Abstract
Sphingosine 1-phosphate (S1P) is a bioactive lipid mediator generated when a cell membrane or its components are damaged by various factors. S1P regulates diverse cell activities via S1P receptors (S1PRs). Keratinocytes express S1PR1-5. Though it is known that S1PRs control keratinocyte differentiation, apoptosis, and wound healing, S1PR functions in keratinocyte infections have not been fully elucidated. We propose that the S1P-S1PR axis in keratinocytes works as a biosensor for bacterial invasion. Indeed, in human impetigo infection, we found high epidermal expression of S1PR1 and 2 in the skin. Furthermore, in normal human epidermal keratinocytes (NHEKs) in vitro, treatment with S. aureus bacterial supernatant not only induced S1P production, but also increased the transcription of S1PR2, confirming our in vivo observation, as well as increased the levels of TNFα, IL36γ, IL6, and IL8 mRNAs. However, direct treatment of NHEKs with S1P increased the expressions of IL36γ, TNFα, and IL8, but not IL6. In both S1P- and S. aureus bacterial supernatant-treated NHEKs, S1PR1 knockdown reduced IL36γ, TNFα, and IL8 transcription, while the S1PR2 antagonist JTE013 blocked the secretion of these cytokines. Overall, we have proven that during infections, keratinocytes communicate damage by using S1P release and tight control of S1PR1 and 2.
INTRODUCTION
Sphingosine 1-phosphate (S1P) is a bioactive lipid mediator (Nema et al., 2016, Park et al., 2016) to regulate a variety of cell activities including cell growth, differentiation, apoptosis, migration, inflammation, metabolism, and angiogenesis (Coant et al., 2017, Nema et al., 2016). As an extracellular signal, S1P can affect many processes through its G-protein coupled receptors, S1PRs (Rivera et al., 2008). In the epidermis, keratinocytes express S1PR1-5 (Allende et al., 2013, Vogler et al., 2003), with S1PR5 being the most abundant, and S1PR1 and 2 being the second and third most-expressed (Supplementary Figure S1).
S1P is the product of the metabolism of sphingomyelin, a component of the cell wall. Sphingomyelinase mediates ceramide synthesis from sphingomyelin. Ceramide is then further metabolized by ceramidase to produce sphingosine, and finally, two sphingosine kinases (SPHK1 and 2) generate S1P (Rivera et al., 2008, Skoura and Hla, 2009, Thieme et al., 2017. Interestingly, some bacteria, such as Staphylococcus aureus (S. aureus) and Pseudomonas aeruginosa, have sphingomyelinase and ceramidase, respectively, as part of their toxins (Oizumi et al., 2014, Salgado-Pabon et al., 2014). Therefore, we hypothesized that during skin infection, the activity of bacterial toxins in the epidermis results in the generation of S1P, which, in this context, signals bacterial invasion and acts as an alarmin.
In the epidermis, it is known that SIP is involved in keratinocyte differentiation, growth arrest, apoptosis, and wound healing (Amano et al., 2004, Lichte et al., 2008, Schmitz et al., 2012, Schuppel et al., 2008). It has also been shown that S1P can play a role in the pathogenesis of some skin disorders such as allergic contact dermatitis, psoriasis, and systemic sclerosis (Castelino and Varga, 2014, Reines et al., 2009, Schaper et al., 2013, Thieme et al., 2017). However, even though S1P-mediated cathelicidin antimicrobial peptide (CAMP) production (Park et al., 2013, Park et al., 2016) and increases in TNFα and IL8 mRNA expressions (Oizumi et al., 2014) have already been shown, the role of S1P and S1PRs in host defense against pathogens in keratinocytes has not been fully elucidated.
The aim of this study was to define the role of S1P and S1PRs in keratinocytes during bacterial skin infection. We have determined that the expressions of S1PR1 and 2 change in response to S. aureus invasion and that S1P induces keratinocyte proinflammatory cytokine expression and secretion that is precisely controlled by S1PR1 and 2.
RESULTS
S1PR2 is increased in impetigo lesional skin and S. aureus bacterial supernatant-treated NHEKs
To assess how S. aureus affects S1PR expression, we incubated primary normal human epidermal keratinocytes (NHEKs) with S. aureus sa113 bacterial supernatant or 3% tryptic soy broth (TSB), as a control, for 4hrs and measured the mRNA expressions of the different S1PRs. In NHEKs, S1PR2 expression was found to be significantly greater in cells incubated with sa113 supernatant than in control cells incubated with TSB. However, while the expressions of the other S1PR isoforms were increased after incubation with sa113 supernatant, they did not reach significance (Figure 1a). Next, to investigate how S. aureus affects the expressions of S1PR1 and 2 in “in vivo” human skin, we performed immunofluorescent staining for these receptors in normal and impetigo lesional skin sections (Figure 1b, c). In normal epidermis, both S1PR1 and 2 showed diffuse cytoplasmic expression in the granular layer and stratum corneum (Figure 1b, Supplementary Figure S2a), but in the basal and squamous layers, they were distributed in a perinuclear pattern (Supplementary Figure S2a, b). In contrast, both S1PR1 and 2 showed increased diffuse cytoplasmic expression in the whole epidermis of impetigo lesional skin (Figure 1c). To confirm these findings, we performed immunofluorescent staining for S1PR1 and 2 in NHEKs stimulated with sa113 supernatant in vitro. NHEKs incubated with TSB for 24 hrs showed perinuclear expression of S1PR1 and 2; however, cells incubated with sa113 supernatant for 24 hrs showed diffuse cytoplasmic expression of these receptors (Figure 1d), similar to the “in vivo” findings (Figure 1c). Ca2+-differentiated NHEKs also showed diffuse cytoplasmic expression (Supplementary Figure S2c) of S1PR1 and 2, similar to the pattern seen in the granular layer and stratum corneum in normal epidermis. Taken together, these data suggest that the expression of S1PRs, particularly S1PR1 and 2, on keratinocytes are increased and mobilized to the surface in response to S. aureus invasion.
