Significance
Functional levels of attention and impulse control allow advanced organisms to behave in a goal-directed manner by decreasing distraction from irrelevant stimuli and by avoiding responses that are premature in a given situation. Attention deficits and impulsivity are often comorbid with the main symptoms of several neuropsychiatric conditions, but their neuroanatomical substrates are still a matter of debate. Here we show that activation of noradrenergic neurons of the mouse locus coeruleus is necessary and sufficient for goal-directed behavior. We found that improvements are mediated by norepinephrine release in separate subregions of the prefrontal cortex. This study provides causal evidence for the modulation of attentional control by locus coeruleus/norepinephrine neurons and specifies the cortical areas involved in such effects.
Keywords: locus coeruleus, prefrontal cortex, norepinephrine, attentional control, response inhibition
Abstract
The attentional control of behavior is a higher-order cognitive function that operates through attention and response inhibition. The locus coeruleus (LC), the main source of norepinephrine in the brain, is considered to be involved in attentional control by modulating the neuronal activity of the prefrontal cortex (PFC). However, evidence for the causal role of LC activity in attentional control remains elusive. Here, by using behavioral and optogenetic techniques, we investigate the effect of LC neuron activation or inhibition in operant tests measuring attention and response inhibition (i.e., a measure of impulsive behavior). We show that LC neuron stimulation increases goal-directed attention and decreases impulsivity, while its suppression exacerbates distractibility and increases impulsive responding. Remarkably, we found that attention and response inhibition are under the control of two divergent projections emanating from the LC: one to the dorso-medial PFC and the other to the ventro-lateral orbitofrontal cortex, respectively. These findings are especially relevant for those pathological conditions characterized by attention deficits and elevated impulsivity.
The attentional control of behavior, which allows the execution of plans and the attainment of goals, represents a distinguishing feature of the most advanced and complex organisms. Attentional control relies on both attentional and inhibitory processes: while the attentional processes subtend the ability to direct or shift the focus of attention to sources of information deemed important for the task at hand (1), the inhibitory processes serve to avoid interference from irrelevant stimuli by inhibiting prepotent behaviors such as orienting toward distractors or impulsively responding to them (2). These highly interacting and mutually constraining cognitive functions are crucial for maintaining goal-directed behavior by allowing the organism to flexibly adapt to continually changing environmental demands (3).
Attentional and inhibitory deficits are manifested as distractibility and impulsivity in several pathological conditions and predict poor academic, professional, and social outcomes in the general population (4, 5). Attention deficit/hyperactivity disorder (ADHD) is the archetypal neurocognitive condition characterized by attentional control deficits. There is evidence that norepinephrine (NE) transmission is altered in ADHD (6–8), which is consistent with the use of NE-boosting drugs as pharmacological therapies for this disorder (9–11).
The locus coeruleus (LC), the main source of NE in the brain, is thought to be involved in attentional control, among other cognitive functions, by modulating neuronal activity in the prefrontal cortex (PFC) (12, 13). Increased neuronal activity in the human PFC and improved performance have been observed during cognitive tasks measuring attention and response inhibition following the administration of noradrenergic drugs (14, 15). However, since NE-boosting drugs both increase extracellular NE (16) and decrease the LC spontaneous firing rate (17, 18), it is not clear which of these effects is responsible for their cognition-enhancing properties, nor whether they are mediated by separate or shared neural substrates in the PFC.
Previous research has sought to investigate in more detail the mechanisms behind the procognitive effects of NE in animal models via translational laboratory tasks measuring attention and response inhibition mainly by physiological recordings, pharmacological interventions, or neurotoxic lesion approaches (19–22). Although these methods possess high neurochemical and neuroanatomical specificity, they do not allow the manipulation of the activity of the noradrenergic system in a direct and temporally specific manner. Such technical limitations are especially important for the LC/NE system, given its crucial role in learning and memory as well as its remarkable plasticity (23, 24). On the other hand, modern optogenetic techniques afford an unprecedented advantage in terms of neuroanatomical and temporal specificity for the study of cognitive functions (25).
In order to better understand the role of the LC/NE system in regulating the attentional control of behavior, we selectively manipulated and recorded LC neuronal activity via temporally targeted optogenetic techniques (26, 27) during operant tests of attention and response inhibition. Crucially, since the effects of perturbing LC neuronal activity are most obvious during difficult tasks (28), we devised three variants of the same operant test in order to separately challenge different aspects of attentional performance. Our results show that LC stimulation increases goal-directed (top-down) attention and response inhibition. Conversely, the inhibition of LC neurons makes the animals more distractible and impulsive as if their attention were controlled prevalently in a stimulus-driven (bottom-up) manner. We then confirmed the physiological relevance of our optogenetic manipulations by recording LC calcium activity during attentional performance. Finally, we tested whether the effects of LC activation on attention and response inhibition could be dissociated in downstream subregions of the PFC. We found that stimulating LC terminals in the dorso-medial PFC (dmPFC) decreases distractibility without affecting response inhibition, whereas stimulation of LC terminals in the ventro-lateral orbitofrontal cortex (vlOFC) specifically reinforces response inhibition without affecting measures of attention. These results are crucial for advancing our understanding of neurocognitive diseases characterized by attentional deficits and impulsivity.
Results
Double-Transgenic Strategy for the Optogenetic Targeting of LC Neurons.
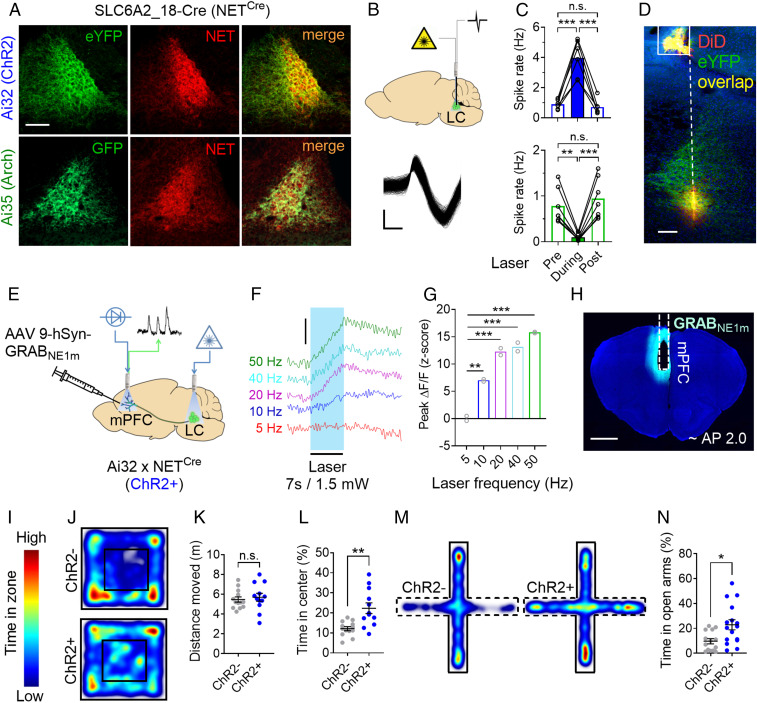
To selectively manipulate LC neuronal activity during specific epochs of behavioral tasks measuring attentional control, we took advantage of neural circuit genetics and optogenetic techniques. By crossing NETCre mice (29) with either Ai32 or Ai35 reporter mice (30), we selectively expressed excitatory (ChR2) and inhibitory (Arch) opsins, respectively, in noradrenergic NET+ neurons (Fig. 1A). We confirmed the interventional efficacy of these opsins by measuring firing rates of NET+ neurons via extracellular electrophysiological recordings and simultaneous optogenetic manipulation of LC neurons (Fig. 1B). In Ai32+ x NETCre+ (ChR2+) animals, a blue laser activation at 20 Hz for 7 s produced a transient increase in neural activity up to ∼5 Hz (Fig. 1 C, Upper: F2,10 = 52.79, P < 0.0001; see also SI Appendix, Fig. S1 B, C, and E). Importantly, this level of optogenetic stimulation is consistent with a physiological “elevated tonic” LC firing rate (31). Continuous green laser light for 7 s in Ai35+ x NETCre+ (Arch+) animals caused an almost total inhibition of LC neuronal activity (Fig. 1 C, Lower: F2,12 = 30.81, P < 0.0001; see also SI Appendix, Fig. S1 D and E). To further support our choice of the stimulation parameters for ChR2+ mice, we assessed NE release in the medial PFC (mPFC) by fiber photometry (FP) recordings of the fluorescent GPCR activation-based NE (GRABNE1m) sensor (32) at different laser frequencies (Fig. 1 E–H) and intensities (SI Appendix, Fig. S1 F–H). We found that 20 Hz stimulation at 1.5 mW produced a moderate but robust increase of extracellular NE in mPFC (Fig. 1G; F4,4 = 521, P < 0.0001).
