Abstract
Background
Sensitisation to moulds and Staphylococcus aureus enterotoxins (SEs) is associated with the pathophysiology of both asthma and chronic rhinosinusitis (CRS). The purpose of this study was to clarify the contribution of sensitisation to these allergens to Type 2 inflammation in the blood, nose and the lower airways, and clinical outcomes in CRS patients.
Methods
We prospectively enrolled 56 CRS patients who underwent endoscopic sinus surgery (ESS) (20 with comorbid asthma) and 28 healthy controls between October 2015 and December 2017. CRS patients were followed up for 12 months after surgery. Type 2 inflammation-related biomarkers were analysed using blood, resected tissue samples and sputum. 10 allergens including Alternaria, Aspergillus and SEs were measured. Type 2 inflammation-related biomarkers and clinical outcomes were compared in the stratification with the presence or absence of allergen sensitisation.
Results
Sensitisation rate to moulds and SEs in asthmatic patients was increased when changing the cut-off value of specific IgE titre from 0.35 UA·mL−1 to 0.10 UA·mL−1 (1.7- and 4.5-fold, respectively). Moulds and SEs affected the prevalence of asthma and eosinophilic CRS by interacting with each other. All Type 2 inflammation-related biomarkers except for eosinophils in sinus tissue were significantly higher in patients with mould or SE (mould/SE) sensitisation (≥0.10 UA·mL−1) (n=19) than in those without (n=37) and healthy subjects (all p<0.05). Meanwhile, mould/SE sensitisation did not affect longitudinal changes in clinical outcomes after ESS. Changes in serum mould/SE-IgE levels after ESS remained unclear.
Conclusion
Mould/SE sensitisation (≥0.10 UA·mL−1) may affect the development of Type 2 inflammation and clinical outcomes in CRS patients.
Short abstract
Alternaria, Aspergillus and S. aureus enterotoxins are important allergens affecting Type 2 inflammation and clinical outcomes in CRS patients. Sensitisation to moulds/SEs (≥0.10 UA·mL−1) would be meaningful in the pathophysiology of CRS. https://bit.ly/3bUG8ZT
Introduction
Sensitisation to allergens is a significant risk factor for adult onset asthma in the general population [1, 2]. It is also common in patients with chronic rhinosinusitis (CRS), its prevalence ranging from 50 to 84% [3]. Generally, we consider patients as having sensitisation to allergens when one or more specific immunoglobulin E (IgE) titres against allergens exceed 0.35 UA·mL−1 [4]. However, several studies indicate the association of a threshold of ≥0.10 UA·mL−1 in serum-specific IgE titres, including Staphylococcus aureus enterotoxins (SEs) and Aspergillus, with onset, severity and poor control of asthma [5–9]. However, the contribution of a lower threshold (≥0.10 UA·mL−1) of serum-specific IgE titres against allergens to Type 2 inflammation and clinical outcomes in allergic diseases remains little known.
Long-term colonisation of the nose and lower airways by moulds and S. aureus plays a role in their sensitisation both systemically and locally [10, 11]. The presence of IgE in the nose and lower airways is associated with local eosinophilic inflammation [12, 13]. Alternaria and Aspergillus are well-known fungal allergens associated with severe asthma [14, 15], along with S. aureus [6–8]. The evidence suggests that environmental microorganism-related allergens may be strong activators of Type 2 inflammation throughout airways and contribute to worse clinical outcomes in CRS patients as compared to other common allergens.
The objective of this study was to explore different cut-off levels of sensitisation to moulds and SEs in relation to Type 2 inflammation-related biomarkers in the blood, nose and lower airways, and the clinical relevance in patients with CRS. Furthermore, we assessed whether mould and SE sensitisation had any effect on the clinical and inflammatory outcomes 12 months after endoscopic sinus surgery (ESS).
Methods
This study is a post hoc analysis of our previous study which evaluated the pathophysiological link between upper and lower airways in CRS patients [16]. We prospectively enrolled 56 CRS patients who agreed to ESS (20 with and 36 without comorbid asthma) and 28 healthy controls between October 2015 and December 2017. The diagnosis of asthma and CRS was made according to guidelines [17, 18]. These were described in our previous report along with inclusion and exclusion criteria used in the study [16]. We excluded current smokers in this study. We confined our patient sample to those who underwent ESS in this study because tissue eosinophilia in the sinuses, particularly in nasal polyps (NPs), is associated with the presence of asthma [19, 20]. We also have suggested that upper airway tissue eosinophilia is pathophysiologically linked to Type 2 lower airway inflammation. Indeed, we have demonstrated the association of tissue eosinophilia in sinus and NPs with Type 2 lower airway inflammation in our previous study [16]. This study was approved by the Ethics Committee of our hospital (1165) and was registered on the University Hospital Medical Information Network (UMIN) Clinical Trials Registry (Registry ID UMIN000018672). Written informed consent was obtained from all participants.
Measurements
All participants underwent spirometry, fractional nitric oxide (FeNO) measurement (oral expiratory flow rate 50 mL·s−1), an olfactory function test as assessed by the Open Essence method (ranging from 0 to 12, and lower scores showing impaired olfaction) [21] and sputum induction, and answered the Sinonasal Outcome Test-22 (SNOT-22) (ranging from 0 to 110, and higher scores showing worse sinonasal-related quality of life (QoL)) [22] before ESS, as did healthy subjects. In one patient we were unable to measure FeNO levels at enrolment due to apparatus failure. Additionally, blood and tissue sample collection and a computed tomography (CT) scan of the sinuses were performed only in CRS patients. Serum Type 2 inflammation-related biomarkers were also determined in 20 healthy subjects using stored serum samples. Inflamed sinus tissue samples and NPs were taken from 54 and 38 patients, respectively, under general anaesthesia by an otorhinolaryngology specialist (M.S.). Eosinophil counts and periostin levels were measured using blood and sputum samples. The number of eosinophils in sinus and NPs (high-power field (HPF), 400×) and the Lund–Mackay score (LMS; ranging from 0 to 24, and higher scores showing severe CRS) from CT of the sinus were determined by a pathologist (A.M.) and a radiologist (Y.O.) in a blinded manner, respectively. We defined eosinophilic CRS when eosinophils in sinus or NP tissue showed ≥70 HPF [19]. We re-evaluated all measurements aside from tissue collection at 12 months after ESS in CRS patients. Patients with asthma also completed the Asthma Quality of Life Questionnaire (AQLQ) [23] (comprising four domains, score ranging from 0 to 7, with lower scores showing worse asthma-related QoL) both before and 12 months after ESS. We obtained permission to use the SNOT-22 and AQLQ for this study from Professor Jay Piccirillo, Washington University, USA, and Professor Elizabeth Juniper, McMaster University, Canada, respectively. Furthermore, an otorhinolaryngologist (M.S.) assessed the recurrence of NPs and three asthma specialists (Y.K., M.T. and A.N.) assessed new asthma onset for 12 months following ESS. Details of these measurements are described in a previous report [16].
