Significance
Many biological processes are based on mechanosensitive channels which are stimulated by mechanical forces. In cell-walled organisms, the large family of MscS-like channels is extremely diverse and often several different versions of these channels are present in the same organism. However, we hardly understand the reason for this diversity because only the simplest known member of this family, MscS, has been studied in some depth. Here, we present the molecular structures of two more complex members of this family, YnaI and YbiO. While the basic architecture of the core is conserved, functional properties and the way they open vary. We ultimately will learn how evolution provides by structural variation a wider range of cellular functions.
Keywords: electron cryomicroscopy, hypoosmotic shock, tension sensing, YbiO, YnaI
Abstract
The mechanosensitive channel of small conductance (MscS) is the prototype of an evolutionarily diversified large family that fine-tunes osmoregulation but is likely to fulfill additional functions. Escherichia coli has six osmoprotective paralogs with different numbers of transmembrane helices. These helices are important for gating and sensing in MscS but the role of the additional helices in the paralogs is not understood. The medium-sized channel YnaI was extracted and delivered in native nanodiscs in closed-like and open-like conformations using the copolymer diisobutylene/maleic acid (DIBMA) for structural studies. Here we show by electron cryomicroscopy that YnaI has an extended sensor paddle that during gating relocates relative to the pore concomitant with bending of a GGxGG motif in the pore helices. YnaI is the only one of the six paralogs that has this GGxGG motif allowing the sensor paddle to move outward. Access to the pore is through a vestibule on the cytosolic side that is fenestrated by side portals. In YnaI, these portals are obstructed by aromatic side chains but are still fully hydrated and thus support conductance. For comparison with large-sized channels, we determined the structure of YbiO, which showed larger portals and a wider pore with no GGxGG motif. Further in silico comparison of MscS, YnaI, and YbiO highlighted differences in the hydrophobicity and wettability of their pores and vestibule interiors. Thus, MscS-like channels of different sizes have a common core architecture but show different gating mechanisms and fine-tuned conductive properties.
Hearing, the sense of touch, and blood pressure regulation are among important biological functions that depend on mechanosensitive (MS) channels (1). The most basic and presumably the earliest function of these channels is osmoregulation under hypoosmotic stress (2). In particular, bacteria and archaea are directly exposed to the environment and thus to sudden changes in osmolarity. The model organism Escherichia coli has, for example, the mechanosensitive channels of large conductance (MscL) and of small conductance (MscS) (3). MscS-like channels form a diverse family (4–6) and, besides the archetypical MscS, five larger paralogs are found in E. coli. In comparison with MscS, which has three transmembrane (TM) helices per subunit, they can be grouped into two classes with either 5 predicted TM helices (YnaI and YbdG) or 11 predicted TM helices (YbiO, MscK, and YjeP). Beyond bacteria, MscS-like channels are also found in archaea, yeast, protozoa, and plants.
It remains unclear why E. coli maintains seven different kinds of MS channels. Protection against hypoosmotic shock is mainly provided by MscL and MscS (7). The other MscS-like channels are also able to protect E. coli but only at elevated expression levels (8). Their native protective function may relate to specific environmental conditions. However, some homologs may have altogether different functions. For example, YbdG, that shows no electrophysiological activity as wild type (WT) but only as a mutational variant (9), has recently been shown to be more important in hyperosmotic stress (10). Functional or conditional diversity is also implied by the fact that they do not have a common regulation (8).
MscS is a well-studied model system for mechanosensation. MscS assembles homoheptameric complexes with a large vestibule enclosing a cytosolic domain and an N-terminal membrane domain (Fig. 1A). The two N-terminal TM helices from each subunit form a bundle, referred to as a paddle, which tilts away from the central axis of the core complex. The third TM helix from each subunit comes together around the central axis of the complex to establish the pore (TM3a) and after a kink at G113 connects to the periplasmic domain (TM3b). This arrangement leaves large cavities between paddles and TM3 helices.
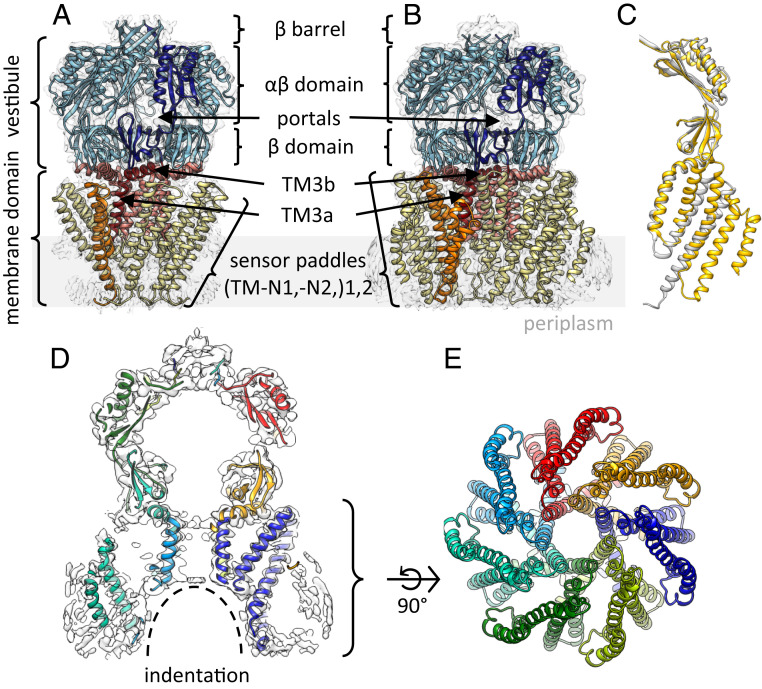
Fig. 1.
Cryo-EM structure of YnaI at 3.0-Å resolution. (A and B) Side views of the atomic models of MscS [A; PDB ID code 6RLD and EMDB ID code 4919 (11–13)] and YnaI (B) in their corresponding EM density. The similarity of the architecture of both heptamers is highlighted by the domain coloring: the cytosolic vestibule (blue), pore helices TM3 (red; separated into TM3a and TM3b), and tilted sensor paddles (yellow, orange). One subunit is highlighted in dark colors. The membrane is indicated as a gray rectangle. (C) Overlay of single subunits of YnaI (yellow) and MscS (gray). The paddle of YnaI is extended by two additional TM helices compared with MscS, while only minor changes are visible in the vestibule. (D) Side-view slice of the map of YnaI-DIBMALPs (white) with the atomic model. The subunits are colored differently. (E) View of the model (membrane domain only) from the periplasmic side.
Several crystal structures depict MscS either in a closed (14–17) or an open (17–20) conformation. Comparisons suggest that the paddles rotate around the central axis and pull and turn the TM3a helices into an open conformation while the cytosolic vestibule is predominantly static (18). Osmotic stress is sensed by MscS through the increase of tension in the membrane so the paddles, which are in direct contact with the membrane, must be of key importance to the sensing mechanism. Recent electron cryomicroscopy (cryo-EM) structures of MscS embedded in the membrane (11, 21) defined the exact contact of the membrane in the closed state which was confirmed by spectroscopic data (22). These studies also showed that lipids are found beyond the membrane in deep pockets between the paddles, which led to the model that extrusion of the lipids from the pockets triggers gating (20).
The paddles play an important role in tension sensing of MscS, which highlights the fact that other homologs of the MscS-like family have additional TM helices N-terminally attached to them. On the basis of a low-resolution cryo-EM structure of YnaI it was speculated that the additional TM helices coalign to and extend the paddles (23). Another cryo-EM structure of YnaI at higher resolution did not resolve the additional TM helices and only could be modeled from helix TM2 (24).
