Abstract
Background/Objective:
Chronic pancreatitis (CP) in children is associated with significant morbidity and can lead to narcotic dependence. Total pancreatectomy (TP) may be indicated in refractory CP to relieve pain; simultaneous islet autotransplant (IAT) may prevent postsurgical diabetes. About half of pediatric patients are insulin independent 1 yr after IAT. Insulin independence correlates best with the number of islets available for transplantation (islet yield). Currently there is no known method to predict islet yield in a given patient. We assessed the ability of preoperative metabolic tests to predict islet yields in 10 children undergoing TP/IAT.
Design/Methods:
Hemoglobin A1c (HbA1c) and mixed meal tolerance tests (MMTT) were obtained prior to surgery in 10 patients age ≤ 18 yr. Fasting glucose, C-peptide, and creatinine were used to calculate the C-peptide to glucose* creatinine ratio (CPGCR). C-peptide peak and area under the curve (AUC) were determined from 2 h MMTT. Linear regressions were performed to predict islet yield from baseline test results.
Results:
Islet yield ranged from 7000 to 434 000 islet equivalents (IE) (mean 222 452 ± 148 697 IE). Islet yield was well predicted from body weight and fasting plasma glucose (R2 = 57%, adjusted for overfitting by bootstrap). Islet yield was positively associated with CPGCR, peak C-peptide, and AUC C-peptide and negatively associated with HbA1c.
Conclusions:
Pilot data from 10 pediatric patients suggest that simple preoperative measurement of fasting plasma glucose may give a useful prediction of islet yield. Islet yield correlates with HbA1c and C-peptide levels. This information allows individual candidates to weigh the specific risk of becoming diabetic against the benefit of pain relief should they undergo TP-IAT.
Keywords: chronic pancreatitis, islet autotransplant, total pancreatectomy
Introduction
Chronic pancreatitis (CP) presents symptomatically as recurrent or persistent abdominal pain which can be disabling, often resulting in dependence on narcotic analgesics (1). This condition is most often seen in adults, where alcoholism and idiopathic disease are the most common causes (2). CP also occurs in childhood, commonly because of genetic mutations (3). Although the prevalence of CP is unknown in children, it is relatively rare. Severely affected children may be unable to attend school or participate in usual childhood activities.
Patients with intractable pain despite medical and endoscopic pancreatic duct drainage interventions are candidates for total pancreatectomy (TP). Data from a cohort that consists mostly of adults demonstrate that simultaneous islet autotransplant (IAT) prevents postsurgical diabetes in about one third of patients and allows euglycemia with minimal insulin requirements in another third (1, 4). Results appear to be somewhat better amongst pediatric patients, of whom approximately half are insulin independent at 1 yr posttransplant and another 22% require only a small amount of exogenous insulin to control blood glucose levels. However, IAT is unsuccessful in one quarter of children, who require complete insulin replacement (5). The most important known predictor of postsurgical islet graft function is the islet mass transplanted (4–8). There is no magic number of islets that guarantees insulin independence, but it is uncommon in patients receiving fewer than 2000–2500 islet equivalents (IE) per kilogram body weight (IE/kg) (5, 6, 8).
CP results in variable progression of damage and fibrosis of the diseased pancreas (2). Thus islet yields are highly variable, even among patients with documented normoglycemia preoperatively. Islet yields in CP patients receiving IAT at the University of Minnesota Medical Center (UMMC) have ranged from nearly zero to over 500 000 IE (unpublished data). Unfortunately, there is not yet any method to reliably predict the islet yield prior to pancreatectomy. Because there is a risk of diabetes mellitus even with IAT, patients and parents are counseled that diabetes must be considered a reasonable trade-off for relief of pain to proceed with surgery. However, many parents desire more specific information on their child’s risk for diabetes.
Metabolic assessments (most often oral glucose tolerance tests, and, in a handful of cases, intravenous glucose tolerance tests and arginine stimulation tests) have been performed before surgical intervention in some adults undergoing TP and IAT. These tests are used to document normoglycemia prior to surgery and/or establish baselines for later follow-up (7, 9). However, the relationship between the results of these tests and the islet yield at TP has not been examined in the published medical literature.
In this preliminary report, we sought to determine if baseline metabolic testing in 10 pediatric patients undergoing TP and IAT was predictive of islet yield. If islet yield can be predicted by simple tests of islet function preoperatively, this would serve two important purposes: (i) it would provide patient-specific information on postoperative diabetes risk, and (ii) it would assist in selection of candidates that are most or least likely to benefit from IAT.
Methods
Subjects
Preoperative laboratory data were prospectively collected on 10 consecutive pediatric patients (age ≤18 yr) with CP and without preexisting diabetes undergoing TP and IAT at the UMMC between December 2006 and June 2008. The study protocol was reviewed and approved by the University of Minnesota Institutional Review Board.