Allergens measurement
We determined serum total IgE and specific IgE antibody against 10 allergens: house dust mite, cat, dog dander, Japanese cedar pollen, mixed Gramineae pollens (orchard grass, sweet vernal grass, Bermuda grass, Timothy grass and reeds), mixed weed pollens (ragweed, mugwort, goldenrod, dandelion and oxeye daisy), Alternaria alternata, Aspergillus fumigatus and Staphylococcus aureus enterotoxins A and B (SEA and SEB, respectively) (ImmunoCAP®; Phadia K.K., Tokyo, Japan). The detection limit for each specific IgE titre was 0.10 UA·mL−1. Table 1 shows the proportion of each sensitised allergen in CRS patients when the cut-off value of serum-specific IgE titre was set at 0.10 UA·mL−1 or 0.35 UA·mL−1. Sensitisation rate to moulds and SEs in asthmatic patients was increased when changing the cut-off value of specific IgE from 0.35 UA·mL−1 to 0.10 UA·mL−1 (1.7- and 4.5-fold, respectively) (table 1). However, sensitisation to other allergens such as mite and pollen was similar between two cut-off values of specific IgE titres. When a cut-off value was set at 0.10 UA·mL−1, there were significant differences in sensitisation to moulds or SEs between CRS patients with and without asthma (table 1). This suggests that sensitisation to moulds or SEs (≥0.10 UA·mL−1) would be meaningful in the pathophysiology of both asthma and CRS. Thus, we considered patients as having sensitisation to moulds or SEs if they showed a serum-specific IgE titre of ≥0.10 UA·mL−1. On the other hand, we considered participants as having sensitisation to other conventional allergens if they showed one or more specific IgE titre against remaining allergens of ≥0.35 UA·mL−1.
TABLE 1.
The proportion of sensitised allergens
| ≥0.35 UA·mL−1 | ≥0.10 UA·mL−1 | |||||||||||
| CRS patients | Asthma | Without asthma | Healthy subjects | p-value# | p-value¶ | CRS patients | Asthma (n=20) | Without asthma | Healthy subjects | p-value# | p-value¶ | |
| Subjects n | 56 | 20 | 36 | 20 | 56 | 20 | 36 | 20 | ||||
| House dust mite | 19 (34) | 10 (50) | 9 (25) | 0.08 | 27 (48) | 13 (65) | 14 (39) | 0.09 | ||||
| Dog dander | 3 (5) | 1 (5) | 2 (6) | >0.99 | 8 (14) | 4 (20) | 4 (11) | 0.44 | ||||
| Cat | 2 (4) | 0 (0) | 2 (6) | 0.53 | 7 (13) | 0 (0) | 7 (19) | 0.04 | ||||
| Japanese cedar | 29 (52) | 12 (60) | 17 (47) | 0.41 | 32 (57) | 13 (65) | 19 (53) | 0.41 | ||||
| Mixed Gramineae | 7 (13) | 4 (20) | 3 (8) | 0.23 | 10 (18) | 5 (25) | 5 (14) | 0.47 | ||||
| Mixed weed | 9 (16) | 5 (25) | 4 (11) | 0.26 | 14 (25) | 6 (30) | 8 (22) | 0.53 | ||||
| Alternaria | 5 (9) | 4 (20) | 1 (3) | 1 (5) | >0.99 | 0.0497 | 12 (21) | 8 (40) | 4 (11) | 1 (5) | 0.16 | 0.02 |
| Aspergillus | 5 (9) | 4 (20) | 1 (3) | 1 (5) | >0.99 | 0.0497 | 8 (14) | 6 (30) | 2 (6) | 2 (10) | >0.99 | 0.02 |
| Moulds | 7 (13) | 6 (30) | 1 (3) | 2 (10) | >0.99 | 0.006 | 14 (25) | 10 (50) | 4 (11) | 2 (10) | 0.21 | 0.003 |
| SEA | 4 (7) | 1 (5) | 3 (8) | 0 (0) | 0.57 | >0.99 | 9 (16) | 5 (25) | 4 (11) | 6 (30) | 0.20 | 0.26 |
| SEB | 5 (9) | 1 (5) | 4 (11) | 0 (0) | 0.32 | 0.64 | 13 (23) | 7 (35) | 6 (17) | 3 (15) | 0.54 | 0.19 |
| SEs (A and/or B) | 6 (11) | 2 (10) | 4 (11) | 0 (0) | 0.33 | >0.99 | 15 (27) | 9 (45) | 6 (17) | 8 (40) | 0.27 | 0.03 |
Categorical data are presented as n (%). CRS: chronic rhinosinusitis; SEA: Staphylococcus aureus enterotoxin A; SEB: Staphylococcus aureus enterotoxin B; SEs: Staphylococcus aureus enterotoxins. #: Compared between all CRS patients and healthy subjects; ¶: Compared between with asthma and without asthma. We could not measure serum-specific IgE titres in eight healthy subjects because of shortage of sample amount.
To compare the prevalence of mould and SE sensitisation between CRS patients and healthy subjects, we also measured serum total IgE levels and specific IgE titres against Alternaria, Aspergillus and SEs in healthy subjects. Sensitisation rates to either moulds or SEs were similar between all CRS patients and healthy subjects (table 1).