While the properties of the vestibule together with the channel formed by TM3 determine ion selectivity and conductivity of MscS-like channels (conductivity module), the paddles rearrange in response to changes in the membrane (sensing module). How the two functionalities are coupled and lead to gating is still controversial. Here we present cryo-EM structures of two larger MscS-like channels, YnaI and YbiO. In both structures, we resolve the conductivity module at ∼3-Å resolution and show that local hydrophobicity together with constrictions are likely to control the conductivity in the different MscS-like channels. We also resolve the complete sensing module of YnaI that lifts the conductivity module out of the plane of the membrane. Conformational changes induced by the addition of lysophosphatidylcholine (LPC), known to open MscS, lead to rearrangements of the sensing module with respect to the conductivity module relocating the conductivity module into the plane of the membrane. The relocation is not tightly coupled to gating. Cryo-EM and structure sorting reveal subpopulations, suggesting that gating requires conformational changes which seem unique to YnaI and its TM3a helix sequence.
Results
The Pore of YnaI Protrudes from the Plane of the Membrane in the Closed-like Conformation.
We obtained a 3.0-Å-resolution map of YnaI embedded in the lipid bilayer of large native nanodiscs, which is directly assembled from E. coli plasma membranes using the diisobutylene/maleic acid (DIBMA) copolymer (25, 26) (SI Appendix, Figs. S1 and S2). The overall structure of YnaI is conserved in comparison with the closed MscS described above with the cytosolic vestibule domain, pore-forming helix TM3, and the paddle (Fig. 1B), in agreement with an earlier EM map of YnaI (24).
Beyond the region of this earlier EM map, our structure resolves two additional TM helices N-terminal to the paddle although only the backbone could be modeled for this region. Following the MscS nomenclature, we named these helices TM-N1 and TM-N2 (for “minus” N-terminal) to allow easy comparison of the conserved TM helices in MscS as well as comparisons with larger homologs having more N-terminal helices (Fig. 1B). They form a helix bundle that lies parallel to and extends the inner helix bundle of TM1 and TM2. With a length of ∼45 Å, helices TM-N1 and TM-N2 span the membrane which is visible as additional density in the EM map (Fig. 1 B and D and SI Appendix, Fig. S3 D–G). Helix TM1 is shorter compared with MscS (11, 21) (∼59 Å; Fig. 1C) but the inner bundle is shifted against the outer bundle, resulting in a similar structural arrangement as in MscS: The periplasmic half of the membrane domain is embedded in the membrane while the loop between helices TM1 and TM2 protrudes into the cytosol by 15 Å. The staggered arrangement lifts the central pore out of the plane of the membrane at the cytosolic side. On the periplasmic side, this leads to an indentation in the membrane which is larger than the funnel in MscS (Fig. 1D). Overall, the membrane domain constitutes a dome-like architecture that encloses the indentation at the periplasmic side.
The inner and outer helical bundles are well-connected between TM1 and TM-N1, with numerous density bridges along the full length of the coaligning helices (SI Appendix, Fig. S3E). In contrast, within the bundles, connections between the helices are predominantly within the loops. While all four TM helices within a paddle are interconnected by contacts to the following and preceding helices, no interactions are seen between the paddles from different subunits. The paddles expand like blades of a propeller around the sevenfold symmetry axis with deep grooves separating them (Fig. 1E). The B factors and the local resolution map indicate that the paddles become gradually more flexible toward the outside of the propeller (SI Appendix, Fig. S3).
YnaI Activation Mechanism Differs from MscS.
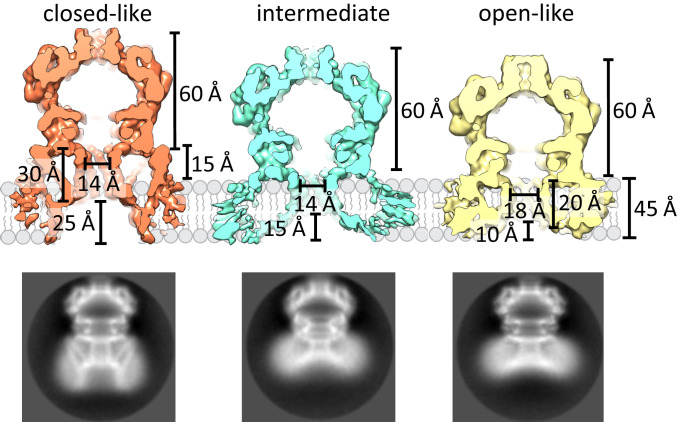
LPC has been shown to activate MscL and MscS in patch-clamp experiments (27–30). Asymmetric insertion of the cone-shaped LPC from the cytosolic side triggers channel opening by creating tension in the membrane (28, 31). Pliotas et al. (20) showed that LPC enters the pockets of MscS and postulated that LPC, because of its smaller hydrophobic volume, destabilizes the closed conformation, which leads to channel opening. We tried to find whether it is possible to extract LPC-activated YnaI directly from membrane vesicles (SI Appendix, Fig. S4). For this, we added 5 µM LPC (30) to YnaI containing proteoliposomes and isolated the channels using DIBMA. It should be noted that LPC as well as phosphatidylcholine, used for the reconstitution, are not native to E. coli and could impact on structure and function. Single-particle cryo-EM analysis displays two-dimensional (2D) class averages with different structural arrangements of the membrane part (Fig. 2 and SI Appendix, Fig. S5). Further 3D classification results in three conformations: One resembles the channel that was isolated directly from the native E. coli membranes and accounts for about 13% of all side-view projections. We will refer to this state as closed-like (map with 3.8-Å resolution; SI Appendix, Fig. S6A). The other two conformations are unchanged in the cytosolic domain (SI Appendix, Fig. S5) but differ in the membrane-embedded part (Fig. 2). The differences mainly relate to the pore diameter and the periplasmic indentation. One of the conformations has a wider pore and the TM3a helices are moved away from the central C7 symmetry axis, thus increasing the minimal diameter between the TM3a helix centers from 14 to 18 Å and shortening the pore length from 30 to 20 Å (Fig. 2). In the following, we will refer to this channel as open-like (map with 4.1-Å resolution; SI Appendix, Fig. S6C). The periplasmic indentation and the protruding elements are diminished.
Fig. 2.
YnaI captured in a closed-like, intermediate, and open-like conformation. Comparison of the dimensions of the closed-like (red), intermediate (blue), and open-like (yellow) conformations of YnaI. Significant changes are revealed in the membrane domain upon opening: In the closed-like form, the membrane domain protrudes from the membrane but is completely buried in the membrane in the open-like conformation accompanied by a diminished indentation on the periplasmic side. Representative 2D classes of the different conformations are shown below. The circle diameter is 160 Å.
