Abstract
Purpose
To assess the functional and refractive outcomes in hyperopia and presbyopia correction by clear lens exchange with the intraocular trifocal artificial lens (IOL) Acrysof IQ Panoptix implant at 1 year.
Materials and Methods
A number of 128 eyes (64 patients) underwent clear lens exchange with placement of the trifocal IOL Acrysof IQ Panoptix implant for hyperopia and presbyopia. Prior to the surgery the patients had a complete ocular examination. In all cases the artificial lens was implanted in the bag without any intraoperative complications. Visual acuity (VA) at distance, intermediate and near and ocular refraction were evaluated at 4 weeks, 6 and 12 months postoperatively.
Results
The mean age was 53.49 ±7.377 years old (range 40–73 years). As high as 51.57% of the patients were males and 48.43% were females. The mean achieved refraction was 0.26 ± 0.73D. Almost 60.93% of patients were within ±0.25D of the target refraction, with 82.03% eyes within ±0.50D of the planned correction. At 1 year after surgery, 96.45% of eyes had a stable refraction (p >0.05). At 1 year, a total of 92.25%, 89.92% and 91.47% achieved a monocular uncorrected distance, intermediate and near visual acuity of 0.2 logarithm of the minimum angle of resolution or better, respectively. At the same time point, a total of 95.35%, 91.47% and 93.80% achieved a binocular uncorrected distance, intermediate and near visual acuity of 0.2 logarithm of the minimum angle of resolution or better, respectively. There was no statistically significant difference (p>0.05) between the postoperative uncorrected and best corrected VA (distance, intermediate, near) at 6 months and postoperative uncorrected and best corrected VA (distance, intermediate, near) at 12 months. None of our patients had any intraoperative complications. Two cases (1.56%) developed posterior capsule opacification. Twelve patients (18.75%) complained about photic phenomena such as glare and haloes, but this symptom disappeared after 6 months postoperatively. As high as93.56% of patients had a high satisfaction with the outcomes of the surgery. Spectacle independence was obtained in 97.65% eyes.
Conclusion
The Acrysof Panoptix trifocal artificial lens offers a good vision at distance, intermediate and near, with a good quality of vision and refraction.
Keywords: trifocal artificial lens, hyperopia, presbyopia, clear lens exchange
Introduction
Presbyopia is the most frequent cause of decreased near vision after the age of 40, regardless of race or gender.1–3 Some authors4 showed the negative effect of presbyopia on the quality of life in people at this age regarding the professional activity or productivity. That is why clear lens exchange (CLE) which consists in displacing the crystalline lens and insert a premium multifocal intraocular lens (IOL) gained a lot of popularity through the world as it offers spectacles independence. Multifocal IOL are available to address this expectation. These IOLs are designed to obtain clear vision at near, intermediate and distant focal points without supplementary spectacle correction.5,6 Trifocal diffractive IOLs have been developed to come up with a better intermediate vision and give a true spectacle independence at all distances.7
Since June 2015, Alcon Acrysof IQ Panoptix trifocal IOL Model TFNT00 (Alcon Fort Worth, TX, USA total size: 13 mm, optical part: 6 mm, constant A: 119.1) was used for patients who underwent lens surgery in order to achieve spectacle independence. It provides clear near, intermediate and distance vision with a single lens. It is a non-apodized, foldable presbyopia correcting IOL that distributes light energy to three focal points in both small and large pupil conditions. The design of the hydrophobic acrylic lens is of such manner that it permits a performance less dependent on the pupil size.8–10 Furthermore, the IOL incorporates in the anterior surface negative spherical aberration to compensate the positive spherical aberration of the average human cornea. It needs 2.2 mm clear corneal incision. Studies showed a low rate of postoperative capsular opacification after the implantation.11 It allows 88% of the light to reach a pupil of 3 mm diameter enhancing the light transmission to the retina.8,12 There is a more physiological transition from different distances because of an Enlighten optical technology13 acting like a trifocal IOL.14
The purpose of our paper was to establish the visual and refractive results at 1, 6 and 12 months after implantation of the trifocal Acrysof IQ Panoptix after clear lens extraction for hyperopia and presbyopia. To our knowledge, no study reporting bilateral Panoptix implantation in clear lens extraction safety and effectiveness in the Romanian population has been published in the scientific literature.
Materials and Methods
This was a retrospective study performed in Oculens Clinic, Cluj-Napoca, Romania that included 128 eyes from 64 patients, that underwent surgery between august 2015 and January 2019 for hyperopia and presbyopia by clear lens extraction with bilateral implantation of Acrysof IQPanoptix (Alcon Fort Worth, TX, USA). The study adhered to the tenets of declaration of Helsinki and was approved by the ethical committee of the Oculens clinic.
Patients over 40 years old, both genders, diagnosed with hyperopia and presbyopia, patients with healthy eyes, clear intraocular media including the lens and uncomplicated surgery were included in the study.
The exclusion criteria were a clinically significant corneal pathology (including keratoconus), astigmatism higher than 1.5 D, ocular trauma, retinal diseases, degenerative eye disorders or colour vision deficiencies, glaucoma or ocular hypertension, pregnancy. The preoperative diagnostic of keratoconus even in a suspect stage is very important in obtaining the expected visual results.15,16 The inflammatory cytokines in the lacrimal tear in patients with keratoconus may alter the postoperative outcomes.17 A special attention must be given to patients with dry eye disease or meibomian gland dysfunction who can be very unsatisfied after the surgery due to subjective symptoms, regardless of multifocal IOL implantation. In all cases, our clinical judgment was to decide the risk/benefit ratio before implanting a lens of this type.
