Significance
Mutagenic translesion synthesis (TLS) increases cell survival after DNA damage by bypassing lesions that normally block DNA replication but introduces mutations. In cancer cells, REV1/POLζ-dependent mutagenic TLS can contribute to intrinsic chemoresistance, while the mutations it introduces can underlie acquired chemoresistance. Interfering with this TLS pathway genetically or with the small molecule inhibitor JH-RE-06 has been shown to improve cisplatin chemotherapy by suppressing tumor growth and enhancing survival in mouse xenograft tumor models. Cisplatin chemotherapy commonly exerts its antitumor effects via DNA damage-mediated apoptosis. However, in two mouse xenograft models and four mammalian cell lines, the JH-RE-06 unexpectedly profoundly alters the biological response to cisplatin. Apoptosis is suppressed and surprisingly numerous hallmarks of senescence are induced prior to cell death.
Keywords: translesion synthesis, Rev1, chemotherapy, senescence, cell death
Abstract
REV1/POLζ-dependent mutagenic translesion synthesis (TLS) promotes cell survival after DNA damage but is responsible for most of the resulting mutations. A novel inhibitor of this pathway, JH-RE-06, promotes cisplatin efficacy in cancer cells and mouse xenograft models, but the mechanism underlying this combinatorial effect is not known. We report that, unexpectedly, in two different mouse xenograft models and four human and mouse cell lines we examined in vitro cisplatin/JH-RE-06 treatment does not increase apoptosis. Rather, it increases hallmarks of senescence such as senescence-associated β-galactosidase, increased p21 expression, micronuclei formation, reduced Lamin B1, and increased expression of the immune regulators IL6 and IL8 followed by cell death. Moreover, although p-γ-H2AX foci formation was elevated and ATR expression was low in single agent cisplatin-treated cells, the opposite was true in cells treated with cisplatin/JH-RE-06. These observations suggest that targeting REV1 with JH-RE-06 profoundly affects the nature of the persistent genomic damage after cisplatin treatment and also the resulting physiological responses. These data highlight the potential of REV1/POLζ inhibitors to alter the biological response to DNA-damaging chemotherapy and enhance the efficacy of chemotherapy.
Mutagenic translesion synthesis (TLS) carried out by REV1 and POLζ (REV3/REV7) allows cells to tolerate DNA damage by bypassing lesions that block normal DNA replication but at the cost of introducing mutations (1). In cancer cells, REV1/POLζ-dependent TLS can contribute to intrinsic chemoresistance (2), while the mutations it introduces can underlie acquired chemoresistance (3). One interface of the critical REV1 C-terminal domain (CTD) recruits POLζ via an interaction with REV7, while a second one recruits other TLS polymerases via an interaction with their REV1-interacting regions (RIR) (1, 4, 5). The small molecule, JH-RE-06, inhibits REV1/POLζ-dependent mutagenic TLS by promoting REV1 CTD dimerization, which prevents POLζ recruitment. JH-RE-06 is the first TLS inhibitor shown to suppress tumor growth and enhance survival in mouse xenograft tumor models (6).
TLS inhibition interferes with the ability of cells to withstand DNA damage. Thus, the predicted consequence of TLS inhibition in response to genotoxic chemotherapy would be an amplification of the normal physiological consequences of damaged DNA. The optimal phenotypic consequence of this genotoxic damage in cancer has generally been viewed as rapid apoptotic tumor cell clearance. More recently, the induction of cellular senescence has been postulated as a desirable therapeutic outcome, as senescent cells can be recognized and cleared by the innate immune system. Moreover, senolytic therapies can specifically target senescent cells (7).
Cisplatin, in chemotherapy, commonly exerts its antitumor effects via DNA damage-mediated cell death (8). However, cisplatin can induce apoptosis, senescence, and other therapeutic consequences with the specific response elicited depending on such factors as cell type, cell state, and dose (9–12). Thus, the use of cisplatin is not, per se, tied to a specific biological outcome. However, given the well-established role for REV1/POLζ-dependent TLS activity in bypassing platinated DNA lesions and in interstrand cross-link repair, we initially expected that, with respect to cell death, inhibitors of this pathway would simply enhance the specific cellular consequences of cisplatin treatment. Surprisingly, in our study of two different mouse xenograft tumor models and four human mouse cell lines, we find that JH-RE-06 not only potentiates cisplatin action but also fundamentally alters context-specific cellular responses to cisplatin.