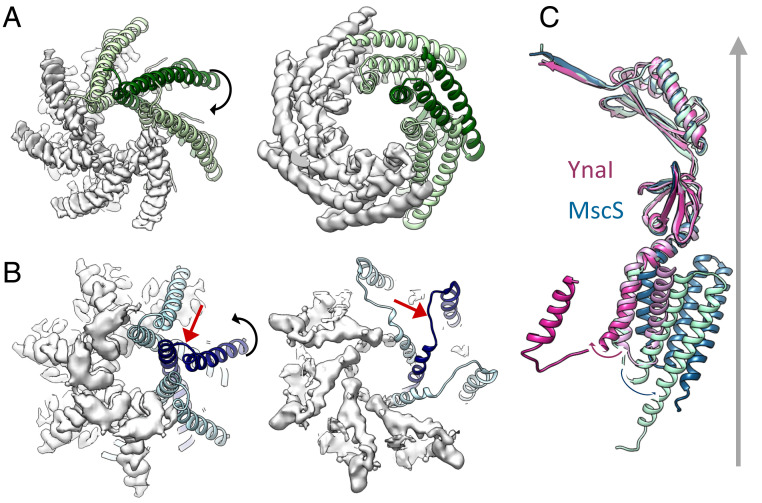
Modeling the pore-forming helices TM3 and locating TM2, which marks one end of the sensor paddle, shows that helix TM3a bends at a GGxGG motif. This, together with stretching of the connecting loop, enabled TM2 of the paddle to shift radially outward by 20 Å and move 60° with respect to its position in the closed-like channel (Fig. 3). In contrast, helix TM2 in MscS moves only 5 Å outward and relocates 20° in the opposite direction. Thus, YnaI opens the pore in a completely different way from that seen for MscS (Fig. 3). Mutagenesis provides independent evidence for the importance of the GGxGG motif in gating: In the mutant G149A/G152A, the motif is changed to the same pattern as in MscS, which should lower the propensity of bending in this region. Western blots show that this mutant has a similar expression as WT (SI Appendix, Fig. S7A). The mutation leads to a loss-of-function (LOF) phenotype in osmotic downshock assays (SI Appendix, Fig. S7B). In electrophysiological experiments, most of the time no activity is seen for this mutant but in 19% of pressure trials (in comparison with 87% for WT) activity is present, which has a similar pressure threshold (PL:PYnaI is the pressure ratio to open MscL relative to YnaI; PL:PYnaI-G149A/G152A = 1.00 ± 0.05 [n = 6] vs. PL:PYnaI-WT = 1.04 ± 0.06 [n = 6]) and conductance as in WT (SI Appendix, Notes and Fig. S7E). At 4.2-Å resolution, the structure of this mutant form shows that the TM3a structure is preserved in comparison with WT (SI Appendix, Fig. S7D and Table S1). A second mutant, G149P, should have a higher propensity of bending: It shows only low expression in the Western blot (SI Appendix, Fig. S7A). This mutant causes severe growth inhibition (SI Appendix, Fig. S7C), which is typical for strong gain-of-function (GOF) mutants of MS channels (22, 32).
Fig. 3.
Opening movement of YnaI differs from MscS. (A) MscS in its closed (Left) and open (Right) conformations viewed from the periplasmic side. Four subunits are shown as density [gray; EMDB ID code 4919 and PDB ID code 6RLD (closed) (11–13); PDB ID code 2VV5 (open) (18, 33)] and three subunits are depicted as a green ribbon. The clockwise movement of helix TM3a is accompanied by a tilting away from the normal vector of the membrane of helices TM1 and TM2. (B) YnaI in its closed-like (Left) and open-like (Right) forms viewed from the periplasmic side; only helix TM2 of the sensor paddle is shown for both. Four subunits are visualized as density (gray) and three subunits are shown as a blue ribbon. For the representation, the model of the closed-like form was fitted into the density of the open-like channel. The loop connecting helices TM3a to TM2 (red arrows) is clearly visible, indicating an unwinding of this loop and a counterclockwise repositioning of helix TM2 farther away from the central axis of the channel. (C) Overlay of one subunit each of the closed-like YnaI (pale pink), open-like YnaI (purple), closed MscS (mint green), and open MscS (cyan). The movements of the TM3a–TM2 helix loops are represented as black arrows; the normal vector of the membrane is shown as a gray arrow.
The third conformation in the dataset represents an intermediate state with a rearranged paddle but without widening of the pore (map with 3.3-Å resolution; Fig. 2 and SI Appendix, Fig. S6B). This state could either be a transition intermediate or an adapted state similar to the one reported for MscS (7, 34, 35). Since adaptation has not yet been tested for YnaI, we performed electrophysiological experiments. Our data indicate that neither YnaI nor the larger YbiO shows adaptation (SI Appendix, Fig. S8). Therefore, we conclude that this conformation is a transition intermediate between the open-like and the closed-like states. In the intermediate state, helix TM3 is less bent than in the open-like state. This suggests that gradual bending precedes pore widening and that paddle rearrangement is not tightly connected to pore widening.
Coordination of Lipids in the Pocket Changes the Pressure Threshold for Opening.
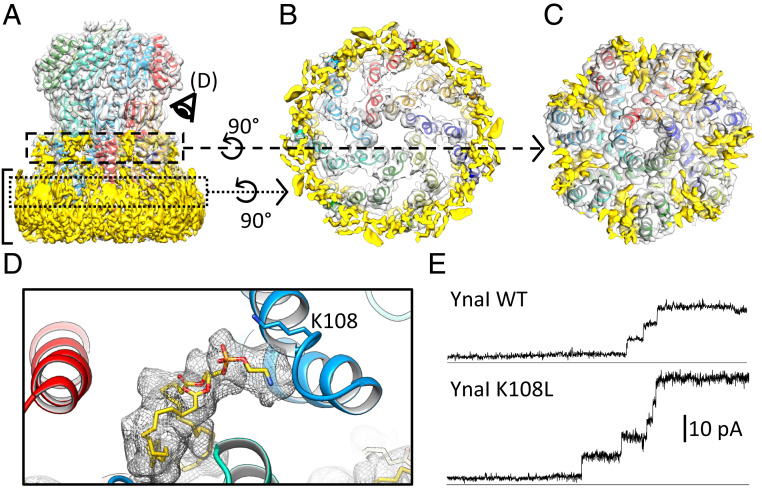
The repositioning of the paddles changes the pockets between helix TM3 and the paddles. In these pockets, lipid-shaped densities are detected in the closed-like state (Fig. 4 A, C, and D). We modeled one phosphatidylethanolamine per subunit as this is the most abundant lipid in E. coli while in MscS two lipids in similar positions and orientations are resolved (11). Previous experiments using collisional quenching of tryptophan fluorescence by brominated lipids have already shown that at least two residues in the pockets, G152W and F168W, are highly accessible to lipids (23). Lipids from the general membrane plane are likely to have access to the pockets through the deep grooves between the well-separated paddles (Fig. 4B).
Fig. 4.
Interaction of YnaI with lipids. (A) Side view of the map of closed-like YnaI (gray) with its atomic model. The subunits are colored differently, and unassigned densities are shown in yellow. The position of the membrane is indicated with a bracket. (B) Slice highlighted in the dotted box in A viewed along the symmetry axis from the periplasmic side. The space between the paddles is empty. (C) Slice highlighted in the dashed box in A viewed along the symmetry axis from the periplasmic side. Elongated densities protruding toward the central axis of the channel are visible. (D) Close-up of a pocket from the cytosolic side as depicted in A. A phosphatidylethanolamine lipid molecule (yellow) and its density are shown (gray mesh). The phosphate headgroup is in close proximity to K108. (E) Electrophysiological experiments indicate that the mutant K108L (Lower) shows normal staircase-like openings with a similar conductivity compared with the WT (Upper) despite opening at a lower tension (see text).
The phosphate headgroup of the resolved lipid forms a salt bridge with the ε-amino group of K108 (Fig. 4D). Mutating K108 to an uncharged leucine (K108L) causes a significant drop in the pressure required to open YnaI: In electrophysiological experiments, the PMscL:PYnaI increases from 1.04 ± 0.06 to 1.24 ± 0.09 (both n = 6; Student’s unpaired t test: P = 0.0018) with normal staircase-like activities (Fig. 4E). This demonstrates that the coordination of a lipid molecule by K108 is important for sensing.
Constrictions in Pores and Portals Vary in Larger Channels with 11 TM Helices.