Fig. 1.
Double-transgenic strategy for the optogenetic targeting of LC neurons. (A) Expression of light-activated opsins in noradrenergic (NET+; red) LC neurons of Ai32+ (Top) or Ai35+ mice (Bottom) crossed with NETCre+ mice. (Scale bar, 100 µm.) (B) (Top) Strategy for optogenetic manipulation and simultaneous electrophysiological recording of LC neurons. (Bottom) Representative waveforms from a LC isolated unit. (Scale bar, 0.2 ms × 0.1 mV.) (C) Effect of blue laser (Top: six neurons, two mice) and green laser (Bottom: seven neurons, two mice) on LC firing rate in Ai32+ x NETCre+ (ChR2+) and Ai35+ x NETCre+ (Arch+) mice, respectively (7-s bins). (D) Histological sample showing the position of the optic fiber (solid line) and electrode track (dashed line). (Scale bar, 100 µm.) (E) Strategy for virally mediated expression of the fluorescent NE sensor GRABNE1m in mPFC and fiber photometry recordings during LC optogenetic stimulation in ChR2+ mice. (F) Representative traces (scale bar, 1% ΔF/F) and quantification (G) of NE release in the mPFC (two mice; three trials each) at different laser activation frequencies (7 s, 1.5 mW, 10-ms pulses). (H) Histological verification of GRABNE1m expression and optic fiber placement in the mPFC. (I) Color scale of heatmaps. (J) Heatmaps of locomotor activity for ChR2 mice in the OF test. (K) Effect of LC stimulation on locomotor activity in the OF test. (L) Effect of LC stimulation on the time spent in the center of the OF apparatus. (M) Heatmaps of ChR2 mice locomotor activity in the EPM test. (N) Effect of LC stimulation on the time spent in the open arms of the EPM apparatus. Data are presented as mean; error bars show ± SEM. Repeated measures (RM) one-way ANOVA followed by Sidak (C) or Dunnett (G; 5 Hz as reference variable) post hoc test. Unpaired t tests (K, L, N) are two-tailed. n.s., not significant; *P < 0.05; **P < 0.005; ***P < 0.0001.
At the behavioral level, we found that our optogenetic stimulation parameters do not alter general locomotor activity levels (Fig. 1 J and K; t23 = 0.48, n.s.). Unexpectedly, we also found that our stimulation protocol increases the time spent by ChR2+ animals in the center of an open field (OF) apparatus (Fig. 1L; t23 = 3.65, P = 0.001; ChR2–: n = 13; ChR2+: n = 12) as well as the time spent in the open arms of the elevated plus maze (EPM) (Fig. 1 M and N; t28 = 2.78, P = 0.009; ChR2–: n = 14; ChR2+: n = 16), suggesting a decrease in anxiety-like behavior (33).
In summary, these results show that our optogenetic approach to LC inhibition strongly silences neural activity, whereas our stimulation parameters mimic physiological elevated levels of tonic LC firing (∼5 Hz) and cause a significant release of NE in PFC. Importantly, our LC stimulation protocol does not alter spontaneous locomotor activity, but it decreases anxiety which is known to impair goal-directed behavior (34, 35).
LC Neurons Activity Modulates Sustained Attention and Response Inhibition.
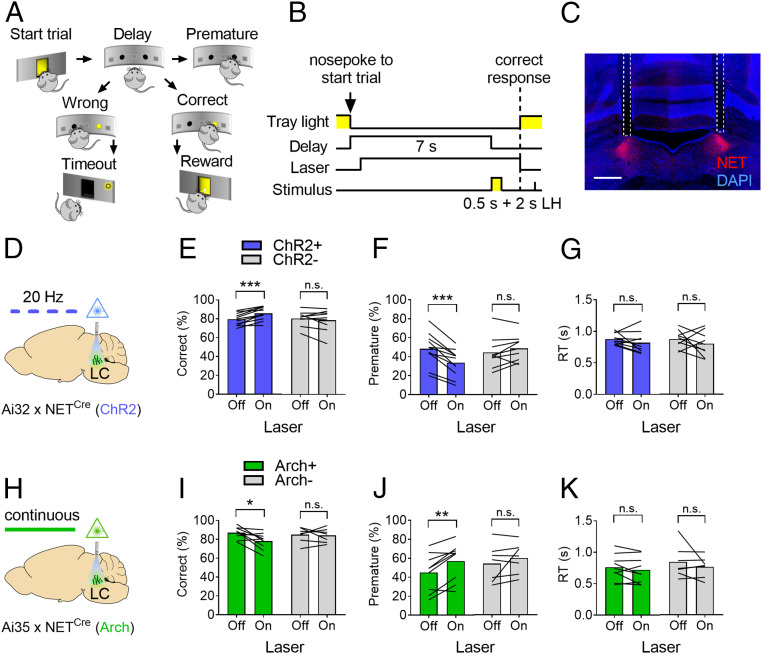
Previous studies have reported marked improvements in cognitive functions by pharmacologically enhancing NE extracellular levels (9, 20, 36). However, evidence for a direct involvement of LC neuronal activity in tasks measuring attention and response inhibition is still lacking. To investigate the causal relationship between LC neuronal activity and attentional control performance, we trained double-transgenic mice and their littermates in a simplified version of the widely used five-choice serial reaction time task (37), the two-choice task (Fig. 2 A and B), designed to measure sustained attention (operationalized as the proportion of correct responses) and response inhibition (defined as the proportion of premature responses, i.e., made before the appearance of the target stimulus). Briefly, food-restricted animals were required to wait for a target stimulus (a light turning on for 0.5 s) appearing in one of two apertures after a delay of 7 s and to select the illuminated aperture by poking in it with the snout in order to receive a food reward. Animals were then implanted with bilateral optic fibers targeting the LC (Fig. 2C) and retrained to criterion performance (Materials and Methods) before being tested on the two-choice task. During test sessions (n = 3, 150 trials/subject), trials with or without laser activation were interleaved in a pseudorandom fashion. Stimulation of LC neurons in ChR2+ animals during the delay period preceding the appearance of the target stimulus (“Laser On”) increased correct responses (Fig. 2E; laser x genotype: F1,18 = 14.38, P = 0.0013; laser: F1,18 = 4.05, P = 0.059, n.s.; genotype: F1,18 = 0.82, n.s.) and decreased premature responses (Fig. 2F; laser x genotype: F1,18 = 8.14, P = 0.01; laser: F1,18 = 26.75, P < 0.0001; genotype: F1,18 = 0.79, n.s.) compared to nonstimulated trials (“Laser Off”), indicating significant improvements in sustained attention and response inhibition, respectively. On the contrary, inhibition of LC neurons in Arch+ animals during the delay decreased correct responses (Fig. 2I; laser x genotype: F1,14 = 3.64, n.s.; laser: F1,14 = 5.09, P = 0.04; genotype: F1,14 = 0.43, n.s.) and increased premature responses (Fig. 2J; laser x genotype: F1,14 = 1.26, n.s.; laser: F1,14 = 10.47, P = 0.006; genotype: F1,14 = 0.43, n.s.). There were no effects of laser activation on correct or premature responses in ChR2– or Arch– mice, nor on other behavioral measures such as reaction time (RT) (Fig. 2 G and K) and reward collection latency (RCL) (SI Appendix, Fig. S2 B and D) in any of the groups, ruling out an effect of LC optogenetic manipulation on motor activity or motivation during the attentional task (38). Overall, these effects are consistent with results obtained across species on the improvement of attention and response inhibition by NET antagonists or NE receptor agonists (9) and demonstrate a causal involvement of LC neuronal activity in these effects.