Metabolic testing
Within 1 wk prior to pancreatectomy, patients had hemoglobin A1c (HbA1c) levels drawn and underwent mixed meal tolerance testing (MMTT, Boost HP 6 mL/kg, maximum 360 mL). Glucose and C-peptide levels were drawn at times 0, 30, 60, 90, and 120 min during MMTT. The C-peptide to glucose* creatinine ratio (CPGCR) was calculated from the fasting labs using the following formula: [C-peptide (ng/mL) * 100]/[glucose (mg/dL) * creatinine (mg/dL)] (10). This measurement accounts for the dependence of C-peptide on concurrent glucose and renal excretion. The area under the curve C-peptide (AUC C-peptide) and glucose (AUC glucose) including baseline were calculated using a trapezoidal method from MMTT results.
HbA1c levels were analyzed by high-performance liquid chromatography. Plasma C-peptide levels were run by chemiluminescent immunoassay (Siemen’s immunolite 2000). Plasma glucose levels were measured by glucose oxidase assay.
Pancreatectomy, islet isolation, and autologous transplantation
The surgical procedure of TP and IAT is described in detail elsewhere (1, 11). Islet isolations were performed in the University of Minnesota Molecular and Cellular Therapeutics GMP Facility in compliance with federal regulations, using previously reported methods (12, 13). Briefly, the pancreas was distended with cold enzyme solution infused through the pancreatic duct using a pressure-controlled pump system (14). Enzymatic digestion was performed with SERVA/Nordmark GMP grade enzymes (SERVA Electrophoresis GmbH, Heidelberg, Germany) supplied in two separate vials (collagenase and neutral protease), using 1600–2709 Wunsch units of collagenase and 100–250 DMC units of neutral protease depending upon the size and quality of pancreas (12). The distended pancreas was then placed in a closed circuit Ricordi digestion chamber (15) containing collagenase solution and shaken until the exocrine and endocrine tissues are free of each other. The switch from digestion phase to dilution phase occurred when most of the islets were free from exocrine tissue.
The number of islets freed by collagenase digestion was counted from an aliquot stained with diphenylthiocarbazone (Sigma, St. Louis, MO, USA) (16). Islet yield was quantified in terms of IE, which is islet mass standardized to an islet size of 150 μm diameter. If the total tissue volume of the digest was small (<0.2 cc/kg of the recipient), the islet preparation was not further purified. If the digest volume was >0.2 cc/kg, the islets were purified by continuous iodixanol (OptiPrep, AxisShield, Oslo, Norway) density gradient on a COBE 2991 cell separator (17). For preparations in which a high percentage of islets were embedded/mantled by exocrine tissue (e.g., 50%), purification was usually not performed because many islets would have been lost. The liberated islets were suspended in Connaught Medical Research Laboratories-1066 medium (Mediatech, Inc., Manassas, VA, USA) supplemented with 25 mM 4-[2-hydroxyethyl]-1-piperazineethanesulfonic acid, antibiotics, and 2.5% human serum albumin.
The islet tissue preparation was infused into the portal vein. Patients received anticoagulation with heparin as prophylaxis against portal vein thrombosis. Portal pressures were monitored during the infusion. The baseline was usually 0–2 cm water. If the pressure elevated to ≥25–30 cm, the infusion was stopped. The remaining tissue was then transplanted into the peritoneal cavity, or elsewhere, at the surgeon’s discretion. Purified islets never elevate portal pressure more than a few centimeters of water. Most impure preparations can be totally infused but if not, the number of IE embolized to the liver is calculated from the proportion of tissue that was infused.
Postoperative care
Postoperatively, all patients are treated with insulin for a minimum of approximately 4 wk to allow the new islets to engraft. Subsequently, subcutaneous insulin doses are adjusted to maintain the following goals: fasting capillary blood glucose <126 mg/dL, postprandial capillary blood glucose <180 mg/dL, and HbA1c ≤ 6.5%. Patients are instructed to monitor blood glucose four times per day. Adjustments in insulin therapy are made by the transplant center or the patient’s local endocrinologist. Postoperative insulin requirements were assessed from study questionnaires and clinical contact between the UMMC physicians and the patient families and/or local physicians.
Data analysis
Baseline demographic and laboratory data are presented as mean ± standard deviation.
Linear regression was used to screen baseline characteristics for predicting IE, and overfitting to the data was corrected by bootstrap methods (18). Logistic regression was used to screen baseline characteristics for predicting IE/kg >3000. Pearson correlations were used to assess association between preoperative test results and islet yield, with 95% confidence intervals estimated by the bootstrap percentile method, with 2000 replications. A composite scoring system was developed based on the significant predictor variables. Differences in proportions were compared using Fisher’s exact test. Statistical analysis was performed using SAS 9.2. Two-tailed p-values ≤0.05 were considered statistically significant.