Statistics
Data were analysed using JMP 14 Start Statics (SAS Institute Inc., Cary, NC, USA) and presented as a median (25th percentile, 75th percentile). We evaluated the interactive effect between moulds and SEs sensitisation for clinical outcomes in CRS patients using ANCOVA. A p-value <0.05 was taken to show that, moulds and SEs affected the development of clinical outcomes by interacting with each other. We also evaluated the interactive effect of smoking or other conventional allergens with mould or SE sensitisation for clinical outcomes. We stratified patients according to the presence or absence of sensitisation to allergens (allergen+ and allergen− groups). We adopted the Kruskal–Wallis test followed by Steel–Dwass analysis or Chi-squared test when comparing indices among patients with and without allergen sensitisation and healthy subjects. We also applied the Wilcoxon rank-sum test or Fisher exact test when comparing between allergen+ and allergen− groups. We analysed changes in indices with ESS using the Wilcoxon single rank test. A p-value ≤0.05 was considered significant when α error was set at 5%.
Results
Characteristics of participants are presented in table 2. The proportion of females was lower in participants with mould or SE sensitisation than in healthy subjects. A history of smoking was less frequent in healthy subjects than CRS patients; however, it was unrelated to sensitisation to either moulds or SEs (≥0.10 UA·mL−1) in CRS patients. There were no obvious differences in age and body mass index among the three groups. When confined to patients with CRS, the prevalence of asthma and sensitisation to other allergens were more frequent in patients with mould or SE sensitisation than in those without (table 2). However, other general indices such as previous history of ESS and disease duration were similar between the two groups (table 2).
TABLE 2.
Participant characteristics
| All participants, except where noted | Moulds+ | Moulds− | SEs+ | SEs− | Healthy subjects | p-value# | p-value¶ | |
| Subjects n | 84 | 14 | 42 | 15 | 41 | 28 | ||
| General indices | ||||||||
| Age years | 60 (50, 67) | 62 (44, 66) | 61 (51, 67) | 63 (50, 66) | 60 (51, 68) | 59 (42, 67) | 0.61 | 0.68 |
| Female sex | 35 (42) | 3 (21)§ | 16 (46) | 2 (13)§ | 17 (41) | 16 (57) | 0.06 | 0.01 |
| Body mass index kg·m−2 | 23.3 (20.6, 25.3) | 24.0 (20.1, 26.3) | 23.5 (21.5, 25.1) | 22.7 (19.9, 26.0) | 23.9 (21.9, 25.5) | 21.6 (20.6, 24.3) | 0.59 | 0.43 |
| Smoking history, never | 45 (54) | 7 (50)§ | 16 (38)ƒ | 5 (33)§ | 18 (44)ƒ | 22 (79) | 0.003 | 0.003 |
| Pack-years## | 15.5 (5.3, 30) | 10 (1.6, 26.3) | 20 (7.3, 30) | 17.5 (8.1, 30.4) | 16.3 (5, 30) | 14 (4.9, 32) | 0.50 | 0.97 |
| Disease indices | ||||||||
| Past history of ESS¶¶ | 11 (20) | 3 (21) | 8 (19) | 4(27) | 7 (17) | |||
| Presence of asthma¶¶ | 20 (36) | 10 (71)+ | 10 (24) | 9 (60)+ | 11 (27) | |||
| Duration of sinusitis years¶¶ | 3 (1, 10) | 2.5 (1, 8) | 3.5(1, 10) | 2 (1, 7.5) | 3.5 (1, 10) | |||
| Duration of asthma years++ | 5 (1, 12) | 11 (4, 13) | 3 (1, 6) | 2.5 (1, 6) | 10 (3, 14) | |||
| ICS dose µg·day−1++ | 450 (340, 640) | 520 (380, 680) | 450 (240, 640) | 500 (360, 840) | 400 (320, 640) | |||
| GINA2015 Treatment step n (2/3, 4)++ | 10, 10 | 4, 6 | 6, 4 | 4, 5 | 6, 5 | |||
| Conventional allergen sensitisation¶¶ | ||||||||
Data are presented as median (25th percentile, 75th percentile) or n (%), unless otherwise stated. ESS: endoscopic sinus surgery; ICS: inhaled corticosteroid; mould: Alternaria and Aspergillus; SEs, Staphylococcus enterotoxins A and B; conventional allergens: house dust mite, dog dander, cat, Japanese cedar, mixed Gramineae and mixed weed; H: healthy subjects. #: Compared among moulds+, moulds− and H using Kruskal–Wallis test or Chi-squared test; ¶: compared among SEs+, SEs− and H using Kruskal–Wallis test or Chi-squared test; +: p<0.05 for moulds or SEs+ versus moulds or SEs−; §: p<0.05 for moulds or SEs+ versus H; ƒ: p<0.05 for moulds or SEs− versus H, analysed by Steel–Dwass analysis, Wilcoxon rank-sum test or Fischer's exact test. ##: n=46 (mould+/–: 7/26, SEs+/–: 10/23, H: 6); ¶¶: n=56; ++: n=20 (mould+/−: 10/10, SEs+/–: 9/11).
Interactive effect between moulds and SEs for clinical outcomes in CRS patients
We evaluated whether moulds and SEs have an interactive effect for the development of clinical outcome in CRS patients. Moulds and SEs significantly increased serum periostin levels and sputum eosinophil counts and affected the prevalence of asthma and eosinophilic CRS by interacting with each other (supplementary table E1). This result suggests that moulds and SEs induce Type 2 inflammation systemically and locally by interacting with each other. Indeed, 10 patients (53%) were sensitised to both moulds and SEs. Therefore, we categorised these allergens into the same group (moulds/SEs) to evaluate their relevance to clinical outcomes in CRS patients. However, other conventional allergens did not have an interactive effect with mould/SE allergens on prevalence of asthma and eosinophilic CRS (data not shown).
The impact of sensitisation to moulds/SEs (≥0.10 UA·mL−1) on clinical outcomes
We evaluated the impact of sensitisation to moulds/SEs (≥0.10 UA·mL−1) on clinical outcome (table 3). All Type 2 inflammation-related biomarkers apart from eosinophil counts in sinus tissue were significantly higher in the moulds/SEs+ group than in the moulds/SEs− group (table 3). These values were not significant except for serum total IgE levels upon setting a cut-off value at 0.35 UA·mL−1 (supplementary table E1). Type 2 lower airways inflammation in the moulds/SEs+ group was also more predominant than in healthy subjects, while a significant difference between the moulds/SEs− group and healthy subjects was only seen in sputum periostin levels (table 3). Conventional allergens had an interactive effect with mould/SE allergens by increasing serum periostin levels and sputum eosinophil counts, whereas smoking did not have any interactive effect with mould/SE sensitisation for the development of clinical outcomes in CRS (data not shown).