Fig. 2.
LC neurons activity modulates sustained attention and response inhibition. (A) Schematic representation of the two-choice task. (B) Single-trial structure of the two-choice task (LH: limited hold). (C) Confocal tile scan of a histological sample of a bilateral optic fiber implant (dashed lines) placed just above the LC. (Scale bar, 500 µm.) (D) Strategy for the optogenetic stimulation of LC neurons in ChR2 mice. Effect of LC stimulation on correct responses (E), premature responses (F), and RTs (G) in ChR2 mice (ChR2+, blue bars, n = 11; ChR2–, gray bars, n = 9). (H) Strategy for the optogenetic inhibition of LC neurons in Arch mice. Effect of LC inhibition on correct responses (I), premature responses (J), and RTs (K) in Arch mice (Arch+, green bars; n = 9; Arch–, gray bars, n = 7). Data are presented as the mean of three sessions (150 trials/subject); Two-way RM ANOVA followed by Sidak post hoc test. Effect of laser: n.s., not significant; *P < 0.05; **P < 0.005; ***P < 0.0001.
LC Neurons Control Goal-Directed Attention.
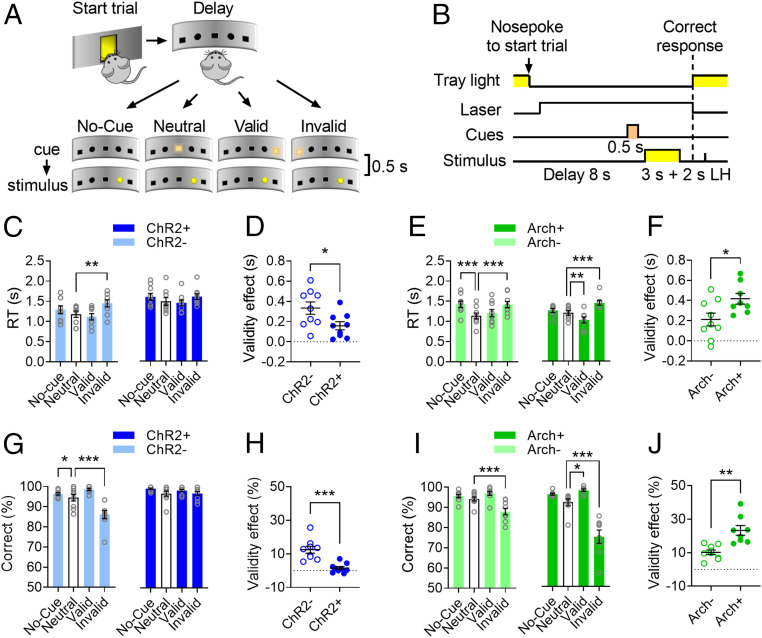
An important aspect of attentional control is the ability to selectively respond to stimuli important for the task at hand and at the same time to ignore irrelevant stimuli that induce distraction and response tendencies potentially conflicting with current goals (39, 40). In order to assess the contribution of LC neurons to maintaining high levels of attention in the presence of irrelevant stimuli (i.e., goal-directed attention), we modified the two-choice task by presenting light cues (turning on for 0.5 s) shortly before the appearance of target stimuli (3 s duration; stimulus onset asynchrony = 0.5 s) (Fig. 3 A and B, Movie S1). In this version of the two-choice task designed after the Posner attentional cueing paradigm (41), the laser is activated during the delay on every trial and the distracting cues are presented on the response panel in a pseudorandomized fashion either centrally (Neutral), on the same side (Valid), or on the opposite side (Invalid) compared to target stimuli or are omitted on a subset of trials (No-cue; Fig. 3A). Crucially, appearance of the distracting cues does not predict where the subsequent target stimuli will be presented. Therefore, although paying attention to the cues might aid performance on a subset of trials (i.e., Valid trials), the most efficient goal-directed strategy is to ignore them while waiting for the target stimulus. In general, if subjects direct their attentional focus to the location of the cues, Valid cues will elicit faster RTs and increase correct responses, whereas Invalid cues will cause slower RTs and decrease correct responses (41).
Fig. 3.
LC neurons control goal-directed attention. (A) Schematic representation of the four trial types in the cued version of the two-choice task (Movie S1). (B) Timeline of events within a cued trial (LH: limited hold). (C) Effects of trial type on the RT of ChR2 mice. (D) Effect of laser stimulation on the RT Validity effect (Invalid RTs – Valid RTs). (E) Effect of trial type on the RT of Arch mice. (F) Effect of laser stimulation on the RT Validity effect in Arch mice. (G) Effect of trial type on the proportion of correct responses in ChR2 mice. (H) Effect of laser stimulation on the Validity effect of response accuracy (Valid % correct – Invalid % correct) in ChR2 mice. (I) Effect of trial type on correct responses in Arch mice. (J) Effect of laser activation on the Validity effect of response accuracy in Arch mice. Data are expressed as averages of six sessions (300 trials/subject) ± SEM. ChR2–, ChR2+, and Arch–: n = 9, Arch+: n = 8. RM ANOVA followed by Dunnett post hoc test (or the equivalent nonparametric Friedman test followed by Dunn post hoc test) with Neutral trials (empty bars) as the reference variable (C, E, G, I). Paired t tests (D, F, H, J) are two-tailed. Lasers were activated on every trial. Effect of trial type (C, E, G, I) or genotype (D, F, H, J): *P < 0.05; **P < 0.005; ***P < 0.0005.
As expected, ChR2– subjects were slower to respond on Invalid trials as compared to Neutral trials (Fig. 3C; Friedman test, P < 0.0006). Although there was a main effect of trial type in ChR2+ mice (F3,24 = 3.72, P = 0.02), Dunnett’s post hoc test (Neutral cue as reference variable) did not reveal any significant difference between trial types. We then calculated the time taken by subjects to resolve the conflict generated by Invalid cues (“Validity effect” = Invalid trials RTs – Valid trials RTs). Laser activation decreased the RT Validity effect in ChR2+ compared to ChR2– subjects (Fig. 3D; t16 = 2. 4, P = 0.02). In both Arch– and Arch+ mice, RTs were significantly affected by trial type compared to the Neutral cue condition (Fig. 3E; Arch–: F3,24 = 13.64, P < 0.0001; Arch+: F3,21 = 23.26, P < 0.0001), whereas the RT Validity effect in Arch+ mice was increased by laser activation compared to Arch– mice (Fig. 3F; t15 = 2.44, P = 0.02). Compared to Neutral cues, Invalid cues decreased correct responses in ChR2– mice (Fig. 3G; F3,24 = 23.5, P < 0.0001). In ChR2+ mice, there was a significant main effect of trial type on correct responses (Fig. 3G; Friedman test = 7.83, P = 0.049), which did not survive after correction for multiple comparisons. In Arch mice, correct responses were significantly affected by trial type (Fig. 3I; Arch–: F3,24 = 19.69, P < 0.0001; Arch+: F3,21 = 36.01, P < 0.0001).