With the established structural features of the small MscS and the middle-sized YnaI in hand, we wondered how this would compare with the large MscS-like channels with 11 TM helices. Thus, YbiO was reconstituted into amphipol A8-35 and cryo-EM provided structural data at 3.0-Å resolution of a large MscS-like homolog with 11 TM helices (SI Appendix, Fig. S9 A–C). The resolution is, however, nonuniform over the complex: While the cytosolic domain is well-resolved, the membrane domain shows only the TM helices homologous to those in MscS but none of the additional eight predicted TM helices. A reliable model can be built from the middle of helix TM2 but density in the map clearly reveals helix TM1 (the backbone is modeled for a 16-amino acid section), indicating that the positions of the paddle helices TM1 and TM2 are similar to MscS in the closed conformation but rotated by ∼10° and slightly tilted relative to TM1 of MscS (SI Appendix, Fig. S9 E and F). Furthermore, a dome-like overall structure is maintained in YbiO and the pore is located on the cytosolic side beyond the plane of the membrane (SI Appendix, Fig. S9D). A prominent ring of additional density surrounds the heptameric arrangement of TM3b helices and is absent in the other MscS-like structures (SI Appendix, Fig. S9D). The density of the ring is strong in comparison with the remaining membrane domain, but we were unable to model this region. The ring density might be formed by an extended loop of the paddle between helices TM1 and TM2, which is predicted by Memsat (36) to be 19 residues long in YbiO in comparison with only 6 residues for MscS.
All three differently sized MscS-like channels, MscS, YnaI, and YbiO, have H bonds and salt bridges between the subunits in the cytosolic vestibule but not in the membrane domain, as far as the local resolution allows us to judge, reflecting the rather static role of the former in contrast to the flexibility of the latter (SI Appendix, Fig. S10). These intersubunit interactions are not conserved in specific residues but are predominantly found in the β-domain, located between the portals and the membrane domain, and in the C-terminal β-barrel. At the given resolutions, we detected no changes in the intersubunit interactions for the closed-like and open-like conformations of YnaI.
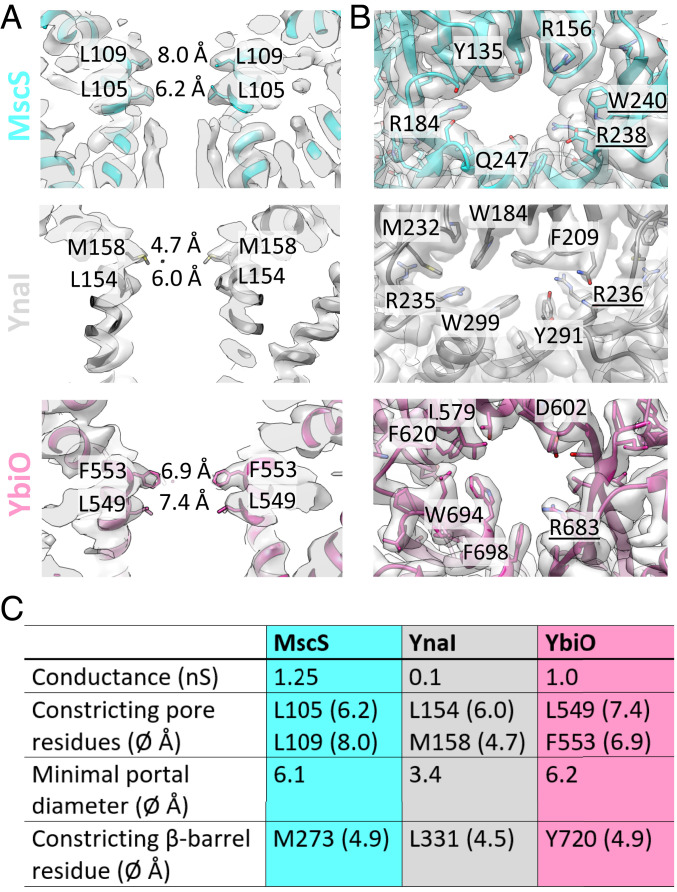
MscS, YnaI, and YbiO display two constrictions at the cytosolic end of the pore located on the TM3a helices. In MscS, it is generally accepted that the responsible residues, L105 and L109, form a hydrophobic seal in the closed conformation (37, 38) (Fig. 5A). While not completely blocking the path in MscS, the narrow and hydrophobic pore causes dewetting (i.e., local desolvation) that prevents exchange of water and solutes as evidenced by the 13 kJ/mol energetic barrier to water (39). In similar positions, we find L154 and M158 in YnaI and L549 and F553 in YbiO (Fig. 5A). Mutational analysis of YnaI showed that M158 is responsible for the potassium/sodium selectivity and an M158A mutation leads to a substantially higher conductance (24). In all three types of channels the side chains of these residues point toward the pore axis and define the narrowest part of the pore.
Fig. 5.
Detailed view of the pores and portals of MscS, YnaI, and YbiO. (A) Side-view slices of the pores of MscS (cyan) (11), YnaI (dark gray), and YbiO (purple) in their respective densities (gray). The side chains of the constricting residues are shown and labeled and the diameter of the pores at these positions are marked. (B) The portals of MscS (cyan), YnaI (dark gray), and YbiO (purple) in their respective densities. Selected residues are labeled; residues from the neighboring subunit contributing to the portals are underlined. (C) Summary of channel properties. The conductance values are taken from the literature (8, 40).
For MscS, the sealing residue, L105, generates the narrowest constriction with 6.2 Å across the pore while the cross-section toward the cytosolic side at L109 is 8.0 Å [Fig. 5 A and C; all diameters were determined with the program CHAP (41)]. For YnaI and YbiO the profile is different as the cytosol-facing sealing residues are the narrowest, M158 with 4.7 Å and F553 with 6.9 Å, respectively. In YbiO the TM3a helices are moved 2 Å farther away from the pore axis in comparison with MscS. However, with the larger phenylalanine side chain in YbiO the constriction has a similar diameter. The phenylalanine is conserved for the large 11-TM MscS-like channels from E. coli and also found in other homologs (SI Appendix, Fig. S11C).
The cytosolic domains of MscS, YnaI, and YbiO emphasize a structural conservation from small to large MscS-like channels. All these cytosolic vestibules start at the N terminus with the well-conserved TM3b helix and β-domain, followed by the αβ-domain showing slightly more variation, and are completed by a β-barrel at the C terminus (Fig. 1 A and B). Of importance for function are the different access gates into the vestibule, the side portals and the β-barrel (16, 42). The side portals in our E. coli paralogs have in common that many bulky, predominantly aromatic residues surround them (Fig. 5B). However, they differ in position and number. W240 and W251 in MscS are important for complex stability (43) while W184 in YnaI is strictly required to retain function (23). The size of the portals in the three channels varies: MscS and YbiO have similarly sized portals while the portals in YnaI are very small because large aromatic residues like W184 and W299 largely block them (Fig. 5 B and C).
The Pores of the MscS-like Channels Differ in Hydrophobicity.