TABLE 3.
The impact of sensitisation to moulds/Staphylococcus aureus enterotoxins (SEs) (≥0.10 UA·mL−1) on clinical outcomes
| All participants, except where noted | Moulds/SEs+ | Moulds/SEs− | Healthy subjects | p-value# | p-value¶ moulds/SEs+versus− |
p-value¶ moulds/SEs+versus H |
p-value¶ moulds/SEs−versus H |
|
| Subjects n | 84 | 19 | 37 | 28 | ||||
| Systemic biomarkers | ||||||||
| Blood eosinophil count per µL++ | 246 (143, 526) | 449 (191, 737) | 198 (117, 361) | 0.01 | ||||
| Serum total IgE IU·mL−++,†+ | 137 (26, 431) | 513 (389, 1020) | 69 (24, 172) | 44 (11, 357) | 0.004 | <0.0001 | 0.0002 | 0.99 |
| Serum periostin ng·mL−1++,+ | 86 (74, 108) | 112 (93, 159) | 80 (73, 97) | 84 (74, 101) | 0.009 | 0.008 | 0.01 | 0.95 |
| Upper airway markers | ||||||||
| Sinus eosinophils per HPF+ | 66 (20, 168) | 100 (56, 178) | 60 (12, 156) | 0.17 | ||||
| Nasal polyps, presence§ | 38 (68) | 15 (79) | 23 (62) | 0.24 | ||||
| Nasal polyps, eosinophils per HPF§ƒ | 85 (6, 145) | 125 (65, 262) | 33 (1, 97) | 0.007 | ||||
| Eosinophilic CRS, presence++ | 33 (59) | 15 (79) | 18 (49) | 0.04 | ||||
| Lund–Mackay score++ | 12 (7, 16) | 14 (9,17) | 11 (7, 16) | 0.41 | ||||
| SNOT-22 score | 15 (3, 35) | 35 (23, 53) | 22 (12, 41) | 2 (0, 4) | <0.0001 | 0.21 | <0.0001 | <0.0001 |
| Open Essence score | 7 (3, 9) | 4 (0, 8) | 5 (1, 8) | 9 (7, 10) | <0.0001 | 0.98 | 0.002 | <0.0001 |
| Lower airway markers | ||||||||
| AQLQ points## | 5.8 (5.5, 6.7) | 5.9 (5.2, 6.7) | 5.6 (5.5, 6.7) | 0.91 | ||||
| Sputum eosinophils %¶¶ | 0 (0, 3.2) | 5.5 (1.8, 57.5) | 0 (0, 2.8) | 0 (0, 0.4) | 0.0005 | 0.01 | 0.0006 | 0.29 |
| Sputum periostin ng·mL−1¶¶ | 7.1 (1.5, 16.3) | 23.0 (11.7, 42.9) | 9.0 (2.0, 14.9) | 1.6 (0.5, 3.4) | <0.0001 | 0.004 | <0.0001 | 0.001 |
| FeNO ppb | 25.8 (17.7, 38.7) | 41.7 (27.9, 73.8) | 26.2 (18.4, 38.0) | 20.6 (16.1, 26.2) | 0.0003 | 0.04 | 0.0001 | 0.13 |
Data are presented as median (25th percentile, 75th percentile) or n (%), unless otherwise stated. Moulds: Alternaria and Aspergillus; SEs, Staphylococcus enterotoxins A and B; H: healthy subjects; HPF: high-power field; eosinophilic CRS: defined when eosinophils in sinus or NP tissue show ≥70 HPF; SNOT-22: Sinonasal Outcome Test-22; AQLQ: Asthma Quality of Life Questionnaire; FeNO: fractional nitric oxide (could not measure FeNO in one patient because of apparatus failure). #: Analysed by Kruskal–Wallis test; ¶: Analysed by Steel–Dwass analysis, Wilcoxon rank-sum test or Fischer's exact test; +: n=56; §: n=54 (moulds/SEs+/–: 18/36); ƒ: n=38 (moulds/SEs+/–: 15/23); ##: n=20 (moulds/SEs+/–: 13/7); ¶¶: n=65 (moulds/SEs+/–: 15/30, H: 20); ++: n=76 (CRS/H: 56/20).
Sensitisation to moulds/SEs (≥0.10 UA·mL−1) did not affect olfaction, radiological CRS severity, or sinonasal- and asthma-related QoL in CRS patients (table 3).
The impact of sensitisation to moulds/SEs (≥0.10 UA·mL−1) on the longitudinal clinical outcomes
We also evaluated the association of sensitisation to moulds/SEs (≥0.10 UA·mL−1) with changes in biomarkers, radiological CRS severity, olfaction and QoL by ESS intervention (table 4). We followed-up 48 CRS patients for 12 months after ESS (15 with mould/SE (≥0.10 UA·mL−1) sensitisation and 33 without). Among 48 patients, 15 had mould or SE sensitisation at enrolment (n=11 for both, respectively). Although serum mould-IgE levels tested negative in only one patient without asthma, none were sensitised 1 year after ESS. On the other hand, serum SE-IgE levels tested negative in four patients (two with asthma and two without asthma), whereas six (two with asthma and four without asthma) were newly sensitised after surgical intervention. Twenty patients had mould or SE sensitisation 1 year after ESS. We could not find any significant difference between patients whose serum mould/SE-IgE levels were changed by ESS.
TABLE 4.