To investigate how Valid and Invalid trials affect response accuracy, we calculated the Validity effect on the relative proportion of correct responses in these trials (% correct on Valid trials – % correct on Invalid trials). Correct responses were less affected by Valid and Invalid cues in ChR2+ mice as compared to ChR2– mice (Fig. 3H; t16 = 4.85, P = 0.0002), while they were more affected by Valid and Invalid cues in Arch+ mice compared to Arch– mice (Fig. 3J; t15 = 4.21, P = 0.0008). There were no effects of trial type on premature or omitted responses in any of the groups (SI Appendix, Fig. S3 A–D). Taken together, these results indicate that activation of LC neurons allows the subjects to ignore irrelevant cues (i.e., decreases distractibility) by increasing goal-directed (top-down) attention. On the other hand, LC inhibition increases stimulus-driven (bottom-up) attention, thus making the subjects more distracted by irrelevant information.
LC Calcium Activity Reflects a Trade-Off between Top-Down and Bottom-Up Attention.
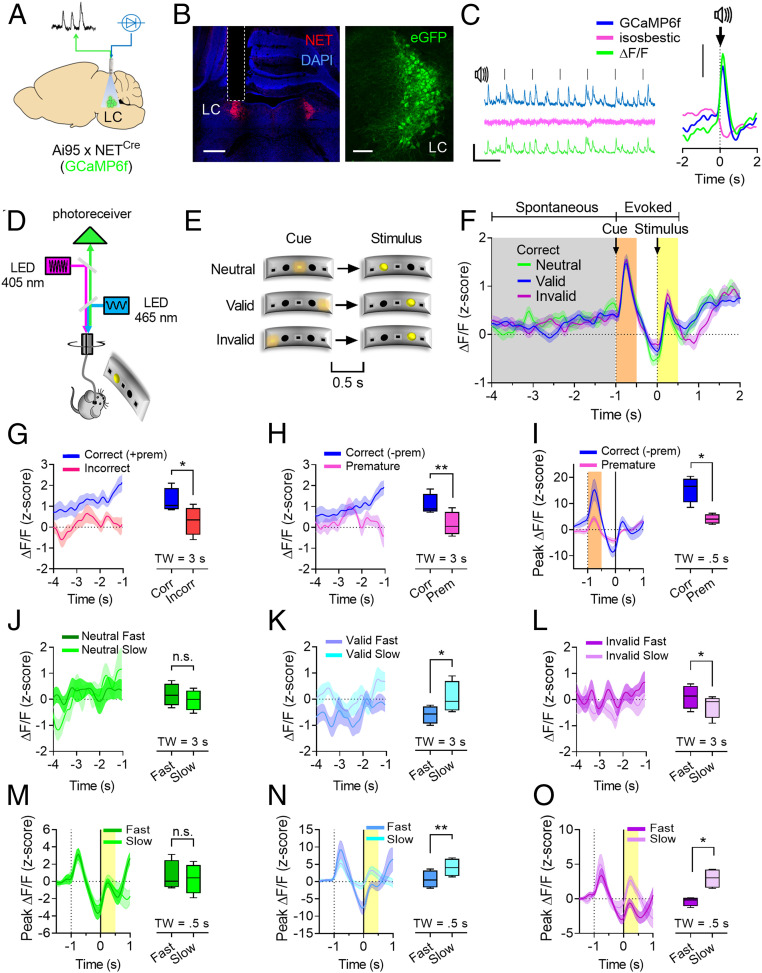
To assess whether the results obtained by the artificial optogenetic manipulation apply to physiologically occurring phenomena, we optically recorded calcium transients in LC neurons during performance in the cued version of the two-choice task. To this end, NETCre mice were crossed with Ai95 reporter mice (42) to target expression of the fluorescent calcium indicator GCaMP6f specifically to NET+ neurons, and the resulting double-transgenic mice were implanted with optic fibers above the LC for FP recordings (Fig. 4 A and B). In the recordings from these mice, auditory stimuli resulted in phasic calcium transients, which are the typical response of LC neurons to sensory stimulation (Fig. 4C).
Fig. 4.
LC calcium activity reflects a trade-off between top-down and bottom-up attention. (A) Approach used for FP recordings of LC calcium activity. (B) Confocal tile scan showing optic fiber placement (Left; scale bar, 500 µm) and GCaMP6f expression in LC neurons (Right; Scale bar, 100 µm). (C) Representative FP traces of LC recordings during white-noise stimulation (Left; scale bar, 10 s × 5% ΔF/F) and FP traces aligned to 1 s white noise stimuli (Right; 144 trial, four mice; scale bar, 1% ΔF/F). (D) Strategy for the recording of calcium transients in Ai95 x NETCre mice during the cued version of the two-choice task. (E) Trial types used in the cued version of the two-choice task during FP recordings. (F) Peri-event histogram showing LC calcium activity during correct trials segregated by trial type and aligned to target stimuli for the analysis of spontaneous (gray, 4 s) or evoked (orange and yellow, 0.5 s) LC activity. (G) Comparison of LC spontaneous activity between correct and incorrect trials. (H) Comparison of LC spontaneous activity between correct and premature trials. (I) Comparison of LC activity evoked by cues during correct and premature trials. LC spontaneous activity divided by Fast and Slow trials during Neutral (J), Valid (K), or Invalid (L) trial types. LC activity evoked by target stimuli divided by Fast and Slow trials during Neutral (M), Valid (N), or Invalid (O) trial types. (n = 4 mice; 150 trials/subject). Correct trials do not include premature trials (– prem) except in G. Data are expressed as mean ± SEM of n = 4 mice. Box and whiskers plots represent the five-number summary. TW: time window analyzed (in seconds). Paired t tests are two-tailed. n.s., not significant; *P < 0.05; **P < 0.005.
Data obtained from four mice (600 trials) were divided according to trial outcome (correct, incorrect, and premature responses), and correct responses were further segregated by trial type (Neutral, Valid, and Invalid; Fig. 4E). No-cue trials and the limited hold (LH) period were not used in order to facilitate data analysis. Calcium activity was aligned to stimulus onset for the analysis of spontaneous LC activity during the second half of the delay period (3 s, gray shaded area; Fig. 4F) or for quantifying LC response evoked by cues and target stimuli (peak activation during a 0.5-s postevent window, orange and yellow shaded areas, respectively; Fig. 4F). The window of analysis for LC spontaneous activity was chosen in order to minimize noise in the data caused by the animals turning toward the response panel.
While there was no difference in LC spontaneous or evoked activity when correct trials were divided according to trial type (SI Appendix, Fig. S4 A–D), LC neurons displayed higher spontaneous activity during the delay on correct trials compared to incorrect trials (Fig. 4G; t3 = 4.35, P = 0.02) or compared to omitted trials (SI Appendix, Fig. S4E). LC calcium activity on correct trials was higher compared to premature trials during the delay (Fig. 4H; t3 = 7.02, P = 0.005) and in response to the cue (Fig. 4I; t3 = 4.43, P = 0.02), but not to the stimulus (SI Appendix, Fig. S4 I and J).
In order to assess whether LC activity during the delay predicts attentional performance on the three trial types, we further subdivided correct responses in “Fast” and “Slow” by median split of the RTs for each animal/session. There was no difference in LC spontaneous activity between Fast and Slow Neutral trials (Fig. 4J; t3 = 0.4, n.s.), while it was higher in Valid Slow compared to Valid Fast trials (Fig. 4K; t3 = 4.13, P = 0.02) and in Invalid Fast compared to Invalid Slow trials (Fig. 4L; t3 = 3.66, P = 0.03). These results indicate that, when LC activity is relatively higher during the delay period, animals are less distracted by the cues since their RTs do not display the typical decrease or increase on Valid and Invalid trials, respectively.