Before the surgery, the patients underwent a complete ocular assessment that included the pre-operative uncorrected distance visual acuity (UDVA) and best-corrected distance visual acuity (CDVA), refractometry, keratometry (Kmax, Kmin) using the autorefractometer (Topcon autorefracto-kerato-meter, KR 8900, Japan), anterior segment slit lamp examination (Slit-Lamp BX 900, Haag-Streit AG), fundus examination, intra-ocular pressure (Haag-Streit AT 900 applanotonometer), corneal tomography using the Oculus Pentacam topographer (Oculus Pentacam Oculus Optikgerate GmbH, Wetzlar, Germany), endothelial cell count (Konan SP-9000, Hyogo, Japan), optical coherence tomography of the macula and optic nerve (Triton Topcon, Japan), Tear Break-up time Test (BUT) and Schirmer test. The function of the pupil was carefully checked. Optical biometry by interferometry was performed with IOL Master 700 (Carl Zeiss Meditec AG, Germany) to establish the axial length and dioptric power of the IOL. The used formulas were Holladay II or Barrett Universal II (Holladay IOL consultant, Houston, TX, USA and www.apacrs.org/barrett_universal2). The “A” constant provided by the company was used. Targeted postoperative refraction was emmetropia for both eyes. After these examinations we had a discussion with each patient regarding the advantages (spectacle independence), limitations (decreasing contrast sensitivity, glare, haloes, and continued needs for optical aids) of trifocal IOL, the patients visual expectations and about the process of neuroadaptation (which may require several months in some cases). Finally, all the patients signed an informed written consent in which they agreed with the surgical procedure and trifocal lens implantation.
The CLE surgery was performed in all cases under topical anaesthesia, 2–3 drops of oxibuprocain solution (Benoxi, Unimed Pharma LTD.- Slovakia) for 3 minutes and consisted of phacoemulsification (Centurion, Alcon, Fort Worth, TX, USA) with manually created clear corneal incision of 2.2 mm, 5.5–5.7mm capsulorhexis, hydro dissection and hydrodeliniation, bimanual irrigation-aspiration of the cortex and nucleus and intrabag insertion of the IOL Panoptix. The IOL was introduced into the cartridge and put into the corneal incision with the Monarch II. By rotational movements the IOL was inserted into the lens bag. The surgery on the fellow eye was performed after 3 or 4 days.
Postoperatively all the patients received local treatment with antibiotics and steroids (Tobradex, Alcon, Fort Worth, TX, USA) 5 times/day, for 4 weeks. Follow-up was performed on the first day after the surgery, at 3 days, at 1, 6 and 12 months. At follow-up visits, ocular refraction, uncorrected (UDVA) and best-corrected (CDVA) at distance (5 m), uncorrected (UIVA) and best-corrected (CIVA) visual acuity at intermediate (60 cm) and near uncorrected (UNVA) and best-corrected (CNVA) visual acuity (30–35cm), monocularly and binocularly were examined. For scientific reasons, we converted the decimal values of VA into Logarithm of Minimum Angle of resolution (logMar scale). The binocular best distance-corrected defocus curve was performed using the Early Treatment Diabetic Retinopathy Study (ETDRS) charts and supplementary lenses from +4D to −4D and with 0.5D additive values. The binocular contrast sensitivity test (CS) was performed under photopic and mesopic conditions using the Pelli-Robson contrast chart. On the last clinical follow-up, patients received a questionnaire in order to grade subjective satisfaction with the surgery outcome. The questionnaire was developed by our clinic and included the following questions: 1. Are you affected by postoperative halos? 2. Do you have a satisfactory visual acuity in dim light conditions postoperatively? 3. Do you have a satisfying visual acuity at a distance, intermediate and near distance postoperatively? 4. Do you have spectacles independence?
Statistics
Data were reported as mean ± standard deviation or number (frequency). Outcomes at different time points were analysed with the paired t-test. P values<0.001 were estimated as high statistically significant, p >0.05- not statistically significant, p=1 equal series.
Results
One hundred twenty-eight eyes of 64 patients underwent CLE with intrabag insertion of an AcrySof IQ Panoptix IOL. The mean age was 53.49 ±7.377 years old (range 40–73 years), 51.57% were males and 48.43% females. The spectrum of IOL powers ranged between 19D and 34D (average 25.7± 3.69D). The preoperatory characteristics are shown in Table 1.
Table 1.
Preoperative Characteristics
| Mean±SD | Min | Max | |
|---|---|---|---|
| Sphere (D) | 3.62± 2.081 | +1 | +9 |
| Cylinder(D) | −0.26±0.304 | −0.75 | +0.75 |
| SE(D) | 3.62± 2.081 | +1 | +9 |
| Kmin (mm) | 7.82±0.242 | 7.39 | 8.33 |
| Kmax(mm) | 7.87±0.226 | 7.3 | 8.50 |
| DeltaK | −0.48±0.430 | −0.5 | +1.5 |
| Axial length (mm) | 22.38±1.052 | 20.13 | 24.9 |
| ACD (mm) | 2.81±0.306 | 2.02 | 3.43 |
| Dioptric IOL power(D) | 25.7±3.692 | 19 | 34 |
| Preoperative VA(logMar) | 0.6±0.325 | 0.1 | 1 |
Refractive results
The mean estimated refraction was −0.0097±0.17D. The mean achieved refraction was 0.26D ±0.73D. Almost 60.93% of patients were between −0.25 and+0.25D of the target refraction, with 82.03% eyes between −0.5 and +0.5D of planned correction.
Mean preoperative spherical equivalent (SE) was 3.62±2.08D (range +1-+9D). At 1 months postoperative SE was 0.26±0.72D (p <0.001). Eighty-two percent eyes were between −0.5D and +0.5D, 91.40% eyes between −1 and +1D. At 6 months postoperative 93.54% of eyes were between −0.5 and +0.5D. At 1 year postoperative 96.45% eyes had a stable refraction (p >0.05). Two cases presented a myopic shift of −1 D Sf. (one eye) and −3.00 D Sf (one eye). In the last case, it was performed corneal refractive surgery with Excimer laser (lasik technique).
Visual Acuity Outcomes
Table 2 includes the monocular visual outcomes during the follow-up period. As it is shown, a significant statistically improvement was noticed in postoperative monocular logMar UDVA, UIVA, UNVA, CDVA, CIVA, CNVA compared with preoperative visual acuities (p<0.0001), with no significant difference between 6 and 12 months after the surgery (p>0.05).
Table 2.