Results
Patient characteristics
Patients underwent TP and IAT at a mean age of 14.8 ± 4.2 yr (range 5–18 yr). The cause of CP was genetic in six cases (PRSS1 gene mutation in four, SPINK1 gene mutation in one, and CFTR gene mutation in one), idiopathic in three, and attributed to congenital pancreatic divisum in one. Onset of pancreatitis occurred 6.5 ± 4.6 yr prior to TP. Two patients had a prior surgical intervention (surgical drainage and/or head resection). Uncontrollable pain and/or recurrent pancreatitis hospitalizations were the indication for TP in all cases.
Prior to surgery, body mass index was normal (10–85th percentile for age) in seven patients and mildly elevated in three (90–95th percentile). Mean BMI Z-score for age was 0.5 ± 0.9 SDS. All patients had normal fasting blood glucose and HbA1c levels (Table 1).
Table 1.
Baseline preoperative characteristics of 10 chronic pancreatitis patients undergoing total pancreatectomy and islet autotransplant
| Case | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Mean ± SD |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 18.4 | 16.3 | 17.0 | 5.1 | 9.9 | 17.5 | 13.3 | 15.6 | 17.4 | 17.6 | 14.8 (±4.2) |
| Gender | M | F | F | F | F | M | M | F | M | F | 6F/4M |
| Hemoglobin A1c (%) | 4.8 | 4.9 | 4.7 | 5.4 | 5.3 | 4.4 | 5.5 | 4.3 | 4.8 | 5.7 | 5.0 ± 0.5 |
| Fasting glucose (mg/dL) | 72 | 74 | 87 | 73 | 64 | 72 | 97 | 68 | 75 | 82 | 76 ± 10 |
| 2-h MMTT glucose (mg/dL) | 71 | 63 | 102 | 72 | 63 | 70 | 106 | 78 | 90 | 93 | 81 ± 16 |
| Fasting C-peptide (ng/mL) | 1.8 | 2 | 1.9 | 0.4 | 1.2 | 2.8 | 1.7 | 2.3 | 2.3 | 2.5 | 1.9 ± 0.7 |
| Peak MMTT C-peptide (ng/mL) | 9.8 | 5.5 | 4.3 | 4.1 | 4 | 6.8 | 2.9 | 13 | 4.5 | 10.7 | 6.6 ±.3.4 |
The total number of islets isolated at the time of pancreatectomy ranged from 7000 to 436 000 IE (mean of 222 452 ± 148 697), and the IE/kg from 280 to 5977 (mean of 3805 ± 1922 IE/kg). In eight patients, all islets were infused intraportally. In the two patients with the greatest islet yield, the majority of the islets were transplanted intraportally with the remainder infused into the intraperitoneal cavity as a result of elevated portal pressures (approximately 17% transplanted intraperitoneal in one case and 45% in the other).
Mean hospitalization time postoperatively was 22 ± 9 days. Four patients required a second surgical procedure during hospitalization–one for splenectomy, one for resection of necrotic bowel, one for repair of biliary leak, and one for removal of an infected spinal cord stimulator device (previously placed for pain management at an outside institution).
Posttransplant insulin requirements and relationship to islet yield
All 10 patients are currently at least 6 months posttransplant. In all, five patients achieved insulin independence at 1–10 months posttransplant (Table 2). However, one of these five resumed low-dose glargine after a 4-month insulin-free interval for intermittent hyperglycemia.
Table 2.