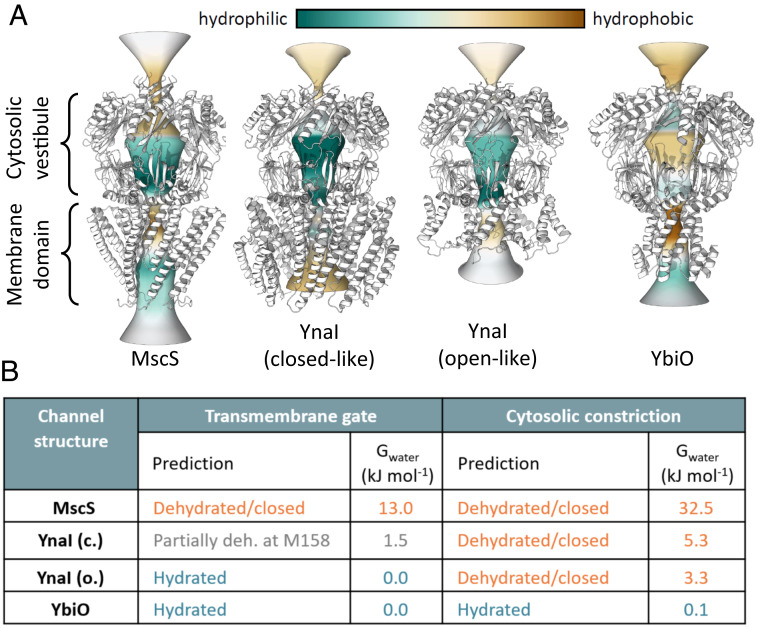
We compared our structures and MscS in more detail using the program CHAP (38, 41) to better understand the predicted conductive properties of the channels (Fig. 6 and SI Appendix, Fig. S12). In the closed-like state of YnaI the degree of local dehydration in the TM pore is less than that in MscS. This suggests that the closed-like state may be from a functional perspective “partially closed.” In computational electrophysiology simulations (3 × 200 ns) no ions are observed to pass through the TM gate for the simulation of the closed-like structure, while for the open-like structure a small number of ions pass, consistent with a conductance of the order of ∼10 pS (V = −200 mV) and ∼40 pS (V = +200 mV) with a Cl− selectivity. In contrast, YbiO has a hydrophobic pore like MscS. However, apart from the constricting residue F553, the pore has a larger diameter than the pore in MscS. Consequently, the pore in YbiO is predicted to be open, and it remains fully hydrated during molecular dynamics (MD) simulations. In computational electrophysiological simulations, YbiO shows a conductance of ∼30 pS and Cl− selectivity.
Fig. 6.
Comparison of the hydrophobicity of the pores. (A) Hydrophobicity of the vestibule openings, β-barrels and portals, and the transmembrane pore were determined with CHAP (41) and is color-coded. The models of MscS (PDB ID code 6RLD) (11–13), closed-like YnaI, open-like YnaI, and YbiO (from Left) are depicted as white ribbons. (B) Summary of the wetting/dewetting simulations along the symmetry axis.
The vestibules in MscS and YnaI are more hydrophilic than in YbiO (Fig. 6A). CHAP predicts closed β-barrels for MscS and YnaI in contrast to the MscS homolog from Thermoanaerobacter tengcongensis (16). The β-barrel in YbiO is hydrated and could conduct. However, since the C terminus of YbiO is not resolved, we do not know whether it is blocked in the unresolved region. Computational electrophysiological simulations suggest that Cl− ions enter the channels via the side portals. The side portals of YnaI, in both conformations, are narrow but are predicted to be open as they remain hydrated during MD simulations though in electrophysiological simulations no conductance was observed. The heuristic predictions for YnaI and YbiO were confirmed by MD simulations where the backbone of the protein was kept static (SI Appendix, Figs. S12 and S13).
In conclusion, MscS, YnaI, and YbiO have similar cytosolic domains. However, at the portals, they are structurally fine-tuned to account for functional differences. Similarly, the pores are constituted of TM3a helices with two consecutive ring-shaped seals toward the cytosol but differ in the sealing residues, their overall hydrophobicity, and wettability.
Discussion
MscS-like channels constitute the largest and most diverse family of mechanosensitive channels known so far. Yet, besides the archetypical MscS from E. coli, little is known about the rest of the family, considering the importance of mechanosensation for life. In the present work, we show structural studies of the MscS paralogs from E. coli, comprising the medium-sized 5-TM helix YnaI and the large-sized 11-TM helix YbiO.
We have determined the high-resolution structure of a larger MscS paralog, YnaI, showing additional TM helices beyond the known paddles of MscS. While the overall architecture is very similar to MscS, YnaI shows a second bundle of TM helices extending the sensor paddles known from MscS. These helices are staggered against the first bundle toward the periplasm and are fully embedded in the membrane. Density bridges connect the inner and the outer bundles with residues T83 and T91 of helix TM1 as likely candidates for H-bond formation with helix TM-N1.
We demonstrate here that the use of the DIBMA copolymer to extract the membrane protein directly from native E. coli membranes forming so-called native nanodiscs can also be applied to cryo-EM structural studies. DIBMA forms larger nanodiscs (25, 26) and is therefore more suitable for large membrane proteins than the more established styrene–maleic acid (44–46). A previously obtained map of YnaI in amphipol A8-35 at 3.3-Å resolution was limited by a low number of side views due to preferential orientation (SI Appendix, Fig. S14 and Table S1). Thus, DIBMA was tested on YnaI, and blob-based autopicking and 2D classification revealed that roughly 33% of YnaI-DIBMA/lipid particles (DIBMALPs) showed side views compared with less than 2% for YnaI in amphipols. Yet, it should be mentioned that the solubilization efficiency of DIBMA is only 30% compared with the solubilization with n-Dodecyl-β-D-Maltopyranoside. While this approach provides the most native-like lipid environment for the channel for structural studies, overexpression of YnaI means that low-abundant lipids could be depleted from the membrane if they are bound to the overexpressed protein. As a consequence, the membrane composition would not be native anymore. DIBMA also allowed us to extract opened YnaI after modifying the membrane properties of proteoliposomes by inserting LPC. Single-particle analysis revealed that the dataset was populated by three conformationally different states: the closed-like state, an open-like state with a flatter membrane domain, and an intermediate state (Fig. 2). The open-like conformation of YnaI is surprisingly different from the open state in MscS (Fig. 3). The position of helix TM2 indicates that the paddle adopts a completely different position in comparison to MscS. The TM3a helix bends at a glycine-rich GGxGG motif on the periplasmic side of the pore-sealing residues M158 and L154 (SI Appendix, Fig. S11A). Such a glycine-rich motif is generally considered as supporting disorder and, therefore, it is surprising to find it in a helix. However, this also makes it plausible that the GGxGG motif in the TM3 helix is metastable and supports conformational switching. Only one or two glycines of the GGxGG motif are found in the paralogs of YnaI in E. coli (SI Appendix, Fig. S11B). In MscS they are part of the gear-like interaction with the neighboring TM3a helices (47). In contrast, we found a wide distribution of the GGxGG motif in homologs of YnaI in other organisms (SI Appendix, Fig. S11C). Variations of this motif are seen; for example, in homologs of archaea a GxGxGG motif is present. Thus, only in a subset of MscS-like channels is this glycine-rich motif seen and perhaps only this subset can bend the TM3a helices and shorten the pore as observed for YnaI. This model of gating is supported by the LOF phenotype of the G149A/G152A mutant (AGxAG), which should stabilize the helix in the GGxGG motif, and the GOF phenotype of the G149P mutant (PGxGG), which should stabilize the kink (SI Appendix, Fig. S7).
In contrast to these differences, we also identified common features of the MscS-like channels which indicate similarities in the mechanism. The eight additional TM helices in YbiO were not resolved in our structure but a large indentation on the periplasmic side is maintained (SI Appendix, Fig. S9D). YnaI shows a narrower indentation than YbiO but larger than the funnel seen in MscS (Fig. 1D). Therefore, size and curvature of the indentation seem to be related to the size of the paddles.
One could speculate that the dome-like arrangement of the TM domains that surround the indentations could function similar to the large, tentacle-like TM domains of the PIEZO channels. These cause a membrane indentation which gets flattened upon opening (48, 49). For YnaI, we see a similar flattening of the dome-like structure upon pore opening (Fig. 2) though on a much smaller scale than in PIEZO. Whether this holds true for all MscS-like channels and whether it varies with the size of the paddles remain to be shown. One should note that the pressure thresholds of the MscS-like channels in E. coli do not correlate with the size of the paddles (8).