Postoperative Monocular Visual Outcome
| VA | Preoperative | 4 Weeks Postoperative | 6 Months Postoperative | 1 Year Postoperative | p value Preoperative vs 4 Weeks Postoperative | p value Preoperative vs 6 Months Postoperative | p value Preoperative vs 1 Year Postoperative | p value 6 Months vs 1 Year Postoperative |
|---|---|---|---|---|---|---|---|---|
| UDVA | 0.595±0.323 | 0.073±0.139 | 0.071±0.135 | 0.070±0.139 | <0.0001 | <0.0001 | <0.0001 | >0.9999 |
| UIVA | 0.603±0.323 | 0.078±0.141 | 0.075±0.138 | 0.075±0.141 | <0.0001 | <0.0001 | <0.0001 | >0.9999 |
| UNVA | 0.600±0.325 | 0.076±0.141 | 0.074±0.136 | 0.074±0.140 | <0.0001 | <0.0001 | <0.0001 | >0.9999 |
| CDVA | 0.144±0.259 | 0.031±0.067 | 0.031±0.066 | 0.029±0.068 | <0.0001 | <0.0001 | <0.0001 | >0.9999 |
| CIVA | 0.150±0.260 | 0.036±0.074 | 0.035±0.072 | 0.034±0.071 | <0.0001 | <0.0001 | <0.0001 | >0.9999 |
| CNVA | 0.146±0.256 | 0.033±0.065 | 0.032±0.068 | 0.031±0.068 | <0.0001 | <0.0001 | <0.0001 | >0.9999 |
Abbreviations: VA, visual acuity; UDVA, uncorrected distance visual acuity; CDVA, best-corrected distance visual acuity; UIVA, uncorrected intermediate visual acuity; CIVA, best-corrected intermediate visual acuity; UNVA, uncorrected near visual acuity; CNVA, best-corrected near visual acuity.
Table 3 summarizes the binocular visual outcomes at all time points. As shown, binocular values of logMar UDVA, UIVA, UNVA, CDVA, CIVA, CNVA were significantly better (p<0.0001) compared with the preoperative values of visual acuity. As expected, binocular values of UDVA, UNVA and UIVA (p=0.014) were significantly better in binocular conditions compared to monocular ones.
Table 3.
Postoperative Binocular Visual Outcome
| VA | Preoperative | 4 Weeks Postoperative | 6 Months Postoperative | 1 Year Postoperative | p value Preoperative vs 4 Weeks Postoperative | p value Preoperative vs 6 Months Postoperative | p value Preoperative vs 1 Year Postoperative | p value 6 Months vs 1 Year Postoperative |
|---|---|---|---|---|---|---|---|---|
| UDVA | 0.462±0.337 | −0.005±0.150 | −0.007±0.130 | −0.007±0.136 | <0.0001 | <0.0001 | <0.0001 | >0.9999 |
| UIVA | 0.469±0.336 | −0.001±0.155 | −0.002±0.134 | −0.002±0.141 | <0.0001 | <0.0001 | <0.0001 | >0.9999 |
| UNVA | 0.467±0.337 | −0.002±0.152 | −0.003±0.130 | −0.003±0.138 | <0.0001 | <0.0001 | <0.0001 | >0.9999 |
| CDVA | 0.016±0.260 | −0.051±0.082 | −0.041±0.077 | −0.042±0.077 | <0.0001 | <0.0001 | <0.0001 | >0.9999 |
| CIVA | 0.020±0.262 | −0.049±0.084 | −0.039±0.080 | −0.039±0.083 | <0.0001 | <0.0001 | <0.0001 | >0.9999 |
| CNVA | 0.018±0.258 | −0.048±0.082 | −0.040±0.077 | −0.041±0.0080 | <0.0001 | <0.0001 | <0.0001 | >0.9999 |
Abbreviations: VA, visual acuity; UDVA, uncorrected distance visual acuity; CDVA, best-corrected distance visual acuity; UIVA, uncorrected intermediate visual acuity; CIVA, best-corrected intermediate visual acuity; UNVA, uncorrected near visual acuity; CNVA, best-corrected near visual acuity.
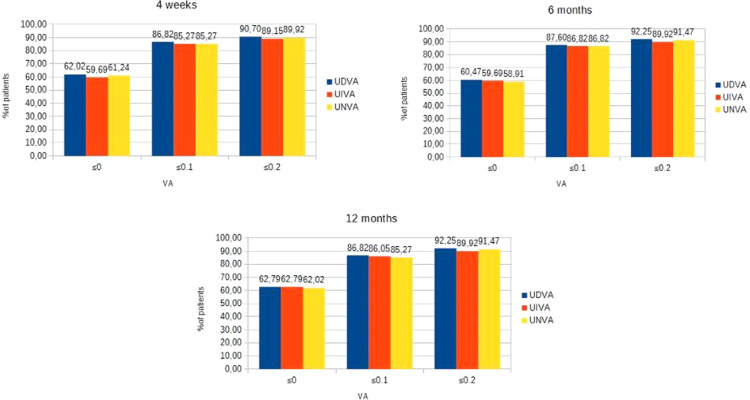
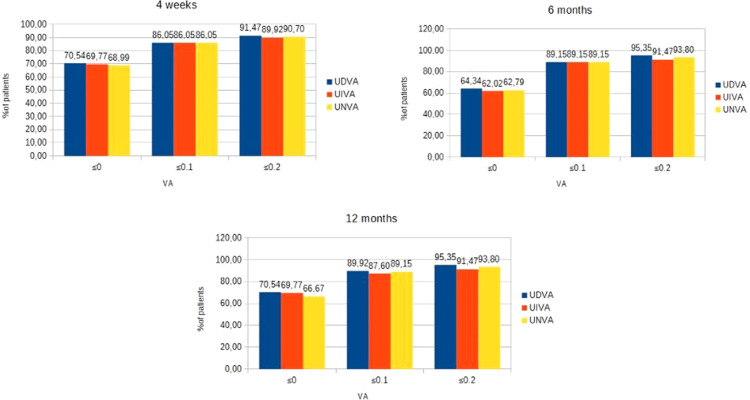
As shown, a total of 90.7%, 89.15% and 89.92% eyes achieved a monocular UDVA, UIVA and UNVA of 0.2 logMar or better, respectively, at 4 weeks, 92.25%, 89.92% and 91.47% eyes achieved a monocular UDVA, UIVA and UNVA of 0.2 logMar or better, respectively, at 6 months and 92.25%, 89.92% and 91.47% eyes achieved a monocular UDVA, UIVA and UNVA of 0.2 logMar or better, respectively, at 1 year (Figure 1). A total of 91.47%, 89.92% and 90.70% eyes achieved a binocular UDVA, UIVA and UNVA of 0.2 logMar or better, respectively, at 4 weeks, 95.35%, 91.47% and 93.80% eyes achieved a binocular UDVA, UIVA and UNVA of 0.2 logMar or better, respectively, at 6 months and 95.35%, 91.47% and 93.80% eyes achieved a binocular UDVA, UIVA and UNVA of 0.2 logMar or better, respectively, at 1 year (Figure 2).