Figure 1. The expression and distribution of sphingosine 1-phosphate receptors (S1PRs) changes in impetigo skin and S. aureus bacterial supernatant-treated normal human epidermal keratinocytes (NHEKs).
(a) S1PR1-5 mRNA expressions in NHEKs incubated with 50 μl/ml TSB or supernatant derived from S. aureus sa113 for 4hrs. Data is shown as the fold of TSB control. (b-d) S1PR1 and 2 immunostaining in normal (b), impetigo lesional (c) skin sections, as well as NHEKs incubated with 50 μl/ml TSB or sa113 supernatant for 24hrs (d). Scale bars: (b, c) 50 μm, (d) 10 μm
S. aureus bacterial supernatant increases SPHK activity and induces S1P production in NHEKs
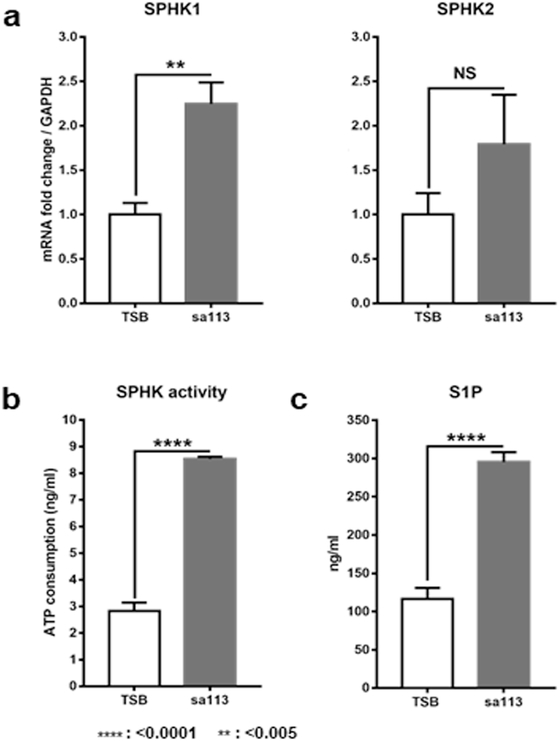
To assess whether S. aureus induces keratinocyte production of S1P, we measured the expression and activities of SPHKs, the rate-limiting enzymes for S1P production (Rivera et al., 2008, Skoura and Hla, 2009). After a 4 hr incubation with sa113 supernatant, both SPHK1 and 2 mRNA expressions were increased in NHEKs, with only the level of SPHK1 mRNA being significantly greater than in TSB-treated control cells (Figure 2a). In addition to SPHK mRNA levels, we also measured SPHK activity in sa113 supernatant-treated NHEKs. Our results showed that sa113 supernatant-treated NHEK lysates had significantly higher SPHK activity than lysates from control TSB-treated cells (Figure 2b). As expected from the increased SPHK activity, ELISA assays for S1P confirmed significantly higher levels of secreted S1P from NHEKs incubated with sa113 supernatant for 48 hrs than TSB-treated cells (Figure 2c).
Figure 2. S. aureus bacterial supernatant increases sphingosine kinase (SPHK) activity and induce S1P production in NHEKs.
(a) mRNA expression levels of SPHK1 and 2 in NHEKs incubated with 50 μl/ml of TSB or sa113 supernatant for 4hrs. Data is shown as the fold of TSB control. (b) ATP consumptions indicating SPHK activity in NHEKs incubated with 50 μl/ml TSB or sa113 supernatant for 24hrs. (c) S1P secreted into culture medium of NHEKs incubated with 50 μl/ml of TSB or sa113 supernatant for 48hrs.
S. aureus induces NHEK production of TNFα, IL6, IL8, and IL36β cytokines
To assess the inflammatory response of NHEKs to bacterial products, we measured the expressions of the proinflammatory cytokines TNFα, IL6, and IL8 following treatment with sa113 supernatant. Our results showed that treatment of NHEKs with sa113 supernatant resulted in the increased expressions of these proinflammatory cytokines (Supplementary Figure S3a). The secretion of TNFα and IL8 from NHEKs was also increased after sa113 supernatant treatment, in accordance with the observed increases in mRNA expression (Supplementary Figure S3b) and previous reports (Aufiero et al., 2007, Krishna and Miller, 2012). Since the IL36 cytokine family has recently been reported to be involved in the response to S. aureus (Liu et al., 2017, Nakagawa et al., 2017, Williams et al., 2017), we also investigated the expression levels of the IL36 family of cytokines. These results showed that only IL36γ mRNA expression was increased in NHEKs after incubation with sa113 supernatant (Supplementary Figure S3a), with a corresponding increase in IL36γ protein secretion (Supplementary Figure S3b). Since Nakagawa et al. (Nakagawa et al., 2017) and Liu et al. (Liu et al., 2017) proved the importance of mouse keratinocyte IL36α production in signaling against S. aureus (USA300 LAC) invasion in the skin, we also tested whether two strains of S. aureus, sa113 and USA300 LAC, supernatant could increase IL36α mRNA levels in human keratinocytes. Unlike mouse keratinocytes, we could not find any detectable IL36α mRNA expression in NHEKs incubated with either TSB, sa113, or USA300 LAC supernatant (Supplementary Figure S3a, USA300 LAC negative data not shown). These data suggest that in NHEKs, IL36γ is the most important member of the IL36 family of cytokines for the anti-S. aureus response.