Islet yield, islet composite score, and insulin independence in 10 pediatric patients undergoing total pancreatectomy and islet autotransplant
| Preadolescents |
Adolescents |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline labs: | Islet equivalents | 88 000 | 139000 | 7000 | 92 000* | 158000 | 245 000 | 296 000 | 346 000 | 422 000 | 434 000 |
| One point awarded if: | |||||||||||
| HbA1c | ≤4.8% | 5.3 | 5.4 | 5.5 | 4.7 | 4.9 | 5.7 | 4.8 | 4.8 | 4.4 | 4.3 |
| Peak C-peptide | ≥5 ng/mL | 4.0 | 4.1 | 2.9 | 4.3 | 5.5 | 10.7 | 4.5 | 9.8 | 6.8 | 13.0 |
| AUC C-peptide | ≥492 ng/mL*min | 389 | 302 | 270 | † | 492 | 858 | 378 | 717 | 602 | 966 |
| CPGCR | ≥3.57 | 2.72 | 1.14 | 1.18 | 3.8 | 3.34 | 5.75 | 4.26 | 3.01 | 5.98 | 5.05 |
| Islet composite score | 0 | 0 | 0 | 2 | 2 | 3 | 2 | 3 | 4 | 4 | |
| IE/kg | 3531 | 6362 | 280 | 1808 | 2904 | 3096 | 4287 | 4013 | 5787 | 5977 | |
| Patient age at transplant (years) | 9.9 | 5.1 | 13.3 | 17.0 | 16.3 | 17.6 | 17.4 | 18.4 | 17.5 | 15.6 | |
| Patient weight (kg) | 24 | 22 | 27 | 51 | 54 | 79 | 69 | 86 | 73 | 73 | |
| Insulin use at 6–12 months‡ | Ind. | Ind. | Dep. | Dep. | Dep. | Min. | Min.§ | Dep. | Ind. | Ind. | |
| Insulin use (units/kg/d) | 0 | 0 | 0.57 | 0.36 | 0.41 | 0.22 | 0.07 | 0.35 | 0 | 0 | |
| HbA1c (%) | 5.7 | 6.0 | 8.0 | 12.1 | 6.9 | 5.8 | 5.4 | 7.5 | 5.4 | 5.4 | |
HbA1c, hemoglobin A1c; AUC C-peptide, area under the curve for C-peptide on mixed meal tolerance test; CPGCR, C-peptide to glucose* creatinine ratio.
Patients who had prior Whipple procedure with IAT of unknown IE at outside institution.
Did not complete mixed meal test due to poor venous access; fasting and 90 min C-peptide draw obtained.
Insulin use status categorized as: independent (Ind.), minimal (Min.) = 0.01 – 0.25 units/kg/day, or dependent (Dep.) => 0.25 units/kg/day.
discontinued insulin at 1 month posttransplant; resumed 0.1 units/kg/day glargine at 5.5 months posttransplant for intermittent postprandial hyperglycemia.
At the 6–12 month posttransplant follow-up, four patients are insulin independent with HbA1c levels of 5.5–6%, two require <0.25 units/kg/day (single injection per day of glargine) with HbA1c levels of 5.4 and 5.8%, and four require >0.25 units/kg/day (basal-bolus regimen) with HbA1c levels of 6.9–12.1%.