Longitudinal changes in clinical outcomes in chronic rhinosinusitis (CRS) patients when stratified according to the presence or absence of sensitisation to moulds/Staphylococcus aureus enterotoxins (SEs) (≥0.10 UA/mL)
| Moulds/SEs+ (n=15) | Moulds/SEs− (n=33) | p-value¶ | |||||
| Before | 12 months after | p-value# | Before | 12 months after | p-value# | ||
| Systemic biomarkers | |||||||
| Blood eosinophil count per µL | 449 (262, 653) | 341 (203, 487) | 0.08 | 178 (85, 310) | 177 (80, 317) | 0.42 | 0.10 |
| Serum periostin ng·mL−1 | 112 (93, 159) | 102 (64, 125) | 0.36 | 80 (72, 100) | 86 (69, 111) | 0.80 | 0.16 |
| Sensitisation to moulds | 11 (73) | 10 (67) | 0 (0) | 0 (0) | |||
| Sensitisation to SEs | 11 (73) | 7 (47) | 0 (0) | 6 (18) | |||
| Upper airway markers | |||||||
| Nasal polyps recurrence, presence, n (%)+ | 4 (27) | 3 (9) | 0.18 | ||||
| Lund–Mackay scores | 14 (10, 15) | 9 (6,13) | 0.004 | 11 (7, 16) | 5 (2, 11) | <0.0001 | 0.81 |
| SNOT-22 scores | 31 (22, 51) | 24 (5, 32) | 0.03 | 20 (12, 33) | 10 (5, 20) | 0.0003 | 0.80 |
| Open Essence scores | 4 (0, 8) | 6 (0, 9) | 0.06 | 5 (0, 8) | 6 (4, 8) | 0.08 | 0.67 |
| Lower airway markers | |||||||
| New asthma onset, presence§ | 1 (25) | 6 (24) | >0.99 | ||||
| AQLQ pointsƒ | 5.9 (4.9, 6.7) | 6.4 (6.0, 6.7) | 0.02 | 5.6 (5.5, 6.57) | 6.2 (6.1, 7) | 0.03 | 0.82 |
| Sputum eosinophils %## | 3.3 (0.9, 27.2) | 3.4 (0.5, 12.1) | 0.76 | 0 (0, 2.0) | 1.0 (0, 4.5) | 0.09 | 0.33 |
| Sputum periostin ng·mL−1## | 27.2 (14, 47.8) | 8.1 (1.7, 20.8) | 0.02 | 9.8 (2.3, 15.6) | 3.3 (1.8, 9.0) | 0.004 | 0.24 |
| FeNO ppb¶¶ | 50.4 (33.6, 73.8) | 32.7 (24.6, 54.6) | 0.56 | 26.4 (17.6, 38.0) | 26.3 (15.4, 46.0) | 0.86 | 0.34 |
Data are presented as median (25th percentile, 75th percentile) or n (%), unless otherwise stated. Moulds: Alternaria and Aspergillus; SEs: Staphylococcus enterotoxins A and B; SNOT-22: Sinonasal Outcome Test-22; AQLQ: Asthma Quality of Life Questionnaire; FeNO: fractional nitric oxide. #: Analysed by Wilcoxon single rank test; ¶: Analysed by Wilcoxon rank-sum test or Fischer's exact test; +: n=34 (moulds/SEs+/–: 12/22; §: n=30 (moulds/SEs+/–: 5/25); ƒ: n=18 (moulds/SEs +/–: 10/8; ##: n=39 (moulds/SEs +/–: 13/26); ¶¶: n=47 (moulds/SEs +/–: 14/33).
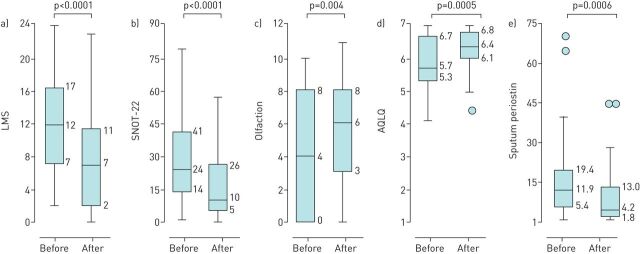
ESS could improve radiological CRS severity, olfaction and sinonasal- and asthma-related QoL in CRS patients (figure 1, table 4). Moreover, levels of sputum periostin also declined significantly with ESS intervention (figure 1, table 4). When patients were divided into those with presence or absence of sensitisation to moulds/SEs (≥0.10 UA·mL−1), however, changes in biomarkers, radiological CRS severity and QoL with ESS intervention were similar between the two groups (table 4). The proportion experiencing recurrence of NPs or new asthma onset over the 12 months following ESS was also not related to mould/SE sensitisation (≥0.10 UA·mL−1) at enrolment.
FIGURE 1.
The efficacy of endoscopic sinus surgery in chronic rhinosinusitis (CRS) patients. Box and whisker plots show the change in a) the Lund–Mackay score (LMS), b) the Sinonasal Outcome Test-22 (SNOT-22) score, c) olfactory score, d) the Asthma Quality of Life Questionnaire (AQLQ) score and e) levels of sputum periostin with endoscopic sinus surgery. The horizontal line in the box interior shows the median values of indices. The length of the box represents the distance between the 25th and 75th percentiles. The circles represent outliers if data were above upper whiskers or below lower whiskers. before: before surgery; 12 m after: 12 months after surgery.
Discussion
To the best of our knowledge, this is the first study to demonstrate that levels of moulds/SEs-IgE of ≥0.10 UA·mL−1 rather than ≥0.35 UA·mL−1 may be more useful as biomarkers that reflect systemic and local Type 2 inflammation and asthma prevalence in CRS patients. Moulds and SEs contributed to the development of clinical outcomes in CRS patients by interacting with each other. However, neither mould nor SE sensitisation (≥0.10 UA·mL−1) affected clinical outcomes and Type 2 inflammation-related biomarkers in ESS.
Specific IgE titres are measured using fluorescence enzyme immunoassay. A cut-off value for this assay was set at 0.35 UA·mL−1 following the discovery of a strong correlation with the radioallergosorbent test in 1990 [4]. The detection limits of specific IgE titres have improved over the last two decades from 0.35 UA·mL−1 to 0.10 UA·mL−1. We have demonstrated the importance of a lower cut-off value of moulds/SEs-IgE (≥0.10 UA·mL−1) on the development of Type 2-predominant inflammation in nose and lower airways. The sensitisation rate to moulds and SEs in asthmatic patients rose from 10–30% to 45–50% when the cut-off value was set at 0.10 UA·mL−1 compared with 0.35 UA·mL−1 (table 1). However, the change in cut-off value from 0.35 UA·mL−1 to 0.10 UA·mL−1 hardly affected the frequency of patients who have sensitisation to other allergens such as house dust mite, cat and pollen (table 1). Moulds and SEs might induce Type 2-predominant airway inflammation to a lesser degree than more conventional allergens. Recent studies have reported a significant association of SE sensitisation determined by a threshold of 0.10 UA·mL−1 with the prevalence [2] and severity of asthma [7]. Thus, a lower cut-off value of 0.10 UA·mL−1 in moulds and SEs would be meaningful in the pathophysiology of allergic diseases such as asthma and CRS. Moulds and SEs may be common allergens in patients with asthma as well as more conventional allergens.