S1P- S1PR1 and 2 activation controls the expression and secretion of proinflammatory cytokines in NHEKs
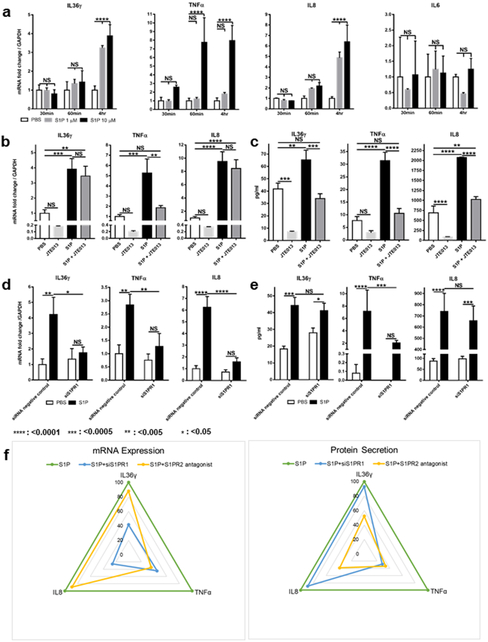
Since S. aureus supernatant induces proinflammatory cytokine synthesis in NHEKs, we examined whether S1P and its receptors are involved in this mechanism of cytokine production. First, we incubated NHEKs with PBS, 1 μM S1P, or 10μM S1P at different time points and found that the expressions of IL36γ, TNFα, and IL8, but not IL6, mRNAs were significantly increased in S1P-treated NHEKs, compared to cells treated with PBS. (Figure 3a). We also observed increases in IL36γ, TNFα, and IL8 secretion from NHEKs following 1μM S1P treatment (Supplementary Figure S4), even though this lower concentration of S1P did not induce a significant increase in TNFα mRNA expression (Figure 3a).
Figure 3. S1P-S1PR1 and 2 controls NHEK cytokine synthesis.
(a) IL36γ, TNFα, IL6, and IL8 mRNA levels were measured in NHEKs treated with PBS, 1 or 10 μM S1P Data is shown as the fold of PBS control at each time point. (b-e) IL36γ, TNFα, and IL8 mRNA levels (b, d) and protein secretion (c, e) in NHEKs incubated with PBS or 10 μM S1P for 4hrs (b, d) and 24hrs (c, e). Before the treatment, NHEK were treated with 10 μM JTE013 (b, c), negative control siRNA or siS1PR1 (d, e). Transcription data (b, d) is shown as the fold of PBS control without inhibitors. (f) Radar charts summarizing the percentage of each cytokine’s mRNA expression and secretion with or without siS1PR1 or JTE013.
Since our data indicates that keratinocyte S1PR1 and 2 are involved in the NHEK response to S. aureus (Figure 1a-d), we next investigated the function of NHEK S1PR2 on cytokine production in response to S1P using the S1PR2 antagonist JTE013. Our results showed that treating NHEKs with 10μM JTE013 blocked S1P-induced TNFα, but not IL36γ or IL8 mRNA expression (Figure 3b). However, the inhibitor was still able to block the S1P-induced secretion of all three cytokines (Figure 3c). For better analysis of S1PR1 function in NHEKs, we transfected cells with siRNA for S1PR1 before S1P treatment. siS1PR1-transfected NHEKs showed reduced S1P-induced IL36γ, TNFα, and IL8 mRNA expression (Figure 3d); however, when we analyzed cytokine secretion, we found that only TNFα was affected by the loss of S1PR1 (Figure 3e). These data suggest that both S1PR1 and 2 are involved in TNFα expression and secretion. However, for IL36γ and IL8 synthesis, S1PR1 and 2 fulfill different roles; namely, S1PR1 only controls IL36γ and IL8 transcription, while S1PR2 only controls IL36γ and IL8 secretion, as summarized in Figure 3f.
S1P production is necessary for S. aureus bacterial supernatant-induced NHEK TNFα, IL8, and IL36γ synthesis
To verify whether S1P production is actually involved in sa113 supernatant-induced cytokine synthesis in NHEKs, we transfected the cells with siRNA for SPHK1 and blocked SPHK2 with its inhibitor, ABC294640. SPHK1 knockdown combined with SPHK2 block resulted in significantly reduced levels of TNFα, IL8, and IL36γ transcription (Figure 4a) and protein secretion (Figure 4b) following sa113 supernatant treatment, while IL6 transcription was unaffected by the reduced S1P production (Figure 4a). These data suggest that for keratinocytes, SPHK1- and 2-mediated S1P production contributes to TNFα, IL8, and IL36γ synthesis in response to S. aureus stimulation.
Figure 4. SPHK1 knockdown and SPHK2 inhibition reduce S. aureus bacterial supernatant-induced TNFα, IL8 and IL36γ cytokine synthesis in NHEKs.
(a, b) IL36γ, TNFα, IL8, and IL6 transcription (a) and IL36γ, TNFα and IL8 protein secretion (b) was measured in NHEKs after incubation with 50 μl/ml TSB or sa113 supernatant for 4hrs (a) and 24hrs (b). Before stimulation, cells were treated with control or SPHK1 siRNAs with or without 1 μM ABC294640, an SPHK2 inhibitor, as indicated. mRNA expression data (a) is shown as the fold of TSB control without inhibitors.