Figure 1.
Distribution of monocular postoperative UDVA, UIVA, UNVA.
Abbreviations: UDVA, uncorrected distance visual acuity; UIVA, uncorrected intermediate visual acuity; UNVA, uncorrected near visual acuity.
Figure 2.
Distribution of binocular postoperative UDVA, UIVA, UNVA.
Abbreviations: UDVA, uncorrected distance visual acuity; UIVA, uncorrected intermediate visual acuity; UNVA, uncorrected near visual acuity.
Defocus Curve
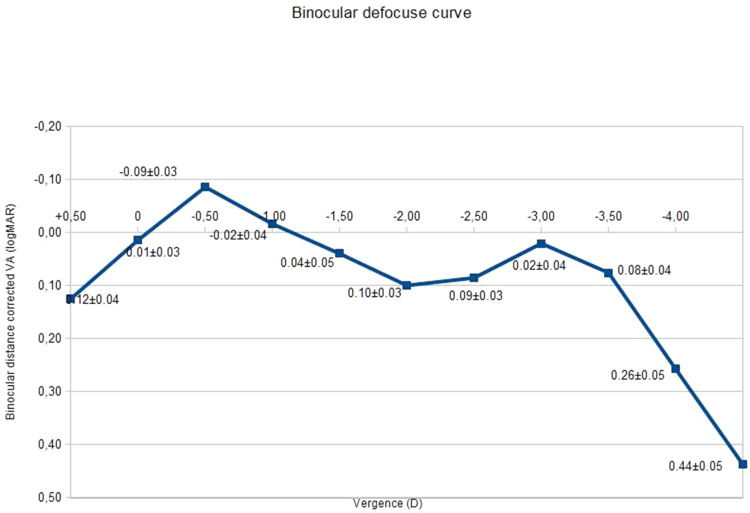
The binocular defocus curve with the distance correction is represented in Figure 3. The best VA (0.01±0.09) was obtained at a vergence of 0.50D, corresponding to the far focus. VA decreased slightly at −1.00D, matching to the intermediate focus and then increased once more at −3.00 D (near focus). VA of more or equal with 0.2 varied between −2.50 and +0.50 D.
Figure 3.
Binocular defocus curve.
Contrast Sensitivity
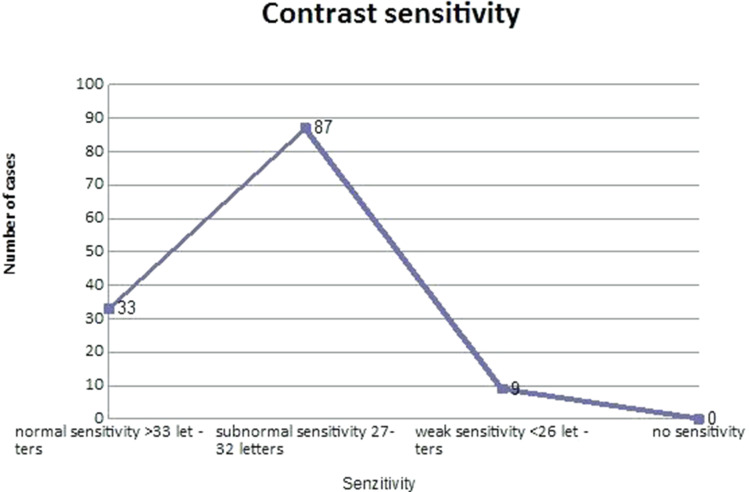
CS in photopic and mesopic state is presented in Figure 4. In 33 eyes (25.78%) we obtained a normal CS (more than 27 letters), in 87 eyes (67.96%) a subnormal CS (27–32 letters) and in 9 eyes (7.03%) a weak CS (under 26 letters). (Figure 4)
Figure 4.
Contrast sensitivity.
Abbreviation: CS, contrast sensitivity.
Complications
None of our patients had any intraoperative complications. Twelve patients (18.75%) complained about photic phenomena such as glare and haloes, but this symptom disappeared after 6 months postoperatively. Two patients (1.56%) developed opacification of posterior capsule requiring Nd Yag Laser. Three patients (4.68%) were not content with the vision quality even if their visual acuity was maximal. We could not find any factors responsible for the symptoms, we only presumed that the neuroadaptation was not acting.
Patient Satisfaction
In the questionnaire given to the patients at 12 months follow-up visit, 93.56% of patients had a high satisfaction with the outcomes of the surgery. Spectacle independence was obtained in 97.65% eyes.
Discussions
As time went by, CLE with implantation of multifocal lenses remained the most prevalent refractive surgery out of corneal laser techniques all over the world.18 The indication for this type of surgery is linked to age (>40 years old), expectations, personality, lifestyle and refractive statement (ideal for hyperopia and presbyopia). AcrySof IQ PanOptix is one of the most recent IOL with a novel design in order to obtain an intermediate visual acuity much easier for presbyopia correction. In several studies8,19–28 showed in Table 4, visual results were noted in lens surgery with different multifocal IOLs.
Table 4.