Evoked LC calcium activity in response to target stimuli did not differ between Fast and Slow Neutral trials (Fig. 4M; t3 = 0.76, n.s.), but it was higher on Slow Valid compared to Fast Valid trials (Fig. 4N; t3 = 7.87, P = 0.004) and on Slow Invalid compared to Fast Invalid trials (Fig. 4O; t3 = 5.17, P = 0.01). These effects likely reflect the increased stimulus-evoked response of LC neurons on trials where the target stimulus did not appear at the expected location (independently of trial type), thus resulting in slower RTs.
In summary, in keeping with the effects of the optogenetic manipulations of LC neurons, we have found that correct responses are consistently preceded by higher LC activity as compared to all other trial outcomes (i.e., incorrect, premature, or omitted responses). These results show that LC spontaneous activity is positively related to goal-directed attention, whereas the activity evoked by target stimuli might reflect the shift between bottom-up and top-down attention when prior expectations are violated (43).
LC Activity Modulates Distractibility and Impulsivity Elicited by Irrelevant Stimuli via Dissociable Coeruleo-Cortical Projections.
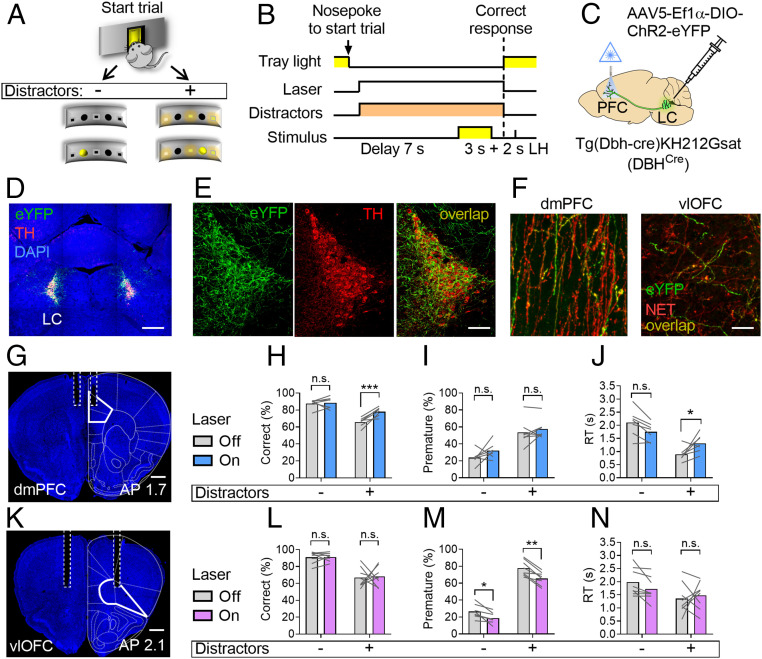
In an attempt to further define the role of LC neurons in attentional control, we sought to dissociate the effects of LC optogenetic manipulation on attention and response inhibition. To this end, we modified the two-choice task by adding distracting lights during the delay between the beginning of the trial and the animals’ response to target stimuli (Fig. 5 A and B). Trials with or without distractors were interleaved pseudorandomly as were trials with or without laser activation. As expected, we found that distractors significantly impaired performance by decreasing accuracy, increasing premature responses, and shortening RTs in all groups of animals.
Fig. 5.
Dissociable roles of divergent coeruleo-cortical projections in attentional control. (A) Schematic representation of the modified version of the of the two-choice task with distractors on a subset of trials. (B) Single-trial structure of the task. (C) Approach used for the expression of ChR2 in coeruleo-cortical terminals and their stimulation (5 Hz). (D) Confocal tile scan showing ChR2-eYFP (green) expression in LC neurons. (Scale bar, 500 µm.) (E) Magnified view of LC neurons expressing ChR2. (Scale bar, 50 µm.) (F) Histological samples showing LC terminals (NET; red) expressing ChR2 (eYFP; green) in the dmPFC (Left) and the vlOFC (Right). (G) Histological sample showing optic fiber placement (dashed lines) in the dmPFC (solid line). (Scale bar, 400 µm.) Effect of LC terminal stimulation in the dmPFC (n = 7) on correct responses (H), premature responses (I), and RTs (J). (K) Histological sample showing optic fiber placement (dashed lines) in vlOFC (solid line). (Scale bar, 400 µm.) Effect of LC terminal stimulation in vlOFC (n = 8) on correct responses (L), premature responses (M), and RTs (N). Data are presented as the mean of three sessions (150 trials/subject). Two-way RM ANOVA followed by Sidak post hoc test. AP: anterior-posterior coordinates. Effects of laser: n.s., not significant; *P < 0.05; **P < 0.005; ***P < 0.0005.
LC neurons stimulation in ChR2+ mice increased correct responses and decreased premature responses (SI Appendix, Fig. S5 E and F) independently of distractors. There was no effect of LC stimulation on RT (SI Appendix, Fig. S5G). On the contrary, LC inhibition in Arch+ mice decreased correct responses only in trials with distractors (SI Appendix, Fig. S5I) and increased premature responses in all trials (SI Appendix, Fig. S5J) with no effect on RTs (SI Appendix, Fig. S5K). There was no effect of laser activation on any of the control animals (SI Appendix, Fig. S6 A–F). Taken together, these results suggest that LC neuronal activity is crucially involved in both selective attention and response inhibition.
Next, to selectively investigate the differential role of LC NE release in PFC subregions in attention and response inhibition, we trained animals on the version of the two-choice task with distractors, as it can best differentiate between attentional and inhibitory processes (SI Appendix, Fig. S5). Then, in order to robustly express ChR2 in LC terminals, we injected DBHCre mice with AAV5-Ef1α-DIO-ChR2 (E123A)-eYFP (or its control version; SI Appendix, Fig. S7) in the proximity of LC neurons (Fig. 5 C–F) and implanted them with optic fibers targeting the dmPFC (Fig. 5G) or the vlOFC (Fig. 5K). Stimulation of LC terminals at 5 Hz in the dmPFC during the delay preceding the appearance of the target stimulus increased correct responses only in trials with distractors compared to Laser Off trials (Fig. 5H; laser x distractors: F1,6 = 50.96, P = 0.0004; laser: F1,6 = 8.61, P = 0.02; distractors: F1,6 = 60.39, P = 0.0002), but it did not affect premature responses (Fig. 5I; laser x distractors: F1,6 = 0.46, n.s.; laser: F1,6 = 3.11, n.s.; distractors: F1,6 = 25.43, P = 0.002). RTs were significantly affected by LC terminal stimulation in dmPFC only in trials with distractors (Fig. 5J; laser x distractors: F1,6 = 16.67, P = 0.006; laser: F1,6 = 0.15, n.s.; distractors: F1,6 = 23.54, P = 0.002). Stimulation of LC terminals in vlOFC had no effect on correct responses (Fig. 5L; laser x distractors: F1,7 = 0.01, n.s.; laser: F1,7 = 0.05, n.s.; distractors: F1,7 = 68.16, P < 0.0001), but decreased premature responses independently of distractors (Fig. 5M; laser x distractors: F1,7 = 1.8, n.s.; laser: F1,7 = 57.15, P = 0.0001; distractors: F1,7 = 369.4, P < 0.0001). There were no effects of LC terminal stimulation in vlOFC on RTs (Fig. 5N; laser x distractors: F1,7 = 1.25, n.s.; laser: F1,7 = 0.3, n.s.; distractors: F1,7 = 9.58, P = 0.01). These results suggest that LC modulation of attentional control is doubly dissociable in its downstream PFC targets.