S1PR1 and 2 inhibition affects S. aureus bacterial supernatant-induced transcription and secretion, respectively, of IL36γ, TNFα, and IL8 in NHEKs.
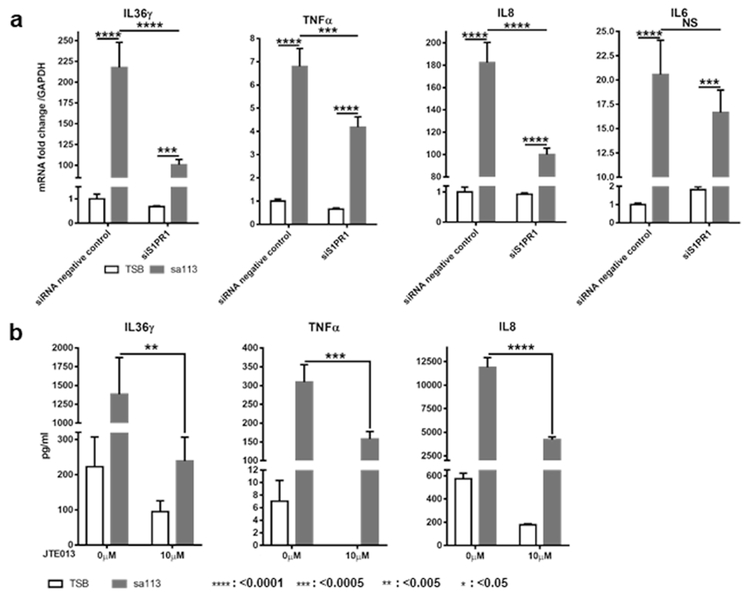
Finally, to confirm that the different roles of keratinocyte S1PR1 and 2 in the cytokine response to S1P (Figure 3b-f) are consistent with those observed during the response to bacteria, we investigated how S1PR1 knockdown and S1PR2 block affect S. aureus-induced IL36γ, TNFα, and IL8 synthesis. Following sa113 supernatant stimulation, siS1PR1-transfected NHEKs showed significantly reduced levels of IL36γ, TNFα, and IL8 mRNAs (Figure 5a). Furthermore, treatment with 10μM JTE013 before stimulation with sa113 supernatant resulted in significantly decreased amounts of secreted IL36γ, TNFα, and IL8 (Figure 5b and Supplementary Figure S5). In siS1PR1-transfected NHEKs, IL6 mRNA expression tended to decrease, but not significantly (Figure 5a). These results confirm that for IL36γ, TNFα, and IL8 synthesis in keratinocytes, S1PR1 mainly controls their transcription and S1PR2 mainly controls their release when stimulated with either S1P (Figure 3b-f) or S. aureus bacterial supernatant (Figure 5a, b).
Figure 5. In NHEKs, S1PR1 knock down affects S. aureus bacterial supernatant-induced IL36γ, TNFα and IL8 transcription and JTE013 blocks their secretion.
(a, b) IL36γ, TNFα, IL8 and IL6 transcription (a) and IL36γ, TNFα and IL8 protein secretion (b) in NHEKs incubated with 50 μl/ml TSB or sa113 supernatant for 4hrs (a) and 24hrs (b). Before stimulation, cells were transfected with control or S1PR1 siRNAs (a) or treated with or without 10 μM JTE013 (b). mRNA expression data (a) is shown as the fold of control siRNA/TSB-treated cells.
DISCUSSION
Keratinocytes are known to synthesize diverse cytokines and chemokines in response to external stimuli. This includes activation by pathogens such as S. aureus, a major cause of skin and soft tissue infections such as impetigo, folliculitis, and cellulitis. During S. aureus infection, cytokines and chemokines produced by keratinocytes, particularly the chemoattractant IL8 (Krishna and Miller, 2012, Ley et al., 2007), are essential for neutrophil recruitment from the circulation to lesional skin sites and are essential for mounting a proper immune response (Krishna and Miller, 2012). Keratinocytes also recognize S. aureus through their pattern recognition receptors, including toll-like receptor 2 (Miller and Cho, 2011), and can produce antimicrobial peptides, such as CAMP, in response to bacterial infection (Ryu et al., 2014). Recently, it was shown that PSMα derived from S. aureus induces IL1α and IL36α production and that these cytokines orchestrate IL17-dependent skin inflammation in mouse epidermis (Nakagawa et al., 2017). Our data confirms these previous reports that S. aureus supernatant-treated NHEKs produce TNFα, IL6, and IL8; however, we could not detect any IL36α production and we only detected IL36γ as an NHEK response to S. aureus supernatant stimulation (Supplementary Figure S3). According to our data, while IL36α is a very important mediator in mouse keratinocytes, IL36γ is dominant in human keratinocytes in response to S. aureus. The role of IL36γ in skin has been well investigated in the pathophysiology of psoriasis to induce chronic skin inflammation (Bassoy et al., 2018, D'Erme et al., 2015, Li et al., 2014, Wang W. et al., 2017). IL36γ can induce human keratinocytes to produce cytokines including IL1β, IL8, and IFNγ (Li et al., 2014, Wang W. et al., 2017). Furthermore, our data indicate that among the four IL36 subtypes (IL36α, β, γ and RN), IL36γ is the most responsive to S. aureus invasion and plays a major role in proinflammatory activity in human skin (Ganesan et al., 2017, Li et al., 2014).