Studies Reporting Visual Outcomes After Bilateral Trifocal Panoptix Implantation
| Study | Follow-Up Period | Surgery | IOL | No Patients/Eyes | UDVA (logMar) |
CDVA (logMar) |
UIVA (logMar) |
CIVA (logMar) |
UNVA (logMar) |
CNVA (logMar) |
|---|---|---|---|---|---|---|---|---|---|---|
| Vilar et al (2017)19 | 1 mo | Cat | Panoptix Blended by focal | 20/40 | 0.01±0.04 0.08±0.05 |
0.01±0.06* 0.04–0.06 |
0.14±0.05* 0.22±0.06 |
- | −0.03±0.04* 0.07±0.03 |
- |
| Lawless et al (2017)20 | 4–9 weeks | Cat/RLE | Panoptix | 33/66 | 0.01±0.01 | - | 0.30±0.14 | - | 0.11±0.04 | - |
| Garcia-Perez (2017)21 | 1 mo | Cat | Panoptix | 58/116 | 0.03±0.046 | - | 0.12±0.143 | - | At 33 cm 0.02±0.099 |
- |
| Gundersen & Potvin (2017)22 | 6–24 mo | Cat | Panoptix Fine Vision |
60/120 | −0.05±0.05 –0.04±0.05 |
- | - | - | 0.07±0.07* 0.11±0.08 |
- |
| Monaco et al (2017)23 | 4 mo | Cat | Panoptix Symfony Acrysof 60WF |
60/120 | 0.00±0.04 0.03±0.05 0.02±0.06 |
−0.01±0.01 –0.01±0.02 –0.01±0.02 |
0.23±0.07* 0.27±0.08 0.42±0.09 |
0.13±0.07* 0.16±0.07* 0.29±0.11 |
0.02±0.06* 0.07±0.08 0.38±0.10 |
0.01±0.04* 0.07±0.07* 0.32±0.09 |
| Kohnen (2017)8 | 3 mo | Cat | Panoptix | 27/54 | 0.00±0.094 | −0.07±0.076 | At 60 cm 0.00±0.11 At 80 cm 0.09±0.107 |
At 60 cm 0.01±0.124At 80 cm 0.10±0.126 |
0.01±0.087 | 0.03±0.113 |
| de Medeiros et al (2017)24 | 180days | Cat | Panoptix Mixed EDOF |
20/40 | 0.01±0.04 –0.01±0.15* |
0.07±0.06* -0.16±0.11 |
0.14±0.05* 0.20±0.05 |
- | −0.03±0.04* 0.11±0.07 |
- |
| Cochener et al (2018)25 | 6 mo | Cat | Panoptix Fine Vision Symfony |
60/120 | 0.976±0.139 0.961±0.100 0.979±0.06 |
- | - | - | 0.66±0.00* 0.64±0.07* 0.57±0.15 |
|
| Ruis Meza et al (2018)26 | 9–24 mo | Cat/RLE | Panoptix Symfony |
34/68 | 0.0±0.03 0.05±0.12 |
−0.03±0.03 –0.02±0.03 |
- | At 60 cm: 0.06±0.10 0.05±0.04 |
- | 0.04±0.06* 0.20±0.06 |
| Alio et al (2018)13 | 6 mo | Cat | Panoptix | 26/52 | 0.07±0.10 | 0.01±0.04 | 0.12±0.13 | 0.12±0.12 | 0.16±0.09 | 0.08±0.06 |
| Mencuci et al (2018)27 | 3 mo | Cat | Panoptix AT Lisa Tri Symfony |
60/120 | −0.02±0.08 0.00±0.02 –0.04±0.05 |
−0.03±0.08 –0.01±0.02 –0.05±0.05 |
At 60cm 0.07±0.04*(P) At 80 cm 0.16±0.06 0.11±0.07 0.07±0.07 |
At 60cm 0.06±0.05*(P) At 80 cm 0.14±0.08 0.10±0.06* 0.06±0.07* |
0.15±0.05* 0.18±0.05* 0.25±-0.08 |
0.12±0.04* 0.13±0.04* 0.20±0.1 |
| Escandon Garcia et al (2018)28 | 39–50days | Cat | Panoptix Symfony Fine Vision |
45/90 | 0.07±0.10 0.08±0.10 0.08±0.09 |
−0.07±0.19 –0.10±0.19 –0.24±0.14 |
– | – | – | – |
Note: *Kruskal–Wallis test.
Abbreviations: UDVA, uncorrected distance visual acuity; CDVA, best-corrected distance visual acuity; UIVA, uncorrected intermediate visual acuity; CIVA, best-corrected intermediate visual acuity; UNVA, uncorrected near visual acuity; CNVA, best-corrected near visual acuity.
The major concerns after clear lens extraction with multifocal intraocular lenses are accuracy, quality of vision, stability and safety.29 In terms of accuracy, our study showed a deviation of less than ±0.325D after CLE with bilateral Panoptix implantation. In our study, 92.25% and 91.47% of patients achieved an UDVA and UNVA of 0.2 or better logMar monocularly at 1 year, respectively. As high as 89.92% of patients achieved a UIVA of 0.2logMar at 1 year. In binocularity, 95.35%, 91.47% and 93.80% achieved a UDVA, UIVA, UNVA, respectively, at 1 year. Our findings are similar with those showed by Kretz30 who obtained a mean UDVA of 20/20 to 20/32 in 8 eyes at 3 months after Panoptix implantation in both eyes. In the same study, the average of UIVA was between 20/20 and 20/40 and the average of UNVA was between 20/25 and 20/40.30 Kohnen et al8 published the initial findings for Acrysof Panoptix, noting excellent uncorrected vision at distance, intermediate (60cm) and near. In the same study, 87% of eyes obtained a monocular UDVA ≤0.10 logMar and 96% of eyes gained a monocular UDVA of ≤0.2 logMar. Furthermore, 85% of eyes obtained a monocular UNVA of ≤0.10 logMar and 91% of eyes obtained ≤0.2 logMar. Fifty percent of eyes obtained a UNVA of at least 0.0 logMar. In total, 83% and 94% of eyes reported an UIVA of at least 0.2 logMar at 80 cm and at 60 cm, respectively.8 Lawless et al20 demonstrated a postoperative UDVA of 0.01±0.10 logMar. In all cases a UDVA of 20/40 or better postoperatively was obtained. Binocularly, 87.9% of patients gained a 0.20 logMar or better at near without correction and 88.9% achieved this level for UIVA uncorrected intermediate visual acuity.20 Garcia-Perez et al21 reported a binocular UDVA and UNVA more than 0.3 logMar, and 94.8% of patients achieved the same result for intermediate vision at 1-month follow-up. Alio et al13 in a 6-month prospective study obtained an important increase in uncorrected and corrected VA results at 1 month after PanOptix implantation. The same author demonstrated the stability of VA at 6-month follow-up. Cochener et al25 and Escandón-García et al28 in comparative studies between Panoptix, Fine Vision (PhysIOL, Liege, Belgium) and Symfony (Abbott Medical Optics, Santa Ana, CA) implantation, had not identified any significant difference in the VA for distance vision (both monocular and binocular) and intermediate VA (monocular) for the three IOLs. Moreover, in both PanOptix and Fine Vision IOLs, a remarkably better near vision compared with Symfony was obtained.28 Mencucci et al27 in a study of 3 months comparing postoperative VA results in patients implanted with PanOptix, AT LISA (Carl Zeiss Meditec), and Symfony showed that PanOptix sustained better VA at 60 cm than the other IOLs; similarly, at 80 cm, Symfony was significantly better than the other IOLs. The near vision was relatively better with PanOptix than AT LISA; both IOLs showed significantly better near vision than Symfony. Moreover, AT LISA and Symphony provided a better contrast sensitivity compared to Panoptix.27 Monaco et al23 in a study comparing the functional outcomes of multifocals (PanOptix and Symfony) IOL with the monofocal AcrySof IOL concluded that both the multifocal and EDOF IOLs performed significantly better for intermediate (60 cm) and near vision than the monofocal IOL. Moreover, with PanOptix a better UIVA, UNVA, and corrected near VA was achieved compared to Symfony.23 The studies of Gundersen & Potvin,22 Mencucci et al27 and Lapid-Gortzak et al31 revealed that PanOptix offered a significantly better near vision compared with Symfony, Fine Vision, and AT LISA. In addition, Gundersen & Potvin22 demonstrated better intermediate VA at 60 cm with PanOptix (P=0.01). Lapid-Gortzak et al31 compared the performance of PanOptix with the AT LISA IOL concluding that the patients with PanOptix obtained significantly better binocular UIVA at 60 cm and binocular UNVA at 40 cm versus AT LISA. de Medeiros et al24 demonstrated better intermediate vision outcomes at (40 cm and 60 cm) with PanOptix in comparison with Symfony and Tecnis ZMBOO. Ruiz-Mesa et al26 revealed that CIVA in patients with PanOptix and Symfony had the same value, both at 80 cm and 60 cm. Nonetheless, UNVA and UDVA were significantly better with PanOptix than Symfony. Lapid-Gortzak et al31 reported in a study that the patients with PanOptix obtained significantly better binocular UIVA at 60 cm and binocular UNVA at 40 cm versus AT LISA.