Alternaria and Aspergillus are classified as moulds, whereas S. aureus are bacteria. Both moulds and S. aureus colonise in particular the NPs of CRS patients [24–26]. We found that moulds and SEs have an interactive effect on the development of clinical outcomes and Type 2 inflammation in CRS patients; however, different mechanisms are associated with the development of Type 2-predominant inflammation. Although enterotoxins produced by S. aureus are well-known inducers of Type 2-related cytokines and eosinophilic inflammation in both nose and lower airways by acting as superantigens [27, 28], S. aureus can directly induce interleukin-33 and thymic stromal lymphoprotein production via airway epithelium through binding to Toll-like receptor 2 [29]. Alternaria alternata and Aspergillus fumigatus have very similar epitopes [30]. This suggests that their sensitisation may elicit a similar immune response. Protease produced by Alternaria and Aspergillus can evoke release of Type 2-related cytokines [31], airway hyperresponsiveness [32] and activation of eosinophils [33]. Furthermore, the humoral immune response to moulds is also thought to be associated with increased production of Type 2-related cytokines [34]. A lesser degree of sensitisation to moulds and SEs may be related to induction of the Type 2 inflammatory cascade in the nose and lower airways, as stated above.
Airways are anatomically continuous from the nose to the lower airways. The sino/nasobronchial reflex is thought to be associated with the development of both upper and lower airway inflammation. Inhalation of allergens through the nose and lower airways can induce Type 2 inflammation of the lower airways and nose, respectively [35, 36]. Nasal colonisation by S. aureus is also related to the development of eosinophilic NPs (i.e. eosinophil cationic protein production) [25] and asthma prevalence [37]. This evidence supports the presence of an interaction between upper and lower airway inflammation by neuronal reflex. However, all Type 2 inflammation-related biomarkers of lower airways except for sputum periostin did not decline after ESS. Furthermore, the presence of moulds/SEs did not improve clinical outcomes or change levels of Type 2 inflammation-related biomarkers 1 year after ESS. Although we did not evaluate levels of moulds/SEs-IgE in NPs, it remains unclear whether ESS produces changes in levels of serum moulds/SEs-IgE. These results indicate that systemic allergen sensitisation induces Type 2 inflammation in the nose and lower airways separately. Neuronal reflex through local allergen sensitisation may not be the major cascade in the development of Type 2 airway inflammation throughout the airways. Indeed, treatment of CRS does not always improve asthma outcomes [3]. Furthermore, most Type 2 inflammation-related biomarkers declined in number after ESS in the moulds/SEs+ group. Sample size may not be sufficient to clarify the impact of moulds/SEs on longitudinal changes in clinical outcomes and Type 2 inflammation-related biomarkers in CRS patients who underwent ESS. Further larger cohort studies are necessary to elucidate how mould/SE sensitisation affects clinical symptoms and inflammation of the nose and lower airways after ESS.
There are some limitations to the present study. First, we did not evaluate local levels of IgE against moulds/SEs in either sputum or sinus samples due to the lack of storage samples. Therefore, the association between lower threshold (≥0.10 UA·mL−1) of serum IgE titres and local sensitisation to allergens remains to be elucidated. However, the present study provides clinicians with data for the clinical importance of sensitisation to moulds/SEs (≥0.10 UA·mL−1) as indicators of the presence of Type 2 inflammation and comorbid asthma in CRS patients. Both otorhinolaryngology and respirology specialists could potentially identify comorbid asthma or eosinophilic CRS by evaluating specific IgE against moulds/SEs. Second, our cohort was enriched with the more severe CRS patients who required ESS. Therefore, the present findings might not be applicable to patients with mild/moderate CRS or those responsive to treatment, who are underrepresented in this study. Therefore, we need to compare the impact of mould/SEs sensitisation (≥0.10 UA·mL−1) on Type 2 inflammation-related biomarkers and clinical outcomes between severe and non-severe CRS patients in future studies. Finally, follow-up of patients ceased 12 months after ESS. Therefore, the association of sensitisation to moulds/SEs (≥0.10 UA·mL−1) with long-term prognosis for asthma and CRS after ESS remains unclear. Further longitudinal studies are necessary to clarify the clinical implications of sensitisation to moulds/SEs determined by ≥0.10 UA·mL−1 in terms of the pathophysiology of asthma and sinusitis.
In conclusion, moulds/SEs are important allergens in the development of comorbid asthma and eosinophilic CRS and in inducing Type 2 inflammation in CRS patients. A lower threshold value of these allergens (≥0.10 UA·mL−1) would be meaningful to evaluate Type 2 inflammation and clinical outcomes in CRS patients. Further studies are warranted to clarify the clinical implications of mould/SE sensitisation as determined by a threshold of ≥0.10 UA·mL−1 in the pathophysiology of asthma and CRS.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00265-2020.SUPPLEMENT (206.5KB, pdf)
Footnotes
This article has supplementary material available from openres.ersjournals.com.
This study is registered in at https://www.umin.ac.jp/ctr/ with identifier number UMIN000018672. We cannot disclose the data sheet of this study.
Author contributions: Y. Kanemitsu established the conception of the whole study, and contributed to the performance of diagnostic tests, the collection of data, the recruitment of patients, disease diagnosis and management, the acquisition and interpretation of data, and drafting the manuscript. K. Fukumitsu, R. Kurokawa and N. Takeda contributed to the performance of diagnostic tests, the collection of data, and the acquisition and interpretation of data. J.M. Yap contributed to the acquisition and interpretation of data, as well as proofreading of the manuscript in English. H. Nishiyama, S. Fukuda, T. Uemura, T. Tajiri, H. Ohkubo, K. Maeno, Y. Ito and T. Oguri contributed to the diagnostic tests, the collection of data and management of patients. J. Ono and K. Izuhara carried out the measurement of periostin. A. Masaki made specimens and assessed the infiltration of eosinophils in upper airway tissues. Y. Ozawa contributed to assessing the radiological severity of CRS. M. Takemura contributed to the recruitment of patients, disease diagnosis and management, and revision of the manuscript. M. Suzuki and A. Niimi contributed to the recruitment of patients, disease diagnosis and management, interpretation of data, and revision of the manuscript.