Keratinocytes produce S1P as a response to various stimulations (Oizumi et al., 2014, Park et al., 2016). Here, we have demonstrated that S. aureus supernatant induces S1P synthesis and release in keratinocytes to signal skin S. aureus invasion (Figure 2). While we did not analyze the precise component of S. aureus supernatant that induces S1P production in NHEKs, S. aureus β-toxin, a specific sphingomyelinase, has the potential to initiate signaling through this pathway (Salgado-Pabon et al., 2014). Furthermore, our transcriptional data shows that NHEK S1PR2 mRNA expression is significantly increased in response to S. aureus supernatant and that the levels of other S1PRs are also induced, although not significantly (Figure 1a). This observation is in line with our immunofluorescent staining data showing that both S1PR1 and 2 change their expression and distribution in the epidermis of impetigo skin and in NHEKs treated with S. aureus supernatant (Figure 1b-d). Moreover, a previous report on S1PR1 showed that this receptor is tightly associated with immune responses in many cell types (Blaho and Hla, 2014, Jiang et al., 2017). These data indicate that S. aureus not only induces S1P production, but also influences the expression of S1PR1 and 2 in human keratinocytes.
Our results have shown that S1P induces keratinocyte TNFα, IL36γ, and IL8 release (Figure 3 and Supplementary Figure S4), which has been shown to result in subsequent neutrophil infiltration (Krishna and Miller, 2012, Li et al., 2014). While direct S1P stimulation resulted in mRNA and secreted protein level inductions that were smaller than what was observed with sa113 supernatant stimulation (Figure 3, Supplementary Figure S3 and S4), we still saw that reduced S1P production caused by SPHK1 knockdown and SPHK2 inhibition significantly decreased TNFα, IL36γ, and IL8 transcription (Figure 4a) and secretion (Figure 4b) in sa113 supernatant-treated NHEKs. This stronger response of NHEKs after sa113 supernatant treatment (Supplementary Figure S3) than S1P stimulation (Figure 3a) could potentially be explained by the presence of different toxins in the supernatant that have the capacity to stimulate many different receptors at the same time. However, TNFα, IL36γ, and IL8 transcription was reduced by S1PR1 knockdown (Figure 3d and 5a) and their protein secretion was decreased by blocking S1PR2 (Figure 3c, 5b and Supplementary Figure S5) similarly in S1P and sa113 supernatant-treated NHEKs. This data ensures that the S1P-S1PR axis plays a significant role in NHEK anti-S. aureus cytokine synthesis.
A previous report has shown that in keratinocytes, S1P-derived IL8 mRNA expression is mediated by S1PR1/3 and NFκB (Oizumi et al., 2014). Our data is consistent with this report in that we have identified S1PR1 in NHEKs as the mediator of TNFα, IL36γ, and IL8 transcription; however, the control of secretion of these cytokines is regulated by S1PR2. More specifically, both S1PR1 and 2 control TNFα secretion, while S1PR2 controls IL36γ and IL8 secretion (Figure 3, 5 and Supplementary Figure S5). In different cell types, S1P binding to S1PRs activates different intracellular signaling pathways and differentially regulates cytokine productions (Brunnert et al., 2015, Chandru and Boggaram, 2007, Hamidi et al., 2014, Oskeritzian et al., 2010, Oz-Arslan et al., 2006, Zhao et al., 2008). In endothelial cells, S1PR1 couples to Gi and activates the phosphatidylinositol-3-kinase (PI3K) pathway. In contrast, S1PR2 antagonizes S1PR1–Gi–PI3K signaling through activation of the G12–Rho pathway (Kim et al., 2015). In trophoblast derived cells, S1PR1 and 2 have distinct functions for transcription and secretion of IL8 (Brunnert et al., 2015). A similar different activation of intracellular pathway by S1PR1 or 2 is possible in NHEKs and these findings support our data that TNFα synthesis seems to be regulated by a different intracellular pathway from IL36γ and IL8 in keratinocytes (Figure 3b-f). Our findings also indicate that S. aureus is a trigger to activate the S1P-S1PR pathway in keratinocytes.
The epidermis uses diverse pathways to recruit neutrophils from the circulation to sites of inflammation (Krishna and Miller, 2012). We provide evidence that there is another pathway centered on the S1P-S1PR axis that contributes to neutrophil recruitment in the epidermis when it is exposed to S. aureus invasion. Although Park et al. reported that S1P induces CAMP production through an S1PR-independent intracellular mechanism in keratinocytes (Park et al., 2013, Park et al., 2016), our findings demonstrate that S1P also has an extracellular anti-bacterial response in keratinocytes that induces proinflammatory cytokine synthesis through an S1PR-dependent mechanism.
In this study, we have proven that S. aureus-induced S1P production in keratinocytes (Figure 2) results in the release of proinflammatory cytokines from keratinocytes as an extracellular signal (Figure 4), but there are many different kinds of cells that express S1PRs on their surface in skin tissue, such as Langerhans cells, mast cells, innate and adaptive immune cells, fibroblasts, and endothelial cells (Bock et al., 2016, Hamidi et al., 2014, Reines et al., 2009, Rivera et al., 2008, Wilkerson and Argraves, 2014), that may have similar mechanisms of activation. We have not investigated the effects of S. aureus-induced, keratinocyte-secreted S1P on other types of S1PR-expressing cells in skin tissue. Our future plan includes investigating the role of keratinocyte-released S1P in orchestrating skin inflammation through S1P receptors on different cell types. To achieve this aim, more precise studies about the communication between keratinocytes and other cell types will be required.