Patient Satisfaction
Our study revealed that 93.56% of patients had no problems in performing daily activities. As high as 18.75% described the presence of halos and glittering during night-time, decreasing as intensity in time. Spectacles independence was obtained in 97.65% cases. Our results are similar with those of Cochener et al25 demonstrating that night-time visual disturbances, dry eye, halos, and glare were present in the same proportion of 1% of patients in Panoptix, Fine Vision and Symfony IOLs group. Garcia-Perez et al21 showed that 84.5% of patients had no problems but 32.8% of patients reported the presence of halos in dim light and the glare was present in 10.3% of patients. As high as 94.8% of his patients achieved complete spectacle independence, 5.1% of patients reported using spectacles for some activities. Monaco et al23 reported that 85% of patients with PanOptix and 70% of patients with Symfony obtained complete spectacle independence. Fifteen percent of patients with PanOptix and 25% of patients with Symfony noted the need to wear spectacles, but rarely. Gundersen & Potvin’s study22 did not find any significant difference between the Panoptix and Fine Vision groups. Mencucci et al27 reported complete satisfaction in all patients with the choice of IOL. Moreover, patients with Symfony needed spectacles in a higher percentage for near vision in comparison with AT LISA and PanOptix. Lapid-Gortzak et al31 in their study demonstrated that more than 95% of patients were satisfied in both Panoptix and AT LISA groups at the 6 months’ visit.
Regarding quality vision, CS was the major feature to take into consideration for the performance of the Panoptix. In our study, CS presented normal values in 25.78% eyes, in 67.96% of eyes subnormal values and in 7.03% weak contrast sensitivity. Similar results were revealed by Kohnen et al9 obtaining the mean contrast sensitivity in photopic, mesopic, and mesopic-with-glare lighting conditions of 1.55 ± 0.35, 0.91 ± 0.26, and 0.86 ± 0.26, respectively. Comparing the contrast sensitivity on Panoptix, Fine Vision and Symfony IOLs, Cochener et al25 showed that the distance contrast sensitivity was similar for all three IOLs, being diminished in mesopic conditions. Garcia-Perez et al21 found out that for PanOptix there was similar distance contrast sensitivity for all spatial frequencies in mesopic and photopic conditions. Escandón-García et al28 demonstrated that the contrast sensitivity performance was equal among Panoptix, Symfony and Fine Vision IOLs in phototopic and scotopic condition. In addition, the CS of PanOptix was diminished in photopic conditions.28 Conflicting results were revealed by Mencucci et al27 showing that the distance CS outcomes were significantly better with Symfony than AT LISA and PanOptix IOLs, in photopic and mesopic conditions. De Medeiros et al24 demonstrated that CS was better at low spatial frequencies in the Symfony and Tecnis ZMBOO group under photopic conditions in comparison with the Panoptix group, but similar results were present between the three IOLs at higher frequencies.
Defocus Curves
In our study we found a defocus curve with a minimal reduction in VA at the intermediate range (at a vergence of −1D) as a result of the additional intermediate focal point. Similar results were shown by Garcia Perez et al21 and Alio13 revealing that VA higher than 0.2 logMar was preserved between −2.50 and +0.50 D and −3 and +0.5D, respectively.
In his study, Kohnen8 demonstrated that the optimum VA was obtained at 0.00 D (4 m) and −2.00 D (50 cm) in both monocular (−0.05 logMar and 0.01 logMar) and binocular (−0.07 logMar and −0.02 logMar) defocus curves. Comparing the defocus curve of Panoptix, Symfony and Fine Vision, Escandon Garcia28 demonstrated superior efficiency at −1.00 D/1 m (P = 0.030). Moreover, PanOptix and Fine Vision offered an excellent provided near vision at −2.5 D (40 cm) and −3.0 D (33 cm), respectively. In addition, PanOptix provided a better VA at −2.00 D (50 cm) defocus compared with Fine Vision and Symfony.28 Another study comparing Panoptix and Symfony demonstrated that PanOptix revealed a statistically significantly better VA, at defocus level −1.5 D, and from −2.5 D to −4.0 D than the Symfony IOL.23 Gundersen and Potvin22 comparing the binocular defocus curves of the Panoptix and Fine Vision trifocals IOLs showed that FineVision IOL proved a better performance at −1.0 D at 80 cm while PanOptix determined better performance at −1.5 D and −2.00 D at 60 cm. Ruiz-Mesa et al26 revealed a comparable pattern in the defocus curve for distance and intermediate vision between the Panoptix and Symfony IOLs, but notably superior near results with PanOptix than Symfony. Lapid-Gortzak et al31 showed similar contrast sensitivity in photopic or mesopic conditions in Panoptix and AT LISA groups.