Results and Discussion
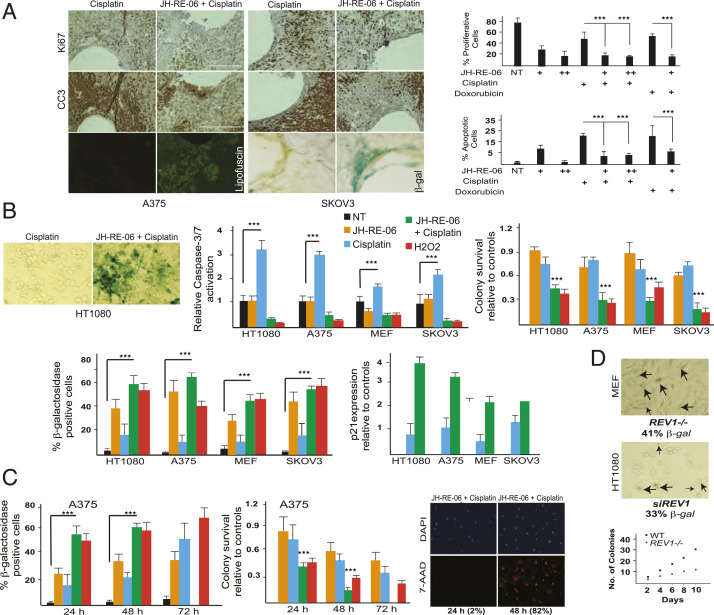
To gain insights into the mechanism of the JH-RE-06–dependent increase in cisplatin efficacy in vivo (6), we implanted a microdevice (13) into two independent mouse xenograft tumor models. The cisplatin and JH-RE-06 doses used were previously shown to suppress tumor volumes in xenograft mouse models (6). Cisplatin/JH-RE-06 combination treatment resulted in significantly reduced Ki67 staining compared to cisplatin alone, consistent with diminished proliferation (Fig. 1 A, Top). Surprisingly, cleaved caspase-3 (CC3) staining, which indicates apoptosis from low-dose cisplatin treatment and might have been anticipated to be enhanced by JH-RE-06, was suppressed in the combination treatment in both xenograft mice models (Fig. 1 A, Middle). Examination of the tissue sections from the combination treatment unexpectedly revealed two hallmarks of senescence (14, 15), lipofuscin accumulation and positive staining for senescence associated β-galactosidase activity (SA-β-Gal) (Fig. 1 A, Bottom).
Fig. 1.
Cisplatin and JH-RE-06 induce senescence phenotypes. (A) Histology sections of the JH-RE-06 (1.5 μM as +; and 3 μM as ++) + cisplatin, and cisplatin (1 mg/kg) treated paraffin-fixed A375 (melanoma cells), or OCT-derived SKOV3 (ovarian cancer cells) tumors from xenograft mice showing differences in Ki67 and CC3 staining. Lipofuscin appears as green autofluorescence, and SA-β-Gal staining is a blue tissue stain. Representative graphs at Right show quantification of Ki67- and CC3-positive cells in the A375 xenograft mice model by ImageQuant software. n = 12 in both Xenograft models. P < 0.001 as determined by a Student t test is represented as ***. Cells that are not Ki67 or CC3 positive either represent stromal cells or nonproliferative, nonapoptotic tumor cells. B shows SA-β-Gal–positive HT1080 (fibrosarcoma) cells, which are quantified in the bar graph for HT1080, A375, MEFs (mouse embryonic fibroblasts), and SKOV3 following 24 h treatments: NT (no treatment with drugs), JH-RE-06 (1.5 μM), cisplatin (1 μM), JH-RE-06+cisplatin, and H2O2 (a positive control for senescence; 0.3 μM). Also seen in bar graphs are the relative levels of Caspase 3/7 activation, colony survivability, and p21 expression levels as quantified via qRT-PCR. (C) Bar graphs quantifying SA-β-Gal–positive cells over the course of 72-h treatment with different drug combinations (same concentrations as in B, except JH-RE-06 is at 0.5 μM) in A375 cells. The graph below shows relative colony survival in A375 cells exposed to same concentration of drugs for 48 and 72 h. Immunofluorescence (IF) panels show DAPI-stained nuclei and 7-AAD–stained necrotic A375 cells over 24- and 48-h incubation time frame within the same experiment. D shows SA-β-Gal–positive REV1−/− MEF and siRNA REV1 knockdown HT1080 cells. Graph shows impaired colony formation in the REV1−/− MEF compared to the WT MEF counterparts.