S1P (molecular weight: 379.47 g/mol) is a small molecular weight lipid (Chun and Hartung, 2010), so S1P and/or its analogues have the capacity to cross the skin barrier and become topical therapeutic agents that may be used to fight S. aureus infections. Our data indicates the importance of controlling S1PR1 and 2 function in the skin, especially in the epidermis, where skin bacterial infection has occurred. Thus, S1PR modulators may contribute to the suppression of excessive inflammation-derived tissue damage and/or activate more selective inflammation to remove bacterial burdens. Though S1P and its receptors have multiple functions in many types of cells, we may be able to limit and control their effects within skin tissue through the use of topical applications of S1P and/or related therapeutics.
MATERIALS AND METHODS
Primary normal human epidermal keratinocytes
Primary normal human epidermal keratinocytes (NHEKs) (Thermo Fisher, Waltham, MA) were cultured in EpiLife medium (60 μM calcium) (Thermo Fisher) supplemented with 1% EpiLife Defined Growth Supplement and 1% Antibiotic-Antimycotic (Thermo Fisher). Subconfluent NHEKs were seeded in 6-well plates, 12-well plates, or chamber slides and grown to sufficient confluence before treatment. For the induction of NHEK differentiation, confluent NHEKs were cultured in EpiLife medium (1.2mM calcium) for 48hrs.
NHEK treatment with bacterial supernatant or S1P
NHEKs were treated with PBS, 1 or 10 μM S1P (TOCRIS, Minneapolis, MN), 50 μl/ml 3% TSB (MilliporeSigma, Burlington, MA) or 50 μl/ml 0.22 μm-filtered (MilliporeSigma) flow of S. aureus (sa113, 35556 ATCC, Manassas, VA) bacterial supernatant in 3% TSB.
Chemical S1PR2 or SPHK2 block in NHEKs
To block S1PR2 or SPHK2, NHEKs were incubated with 10 μM JTE013 (Cayman, Ann Arbor, MI) or 1 μM ABC294640 (Cayman) at 37 °C for 2hrs or 4hrs, respectively, prior to S1P or S. aureus bacterial supernatant treatment, according to previous reports (Schaper et al., 2014, Terashita et al., 2016, Zhou et al., 2018).
RNA interference
Prior to S1P or S. aureus bacterial supernatant treatment, NHEKs were transfected with 10nM siRNA for SPHK1 (Silencer® Select s16957, Thermo Fisher), S1PR1 (Silencer® Select s4448, Thermo Fisher) or negative control siRNA (Silencer® Select Negative control #1, Thermo Fisher) using Lipofectamine RNAiMAX (Thermo Fisher).
Histology and immunofluorescent staining
Human normal and impetigo skin samples were kindly provided from Asahikawa Medical University, de-identified. All samples were collected under the written informed consent and the protocol approved by the medical ethics committee of the Asahikawa Medical University. The study was conducted according to the principles of the Helsinki declaration. Immunofluorescent staining of skin sections and NHEKs was performed, as described previously (Wang Z. et al., 2017), with the primary and secondary antibodies listed in Supplementary Table S1. Incubation with secondary antibodies only served as a negative control (Supplementary Figure S6). Fluorescence images were detected using an immunofluorescent microscope.
Real-time quantitative RT-PCR
Total RNA from NHEKs was isolated by RNeasy Mini Kit (QIAGEN, Hilden, Germany). At least 1 μg RNA was converted to cDNA using the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA), according to the manufacturer’s instructions. Real-time RT-qPCR was performed using iTaq Universal SYBR® Green Supermix (Bio-Rad) or TaqMan Gene Expression Master Mix (Thermo Fisher) on a CFX96 Touch Real-Time PCR Detection System (Bio-Rad). The hS1PR1 – 5 primers (Gandy et al., 2013) and the other probes used for real-time RT-qPCR are listed in Supplementary Tables S2 and S3, respectively. The expression of target genes was normalized to GAPDH expression and analyzed by the 2– ΔΔct method.
ELISA
Supernatants from NHEKs were collected after 4, 24, or 48 hrs incubation with sa113 supernatant or S1P, and S1P and/or proinflammatory cytokine concentrations were detected using the ELISA kits listed in Supplementary Table S4, according to the manufacturer’s instructions. Optical density was determined using a DTX 880 multimode detector (Beckman Coulter, Brea, CA).
SPHK activity assay
To measure SPHK activity in NHEKs, we used the ATP depletion assay. SPHK activity was measured using a sphingosine kinase activity assay kit (Echelon, Salt Lake City, UT), according to the manufacturer’s instructions. Briefly, NHEKs were incubated with 50 μl/ml TSB or sa113 supernatant for 24 hrs and lysed in reaction buffer. Cell lysates were incubated with 100 μM sphingosine and 10 μM ATP for 90 minutes at room temperature. Reaction buffer and 500 ng/ml recombinant SPHK1 (Cayman) and 2 (Cayman) were also incubated, instead of cell lysates, as negative and positive controls, respectively (Supplementary Figure S7). ATP detector was added to stop the reaction, and luminescence was measured using the SpectraMax Gemini EM (Molecular Devices, San Jose, CA). ATP concentrations were calculated by linear regression analysis, and the data is represented as the amount of ATP consumed.
Radar charts
Radar charts were used to compare the percentage of cytokine mRNA expression and protein secretion from 10 μM S1P-treated NHEKs with or without siS1PR1 or 10 μM JTE013 treatment.