An alternative sensing model is based on the lipids found in the cytosolic pockets in between the paddles (20). We observe lipids in YnaI at similar positions and in similar orientations as for MscS (11) (Fig. 4 C and D). Weakening of the lipid binding in YnaI by mutating K108 (K108L) that forms a salt bridge to the phosphate headgroup of the lipids decreases the pressure required for opening. Similarly, mutation of R59 at a comparable position in MscS causes a severe GOF phenotype (22), which is in agreement with a model of mechanosensation where lipid extrusion of the pockets triggers gating (20). On a sequence level, no strict conservation for this position is seen for MscS-like channels, but often positively charged amino acids are found in the region. The structural conservation of the lipid binding in the pockets of MscS-like channels and the GOF phenotype linked to changes in the lipid coordination suggests that sensing is conserved among the channels despite the different gating mechanisms.
Structural alignment of closed MscS with one of our resolved YnaI conformations suggests that this structure represents a closed state we termed “closed-like” (Fig. 1C). However, in silico analysis of this structure indicates that the pore in this form undergoes only partial dehydration but seems not to be conductive to ions. Interestingly, the pore in YnaI is more hydrophilic than in MscS, another variation between MscS-like channels (Fig. 6A). Based on the MD simulations it seems likely that the fully closed state of YnaI, if it exists at all, is somewhat more compressed than the conformation we captured in DIBMA nanodiscs. Another possible explanation is that lipid molecules block the pore in the closed state which are not resolved in our structure and not considered for the simulation. Indeed, for MscS, densities, likely to be lipids, were resolved within the pore (11, 21) (Fig. 5A). Furthermore, spectroscopic data indicated that L154 in YnaI is in contact with lipids (23).
These closed-like and open-like structures should be informative to understand the gating transition of YnaI even if they perhaps do not represent the fully open and closed conformations. YbiO seems to be, like YnaI, upon first visual inspection in a closed-like conformation (SI Appendix, Fig. S9 E and F). In comparison with MscS, the pore is similarly hydrophobic but overall wider so that MD simulations show hydration and conductance. Here, a wider pore is accompanied by slight rotation of the paddle, which is seen for the open transition in MscS (18). Thus, it is likely that our structure of YbiO in amphipols represents not the completely closed conformation of the channel.
For YnaI, our data do not show any obvious conduction path through the vestibule. The β-barrel has a hydrophobic seal, while the portals are largely occluded by the aromatic residues which do not change their position upon pore opening (Fig. 5B). MD simulations indicated that the portals are hydrated and, with a diameter of 3.4 Å, may just allow the passage of hydrated potassium, sodium, or chloride ions. Computational electrophysiological simulations show no conductance. Experimentally, YnaI has a far lower conductance than MscS and YbiO (8) (Fig. 5C). The mutation F209A in the portal of YnaI, which is likely to change the open cross-section of the portals, almost quadruples the conductance while a substituted β-barrel for the T. tengcongensis MscS region does not change conductance (24).
In conclusion, YnaI follows a distinct opening mechanism which is different from MscS. It is characterized by gradual bending of the TM3a helix at a flexible GGxGG motif, which extends the connecting loop to the TM2 helix and enables an outward shift. Sequence comparison suggests that this mechanism is restricted to a subgroup of MscS-like channels. In contrast, the closed-like state is very similar to MscS and YbiO. Structural fine-tuning of the pores and portals in the conductivity module adjusts functional properties for the different channels, like conductance and specificity. Thus, our study revealed common features as well as variations in gating of MscS-like channels across all size classes.
Materials and Methods
A detailed description of all materials and methods is given in SI Appendix.
Purification and Reconstitution of YnaI and YbiO.
YnaI and YbiO were expressed with a C-terminal His6 tag and purified as described previously (8) with immobilized metal affinity chromatography (IMAC). The integrity of the protein was confirmed by matrix-assisted laser desorption/ionization mass spectrometry (SI Appendix, Fig. S15) (50). After incubation with an eightfold excess (mass/mass) of amphipol A8-35 (Jena Bioscience) for 1 h at 4 °C, the detergent was removed with Bio-Beads SM-2 (Bio-Rad) and the channels were further purified by size-exclusion chromatography. Purified YnaI was also reconstituted in preformed 1,2-dioleoyl-sn-glycero-3-phosphocholine/1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-(1'-rac-glycerol) (sodium salt) (DOPC:POPG) (3:1) liposomes at a 1:1,000 protein-to-lipid molar ratio. Furthermore, YnaI was directly extracted from cells using the copolymer diisobutylene/maleic acid (25) (kindly provided by BASF) at a concentration of 2.5% (weight/volume; wt/vol) and purified by IMAC and size-exclusion chromatography. For channel opening, YnaI-containing proteoliposomes (1 mg/mL) were incubated with 5 µM lysophosphatidylcholine (from egg yolk; Sigma) for 40 min at room temperature before addition of 0.2% (wt/vol) DIBMA. After incubation at room temperature with gentle rotation for 20 h, the samples were further purified via IMAC and size-exclusion chromatography.
Electron Cryomicroscopy and Structural Analysis.
Samples (3.5 µL) were applied on glow-discharged Quantifoil 400 mesh 1.2/1.3 grids and plunge-frozen in liquid ethane by use of a Vitrobot IV (FEI/Thermo Fisher). Samples were transferred to a Thermo Fisher Titan Krios G3 transmission electron microscope at the Würzburg cryo-EM facility (51). Movies were acquired at 300 kV with a Falcon III camera at a nominal magnification of 75,000, which corresponds to a calibrated pixel size of 1.0635 Å. Data were collected with EPU acquisition software (Thermo Fisher) either in integrating mode (closed YnaI) or in counting mode (LPC-treated YnaI and YbiO). Details for the individual datasets are listed in SI Appendix, Table S1. Data were analyzed with RELION 3.0 (52) as described further in SI Appendix. De novo model building for YnaI and YbiO was carried out using Coot version 0.8.9.1 (53) and real-space refinement was performed in PHENIX version 1.17.1 using secondary-structure restraints (54) (SI Appendix, Table S2). All images of models and densities were prepared using UCSF Chimera version 1.12 (55).
Electrophysiological Experiments.
Patch-clamp recordings were conducted on membrane patches derived from giant protoplasts using the strains MJF429 (ΔmscS, ΔmscK) and for controls MJF641 (ΔmscS, ΔmscK, ΔynaI, ΔybdG, ΔybiO, ΔyjeP, ΔmscL) transformed with plasmids (7, 8). The detailed description of protoplast generation has been reported previously (56). The pressure threshold for activation of the YnaI or YbiO channels was referenced against the activation threshold of MscL (PMscL:PYnaI or PMscL:PYbiO, respectively) to determine the pressure ratio for gating, as previously described (57). Reference measurements with the WT channels resulted in PMscL:PYnaI = 1.04 ± 0.06 (n = 6) for YnaI WT and PMscL:PYbiO: 1.16 ± 0.19 (n = 6) for YbiO WT and are in agreement with published values (8).
Prediction and Simulations of Pore Hydration.
The pore size and hydrophobicity along the channel axis of each structure and through its cytosolic side portals were determined using CHAP (41). The likelihood of observing a hydrophobic barrier to permeation was predicted on the basis of the structures alone according to a previously described model, derived from analyzing ∼200 ion-channel structures (38). MD simulations were performed using GROMACS 2018 (58), with each protein structure embedded in a 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine lipid bilayer membrane (59), solvated on either side with the TIP4P/2005 water model (60) and 0.15 M NaCl. Computational electrophysiological simulations were conducted, in triplicates of 200 ns, by applying an external uniform electric field in the membrane normal direction to generate a transmembrane potential difference of −200 or +200 mV, corresponding to negative or positive potential on the cytosolic side, respectively.