Of the five patients with islet yields >200000 IE, three became insulin independent, one maintained euglycemia on once daily insulin (0.2 units/kg/d at 6 months posttransplant), and one required a basal-bolus insulin regimen. The two patients with the highest islet yields achieved insulin independence. Of note, two patients with <200 000 IE became insulin independent. These were the only two preadolescents in this series, an age group that has historically had good glycemic outcomes (5).
In terms of relative islet yield, six of seven patients with > 3000 IE/kg transplanted are insulin independent or require minimal insulin (once daily glargine, <0.25 units/kg/d). All three patients with fewer than 3000 IE/kg transplanted require a basal-bolus regimen.
Predicting total islet equivalents from baseline test results
The best prediction equation from baseline measurements was:
Measured vs. predicted values are shown in Fig. 1. The prediction equation explained most of the variability in total IE (R2 = 57%) adjusted for overfitting.
Fig. 1.
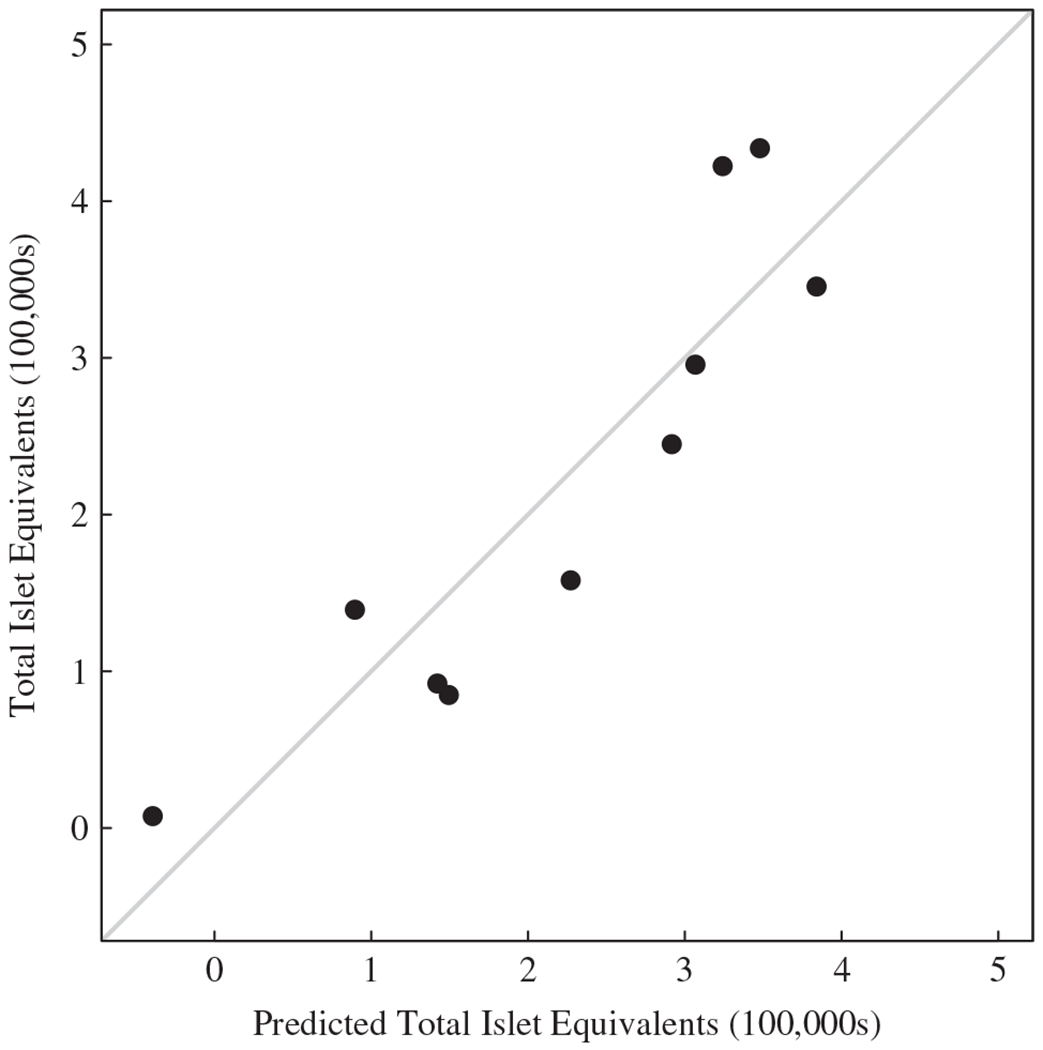
Total islet equivalents (100 000 IEs) vs. predicted total IE (100 000s) from the regression equation based on body weight and preoperative fasting plasma glucose. The diagonal line is the line of identity (x = y).
Predicting IE/kg >3000
None of the baseline measurements alone or in combination were significant predictors of IE/kg >3000.
Correlation of test results with total IE and IE/kg
Based on the data from all 10 patients, total IE was also positively associated with peak C-peptide, AUC C-peptide, CPGCR, and fasting C-peptide; total IE was negatively associated with preoperative HbA1c level (Table 3, Fig. 2).
Table 3.
Correlation coefficient and 95% confidence intervals for baseline laboratory values with total islet equivalents (IE) and IE/kg
| Total IE |
IE/kg |
|||
|---|---|---|---|---|
| Baseline test | r | 95% CI | r | 95% CI |
| HbA1c | −0.68 | (−0.97, −0.06)* | −0.42 | (−0.91, 0.31) |
| Peak C-peptide | 0.75 | (0.43, 0.98)* | 0.40 | (−0.28, 0.81) |
| AUC C-peptide | 0.73 | (0.33, 0.96)* | 0.31 | (−0.58, 0.82) |
| CPGCR | 0.72 | (0.29, 0.93)* | 0.30 | (−0.53, 0.91) |
| Fasting C-peptide | 0.60 | (0.26, 0.88)* | −0.05 | (−0.71, 0.81) |
| AUC glucose | −0.45 | (−0.89, 0.47) | −0.80 | (−0.98, 0.03) |
| Fasting glucose | −0.52 | (−0.95, 0.32) | −0.77 | (−0.97, −0.09)* |
r, correlation coefficient; 95% CI, 95% confidence interval; IE, islet equivalents; IE/kg, islet equivalents per kilogram body weight; HbA1c, hemoglobin A1c; AUC C-peptide, area under the curve for C-peptide on mixed meal tolerance test; CPGCR, C-peptide to glucose* creatinine ratio.
Indicates statistically significant correlation (p < 0.05).
Fig. 2.