Statistical analysis
In all in vitro experiments, all samples were in triplicate, and values are expressed as means ± SD. Student’s or Welch’s t-tests were applied to analyze the differences between two groups. One- or two-way ANOVA and Tukey’s tests were applied to analyze the differences among more than two groups. P < 0.05 was considered significant.
DATA AVAIABILITY STATEMENT
Datasets related to this article can be found at https://data.mendeley.com/datasets/cjhgx6wbxy/1, hosted at Mendeley.
Supplementary Material
ACKNOWLEDGMENTS
We thank Ms. K. Nishikura for providing normal and impetigo human skin sections and Dr. Amy Sullivan at Obrizus Communication for her help in editing the manuscript.
FUNDING
This research was funded by National Institute of Health, grant number R01 AI106874-05
1. Abbreviations:
- CAMP
cathelicidin antimicrobial peptide
- NHEK
normal human epidermal keratinocyte
- PI3K
phosphatidylinositol-3-kinase
- S1P
sphingosine 1-phosphate
- S1PR
sphingosine 1-phosphate receptor
- S. aureus
Staphylococcus aureus
- siRNA
small interfering RNA
- SPHK
sphingosine kinase
- TSB
3% tryptic soy broth
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Allende ML, Sipe LM, Tuymetova G, Wilson-Henjum KL, Chen W, Proia RL. Sphingosine-1-phosphate phosphatase 1 regulates keratinocyte differentiation and epidermal homeostasis. J Biol Chem 2013;288(25):18381–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano S, Akutsu N, Ogura Y, Nishiyama T. Increase of laminin 5 synthesis in human keratinocytes by acute wound fluid, inflammatory cytokines and growth factors, and lysophospholipids. Br J Dermatol 2004;151(5):961–70. [DOI] [PubMed] [Google Scholar]
- Aufiero B, Guo M, Young C, Duanmu Z, Talwar H, Lee HK, et al. Staphylococcus aureus induces the expression of tumor necrosis factor-alpha in primary human keratinocytes. Int J Dermatol 2007;46(7):687–94. [DOI] [PubMed] [Google Scholar]
- Bassoy EY, Towne JE, Gabay C. Regulation and function of interleukin-36 cytokines. Immunol Rev 2018;281(1):169–78. [DOI] [PubMed] [Google Scholar]
- Bock S, Pfalzgraff A, Weindl G. Sphingosine 1-phospate differentially modulates maturation and function of human Langerhans-like cells. J Dermatol Sci 2016;82(1):9–17. [DOI] [PubMed] [Google Scholar]
- Brunnert D, Piccenini S, Ehrhardt J, Zygmunt M, Goyal P. Sphingosine 1-phosphate regulates IL-8 expression and secretion via S1PR1 and S1PR2 receptors-mediated signaling in extravillous trophoblast derived HTR-8/SVneo cells. Placenta 2015;36(10): 1115–21. [DOI] [PubMed] [Google Scholar]
- Castelino FV, Varga J. Emerging cellular and molecular targets in fibrosis: implications for scleroderma pathogenesis and targeted therapy. Curr Opin Rheumatol 2014;26(6):607–14. [DOI] [PubMed] [Google Scholar]
- Chandru H, Boggaram V. The role of sphingosine 1-phosphate in the TNF-alpha induction of IL-8 gene expression in lung epithelial cells. Gene 2007;391(1-2): 150–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun J, Hartung HP. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin Neuropharmacol 2010;33(2):91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coant N, Sakamoto W, Mao C, Hannun YA. Ceramidases, roles in sphingolipid metabolism and in health and disease. Adv Biol Regul 2017;63:122–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Erme AM, Wilsmann-Theis D, Wagenpfeil J, Holzel M, Ferring-Schmitt S, Sternberg S, et al. IL-36gamma (IL-1F9) is a biomarker for psoriasis skin lesions. J Invest Dermatol 2015;135(4):1025–32. [DOI] [PubMed] [Google Scholar]
- Gandy KA, Canals D, Adada M, Wada M, Roddy P, Snider AJ, et al. Sphingosine 1-phosphate induces filopodia formation through S1PR2 activation of ERM proteins. Biochem J 2013;449(3):661–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan R, Raymond EL, Mennerich D, Woska JR Jr., Caviness G, Grimaldi C, et al. Generation and functional characterization of anti-human and anti-mouse IL-36R antagonist monoclonal antibodies. MAbs 2017;9(7):1143–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamidi S, Schafer-Korting M, Weindl G. TLR2/1 and sphingosine 1-phosphate modulate inflammation, myofibroblast differentiation and cell migration in fibroblasts. Biochim Biophys Acta 2014;1841(4):484–94. [DOI] [PubMed] [Google Scholar]
- Kim GS, Yang L, Zhang G, Zhao H, Selim M, McCullough LD, et al. Critical role of sphingosine-1-phosphate receptor-2 in the disruption of cerebrovascular integrity in experimental stroke. Nat Commun 2015;6:7893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna S, Miller LS. Innate and adaptive immune responses against Staphylococcus aureus skin infections. Semin Immunopathol 2012;34(2):261–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol 2007;7(9):678–89. [DOI] [PubMed] [Google Scholar]
- Li N, Yamasaki K, Saito R, Fukushi-Takahashi S, Shimada-Omori R, Asano M, et al. Alarmin function of cathelicidin antimicrobial peptide LL37 through IL-36gamma induction in human epidermal keratinocytes. J Immunol 2014;193(10):5140–8. [DOI] [PubMed] [Google Scholar]