We then examined combination treatment with JH-RE-06 plus cisplatin versus cisplatin alone for four different human and mouse cell lines in vitro. Here, we chose a low dose of cisplatin (1 μM) that is clinically achievable in patients treated with systemic chemotherapy (16). In all cases, we observed an increase in SA-β-Gal activity in combination versus cisplatin monotherapy-treated cells; H2O2 was used as a control senescence-inducing agent (Fig. 1B). We also observed reduced apoptosis, as visualized by CC3 staining, and increased p21 expression in combination-treated cells. p21 is a well-established inducer of cell cycle arrest and senescence (14, 15) The induction of senescence hallmarks by cisplatin/JH-RE-06 treatment was maximal for 48 h, after which the cells lost plasma membrane integrity, as demonstrated by the ability of membrane-impermeable 7-AAD (7-aminoactinomycin D) to label the nucleus and exhibited decreased colony survival (Fig. 1 B and C). Indeed, by 72 h following combination treatment, we observed a complete elimination of SA-β-Gal–positive cells. Notably, while some SA-β-Gal positivity emerges over time in cells treated with cisplatin monotherapy, senescence marker-associated cell clearance was never seen in this context. Thus, a dose of cisplatin that induces a low level of apoptosis mediated cell clearance as single agent synergizes with JH-RE-06 to promote nonapoptotic cell elimination.
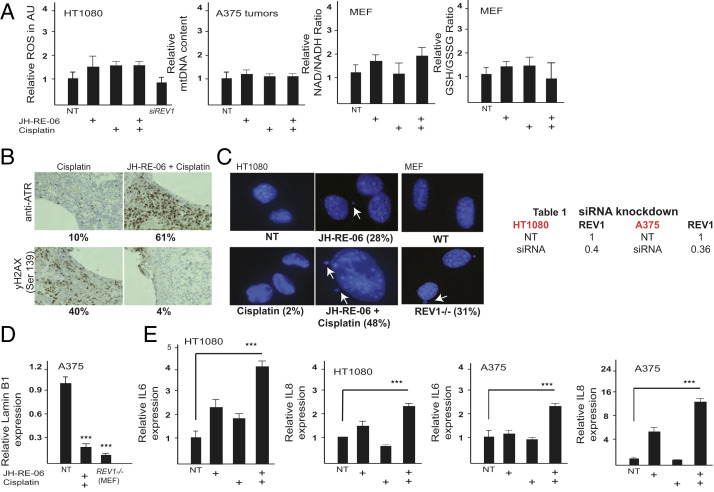
Two other hallmarks of senescence, increased micronuclei formation (Fig. 2C) and reduced Lamin B1 expression (Fig. 2D), were altered in the combination treatment compared to cisplatin alone. Strikingly, cells treated with cisplatin/JH-RE-06, but not with cisplatin alone, also exhibited enhanced expression of the immune factors IL6 and IL8 (Fig. 2E), which are induced as part of the senescence-associated secretory phenotype (14, 15). Collectively, these observations suggest that inhibition of REV1’s functions by JH-RE-06 switches the cisplatin-dependent commitment to apoptotic cell death in cancer cells to a different mode of lethality associated with the induction of multiple hallmarks of senescence.
Fig. 2.
Cisplatin and JH-RE-06 induce senescence phenotypes. (A) Graphs showing relative levels of reactive oxygen species (ROS) in HT1080 cells, relative mtDNA content in A375 tumor tissue, and LC/MS analysis to determine the relative change in NAD+/NADH and GSH/GSSG ratios in MEF cells. Cell culture drug treatments were the same as in Fig. 1B. (B) Histology sections showing ATR and gH2AX staining in JH-RE-06+cisplatin and cisplatin treated tissue sections. Drug concentrations were same as in Fig. 1A. (C) DAPI stained HT1080 nuclei show micronuclei formation in both JH-RE-06 and JH-RE-06+cisplatin treatment (same drug dose as in Fig. 1B). REV1−/− MEFs show micronuclei formation with no drug treatment. (D) Graph shows relative mRNA levels of Lamin B1 in JH-RE-06+cisplatin treatment in A375 cells and in native REV1−/− MEFs. (E) Graphs showing relative IL6 and IL8 expression in HT1080 and A375 cells treated with different drug combinations as in Fig. 1B. The table shows fraction of REV1 in HT1080 and A375 cells post siRNA knockdown. siRNAs were used at 100 nM concentration.