Conflict of interest: Y. Kanemitsu reports research grants from Novartis Pharma and Tanabe Mitsubishi Pharma for the submitted work, and grants from MSD and Kyowa-Kirin corporations outside the submitted work.
Conflict of interest: K. Fukumitsu has received a grant from Novartis for 500 000 JPY and GSK for 2 000 000 JPY outside the submitted work.
Conflict of interest: R. Kurokawa has nothing to disclose.
Conflict of interest: N. Takeda has nothing to disclose.
Conflict of interest: Y. Ozawa has nothing to disclose.
Conflict of interest: A. Masaki has nothing to disclose.
Conflict of interest: J. Ono is an employee of Shino-test corporation.
Conflict of interest: K. Izuhara reports research grants from Shino-Test Corporation for the submitted work.
Conflict of interest: J-M. Yap has nothing to disclose.
Conflict of interest: H. Nishiyama has nothing to disclose.
Conflict of interest: S. Fukuda reports speaker honoraria from AstraZeneca and Eli Lilly Japan outside the submitted work.
Conflict of interest: T. Uemura has nothing to disclose.
Conflict of interest: T. Tajiri has nothing to disclose.
Conflict of interest: H Ohkubo has received a research grant from Boehringer Ingelheim outside the submitted work.
Conflict of interest: K. Maeno reports speaker honoraria from Pfizer and Chugai Pharmaceutical outside the submitted work.
Conflict of interest: Y. Ito has nothing to disclose.
Conflict of interest: T. Oguri has received a research grant from Ono outside the submitted work.
Conflict of interest: M. Takemura has received a research grant from Pfizer outside the submitted work.
Conflict of interest: M. Suzuki reports a research grant from Kobayashi Foundation for the submitted work.
Conflict of interest: A. Niimi reports personal fees from Astellas, AstraZeneca, Kyorin, GSK, MSD, Shionogi and Boehringer Ingelheim, and grants from Astellas outside the submitted work.
Support statement: This study was supported, in part, by research grants from Novartis Pharma, Mitsubishi Tanabe Pharma and the Kobayashi Foundation. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Antó JM, Sunyer J, Basagaña X, et al. Risk factors of new-onset asthma in adults: a population-based international cohort study. Allergy 2010; 65: 1021–1030. doi: 10.1111/j.1398-9995.2009.02301.x [DOI] [PubMed] [Google Scholar]
- 2.Tomassen P, Jarvis D, Newson R, et al. Staphylococcus aureus enterotoxin-specific IgE is associated with asthma in the general population: a GA(2)LEN study. Allergy 2013; 68: 1289–1297. doi: 10.1111/all.12230 [DOI] [PubMed] [Google Scholar]
- 3.Rosati MG, Peters AT. Relationships among allergic rhinitis, asthma, and chronic rhinosinusitis. Am J Rhinol Allergy 2016; 30: 44–47. doi: 10.2500/ajra.2016.30.4252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ewan PW, Coote D. Evaluation of a capsulated hydrophilic carrier polymer (the ImmunoCAP) for measurement of specific IgE antibodies. Allergy 1990; 45: 22–29. doi: 10.1111/j.1398-9995.1990.tb01080.x [DOI] [PubMed] [Google Scholar]
- 5.Matsui EC, Sampson HA, Bahnson HT, et al. Allergen-specific IgE as a biomarker of exposure plus sensitization in inner-city adolescents with asthma. Allergy 2010; 65: 1414–1422. doi: 10.1111/j.1398-9995.2010.02412.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bachert C, Gevaert P, Howarth P, et al. IgE to Staphylococcus aureus enterotoxins in serum is related to severity of asthma. J Allergy Clin Immunol 2003; 111: 1131–1132. doi: 10.1016/S0091-6749(03)70044-X [DOI] [PubMed] [Google Scholar]
- 7.Bachert C, van Steen K, Zhang N, et al. Specific IgE against Staphylococcus aureus enterotoxins: an independent risk factor for asthma. J Allergy Clin Immunol 2012; 130: 376–381.e378. doi: 10.1016/j.jaci.2012.05.012 [DOI] [PubMed] [Google Scholar]
- 8.Song WJ, Sintobin I, Sohn KH, et al. Staphylococcal enterotoxin IgE sensitization in late-onset severe eosinophilic asthma in the elderly. Clin Exp Allergy 2016; 46: 411–421. doi: 10.1111/cea.12652 [DOI] [PubMed] [Google Scholar]
- 9.Tanaka A, Fujiwara A, Uchida Y, et al. Evaluation of the association between sensitization to common inhalant fungi and poor asthma control. Ann Allergy Asthma Immunol 2016; 117: 163–168.e161. doi: 10.1016/j.anai.2016.06.001 [DOI] [PubMed] [Google Scholar]
- 10.Fairs A, Agbetile J, Hargadon B, et al. IgE sensitization to Aspergillus fumigatus is associated with reduced lung function in asthma. Am J Respir Crit Care Med 2010; 182: 1362–1368. doi: 10.1164/rccm.201001-0087OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gevaert P, Holtappels G, Johansson SG, et al. Organization of secondary lymphoid tissue and local IgE formation to Staphylococcus aureus enterotoxins in nasal polyp tissue. Allergy 2005; 60: 71–79. doi: 10.1111/j.1398-9995.2004.00621.x [DOI] [PubMed] [Google Scholar]
- 12.Matsuwaki Y, Uno K, Okushi T, et al. Total and antigen- (fungi, mites and staphylococcal enterotoxins) specific IgEs in nasal polyps is related to local eosinophilic inflammation. Int Arch Allergy Immunol 2013; 161 Suppl 2: 147–153. doi: 10.1159/000350387 [DOI] [PubMed] [Google Scholar]
- 13.Manise M, Holtappels G, Van Crombruggen K, et al. Sputum IgE and cytokines in asthma: relationship with sputum cellular profile. PLoS One 2013; 8: e58388. doi: 10.1371/journal.pone.0058388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zureik M, Neukirch C, Leynaert B, et al. Sensitisation to airborne moulds and severity of asthma: cross sectional study from European Community respiratory health survey. BMJ 2002; 325: 411–414. doi: 10.1136/bmj.325.7361.411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knutsen AP, Bush RK, Demain JG, et al. Fungi and allergic lower respiratory tract diseases. J Allergy Clin Immunol 2012; 129: 280–291; quiz 292–283. doi: 10.1016/j.jaci.2011.12.970 [DOI] [PubMed] [Google Scholar]
- 16.Kanemitsu Y, Suzuki M, Fukumitsu K, et al. A novel pathophysiologic link between upper and lower airways in patients with chronic rhinosinusitis: association of sputum periostin levels with upper airway inflammation and olfactory function. World Allergy Organ J 2020; 13: 100094. doi: 10.1016/j.waojou.2019.100094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Global Initiative for Asthma. 2015 GINA Report, Global Strategy for Asthma Management and Prevention. https://ginasthma.org Date last accessed:. Date last updated:.