Discussion
The LC/NE system has long been recognized to modulate attentional processes (12, 28, 31). However, a causal link between LC/NE neuronal activity and the attentional control of behavior, although inferred, has been difficult to demonstrate directly. Here we have applied behavioral, optogenetic, and neural circuit genetic techniques, which afford a high degree of temporal and cell-type specificity, for the manipulation and recording of noradrenergic neuron activity in the LC and demonstrate a causal link between temporal-specific LC/NE modulation and attentional control. We show that optognetic activation of LC/NE neurons improves attention and response inhibition by decreasing distractibility, while their inactivation elicits the opposite effect. Consistent with these results, our calcium-based photometric recordings suggest that LC/NE neuronal activity controls the trade-off between goal-directed (top-down) and stimulus-driven (bottom-up) attention. Moreover, our results reveal that the attentional control of behavior is modulated by the synergistic effects of two dissociable coeruleo-cortical pathways, with LC projections to dmPFC enhancing attention and LC projections to vlOFC reducing impulsivity.
Optogenetic techniques allow the perturbation of genetically defined neuronal populations, and their effects are fully reversible, thus representing an ideal method for the study of cognition using trial-based operant tasks (25).
Here we initially used a double-transgenic approach to the optogenetic targeting of LC neurons (Fig. 1A) because it allows a less invasive and more homogeneous long-term expression of transgenes compared to virus-assisted opsin delivery (44). On the other hand, one potential limitation to the anatomical specificity of the double-transgenic approach is that a subset of medullary NE neurons outside of the LC also expresses opsins and could be affected by our optogenetic manipulation via their processes in the peri-coerulear space—a problem shared with the use of viral vectors, which could be taken up by non-LC NE fibers in the PFC. Although we cannot completely rule out that our optogenetic approach does not affect non-LC NE neurons, a few observations make us confident about the anatomical specificity of our approach: 1) our double-transgenic approach is based on the NET promoter, which is very specific for the LC/NE system as compared to other NE nuclei (45) and compared to other often-used catecholaminergic promoters (46, 47); 2) we have not observed any of the effects expected by the stimulation of medullary NE neurons such as those related to food intake (SI Appendix, Fig. S2B) or spontaneous locomotor activity (Fig. 1K) (48, 49); and 3) LC projections to the PFC do not express visible levels of opsins in our double-transgenic animals (hence the use of a virus-mediated approach when targeting LC projections there; see below), and a similar absence of GFP immunofluorescence can be seen lateral to the LC where fibers of the medullary NE neurons are present (e.g., compare Fig. 1A with Fig. 5E).
Our stimulation protocol causes LC neurons to increase their firing rate up to ∼5 Hz over 7 s of laser activation (Fig. 1 B–D and SI Appendix, Fig. S1 B and C), which could be best described as a transient high tonic activity (31). Importantly, we did not observe any change in locomotor activity caused by LC optogenetic stimulation (Fig. 1K), but we found reduced anxiety-like behavior (Fig. 1 L and N), in contrast to the conclusions of some previous studies using similar levels of LC optogenetic stimulation (50, 51). Although unexpected, these findings are consistent with other reports which found that increasing LC output has anxiolytic effects, while decreasing it results in anxiety-like behavior (52–57). Moreover, since anxiety is known to impair attentional control (34), our results are consistent with the improved attention and response inhibition by LC stimulation discussed below.
LC/NE Neuron Stimulation Improves Attention and Impulsivity.
Previous studies have shown that increasing NE in forebrain areas by blocking its reuptake improves sustained attention and response inhibition (58–62). However, pharmacological strategies aimed at blocking NET strongly decrease the LC neuron spontaneous firing rate (17, 18) due to local autoinhibition. Thus, it is not clear how LC neuron activity affects performance during cognitive tasks. The results of the present study indicate that LC/NE stimulation during a task of sustained attention (Fig. 2 A and B) with a short target stimulus (0.5 s) significantly improves attentional control by increasing response accuracy and decreasing premature responses (Fig. 2 E and F), which are widely used laboratory measures of attention and response inhibition, respectively (38). Conversely, silencing LC neurons specifically during the delay before the appearance of the target stimulus impaired attention and response inhibition (Fig. 2 I and J). Importantly, none of the LC optogenetic manipulations altered measures of response speed (Fig. 2 G and K) or motivation for the food reward (SI Appendix, Fig. S2 B and D) during the attentional task.
LC/NE Neuron Activation Improve Attentional Control by Decreasing Distractibility.
Attentional control is fundamental for the selection of information relevant for current plans and goals (39, 63). However, it is not clear how the mammalian nervous system flexibly switches between prioritizing and ignoring distinct types of information. Here, we devised a version of the Posner attentional cueing task (41) adapted to be used in rodents (Fig. 3 A and B, Movie S1). We found that activation of LC neurons is necessary and sufficient for directing attention to goal-relevant information while ignoring irrelevant cues (Fig. 3 C, D, G, and H). Conversely, upon inhibition of LC neuronal activity, animal responses were affected more by the irrelevant cues than by the rules of the task, displaying a pattern of behavior suggestive of increased stimulus-driven attention (Fig. 3 E, F, I, and J). Consistent with our results, one study has found decreased accuracy (but no effects on impulsivity) in response to distractors after forebrain NE depletion (64).
By recording calcium activity in the LC of freely moving animals, we confirmed that the effects that we observed following optogenetic manipulations of LC neurons are also relevant when the LC/NE system is not artificially perturbed. We found that spontaneous LC activity was consistently higher during the delay period preceding correct responses, while lower activity preceded wrong or premature responses (Fig. 4 G and H). LC neuronal activity during the delay period was inversely related to distractibility, which can be inferred from fast responses on validly cued trials and slow responses on invalidly cued ones (Fig. 4 K and L). Since we did not find any difference in tonic LC activity between fast and slow Neutral trials (Fig. 4J), it is unlikely that our results are merely due to a generalized increase in arousal (65).
Stimulus-evoked LC calcium activity was higher for slow responses to target stimuli in both Valid and Invalid trials, but not in Neutral ones (Fig. 4 M–O). While Neutral cues are devoid of spatial information, both Valid and Invalid cues generate some expectation regarding the location of the upcoming stimulus. Therefore, slower responses in these spatially cued trial types likely reflect the extra time needed for the animals to shift attention to the unattended location when their expectations are violated. Higher stimulus-evoked activity in these slower trials may thus signal prediction errors that cause the fast reorienting toward unexpected sources of information (66, 67). These results strongly suggest that, whereas spontaneous LC activity controls the trade-off between stimulus-driven and goal-directed attention, LC activation by salient stimuli reflects the accuracy of the animals’ prior expectations and promotes a shift of the attentional focus toward relevant sources of information when such expectations are violated.
Dissociable Effects of LC/NE Projections to the PFC on Attentional Control.
Previous research has shown that distinct PFC subregions of the primate brain are differentially involved in attentional control processes (5, 9, 68–70). Moreover, noradrenergic modulation of PFC neurons is thought to affect different cognitive processes in a region-specific manner (71, 72). Based on this evidence, we asked whether the effects of LC neural activity on the two main behavioral components of attentional control, namely attention and response inhibition, can be dissociated at the level of LC projection targets.
To investigate the potential differential contribution of LC neurons to measures of attention and response inhibition, we used a version of the two-choice task with distractors presented on a subset of trials (SI Appendix, Fig. S5 A and B). We first confirmed that both attention and response inhibition are impaired by the presentation of distractors and that both measures are similarly improved by LC optogenetic stimulation and impaired by its inhibition (SI Appendix, Fig. S5 D–K). On the other hand, we found that stimulation of LC terminals in dmPFC specifically improved measures of attention with no effects on response inhibition (Fig. 5 G–J), whereas stimulation of LC terminals in vlOFC significantly improved response inhibition without affecting attention (Fig. 5 K–N). We also found that stimulation of LC terminals in the dmPFC in trials with distractors increases RTs (Fig. 5J). This latter effect might be due to the extra time needed for the successful discrimination of the target stimuli among distractors. Taken together, these results reveal a double dissociation of the effects of NE release in PFC subregions on separate aspects of attentional control.
In summary, our results demonstrate a fundamental causal role of LC neuronal activation in the implementation of attentional control by the selective modulation of neural activity in its target areas. These findings are especially relevant for our understanding of pathological states where cognitive impairments are known to be critically contributed by NE imbalance. The results reported above may help to devise treatment avenues for these diseases.