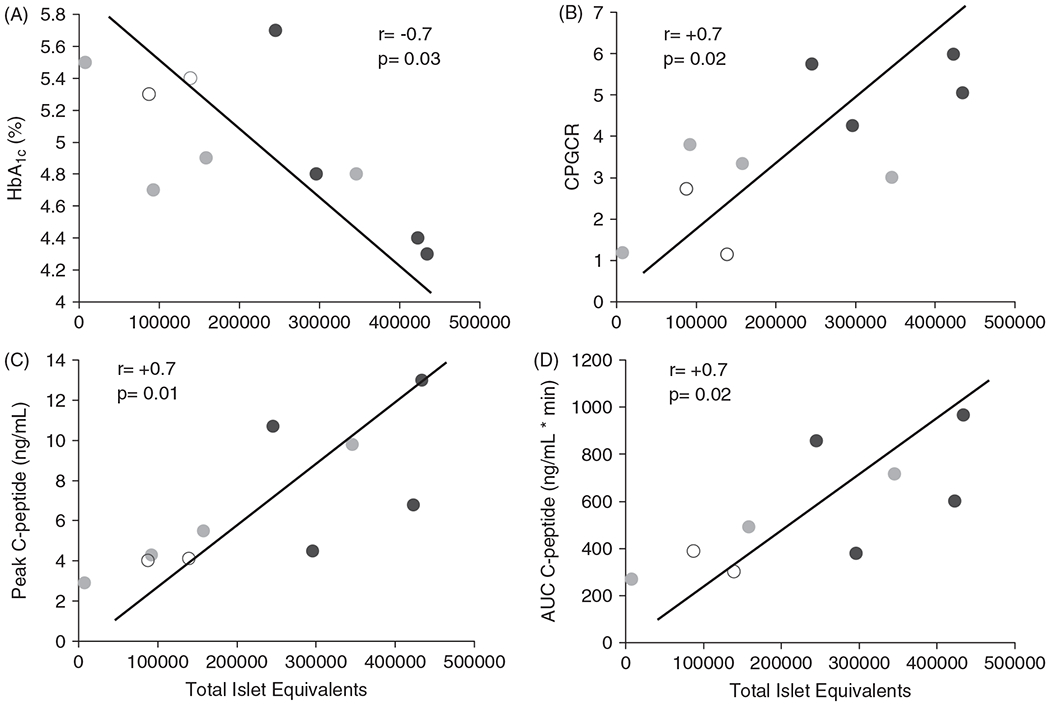
Correlation of total islet equivalents isolated at the time of total pancreatectomy with preoperative hemoglobin A1c (HbA1c) levels (A), C-peptide to glucose* creatinine ratio (CPGCR) (B), peak C-peptide (C), and area under the curve C-peptide (AUC C-peptide) (D). Blue circles indicate insulin independent patients or those on minimal insulin (glargine daily, <0.25 units/kg/d) postoperatively; green circles indicate fully insulin-dependent patients (basal-bolus regimen); open circles indicate preadolescent patients (both insulin independent).
We also examined the correlation between baseline results and the number of islet equivalents isolated relative to the patient’s size (IE/kg). IE/kg weakly correlated with fasting plasma glucose, and trended strongly towards a negative correlation with AUC glucose. There was no association between IE/kg and HbA1c, fasting C-peptide, peak C-peptide, AUC C-peptide, or CPGCR. Both of the two youngest and smallest children in this series had a low total IE but relatively high IE/kg because of their small body weight. If we excluded these two individuals from the correlation analysis, there was a statistically significant negative association between IE/kg and HbA1c and positive association between IE/kg and CPGCR and fasting C-peptide. However, IE/kg was not significantly associated with C-peptide response to the mixed meal, either for peak C-peptide or AUC C-peptide.
Of note, nearly all patients had a brisk C-peptide response to the mixed meal, with peak C-peptide observed at 30–60 min in all but one patient. This patient, who received very few islets (~7000 IE, 280 IE/kg), had a delayed peak at 120 min.
Islet composite score calculated from significant predictors of IE
We developed a scoring algorithm incorporating the four baseline factors which most strongly correlated with total islet yield. Patients were awarded one point for HbA1c level at or below the median and one point each for stimulated C-peptide, AUC C-peptide, and CPGCR at or above the median. Composite scores ranged from 0 to 4 (Table 2).
The total composite score correlated with total islet yield (r = 0.9, p < 0.05). Four of the five patients with total islet yield greater than the median (roughly >200 000 IE) had a composite score of ≥3, whereas none of the five patients with the lowest islet yields (<200 000 IE) had a composite score of ≥3 (Fisher’s exact test, p = 0.05). The two patients with a composite score of 4 had the greatest islet yields (total IE) and both achieved insulin independence.
In terms of relative islet yield, all four patients with a composite score >3 had >3000 IE/kg isolated. However, composite score was <3 in three of six patients with >3000 IE/kg, including the two preadolescent patients. Only one adolescent patient with >3000 IE/kg had a composite score <3. These differences were not statistically significant.
Discussion
The goal of IAT is to prevent or minimize the impact of postoperative diabetes in those patients undergoing TP for severe, intractably painful CP. Approximately half of pediatric patients are insulin independent at 1 yr posttransplant (5). The most critical factor underlying posttransplant islet graft function is the islet mass transplanted (1, 4, 5, 8). However, islet yield is determined only after resection and processing of the pancreas. Development of a method to predict islet yield would aid in appropriate preoperative counseling of surgical candidates. In this report, we present data from 10 pediatric patients that suggest a correlation between simple metabolic testing (fasting glucose, HbA1c, MMTT) and islet yield.