Regarding stability, CLE probably represents the most stable refractive procedure.10,13 Our study confirms the predictability of the procedure showing the same value of SE between 6 and 12 months (p>0.05).
In terms of safety, 18.75% of our patients complained about visual disturbance, which is due to the effect produced by the multifocal IOL.10 Our study revealed that 93.56% of patients had no problems in performing daily tasks. Similarly, Cochener et al25 demonstrated that darkness visual disorders, dry eye, halos, and glare were present in the same proportion of 1% of patients in Panoptix, Fine Vision and Symfony IOLs group. Garcia-Perez et al21 showed that 84.5% of patients had no problems but 32.8% of patients reported the presence of halos often in dim light and 10.3% reported glare. The reported incidence of halos among several of studies showed a large variation (<1% to 89%)9,21–23,27 but without any negative impact on patients’ quality of life. None of this study reported any reason coming from patients to exchange Panoptix for photic phenomena. In his study, Kohnen8 revealed that 93% of patients presented photopic phenomena, especially halos (89%) and in low percentage glare (11%), double vision (7%), ghosting and distorted vision (4%). Lawless et al20 reported the presence of moderate halos in 15% of patients after the surgery without affecting daily activities. Moreover, the complaints diminished in 2–3 months after the surgery.20 Mencucci et al27 showed in his comparative study that visual disturbances were present in 50 to 70% of patients, although the symptoms were mild without disturbing the patients.27 On the opposite side, Monaco et al23 reported the presence of severe or bothering haloes in 15% of patients with PanOptix and in 25% in the Symfony group. Rosen et al10 in a meta-analyse on clear lens extraction with multifocal lenses showed that even the photopic phenomena are usually present in a high frequency in trifocal IOL compared with bifocal ones, but after 6 months the patients become more tolerant with them. Monaco et al23 revealed that there was no difference of dysphotopsia score between PanOptix and Symfony IOLs.
In our study, we had only two cases (1.56%) of posterior capsule opacification (PCO) which appeared after 1 year after the surgery. They were scheduled to Nd: YAG capsulotomy. It is known that frequency of PCO and Nd: YAG capsulotomy had an inferior level with PanOptix being a part of the AcrySof hydrophobic IOLs group with a low incidence of PCO. Beyond published studies, only 1 case was reported.21 Similarly, Kacerovsky,32 in a short-term comparative study revealed a rate of PCO of barely 0.5% with PanOptix in contrast with 6% with AT LISA. Other studies showed a higher frequency of PCO after AT LISA on long term follow-up (34%)33 in comparison with Fine Vision (14%) on the same period of evaluation.34 Therefore, long-term follow-up studies with Panoptix are required in order to assess the real frequency of PCO.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
- 1.Bourne RR, Jonas JB, Bron AM, et al. Prevalence and causes of vision loos in high-income countries and in Eastern and central Europe in 2015: magnitude, temporal trends and projections. Br J Ophthalmol. 2018;102:575–585. doi: 10.1136/bjophthalmol-2017-311258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bourne RR, Flaxman SR, Braithwaite T, et al. Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment: a systematic review and meta-analysis. Lancet Glob Health. 2017;5:e888–97. doi: 10.1016/S2214-109X(17)30293-0 [DOI] [PubMed] [Google Scholar]
- 3.Ajibode HA, Fakolujo VO, Onabolu OO, et al. A community-based prevalence of presbyopia and spectacle coverage in Southwest Nigeria. J West Afr Coll Surg. 2016;6:66–82. [PMC free article] [PubMed] [Google Scholar]
- 4.Goertz AD, Stewart WC, Burns W, Stewart JA, Nelson LA. Review of the impact of presbyopia on quality of life in the developing and developed world. Acta Ophthalmol. 2014;92:497–500. doi: 10.1111/aos.12308 [DOI] [PubMed] [Google Scholar]
- 5.Wang SY, Stem MS, Oren G, Shtein R, Lichter PR. Patient-centred and visual quality outcomes of premium cataract surgery: a systematic review. Eur J Ophthalmol. 2017;27(4):387–401. doi: 10.5301/ejo.5000978 [DOI] [PubMed] [Google Scholar]
- 6.Zvorničanin J, Zvorničanin E. Premium intraocular lenses: the past, present and future. J Curr Ophthalmol. 2018;30(4):287–296. doi: 10.1016/j.joco.2018.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cochener B. Prospective clinical comparison of patient outcomes following implantation of trifocal or bifocal intraocular lenses. J Refract Surg. 2016;32:146–151. doi: 10.3928/1081597X-20160114-01 [DOI] [PubMed] [Google Scholar]
- 8.Kohnen T. First implantation of a diffractive quadrifocal (trifocal) intraocular lens. J Cataract Refract Surg. 2015;41:2330–2332. doi: 10.1016/j.jcrs.2015.11.012 [DOI] [PubMed] [Google Scholar]
- 9.Kohnen T, Herzog M, Hemkeppler E, et al. Visual performance of a quadrifocal (trifocal) intraocular lens following removal of the crystalline lens. Am J Ophthalmol. 2017;184:52–62. doi: 10.1016/j.ajo.2017.09.016 [DOI] [PubMed] [Google Scholar]
- 10.Rosen E, Alió JL, Dick HB, Dell S, Slade S. Efficacy and safety of multifocal intraocular lenses following cataract and refractive lens exchange: metanalysis of peer-reviewed publications. J Cataract Refract Surg. 2016;42:310–328. doi: 10.1016/j.jcrs.2016.01.014 [DOI] [PubMed] [Google Scholar]
- 11.Scorsetti DH. Trifocal lenses for cataract surgery. Ophthalmol Open J. 2020. doi: 10.17140/OOJ-2-e006 [DOI] [Google Scholar]
- 12.Lee S, Choi M, Xu Z, Zhao Z, Alexander E, Liu Y. Optical bench performance of a novel trifocal intraocular lens compared with a multifocal intraocular lens. Clin Ophthalmol. 2016;10:1031–1038. doi: 10.2147/OPTH.S106646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alio JL, Plaza-Puche AB, Alio Del Barrio JL, et al. Clinical outcomes with a diffractive trifocal intraocular lens. Eur J Ophthalmol. 2018;28:419–424. doi: 10.1177/1120672118762231 [DOI] [PubMed] [Google Scholar]