The induction of the senescence markers did not appear to involve an intermediate oxidative stress component since quantification of reactive oxygen species (ROS) in cultured cells, mitochondrial DNA (mtDNA) content both in cells or tumor samples, and the NAD/NADH or the GSH/GSSH ratios by liquid chromatography/mass spectrometry (LC/MS) analysis (17) were unchanged following cisplatin/JH-RE-06 treatment compared to cisplatin controls (Fig. 2A). Remarkably, although p-γ-H2AX foci formation was elevated and ATR expression was low in single agent cisplatin-treated cells, the opposite was true in cells treated with cisplatin/JH-RE-06 (Fig. 2B). These observations suggest that targeting REV1 with JH-RE-06 profoundly affects the nature of the persistent genomic damage after cisplatin treatment and also the resulting physiological responses, as p-γ-H2AX foci form at DNA double-strand breaks, whereas ATR expression reflects the presence of single-stranded DNA. Additionally, given that the inhibition of mutagenic TLS by JH-RE-06 is REV1-dependent (6), our results suggest that REV1 normally suppresses the induction of senescence markers after cisplatin damage. This seems to be true even in the absence of exogenous DNA damage, since REV1−/− mouse embryonic fibroblasts (MEF) cells (18) exhibited a senescence phenotype of large flattened cells, slower growth, SA-β-Gal staining, and increased IL6 and IL8 transcription compared to normal REV1+/+ MEFs (Figs. 1D and 2D) and the REV1 inhibitor JH-RE-06 alone partially induces senescence markers. It is important to note that JH-RE-06 treatment or loss of REV1 result in induction of senescence hallmarks but do not promote cell killing in vitro or clearance and elimination of cancer cells in vivo. The potentially useful therapeutic effect is only observed when cisplatin is present, and it is possible that cisplatin is acting as a senolytic therapy under such conditions.
The altered cisplatin response of cells in the context of Rev1 inhibition provides a rationale for the use of TLS inhibitors as senescence-based therapeutics. Indeed, in a companion to this study, effective cisplatin-mediated tumor clearance was achieved in treatment refractory lung cancers lacking the POLζ- component Rev7 (19). In this setting, cisplatin treatment of Rev7-deficient cells also induced a senescence-like phenotype, while cisplatin treatment of Rev7-proficient tumors was largely ineffective and induced apoptosis. The precise etiology of both this senescence-like phenotype and the phenotype elicited by JH-RE-06/cisplatin combination therapy remain unclear, as are the differences between these therapeutic outcomes and established forms of cellular senescence. However, the ability of TLS inhibition to alter the response to low-dose cisplatin suggests that this approach may represent a strategy to achieve desirable therapeutic outcomes while minimizing toxicity associated with high-dose chemotherapy.
Materials and Methods
Antibodies included Ki67 (ab15580; Cell Signaling), CC3 (ab13847; Cell Signaling), ATR (ab-428, Sigma), and γH2A.X (Ser-139) (ab9718; Cell Signaling). JH-RE-06 (synthesized in J.H. Laboratory, Duke University), Cisplatin (catalog No. S1166; Selleckchem), H2O2 (H1009; Sigma), DAPI (F6057; Sigma), DHE (dihydroethidium) (D1168; Invitrogen). REV1 small interfering RNA (siRNA) (L-003551-00-0005; Dharmacon), SA-β-Gal staining kit (9860; Cell Signaling); Caspase-Glo 3/7 Assay system (G8091, Promega), GFP-CERTIFIED Apoptosis/Necrosis detection kit (ENZ-51002; Enzo Lifesciences). qPCR primer sequences are available on request. Cell culture, immunofluorescence, and qPCR were performed as described before (6). Experiments involving kits were conducted per the manufacturer’s recommendations. mtDNA content was quantified as described in (20). LC/MS experiments were done as described before (17). Mouse work was conducted as described before (13). The Committee on Animal Care approval no. is 0318-017-21.
Acknowledgments
This work was supported by National Institute of Environmental Health Sciences Grants ES028303 (to G.C.W.) and P30 ES002109 (to the Massachusetts Institute of Technology (MIT) Center of Environmental Health Sciences); National Cancer Institute Grants CA191448 (to P.Z. and J.H.), CA224144 (to O.J.), and R35CA242379 (to M.G.V.H.); National Institute of General Medical Sciences Grant T32GM007287 (to A.L.); NSF Grant GRFP DGE-1122374 (to A.L.); and Duke CTSA Grant UL1TR002553 through a subaward (to P.Z. and J.H.). E.C.L. was supported by Damon Runyon Cancer Research Foundation Grant DRG-2299-17. Additional support was received by a faculty scholars award from HHMI, support from the Ludwig Center at MIT, and support from the Emerald Foundation (M.G.V.H.) and the Center for Precision Cancer Medicine at MIT (M.G.V.H. and M.T.H.). G.C.W. is an American Cancer Society Professor.