Materials and Methods
Methods of mouse generation, surgery, immunohistochemistry, optogenetics, behavior, fiber photometry, electrophysiology, and statistical analysis are described in SI Appendix, SI Materials and Methods. Animal experiments were performed in accordance with NIH guidelines and the Massachusetts Institute of Technology Department of Comparative Medicine and Committee on Animal Care. Materials requests may be submitted to tonegawa@mit.edu; all reasonable requests will be granted through a materials transfer agreement.
Supplementary Material
Acknowledgments
We thank F. Bushard, A. Hamalian, S. Y. Huang, D. King, S. LeBlanc, T. O’Connor, L. Smith, W. Yu, and X. Zhu for help with experiments; T. W. Robbins, B. Queenan, and T. J. Ryan for comments and suggestions on an earlier version of the manuscript; and all the members of the Tonegawa Laboratory for helpful discussion. This work was supported by the RIKEN Center for Brain Science, the HHMI, and the JPB Foundation (S.T.); a Human Frontier Science Program Fellowship (to D.T.); the General Research Program of the National Natural Science Foundation of China (Project 31871087 and 31671118); the NIH BRAIN Initiative (Grant U01NS103558); and Grant Z181100001518004 from the Beijing Brain Initiative of Beijing Municipal Science & Technology Commission (to Y.L.).
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2015635117/-/DCSupplemental.
Data Availability.
All study data are included in the article and supporting information.
References
- 1.Miyake A., et al. , The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognit. Psychol. 41, 49–100 (2000). [DOI] [PubMed] [Google Scholar]
- 2.Friedman N. P., Miyake A., The relations among inhibition and interference control functions: A latent-variable analysis. J. Exp. Psychol. Gen. 133, 101–135 (2004). [DOI] [PubMed] [Google Scholar]
- 3.Yantis S., “Control of visual attention” in Attention, Pashler H., Ed. (Psychology Press, Hove, England, 1998), pp. 223–256. [Google Scholar]
- 4.Bari A., Robbins T. W., Inhibition and impulsivity: Behavioral and neural basis of response control. Prog. Neurobiol. 108, 44–79 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Whelan R. et al.; IMAGEN Consortium , Adolescent impulsivity phenotypes characterized by distinct brain networks. Nat. Neurosci. 15, 920–925 (2012). [DOI] [PubMed] [Google Scholar]
- 6.Liao C., et al. , Transcriptome-wide association study of attention deficit hyperactivity disorder identifies associated genes and phenotypes. Nat. Commun. 10, 4450 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim C. H., Waldman I. D., Blakely R. D., Kim K. S., Functional gene variation in the human norepinephrine transporter: Association with attention deficit hyperactivity disorder. Ann. N. Y. Acad. Sci. 1129, 256–260 (2008). [DOI] [PubMed] [Google Scholar]
- 8.Sigurdardottir H. L., et al. , Association of norepinephrine transporter methylation with in vivo NET expression and hyperactivity-impulsivity symptoms in ADHD measured with PET. Mol. Psychiatry, 10.1038/s41380-019-0461-x (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robbins T. W., Arnsten A. F., The neuropsychopharmacology of fronto-executive function: Monoaminergic modulation. Annu. Rev. Neurosci. 32, 267–287 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asherson P., Buitelaar J., Faraone S. V., Rohde L. A., Adult attention-deficit hyperactivity disorder: Key conceptual issues. Lancet Psychiatry 3, 568–578 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Biederman J., Spencer T., Attention-deficit/hyperactivity disorder (ADHD) as a noradrenergic disorder. Biol. Psychiatry 46, 1234–1242 (1999). [DOI] [PubMed] [Google Scholar]
- 12.Aston-Jones G., Cohen J. D., An integrative theory of locus coeruleus-norepinephrine function: Adaptive gain and optimal performance. Annu. Rev. Neurosci. 28, 403–450 (2005). [DOI] [PubMed] [Google Scholar]
- 13.Sara S. J., Bouret S., Orienting and reorienting: The locus coeruleus mediates cognition through arousal. Neuron 76, 130–141 (2012). [DOI] [PubMed] [Google Scholar]
- 14.Chamberlain S. R., et al. , Atomoxetine modulates right inferior frontal activation during inhibitory control: A pharmacological functional magnetic resonance imaging study. Biol. Psychiatry 65, 550–555 (2009). [DOI] [PubMed] [Google Scholar]
- 15.Nagashima M., et al. , Acute neuropharmacological effects of atomoxetine on inhibitory control in ADHD children: A fNIRS study. Neuroimage Clin. 6, 192–201 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bymaster F. P., et al. , Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: A potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology 27, 699–711 (2002). [DOI] [PubMed] [Google Scholar]
- 17.Bari A., Aston-Jones G., Atomoxetine modulates spontaneous and sensory-evoked discharge of locus coeruleus noradrenergic neurons. Neuropharmacology 64, 53–64 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devilbiss D. M., Berridge C. W., Low-dose methylphenidate actions on tonic and phasic locus coeruleus discharge. J. Pharmacol. Exp. Ther. 319, 1327–1335 (2006). [DOI] [PubMed] [Google Scholar]
- 19.Dalley J. W., Mar A. C., Economidou D., Robbins T. W., Neurobehavioral mechanisms of impulsivity: Fronto-striatal systems and functional neurochemistry. Pharmacol. Biochem. Behav. 90, 250–260 (2008). [DOI] [PubMed] [Google Scholar]
- 20.Pattij T., Vanderschuren L. J., The neuropharmacology of impulsive behaviour. Trends Pharmacol. Sci. 29, 192–199 (2008). [DOI] [PubMed] [Google Scholar]
- 21.Robbins T. W., Cross-species studies of cognition relevant to drug discovery: A translational approach. Br. J. Pharmacol. 174, 3191–3199 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aston-Jones G., Rajkowski J., Cohen J., Role of locus coeruleus in attention and behavioral flexibility. Biol. Psychiatry 46, 1309–1320 (1999). [DOI] [PubMed] [Google Scholar]
- 23.Bouret S., Sara S. J., Reward expectation, orientation of attention and locus coeruleus-medial frontal cortex interplay during learning. Eur. J. Neurosci. 20, 791–802 (2004). [DOI] [PubMed] [Google Scholar]
- 24.Uematsu A., et al. , Modular organization of the brainstem noradrenaline system coordinates opposing learning states. Nat. Neurosci. 20, 1602–1611 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Carr M. R., de Vries T. J., Pattij T., Optogenetic and chemogenetic approaches to manipulate attention, impulsivity and behavioural flexibility in rodents. Behav. Pharmacol. 29, 560–568 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Lin M. Z., Schnitzer M. J., Genetically encoded indicators of neuronal activity. Nat. Neurosci. 19, 1142–1153 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deisseroth K., Schnitzer M. J., Engineering approaches to illuminating brain structure and dynamics. Neuron 80, 568–577 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robbins T. W., Cortical noradrenaline, attention and arousal. Psychol. Med. 14, 13–21 (1984). [DOI] [PubMed] [Google Scholar]
- 29.Wagatsuma A., et al. , Locus coeruleus input to hippocampal CA3 drives single-trial learning of a novel context. Proc. Natl. Acad. Sci. U.S.A. 115, E310–E316 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madisen L., et al. , A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat. Neurosci. 15, 793–802 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berridge C. W., Waterhouse B. D., The locus coeruleus-noradrenergic system: Modulation of behavioral state and state-dependent cognitive processes. Brain Res. Brain Res. Rev. 42, 33–84 (2003). [DOI] [PubMed] [Google Scholar]
- 32.Feng J., et al. , A genetically encoded fluorescent sensor for rapid and specific in vivo detection of norepinephrine. Neuron 102, 745–761.e8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gould T. D., Ed., Mood and Anxiety Related Phenotypes in Mice (Humana Press, Totowa, NJ, 2009). [Google Scholar]