Supplementary Material
Acknowledgments
We thank Cristian Kraft for technical assistance and Bernhard Fröhlich for information-technology support. DIBMA was a kind gift of BASF. The work was supported by the Deutsche Forschungsgemeinschaft (DFG) (to B.B.) (DFG Project Grant Bo1150/15-1 and DFG Equipment Grant INST 93/903-1 FUGG) and Biotechnology and Biological Sciences Research Council (Grant BB/N000145/1), Engineering and Physical Sciences Research Council (Grant EP/R004722/1), and Wellcome Trust (Grant 208361/Z/17/Z) (to M.S.P.S.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2005641117/-/DCSupplemental.
Data Availability.
Cryo-EM maps reported in this article have been deposited in the Electron Microscopy Data Bank (EMDB) for closed-like YnaI in DIBMA (ID code EMD-11557) (61), YbiO (EMD-11629) (62), YnaI mutant G149A/G152A (EMD-11556) (63), and closed-like YnaI in amphipols (EMD-11558) (64). Cryo-EM maps derived from LPC-treated YnaI are available for the open-like (EMD-11560) (65), intermediate (EMD-11561) (66), and closed-like conformations (EMD-11559) (67). The molecular models of closed-like YnaI, open-like YnaI, and YbiO were deposited in the Protein Data Bank (PDB) with ID codes 6ZYD (68), 6ZYE (69), and 7A46 (70), respectively.
References
- 1.Dance A., The quest to decipher how the body’s cells sense touch. Nature 577, 158–160 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Booth I. R., Bacterial mechanosensitive channels: Progress towards an understanding of their roles in cell physiology. Curr. Opin. Microbiol. 18, 16–22 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rasmussen T., Rasmussen A., “Bacterial mechanosensitive channels” in Membrane Protein Complexes: Structure and Function. Subcellular Biochemistry, Harris J., Boekema E., Eds. (Springer, 2018), vol. 87, pp. 83–116. [DOI] [PubMed] [Google Scholar]
- 4.Pivetti C. D., et al. , Two families of mechanosensitive channel proteins. Microbiol. Mol. Biol. Rev. 67, 66–85 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malcolm H. R., Maurer J. A., The mechanosensitive channel of small conductance (MscS) superfamily: Not just mechanosensitive channels anymore. ChemBioChem 13, 2037–2043 (2012). [DOI] [PubMed] [Google Scholar]
- 6.Cox C. D., Nakayama Y., Nomura T., Martinac B., The evolutionary ‘tinkering’ of MscS-like channels: Generation of structural and functional diversity. Pflugers Arch. 467, 3–13 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Levina N., et al. , Protection of Escherichia coli cells against extreme turgor by activation of MscS and MscL mechanosensitive channels: Identification of genes required for MscS activity. EMBO J. 18, 1730–1737 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards M. D., et al. , Characterization of three novel mechanosensitive channel activities in Escherichia coli. Channels (Austin) 6, 272–281 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schumann U., et al. , YbdG in Escherichia coli is a threshold-setting mechanosensitive channel with MscM activity. Proc. Natl. Acad. Sci. U.S.A. 107, 12664–12669 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amemiya S., et al. , The mechanosensitive channel YbdG from Escherichia coli has a role in adaptation to osmotic up-shock. J. Biol. Chem. 294, 12281–12292 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rasmussen T., Flegler V. J., Rasmussen A., Böttcher B., Structure of the mechanosensitive channel MscS embedded in the membrane bilayer. J. Mol. Biol. 431, 3081–3090 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Rasmussen T., Flegler V. J., Rasmussen A., Boettcher B., Structure of the mechanosensitive channel MscS embedded in the membrane bilayer. Protein Data Bank. https://www.rcsb.org/structure/6RLD. Deposited 2 May 2019. [DOI] [PubMed]
- 13.Rasmussen T., Flegler V. J., Rasmussen A., Boettcher B., Structure of the mechanosensitive channel MscS embedded in the membrane bilayer. Electron Microscopy Data Bank. https://www.ebi.ac.uk/pdbe/entry/emdb/EMD-4919. Deposited 2 May 2019. [DOI] [PubMed]
- 14.Bass R. B., Strop P., Barclay M., Rees D. C., Crystal structure of Escherichia coli MscS, a voltage-modulated and mechanosensitive channel. Science 298, 1582–1587 (2002). [DOI] [PubMed] [Google Scholar]
- 15.Steinbacher S., Bass R., Strop P., Rees D. C., Mechanosensitive Ion Channels, Part A (Elsevier, 2007). [Google Scholar]
- 16.Zhang X., et al. , Structure and molecular mechanism of an anion-selective mechanosensitive channel of small conductance. Proc. Natl. Acad. Sci. U.S.A. 109, 18180–18185 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai J. Y., Poon Y. S., Kaiser J. T., Rees D. C., Open and shut: Crystal structures of the dodecylmaltoside solubilized mechanosensitive channel of small conductance from Escherichia coli and Helicobacter pylori at 4.4 Å and 4.1 Å resolutions. Protein Sci. 22, 502–509 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang W., et al. , The structure of an open form of an E. coli mechanosensitive channel at 3.45 Å resolution. Science 321, 1179–1183 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pliotas C., et al. , Conformational state of the MscS mechanosensitive channel in solution revealed by pulsed electron-electron double resonance (PELDOR) spectroscopy. Proc. Natl. Acad. Sci. U.S.A. 109, E2675–E2682 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pliotas C., et al. , The role of lipids in mechanosensation. Nat. Struct. Mol. Biol. 22, 991–998 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reddy B., Bavi N., Lu A., Park Y., Perozo E., Molecular basis of force-from-lipids gating in the mechanosensitive channel MscS. eLife 8, e50486 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rasmussen T., et al. , Interaction of the mechanosensitive channel, MscS, with the membrane bilayer through lipid intercalation into grooves and pockets. J. Mol. Biol. 431, 3339–3352 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Böttcher B., Prazak V., Rasmussen A., Black S. S., Rasmussen T., The structure of YnaI implies structural and mechanistic conservation in the MscS family of mechanosensitive channels. Structure 23, 1705–1714 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu J., et al. , A binding-block ion selective mechanism revealed by a Na/K selective channel. Protein Cell 9, 629–639 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oluwole A. O., et al. , Solubilization of membrane proteins into functional lipid-bilayer nanodiscs using a diisobutylene/maleic acid copolymer. Angew. Chem. Int. Ed. Engl. 56, 1919–1924 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oluwole A. O., et al. , Formation of lipid-bilayer nanodiscs by diisobutylene/maleic acid (DIBMA) copolymer. Langmuir 33, 14378–14388 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Perozo E., Cortes D. M., Sompornpisut P., Kloda A., Martinac B., Open channel structure of MscL and the gating mechanism of mechanosensitive channels. Nature 418, 942–948 (2002). [DOI] [PubMed] [Google Scholar]
- 28.Perozo E., Kloda A., Cortes D. M., Martinac B., Physical principles underlying the transduction of bilayer deformation forces during mechanosensitive channel gating. Nat. Struct. Biol. 9, 696–703 (2002). [DOI] [PubMed] [Google Scholar]
- 29.Vásquez V., Sotomayor M., Cordero-Morales J., Schulten K., Perozo E., A structural mechanism for MscS gating in lipid bilayers. Science 321, 1210–1214 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nomura T., et al. , Differential effects of lipids and lyso-lipids on the mechanosensitivity of the mechanosensitive channels MscL and MscS. Proc. Natl. Acad. Sci. U.S.A. 109, 8770–8775 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoo J., Cui Q., Curvature generation and pressure profile modulation in membrane by lysolipids: Insights from coarse-grained simulations. Biophys. J. 97, 2267–2276 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller S., et al. , Domain organization of the MscS mechanosensitive channel of Escherichia coli. EMBO J. 22, 36–46 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang W., Dong C., Johnson K. A., Naismith J. H., The open structure of MscS. Protein Data Bank. https://www.rcsb.org/structure/2VV5. Deposited 3 June 2008.