Footnotes
Competing interest statement: M.G.V.H. is a consultant and SAB member for Agios Pharmaceuticals, Aeglea Biotherapeutics, iTeos Therapeutics, and Auron Therapeutics. A.L. is a current employee of a Flagship Pioneering biotechnology startup company.
Data Availability.
All study data are included in the article and supporting information.
References
- 1.Yamanaka K., Chatterjee N., Hemann M. T., Walker G. C., Inhibition of mutagenic translesion synthesis: A possible strategy for improving chemotherapy? PLoS Genet. 13, e1006842 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doles J., et al. , Suppression of Rev3, the catalytic subunit of Pol{zeta}, sensitizes drug-resistant lung tumors to chemotherapy. Proc. Natl. Acad. Sci. U.S.A. 107, 20786–20791 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xie K., Doles J., Hemann M. T., Walker G. C., Error-prone translesion synthesis mediates acquired chemoresistance. Proc. Natl. Acad. Sci. U.S.A. 107, 20792–20797 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sail V., et al. , Identification of small molecule translesion synthesis inhibitors that target the Rev1-CT/RIR protein-protein interaction. ACS Chem. Biol. 12, 1903–1912 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wojtaszek J., et al. , Multifaceted recognition of vertebrate Rev1 by translesion polymerases zeta and kappa. J. Biol. Chem. 287, 26400–26408 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wojtaszek J. L., et al. , A small molecule targeting mutagenic translesion synthesis improves chemotherapy. Cell 178, 152–159.e11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Short S., Fielder E., Miwa S., von Zglinicki T., Senolytics and senostatics as adjuvant tumour therapy. EBioMedicine 41, 683–692 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang D., Lippard S. J., Cellular processing of platinum anticancer drugs. Nat. Rev. Drug Discov. 4, 307–320 (2005). [DOI] [PubMed] [Google Scholar]
- 9.Childs B. G., Baker D. J., Kirkland J. L., Campisi J., van Deursen J. M., Senescence and apoptosis: Dueling or complementary cell fates? EMBO Rep. 15, 1139–1153 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murray D., Mirzayans R., Cellular responses to platinum-based anticancer drugs and UVC: Role of p53 and implications for cancer therapy. Int. J. Mol. Sci. 21, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mikula-Pietrasik J., et al. , Comprehensive review on how platinum- and taxane-based chemotherapy of ovarian cancer affects biology of normal cells. Cell. Mol. Life Sci. 76, 681–697 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marcozzi S., et al. , Distinct effects of epirubicin, cisplatin and cyclophosphamide on ovarian somatic cells of prepuberal ovaries. Aging (Albany NY) 11, 10532–10556 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jonas O., et al. , An implantable microdevice to perform high-throughput in vivo drug sensitivity testing in tumors. Sci. Transl. Med. 7, 284ra257 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorgoulis V., et al. , Cellular senescence: Defining a path forward. Cell 179, 813–827 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Hernandez-Segura A., Nehme J., Demaria M., Hallmarks of cellular senescence. Trends Cell Biol. 28, 436–453 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Ikeda K., et al. , Pharmacokinetics of cisplatin in combined cisplatin and 5-fluorouracil therapy: A comparative study of three different schedules of cisplatin administration. Jpn. J. Clin. Oncol. 28, 168–175 (1998). [DOI] [PubMed] [Google Scholar]
- 17.Sullivan L. B., et al. , Supporting aspartate biosynthesis is an essential function of respiration in proliferating cells. Cell 162, 552–563 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jansen J. G., et al. , Strand-biased defect in C/G transversions in hypermutating immunoglobulin genes in Rev1-deficient mice. J. Exp. Med. 203, 319–323 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vassel F.-M., Bian K., Walker G. C., Hemann M. T., Rev7 loss alters cisplatin response and increases drug efficacy in chemotherapy-resistant lung cancer. Proc. Natl. Acad. Sci. U.S.A. 117, 28922–28924 (2020).33144509 [Google Scholar]
- 20.Fakouri N. B., et al. , Rev1 contributes to proper mitochondrial function via the PARP-NAD(+)-SIRT1-PGC1alpha axis. Sci. Rep. 7, 12480 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All study data are included in the article and supporting information.