 10.1007/s00247-015-3472-z [DOI] [PubMed] [Google Scholar]
- McCrindle BW (1994). Independent predictors of long-term results after balloon pulmonary valvuloplasty. Valvuloplasty and Angioplasty of Congenital Anomalies (VACA) Registry Investigators. Circulation, 89(4), 1751–1759. 10.1161/01.cir.89.4.1751 [DOI] [PubMed] [Google Scholar]
- Morice A, Harroche A, Cairet P, & Khonsari RH (2018). Preoperative detailed coagulation tests are required in patients with Noonan syndrome. Journal of Oral and Maxillofacial Surgery, 76(7), 1553–1558. 10.1016/j.joms.2017.12.012 [DOI] [PubMed] [Google Scholar]
- Noonan JA (1968). Hypertelorism with turner phenotype. A new syndrome with associated congenital heart disease. American Journal of Diseases of Children, 116(4), 373–380. [DOI] [PubMed] [Google Scholar]
- Pandit B, Sarkozy A, Pennacchio LA, Carta C, Oishi K, Martinelli S, … Gelb BD (2007). Gain-of-function RAF1 mutations cause Noonan and LEOPARD syndromes with hypertrophic cardiomyopathy. Nature Genetics, 39(8), 1007–1012. 10.1038/ng2073 [DOI] [PubMed] [Google Scholar]
- Perez Botero J, Ho TP, Rodriguez V, Khan SP, Pruthi RK, & Patnaik MM (2017). Coagulation abnormalities and haemostatic surgical outcomes in 142 patients with Noonan syndrome. Haemophilia, 23(3), e237–e240. 10.1111/hae.13225 [DOI] [PubMed] [Google Scholar]
- Pradhan AK, Pandey S, Usman K, Kumar M, & Mishra R. (2013). Noonan syndrome with complete atrioventricular canal defect with pulmonary stenosis. Journal of the American College of Cardiology, 62 (20), 1905 10.1016/j.jacc.2013.06.062 [DOI] [PubMed] [Google Scholar]
- Prendiville TW, Gauvreau K, Tworog-Dube E, Patkin L, Kucherlapati RS, Roberts AE, & Lacro RV (2014). Cardiovascular disease in Noonan syndrome. Archives of Disease in Childhood, 99(7), 629–634. 10.1136/archdischild-2013-305047 [DOI] [PubMed] [Google Scholar]
- Roberts AE, Allanson JE, Tartaglia M, & Gelb BD (2013). Noonan syndrome. Lancet, 381(9863), 333–342. 10.1016/S0140-6736(12)61023-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkozy A, Conti E, Seripa D, Digilio MC, Grifone N, Tandoi C, … Dallapiccola B. (2003). Correlation between PTPN11 gene mutations and congenital heart defects in Noonan and LEOPARD syndromes. Journal of Medical Genetics, 40(9), 704–708. 10.1136/jmg.40.9.704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savla JJ, Itkin M, Rossano JW, & Dori Y. (2017). Post-operative chylothorax in patients with congenital heart disease. Journal of the American College of Cardiology, 69(19), 2410–2422. 10.1016/j.jacc.2017.03.021 [DOI] [PubMed] [Google Scholar]
- Sboner A, Mu XJ, Greenbaum D, Auerbach RK, & Gerstein MB (2011). The real cost of sequencing: Higher than you think! Genome Biology, 12(8), 125 10.1186/gb-2011-12-8-125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw AC, Kalidas K, Crosby AH, Jeffery S, & Patton MA (2007). The natural history of Noonan syndrome: A long-term follow-up study. Archives of Disease in Childhood, 92(2), 128–132. 10.1136/adc.2006.104547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smpokou P, Tworog-Dube E, Kucherlapati RS, & Roberts AE (2012). Medical complications, clinical findings, and educational outcomes in adults with Noonan syndrome. American Journal of Medical Genetics. Part A, 158A(12), 3106–3111. 10.1002/ajmg.a.35639 [DOI] [PubMed] [Google Scholar]
- Sznajer Y, Keren B, Baumann C, Pereira S, Alberti C, Elion J, … Verloes A. (2007). The spectrum of cardiac anomalies in Noonan syndrome as a result of mutations in the PTPN11 gene. Pediatrics, 119(6), e1325–e1331. 10.1542/peds.2006-0211 [DOI] [PubMed] [Google Scholar]
- Tahir RA, Asmaro K, Pabaney A, Kole M, Nypaver T, & Marin H. (2017). Separate origins of the left internal and external carotid arteries from the aortic arch and cervical internal carotid artery aneurysm in a patient with Noonan syndrome. Journal of Neurointerventional Surgery, 9(4), e11. 10.1136/neurintsurg-2016-012482.rep [DOI] [PubMed] [Google Scholar]
- Tajan M, Paccoud R, Branka S, Edouard T, & Yart A. (2018). The RASopathy family: Consequences of germline activation of the RAS/MAPK pathway. Endocrine Reviews, 39(5), 676–700. 10.1210/er.2017-00232 [DOI] [PubMed] [Google Scholar]
- Tartaglia M, Gelb BD, & Zenker M. (2011). Noonan syndrome and clinically related disorders. Best Practice & Research Clinical Endocrinology & Metabolism, 25(1), 161–179. 10.1016/j.beem.2010.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia M, Kalidas K, Shaw A, Song X, Musat DL, van der Burgt I, … Gelb BD (2002). PTPN11 mutations in Noonan syndrome: Molecular spectrum, genotype-phenotype correlation, and phenotypic heterogeneity. American Journal of Human Genetics, 70(6), 1555–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia M, Mehler EL, Goldberg R, Zampino G, Brunner HG, Kremer H, … Gelb BD (2001). Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nature Genetics, 29(4), 465–468. 10.1038/ng772 [DOI] [PubMed] [Google Scholar]
- Tidyman WE, & Rauen KA (2016). Expansion of the RASopathies. Current Genetic Medicine Reports, 4(3), 57–64. 10.1007/s40142-016-0100-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weismann CG, Hager A, Kaemmerer H, Maslen CL, Morris CD, Schranz D, … Gelb BD (2005). PTPN11 mutations play a minor role in isolated congenital heart disease. American Journal of Medical Genetics, Part A, 136(2), 146–151. 10.1002/ajmg.a.30789 [DOI] [PubMed] [Google Scholar]
- Wilkinson JD, Lowe AM, Salbert BA, Sleeper LA, Colan SD, Cox GF, … Lipshultz SE (2012). Outcomes in children with Noonan syndrome and hypertrophic cardiomyopathy: A study from the pediatric cardiomyopathy registry. American Heart Journal, 164(3), 442–448. 10.1016/j.ahj.2012.04.018 [DOI] [PubMed] [Google Scholar]
- Yamamoto GL, Aguena M, Gos M, Hung C, Pilch J, Fahiminiya S, … Bertola DR (2015). Rare variants in SOS2 and LZTR1 are associated with Noonan syndrome. Journal of Medical Genetics, 52(6), 413–421. 10.1136/jmedgenet-2015-103018 [DOI] [PubMed] [Google Scholar]
- Yu KPT, Luk H-M, Leung GKC, Mak CCY, Cheng SSW, Hau EWL, … Lo IFM (2019). Genetic landscape of RASopathies in Chinese: Three decades’ experience in Hong Kong. American Journal of Medical Genetics Part C: Seminars in Medical Genetics, 181(2), 208–217. 10.1002/ajmg.c.31692 [DOI] [PubMed] [Google Scholar]