- 34.Sukharev S. I., Blount P., Martinac B., Kung C., Mechanosensitive channels of Escherichia coli: The MscL gene, protein, and activities. Annu. Rev. Physiol. 59, 633–657 (1997). [DOI] [PubMed] [Google Scholar]
- 35.Koprowski P., Kubalski A., Voltage-independent adaptation of mechanosensitive channels in Escherichia coli protoplasts. J. Membr. Biol. 164, 253–262 (1998). [DOI] [PubMed] [Google Scholar]
- 36.Jones D. T., Taylor W. R., Thornton J. M., A model recognition approach to the prediction of all-helical membrane protein structure and topology. Biochemistry 33, 3038–3049 (1994). [DOI] [PubMed] [Google Scholar]
- 37.Anishkin A., Sukharev S., Water dynamics and dewetting transitions in the small mechanosensitive channel MscS. Biophys. J. 86, 2883–2895 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rao S., Klesse G., Stansfeld P. J., Tucker S. J., Sansom M. S. P., A heuristic derived from analysis of the ion channel structural proteome permits the rapid identification of hydrophobic gates. Proc. Natl. Acad. Sci. U.S.A. 116, 13989–13995 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rasaiah J. C., Garde S., Hummer G., Water in nonpolar confinement: From nanotubes to proteins and beyond. Annu. Rev. Phys. Chem. 59, 713–740 (2008). [DOI] [PubMed] [Google Scholar]
- 40.Sukharev S., Purification of the small mechanosensitive channel of Escherichia coli (MscS): The subunit structure, conduction, and gating characteristics in liposomes. Biophys. J. 83, 290–298 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klesse G., Rao S., Sansom M. S. P., Tucker S. J., CHAP: A versatile tool for the structural and functional annotation of ion channel pores. J. Mol. Biol. 431, 3353–3365 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cox C. D., et al. , Selectivity mechanism of the mechanosensitive channel MscS revealed by probing channel subconducting states. Nat. Commun. 4, 2137 (2013). [DOI] [PubMed] [Google Scholar]
- 43.Rasmussen A., et al. , The role of tryptophan residues in the function and stability of the mechanosensitive channel MscS from Escherichia coli. Biochemistry 46, 10899–10908 (2007). [DOI] [PubMed] [Google Scholar]
- 44.Sun C., et al. , Structure of the alternative complex III in a supercomplex with cytochrome oxidase. Nature 557, 123–126 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qiu W., et al. , Structure and activity of lipid bilayer within a membrane-protein transporter. Proc. Natl. Acad. Sci. U.S.A. 115, 12985–12990 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parmar M., et al. , Using a SMALP platform to determine a sub-nm single particle cryo-EM membrane protein structure. Biochim. Biophys. Acta Biomembr. 1860, 378–383 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Edwards M. D., et al. , Pivotal role of the glycine-rich TM3 helix in gating the MscS mechanosensitive channel. Nat. Struct. Mol. Biol. 12, 113–119 (2005). [DOI] [PubMed] [Google Scholar]
- 48.Wang L., et al. , Structure and mechanogating of the mammalian tactile channel PIEZO2. Nature 573, 225–229 (2019). [DOI] [PubMed] [Google Scholar]
- 49.Lin Y.-C., et al. , Force-induced conformational changes in PIEZO1. Nature 573, 230–234 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen F., et al. , High-mass matrix-assisted laser desorption ionization-mass spectrometry of integral membrane proteins and their complexes. Anal. Chem. 85, 3483–3488 (2013). [DOI] [PubMed] [Google Scholar]
- 51.Song B., et al. , Capabilities of the Falcon III detector for single-particle structure determination. Ultramicroscopy 203, 145–154 (2019). [DOI] [PubMed] [Google Scholar]
- 52.Scheres S. H. W., RELION: Implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Emsley P., Lohkamp B., Scott W. G., Cowtan K., Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Adams P. D., et al. , PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pettersen E. F., et al. , UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004). [DOI] [PubMed] [Google Scholar]
- 56.Blount P., Sukharev S. I., Moe P. C., Martinac B., Kung C., Mechanosensitive channels of bacteria. Methods Enzymol. 294, 458–482 (1999). [DOI] [PubMed] [Google Scholar]
- 57.Blount P., Sukharev S. I., Schroeder M. J., Nagle S. K., Kung C., Single residue substitutions that change the gating properties of a mechanosensitive channel in Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 93, 11652–11657 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abraham M. J., et al. , GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1–2, 19–25 (2015). [Google Scholar]
- 59.Stansfeld P. J., et al. , MemProtMD: Automated insertion of membrane protein structures into explicit lipid membranes. Structure 23, 1350–1361 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abascal J. L. F., Vega C., A general purpose model for the condensed phases of water: TIP4P/2005. J. Chem. Phys. 123, 234505 (2005). [DOI] [PubMed] [Google Scholar]
- 61.Flegler V. J., et al. , YnaI. Electron Microscopy Data Bank. https://www.ebi.ac.uk/pdbe/entry/emdb/EMD-11557. Deposited 31 July 2020.
- 62.Flegler V. J., et al. , Small conductance mechanosensitive channel YbiO. Electron Microscopy Data Bank. https://www.ebi.ac.uk/pdbe/entry/emdb/EMD-11629. Deposited 19 August 2020.
- 63.Flegler V. J., et al. , Small conductance mechanosensitive channel YnaI mutant G149A/G152A. Electron Microscopy Data Bank. https://www.ebi.ac.uk/pdbe/entry/emdb/EMD-11556. Deposited 31 July 2020.
- 64.Flegler V. J., et al. , Mechanosensitive channel YnaI in amphipol A8-35. Electron Microscopy Data Bank. https://www.ebi.ac.uk/pdbe/entry/emdb/EMD-11558. Deposited 3 August 2020.
- 65.Flegler V. J., et al. , YnaI in an open-like conformation. Electron Microscopy Data Bank. https://www.ebi.ac.uk/pdbe/entry/emdb/EMD-11560. Deposited 31 July 2020.
- 66.Flegler V. J., et al. , Mechanosensitive channel YnaI in an intermediate conformation. Electron Microscopy Data Bank. https://www.ebi.ac.uk/pdbe/entry/emdb/EMD-11561. Deposited 5 August 2020.
- 67.Flegler V. J., et al. , Mechanosensitive channel YnaI in a closed-like conformation. Electron Microscopy Data Bank. https://www.ebi.ac.uk/pdbe/entry/emdb/EMD-11559. Deposited 5 August 2020.
- 68.Flegler V. J., et al. , YnaI. Protein Data Bank. https://www.rcsb.org/structure/6ZYD. Deposited 31 July 2020.
- 69.Flegler V. J., et al. , YnaI in an open-like conformation. Protein Data Bank. https://www.rcsb.org/structure/6ZYE. Deposited 31 July 2020.
- 70.Flegler V. J., et al. , Small conductance mechanosensitive channel YbiO. Protein Data Bank. https://www.rcsb.org/structure/7A46. Deposited 19 August 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Cryo-EM maps reported in this article have been deposited in the Electron Microscopy Data Bank (EMDB) for closed-like YnaI in DIBMA (ID code EMD-11557) (61), YbiO (EMD-11629) (62), YnaI mutant G149A/G152A (EMD-11556) (63), and closed-like YnaI in amphipols (EMD-11558) (64). Cryo-EM maps derived from LPC-treated YnaI are available for the open-like (EMD-11560) (65), intermediate (EMD-11561) (66), and closed-like conformations (EMD-11559) (67). The molecular models of closed-like YnaI, open-like YnaI, and YbiO were deposited in the Protein Data Bank (PDB) with ID codes 6ZYD (68), 6ZYE (69), and 7A46 (70), respectively.