Low islet yields are common in patients with CP, as inflammation and fibrosis lead to pancreatic endocrine failure over time; by 20 yr after onset of disease, over 18% of patients with hereditary pancreatitis have diabetes (19). While alla 10 patients in this series had normal glucose and HbA1c levels preoperatively, they had a highly variable islet yield (7400–434 000 IE).
The preliminary results presented here suggest that preoperative testing of glycemic control and islet function may be valuable in predicting islet yield. Notably, a single fasting glucose and the patient’s weight were reasonably accurate in predicting total IE in these patients. This finding, if replicated in a larger number of pediatric patients, would allow for a very easy estimate of islet mass preoperatively. In addition, total IE correlated well with C-peptide response to a mixed meal test (peak C-peptide and AUC C-peptide), CPGCR, and HbA1c. A composite scoring index incorporating these measures distinguished between patients who had more than and less than 200 000 IE isolated. Interestingly, the only patient with a delayed rise in the C-peptide on mixed meal testing (peak value at 120 min) was the patient with the lowest islet yield (<10 000 IE). More patients are needed, but this may be a parameter that is particularly indicative of few islets.
IE/kg correlated marginally with fasting glucose and glycemic response to a mixed meal. However, these results may have been skewed by the inclusion of the two youngest patients (5 and 9 yr), both of whom had a low IE but, because of their small size, a high IE/kg. When these two patients are excluded from the analysis, there is a significant correlation of IE/kg with fasting parameters (glucose, C-peptide, CPGCR), but not with C-peptide response to an MMTT. Continued monitoring in a larger number of patients is needed to determine whether IE or IE/kg is better predicted from preoperative testing.
Insulin requirements were minimal (none or <0.25 units/kg/day) in 6 of our 10 pediatric patients during the first year posttransplant. Three of the five patients with the highest islet yields (>200 000 IE) became insulin independent and one maintains a normal HbA1c (5.8%) on once daily glargine (0.2 units/kg/day) alone.
Interestingly, the two youngest patients in this series were both insulin independent, despite relatively low total islet yields. Because of their small size, they received a relatively high islet yield for body weight (IE/kg), which may explain their good outcome. Prior histopathologic studies have shown less severe fibrosis in younger patients with a shorter duration of CP, which may explain the higher IE/kg (20). In addition, historic data supports that patients in this preadolescent age group (<13 yr old) are more likely to be insulin independent after IAT than their adolescent (age 13–18 yr) counterparts (5). The reason for this difference is unclear. It could be that the less insulin-resistant environment in the younger children is more favorable to islet engraftment, or perhaps there is better capacity for beta cell replication.
The data presented here are from a small number of patients. These findings need to be replicated before reliable predictions or risk estimates can be made. Additional testing will better define the relative contributions of fasting glucose, HbA1c, and mixed meal testing to predicting islet yield. There may be a role for more sensitive tests of islet function, such as the intravenous glucose tolerance test or arginine stimulation test, in predicting islet mass more precisely. Furthermore, these patients are being longitudinally followed so that baseline islet function tests can be correlated with the long-term success of IAT.
In conclusion, we demonstrate here that simple metabolic testing prior to TP may be useful in predicting islet yield, an important contributor to posttransplant glucose control. In this preliminary data set, total IE isolated was predicted from fasting glucose and the patient’s weight. In addition, patients with the most favorable HbA1c levels, peak C-peptide, AUC C-peptide, and CPGCR were most likely to have islet yields above 200 000 IE. Predicting islet yield prior to surgery would allow for more patient-specific counseling about diabetes risk. It could also determine which candidates have the best risk−benefit ratio. Candidates most likely to benefit may choose to proceed with surgical intervention soon rather than later, to avoid the progressive islet damage from CP. If the results are confirmed in a larger number of pediatric patients, the preoperative tests will clearly be a clinically useful tool.