- 18.Fokkens WJ, Lund VJ, Mullol J, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinology 2012; 23: Suppl., 1–298. [PubMed] [Google Scholar]
- 19.Tokunaga T, Sakashita M, Haruna T, et al. Novel scoring system and algorithm for classifying chronic rhinosinusitis: the JESREC Study. Allergy 2015; 70: 995–1003. doi: 10.1111/all.12644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomassen P, Vandeplas G, Van Zele T, et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J Allergy Clin Immunol 2016; 137: 1449–1456.e1444. doi: 10.1016/j.jaci.2015.12.1324 [DOI] [PubMed] [Google Scholar]
- 21.Asano T, Takemura M, Kanemitsu Y, et al. Combined measurements of fractional exhaled nitric oxide and nasal nitric oxide levels for assessing upper airway diseases in asthmatic patients. J Asthma 2018; 55: 300–309. doi: 10.1080/02770903.2017.1332203 [DOI] [PubMed] [Google Scholar]
- 22.Kennedy JL, Hubbard MA, Huyett P, et al. Sino-nasal outcome test (SNOT-22): a predictor of postsurgical improvement in patients with chronic sinusitis. Ann Allergy Asthma Immunol 2013; 111: 246–251.e242. doi: 10.1016/j.anai.2013.06.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Juniper EF, Buist AS, Cox FM, et al. Validation of a standardized version of the Asthma Quality of Life Questionnaire. Chest 1999; 115: 1265–1270. doi: 10.1378/chest.115.5.1265 [DOI] [PubMed] [Google Scholar]
- 24.Gawlik R, Czecior E, Jarząb J, et al. Frequency of IgE-dependent hypersensitivity to moulds in patients with chronic rhinosinusitis with polyps. Postepy Dermatol Alergol 2014; 31: 159–163. doi: 10.5114/pdia.2014.40976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Zele T, Gevaert P, Watelet JB, et al. Staphylococcus aureus colonization and IgE antibody formation to enterotoxins is increased in nasal polyposis. J Allergy Clin Immunol 2004; 114: 981–983. doi: 10.1016/j.jaci.2004.07.013 [DOI] [PubMed] [Google Scholar]
- 26.Corriveau MN, Zhang N, Holtappels G, et al. Detection of Staphylococcus aureus in nasal tissue with peptide nucleic acid-fluorescence in situ hybridization. Am J Rhinol Allergy 2009; 23: 461–465. doi: 10.2500/ajra.2009.23.3367 [DOI] [PubMed] [Google Scholar]
- 27.Hellings PW, Hens G, Meyts I, et al. Aggravation of bronchial eosinophilia in mice by nasal and bronchial exposure to Staphylococcus aureus enterotoxin B. Clin Exp Allergy 2006; 36: 1063–1071. doi: 10.1111/j.1365-2222.2006.02527.x [DOI] [PubMed] [Google Scholar]
- 28.Huvenne W, Hellings PW, Bachert C. Role of staphylococcal superantigens in airway disease. Int Arch Allergy Immunol 2013; 161: 304–314. doi: 10.1159/000350329 [DOI] [PubMed] [Google Scholar]
- 29.Lan F, Zhang N, Holtappels G, et al. Staphylococcus aureus induces a mucosal type 2 immune response via epithelial cell-derived cytokines. Am J Respir Crit Care Med 2018; 198: 452–463. doi: 10.1164/rccm.201710-2112OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rick EM, Woolnough K, Pashley CH, et al. Allergic fungal airway disease. J Investig Allergol Clin Immunol 2016; 26: 344–354. doi: 10.18176/jiaci.0122 [DOI] [PubMed] [Google Scholar]
- 31.Porter P, Susarla SC, Polikepahad S, et al. Link between allergic asthma and airway mucosal infection suggested by proteinase-secreting household fungi. Mucosal Immunol 2009; 2: 504–517. doi: 10.1038/mi.2009.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balenga NA, Klichinsky M, Xie Z, et al. A fungal protease allergen provokes airway hyper-responsiveness in asthma. Nat Commun 2015; 6: 6763. doi: 10.1038/ncomms7763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsuwaki Y, Wada K, Moriyama H, et al. Human eosinophil innate response to Alternaria fungus through protease-activated receptor-2. Int Arch Allergy Immunol 2011; 155: Suppl 1, 123–128. doi: 10.1159/000327498 [DOI] [PubMed] [Google Scholar]
- 34.Shin SH, Ponikau JU, Sherris DA, et al. Chronic rhinosinusitis: an enhanced immune response to ubiquitous airborne fungi. J Allergy Clin Immunol 2004; 114: 1369–1375. doi: 10.1016/j.jaci.2004.08.012 [DOI] [PubMed] [Google Scholar]
- 35.Braunstahl GJ, Overbeek SE, Kleinjan A, et al. Nasal allergen provocation induces adhesion molecule expression and tissue eosinophilia in upper and lower airways. J Allergy Clin Immunol 2001; 107: 469–476. doi: 10.1067/mai.2001.113046 [DOI] [PubMed] [Google Scholar]
- 36.Braunstahl GJ, Kleinjan A, Overbeek SE, et al. Segmental bronchial provocation induces nasal inflammation in allergic rhinitis patients. Am J Respir Crit Care Med 2000; 161: 2051–2057. doi: 10.1164/ajrccm.161.6.9906121 [DOI] [PubMed] [Google Scholar]
- 37.Kim YC, Won HK, Lee JW, et al. Staphylococcus aureus nasal colonization and asthma in adults: systematic review and meta-analysis. J Allergy Clin Immunol Pract 2019; 7: 606–615.e609. doi: 10.1016/j.jaip.2018.08.020 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00265-2020.SUPPLEMENT (206.5KB, pdf)