- 14.Carballo-Alvarez J, Vazquez-Molini JM, Sanz-Fernandez JC, et al. Visual outcomes after bilateral trifocal diffractive intraocular lens implantation. BMC Ophthalmol. 2015;15:26. doi: 10.1186/s12886-015-0012-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicula C, Nicula D, Pop RN. Results at 7 years after cross-linking procedure in keratoconus patients (Les resultants a 7 Ans après la procedure de reticulation chez les patients attaints de keratoconus). J Ophthalmol. 2017;40(7):535–541. [Google Scholar]
- 16.Nicula C, Pop R, Nicula D. Comparative results in a combined procedure of intrastromal corneal rings implantation and cross-linking in patients with keratoconus; a retrospective study. Ophthalmol Ther. 2017;6(2):313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ionescu IC, Corbu CG, Tanase C, et al. Overexpression of tear inflammatory cytokines as additional findings in keratoconus patients and their first degree family members. Hindawi Mediators Inflamm. 2018;2018:4285268. doi: 10.1155/2018/4285268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmack I, Auffarth GU, Epstein D, Holzer MP. Refractive surgery trends and practice style changes in Germany over a 3-year period. J Refract Surg. 2010;26:202–208. doi: 10.3928/1081597X-20090515-05 [DOI] [PubMed] [Google Scholar]
- 19.Vilar C, Hida WT, de Medeiros AL, et al. Comparison between bilateral implantation of a trifocal intraocular lens and blended implantation of two bifocal intraocular lenses. Clin Ophthalmol. 2017;11:1393–1397. doi: 10.2147/OPTH.S139909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lawless M, Hodge C, Reich J, et al. Visual and refractive outcomes following implantation of a new trifocal intraocular lens. Eye Vis (Lond). 2017;4:10. doi: 10.1186/s40662-017-0076-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia-Perez JL, Gros-Otero J, Sanchez-Ramos C, et al. Short-term visual outcomes of a new trifocal intraocular lens. BMC Ophthalmol. 2017;17:72. doi: 10.1186/s12886-017-0462-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gundersen KG, Potvin R. Trifocal intraocular lenses: a comparison of the visual performance and quality of vision provided by two different lens designs. Clin Ophthalmol. 2017;11:1081–1087. doi: 10.2147/OPTH.S136164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monaco G, Gari M, Di Censo F, et al. Visual performance after bilateral implantation of 2 new presbyopia-correcting intraocular lenses: trifocal versus extended range of vision. J Cataract Refract Surg. 2017;43:737–747. doi: 10.1016/j.jcrs.2017.03.037 [DOI] [PubMed] [Google Scholar]
- 24.de Medeiros AL, de Araujo Rolim AG, Motta AFP, et al. Comparison of visual outcomes after bilateral implantation of a diffractive trifocal intraocular lens and blended implantation of an extended depth of focus intraocular lens with a diffractive bifocal intraocular lens. Clin Ophthalmol. 2017;11:1911–1916. doi: 10.2147/OPTH.S145945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cochener B, Boutillier G, Lamard M, et al. A comparative evaluation of a new generation of diffractive trifocal and extended depth of focus intraocular lenses. J Refract Surg. 2018;34:507–514. doi: 10.3928/1081597X-20180530-02 [DOI] [PubMed] [Google Scholar]
- 26.Ruiz-Mesa R, Abengozar-Vela A, Ruiz-Santos M. A comparative study of the visual outcomes between a new trifocal and an extended depth of focus intraocular lens. Eur J Ophthalmol. 2018;28:182–187. doi: 10.5301/ejo.5001029 [DOI] [PubMed] [Google Scholar]
- 27.Mencucci R, Favuzza E, Caporossi O, et al. Comparative analysis of visual outcomes, reading skills, contrast sensitivity, and patient satisfaction with two models of trifocal diffractive intraocular lenses and an extended range of vision intraocular lens. Graefes Arch Clin Exp Ophthalmol. 2018;256:1913–1922. doi: 10.1007/s00417-018-4052-3 [DOI] [PubMed] [Google Scholar]
- 28.Escandón-García S, Ribeiro FJ, McAlinden C, et al. Through-focus vision performance and light disturbances of 3 new intraocular lenses for presbyopia correction. J Ophthalmol. 2018;2018:6165493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knezović I, Parać A, Raguž H, Gegović M, Levak L, Marinić D. Clinical Results After Clear Lens Extraction (CLE) and Bilateral Implantation of Extended-Range of- Vision Presbyopia-Correcting Intraocular Lenses (IOL) in 30 Patients. Med Surg Ophthalmol Res. 2018;2:4. [Google Scholar]
- 30.Kretz F, Choi C, Müller M, et al. Visual outcomes, patient satisfaction and Spectacle independence with a trifocal diffractive intraocular lens. Korean J Ophthalmol. 2016;30(3):180–191. doi: 10.3341/kjo.2016.30.3.180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lapid-Gortzak L, Martines A Multicentre visual outcomes comparison of two trifocal presbyopia correcting intraocular lenses: 6-month postoperative results. Oral presentation at: XXXVI European Society of Cataract and Refractive Surgeons Meeting; September22–26th, 2018, Vienna, Austria. [Google Scholar]
- 32.Kacerovsky M. PanOptix and AT LISA tri in presbyopia surgery. Presented at European Society of Cataract and Refractive Surgeons education forum. Vienna, Austria; 2018. Available from: http://forum.escrs.org/escrs-presentations-and-videos/panoptix-and-at-lisa-tri-in-presbyopic-surgery. Accessed November12, 2020. [Google Scholar]
- 33.Mojzis P, Majerova K, Hrckova L, et al. Implantation of a diffractive trifocal intraocular lens: one-year follow-up. J Cataract Refract Surg. 2015;41:1623–1630. doi: 10.1016/j.jcrs.2014.11.050 [DOI] [PubMed] [Google Scholar]
- 34.Bilbao-Calabuig R, Llovet-Osuna F, Gonzalez-Lopez F, et al. Nd: YAG capsulotomy rates with two trifocal intraocular lenses. J Refract Surg. 2016;32:748–752. doi: 10.3928/1081597X-20160803-02 [DOI] [PubMed] [Google Scholar]