- 34.Eysenck M. W., Derakshan N., Santos R., Calvo M. G., Anxiety and cognitive performance: Attentional control theory. Emotion 7, 336–353 (2007). [DOI] [PubMed] [Google Scholar]
- 35.Park J., Moghaddam B., Impact of anxiety on prefrontal cortex encoding of cognitive flexibility. Neuroscience 345, 193–202 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arnsten A. F., Stimulants: Therapeutic actions in ADHD. Neuropsychopharmacology 31, 2376–2383 (2006). [DOI] [PubMed] [Google Scholar]
- 37.Bari A., Dalley J. W., Robbins T. W., The application of the 5-choice serial reaction time task for the assessment of visual attentional processes and impulse control in rats. Nat. Protoc. 3, 759–767 (2008). [DOI] [PubMed] [Google Scholar]
- 38.Robbins T. W., The 5-choice serial reaction time task: Behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl.) 163, 362–380 (2002). [DOI] [PubMed] [Google Scholar]
- 39.Desimone R., Duncan J., Neural mechanisms of selective visual attention. Annu. Rev. Neurosci. 18, 193–222 (1995). [DOI] [PubMed] [Google Scholar]
- 40.Moore T., The neurobiology of visual attention: Finding sources. Curr. Opin. Neurobiol. 16, 159–165 (2006). [DOI] [PubMed] [Google Scholar]
- 41.Petersen S. E., Posner M. I., The attention system of the human brain: 20 years after. Annu. Rev. Neurosci. 35, 73–89 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Madisen L., et al. , Transgenic mice for intersectional targeting of neural sensors and effectors with high specificity and performance. Neuron 85, 942–958 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corbetta M., Patel G., Shulman G. L., The reorienting system of the human brain: From environment to theory of mind. Neuron 58, 306–324 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Allen B. D., Singer A. C., Boyden E. S., Principles of designing interpretable optogenetic behavior experiments. Learn. Mem. 22, 232–238 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schroeter S., et al. , Immunolocalization of the cocaine- and antidepressant-sensitive l-norepinephrine transporter. J. Comp. Neurol. 420, 211–232 (2000). [PubMed] [Google Scholar]
- 46.Lammel S., et al. , Diversity of transgenic mouse models for selective targeting of midbrain dopamine neurons. Neuron 85, 429–438 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lonergan T., et al. , Targeting brain stem centers of cardiovascular control using adenoviral vectors: Impact of promoters on transgene expression. Physiol. Genomics 20, 165–172 (2005). [DOI] [PubMed] [Google Scholar]
- 48.Roman C. W., Derkach V. A., Palmiter R. D., Genetically and functionally defined NTS to PBN brain circuits mediating anorexia. Nat. Commun. 7, 11905 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rinaman L., Hindbrain noradrenergic A2 neurons: Diverse roles in autonomic, endocrine, cognitive, and behavioral functions. Am. J. Physiol. Regul. Integr. Comp. Physiol. 300, R222–R235 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCall J. G., et al. , CRH engagement of the locus coeruleus noradrenergic system mediates stress-induced anxiety. Neuron 87, 605–620 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li L., et al. , Stress accelerates defensive responses to looming in mice and involves a locus coeruleus-superior colliculus projection. Curr. Biol. 28, 859–871.e5 (2018). [DOI] [PubMed] [Google Scholar]
- 52.Pohl R., Rainey J. M., Ortiz A., Yeragani V. K., Locus coeruleus and anxiety. Biol. Psychiatry 22, 116–117 (1987). [DOI] [PubMed] [Google Scholar]
- 53.Weiss J. M., et al. , Depression and anxiety: Role of the locus coeruleus and corticotropin-releasing factor. Brain Res. Bull. 35, 561–572 (1994). [DOI] [PubMed] [Google Scholar]
- 54.Britton D. R., Ksir C., Britton K. T., Young D., Koob G. F., Brain norepinephrine depleting lesions selectively enhance behavioral responsiveness to novelty. Physiol. Behav. 33, 473–478 (1984). [DOI] [PubMed] [Google Scholar]
- 55.Ferrazzo S., et al. , Increased anxiety-like behavior following circuit-specific catecholamine denervation in mice. Neurobiol. Dis. 125, 55–66 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harro J., Oreland L., Vasar E., Bradwejn J., Impaired exploratory behaviour after DSP-4 treatment in rats: Implications for the increased anxiety after noradrenergic denervation. Eur. Neuropsychopharmacol. 5, 447–455 (1995). [DOI] [PubMed] [Google Scholar]
- 57.Mason S. T., Fibiger H. C., Current concepts. I. Anxiety: The locus coeruleus disconnection. Life Sci. 25, 2141–2147 (1979). [DOI] [PubMed] [Google Scholar]
- 58.Robinson E. S., et al. , Similar effects of the selective noradrenaline reuptake inhibitor atomoxetine on three distinct forms of impulsivity in the rat. Neuropsychopharmacology 33, 1028–1037 (2008). [DOI] [PubMed] [Google Scholar]
- 59.Bari A., et al. , Prefrontal and monoaminergic contributions to stop-signal task performance in rats. J. Neurosci. 31, 9254–9263 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chamberlain S. R., et al. , Neurochemical modulation of response inhibition and probabilistic learning in humans. Science 311, 861–863 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pattij T., Schetters D., Schoffelmeer A. N., van Gaalen M. M., On the improvement of inhibitory response control and visuospatial attention by indirect and direct adrenoceptor agonists. Psychopharmacology (Berl.) 219, 327–340 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Navarra R., et al. , Effects of atomoxetine and methylphenidate on attention and impulsivity in the 5-choice serial reaction time test. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 32, 34–41 (2008). [DOI] [PubMed] [Google Scholar]
- 63.Miller E. K., Cohen J. D., An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 24, 167–202 (2001). [DOI] [PubMed] [Google Scholar]
- 64.Carli M., Robbins T. W., Evenden J. L., Everitt B. J., Effects of lesions to ascending noradrenergic neurones on performance of a 5-choice serial reaction task in rats: Implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behav. Brain Res. 9, 361–380 (1983). [DOI] [PubMed] [Google Scholar]
- 65.Carter M. E., et al. , Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat. Neurosci. 13, 1526–1533 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Corbetta M., Shulman G. L., Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 3, 201–215 (2002). [DOI] [PubMed] [Google Scholar]
- 67.Bouret S., Sara S. J., Network reset: A simplified overarching theory of locus coeruleus noradrenaline function. Trends Neurosci. 28, 574–582 (2005). [DOI] [PubMed] [Google Scholar]
- 68.Brennan A. R., Arnsten A. F., Neuronal mechanisms underlying attention deficit hyperactivity disorder: The influence of arousal on prefrontal cortical function. Ann. N. Y. Acad. Sci. 1129, 236–245 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aron A. R., Cai W., Badre D., Robbins T. W., Evidence supports specific braking function for inferior PFC. Trends Cogn. Sci. 19, 711–712 (2015). [DOI] [PubMed] [Google Scholar]
- 70.Aron A. R., Sahakian B. J., Robbins T. W., Distractibility during selection-for-action: Differential deficits in Huntington’s disease and following frontal lobe damage. Neuropsychologia 41, 1137–1147 (2003). [DOI] [PubMed] [Google Scholar]
- 71.Arnsten A. F., Catecholamine influences on dorsolateral prefrontal cortical networks. Biol. Psychiatry 69, e89–e99 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Del Campo N., Chamberlain S. R., Sahakian B. J., Robbins T. W., The roles of dopamine and noradrenaline in the pathophysiology and treatment of attention-deficit/hyperactivity disorder. Biol. Psychiatry 69, e145–e157 (2011). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and supporting information.