References
- 1.Blondet JJ, Carlson AM, Kobayashi T, Jie T, Bellin M, Hering BJ et al. The role of total pancreatectomy and islet autotransplantation for chronic pancreatitis. Surg Clin North Am 2007: 87: 1477–1501. [DOI] [PubMed] [Google Scholar]
- 2.Ammann RW. Diagnosis and management of chronic pancreatitis: current knowledge. Swiss Med Wkly 2006: 136: 166–174. [DOI] [PubMed] [Google Scholar]
- 3.Lowe ME. Pancreatitis in childhood. Curr Gastroenterol Rep 2004: 6: 240–246. [DOI] [PubMed] [Google Scholar]
- 4.Jie T, Hering BJ, Ansite JD, Gilmore TR, Fraga DW, Beilman GJ et al. Pancreatectomy and auto-islet transplant in patients with chronic pancreatitis. ACS 2005: 201(Suppl.): S14. [Google Scholar]
- 5.Md Bellin, Carlson AM, Kobayashi T, Gruessner AC, Hering BJ, Moran A et al. Outcome after pancreatectomy and islet autotransplantation in a pediatric population. J Pediatr Gastroenterol Nutr 2008: 47: 37–44. [DOI] [PubMed] [Google Scholar]
- 6.Gruessner RW, Sutherland DE, Dunn DL, Najarian JS, Jie T, Hering BJ et al. Transplant options for patients undergoing total pancreatectomy for chronic pancreatitis. J Am Coll Surg 2004: 198: 559–567; discussion 568–569. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez Rilo HL, Ahmad SA, D’Alessio D, Iwanaga Y, Kim J, Choe KA et al. Total pancreatectomy and autologous islet cell transplantation as a means to treat severe chronic pancreatitis. J Gastrointest Surg 2003: 7: 978–989. [DOI] [PubMed] [Google Scholar]
- 8.Sutherland DE, Gruessner AC, Carlson AM, Blondet JJ, Balamurugan AN, Reigstad KF et al. Islet autotransplant outcomes after total pancreatectomy: a contrast to islet allograft outcomes. Transplantation 2008: 86: 1799–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Webb MA, Illouz SC, Pollard CA, Gregory R, Mayberry JF, Tordoff SG et al. Islet auto transplantation following total pancreatectomy: a long-term assessment of graft function. Pancreas 2008: 37: 282–287. [DOI] [PubMed] [Google Scholar]
- 10.Faradji RN, Monroy K, Messinger S, Pileggi A, Froud T, Baidal DA et al. Simple measures to assess beta cell mass and monitor islet graft dysfunction. Am J Transplant 2007: 7: 303–308. [DOI] [PubMed] [Google Scholar]
- 11.Farney AC, Najarian JS, Nakhleh RE, Lloveras G, Field MJ, Gores PF et al. Autotransplantation of dispersed pancreatic islet tissue combined with total or near-total pancreatectomy for treatment of chronic pancreatitis. Surgery 1991: 110: 427–437; discussion 437–439. [PubMed] [Google Scholar]
- 12.Anazawa T, Balamurugan AN, Bellin M, Zhang H, Matsumoto S, Yonekawa Y et al. Human islet isolation for autologous transplantation: comparison of yield and function using SERVA/Nordmark versus Roche enzymes. Am J Transplant 2009: In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hering BJ, Kandaswamy R, Harmon JV, Ansite JD, Clemmings SM, Sakai T et al. Transplantation of cultured islets from two-layer preserved pancreases in type 1 diabetes with anti-CD3 antibody. Am J Transplant 2004: 4: 390–401. [DOI] [PubMed] [Google Scholar]
- 14.Lakey JR, Warnock GL, Shapiro AM, Korbutt GS, Ao Z, Kneteman NM et al. Intraductal collagenase delivery into the human pancreas using syringe loading or controlled perfusion. Cell Transplant 1999: 8: 285–292. [DOI] [PubMed] [Google Scholar]
- 15.Ricordi C, Lacy PE, Scharp DW. Automated islet isolation from human pancreas. Diabetes 1989: 38(Suppl. 1): 140–142. [DOI] [PubMed] [Google Scholar]
- 16.Ricordi C, Gray DW, Hering BJ, Kaufman DB, Warnock GL, Kneteman NM et al. Islet isolation assessment in man and large animals. Acta Diabetol Lat 1990: 27: 185–195. [DOI] [PubMed] [Google Scholar]
- 17.Lake SP, Bassett PD, Larkins A, Revell J, Walczak K, Chamberlain J et al. Large-scale purification of human islets utilizing discontinuous albumin gradient on IBM 2991 cell separator. Diabetes 1989: 38(Suppl. 1): 143–145. [DOI] [PubMed] [Google Scholar]
- 18.Efron B, Tibshirani R. An Introduction to the Bootstrap. New York: Chapman and Hall, 1993. [Google Scholar]
- 19.Howes N, Lerch MM, Greenhalf W, Stocken DD, Ellis I, Simon P et al. Clinical and genetic characteristics of hereditary pancreatitis in Europe. Clin Gastroenterol Hepatol 2004: 2: 252–261. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi T, Bellin M, Manivel C, Carlson A, Moran A, Jie T et al. Correlation of histopathology of excised pancreas and islet autotransplant outcome in children with chronic pancreatitis. Am J Transplant 2007: 7(Suppl. 2): 567–568. [Google Scholar]