- Lichte K, Rossi R, Danneberg K, ter Braak M, Kurschner U, Jakobs KH, et al. Lysophospholipid receptor-mediated calcium signaling in human keratinocytes. J Invest Dermatol 2008;128(6):1487–98. [DOI] [PubMed] [Google Scholar]
- Liu H, Archer NK, Dillen CA, Wang Y, Ashbaugh AG, Ortines RV, et al. Staphylococcus aureus Epicutaneous Exposure Drives Skin Inflammation via IL-36-Mediated T Cell Responses. Cell Host Microbe 2017;22(5):653–66 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LS, Cho JS. Immunity against Staphylococcus aureus cutaneous infections. Nat Rev Immunol 2011;11(8): 505–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Matsumoto M, Katayama Y, Oguma R, Wakabayashi S, Nygaard T, et al. Staphylococcus aureus Virulent PSMalpha Peptides Induce Keratinocyte Alarmin Release to Orchestrate IL-17-Dependent Skin Inflammation. Cell Host Microbe 2017;22(5):667–77 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nema R, Vishwakarma S, Agarwal R, Panday RK, Kumar A. Emerging role of sphingosine-1-phosphate signaling in head and neck squamous cell carcinoma. Onco Targets Ther 2016;9:3269–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oizumi A, Nakayama H, Okino N, Iwahara C, Kina K, Matsumoto R, et al. Pseudomonas-derived ceramidase induces production of inflammatory mediators from human keratinocytes via sphingosine-1-phosphate. PLoS One 2014;9(2):e89402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskeritzian CA, Price MM, Hait NC, Kapitonov D, Falanga YT, Morales JK, et al. Essential roles of sphingosine-1-phosphate receptor 2 in human mast cell activation, anaphylaxis, and pulmonary edema. J Exp Med 2010;207(3):465–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oz-Arslan D, Ruscher W, Myrtek D, Ziemer M, Jin Y, Damaj BB, et al. IL-6 and IL-8 release is mediated via multiple signaling pathways after stimulating dendritic cells with lysophospholipids. J Leukoc Biol 2006;80(2):287–97. [DOI] [PubMed] [Google Scholar]
- Park K, Elias PM, Shin KO, Lee YM, Hupe M, Borkowski AW, et al. A novel role of a lipid species, sphingosine-1-phosphate, in epithelial innate immunity. Mol Cell Biol 2013;33(4):752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K, Ikushiro H, Seo HS, Shin KO, Kim YI, Kim JY, et al. ER stress stimulates production of the key antimicrobial peptide, cathelicidin, by forming a previously unidentified intracellular S1P signaling complex. Proc Natl Acad Sci U S A 2016;113(10):E1334–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reines I, Kietzmann M, Mischke R, Tschernig T, Luth A, Kleuser B, et al. Topical application of sphingosine-1-phosphate and FTY720 attenuate allergic contact dermatitis reaction through inhibition of dendritic cell migration. J Invest Dermatol 2009;129(8): 1954–62. [DOI] [PubMed] [Google Scholar]
- Rivera J, Proia RL, Olivera A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat Rev Immunol 2008;8(10):753–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu S, Song PI, Seo CH, Cheong H, Park Y. Colonization and infection of the skin by S. aureus: immune system evasion and the response to cationic antimicrobial peptides. Int J Mol Sci 2014;15(5):8753–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado-Pabon W, Herrera A, Vu BG, Stach CS, Merriman JA, Spaulding AR, et al. Staphylococcus aureus beta-toxin production is common in strains with the beta-toxin gene inactivated by bacteriophage. J Infect Dis 2014;210(5):784–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaper K, Dickhaut J, Japtok L, Kietzmann M, Mischke R, Kleuser B, et al. Sphingosine-1-phosphate exhibits anti-proliferative and anti-inflammatory effects in mouse models of psoriasis. J Dermatol Sci 2013;71(1):29–36. [DOI] [PubMed] [Google Scholar]
- Schaper K, Kietzmann M, Baumer W. Sphingosine-1-phosphate differently regulates the cytokine production of IL-12, IL-23 and IL-27 in activated murine bone marrow derived dendritic cells. Mol Immunol 2014;59(1):10–8. [DOI] [PubMed] [Google Scholar]
- Schmitz EI, Potteck H, Schuppel M, Manggau M, Wahydin E, Kleuser B. Sphingosine 1-phosphate protects primary human keratinocytes from apoptosis via nitric oxide formation through the receptor subtype S1P(3). Mol Cell Biochem 2012;371(1-2):165–76. [DOI] [PubMed] [Google Scholar]
- Schuppel M, Kurschner U, Kleuser U, Schafer-Korting M, Kleuser B. Sphingosine 1-phosphate restrains insulin-mediated keratinocyte proliferation via inhibition of Akt through the S1P2 receptor subtype. J Invest Dermatol 2008;128(7):1747–56. [DOI] [PubMed] [Google Scholar]
- Skoura A, Hla T. Regulation of vascular physiology and pathology by the S1P2 receptor subtype. Cardiovasc Res 2009;82(2):221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashita T, Kobayashi K, Nagano T, Kawa Y, Tamura D, Nakata K, et al. Administration of JTE013 abrogates experimental asthma by regulating proinflammatory cytokine production from bronchial epithelial cells. Respir Res 2016;17(1):146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thieme M, Zillikens D, Sadik CD. Sphingosine-1-phosphate modulators in inflammatory skin diseases - lining up for clinical translation. Exp Dermatol 2017;26(3):206–10. [DOI] [PubMed] [Google Scholar]
- Vogler R, Sauer B, Kim DS, Schafer-Korting M, Kleuser B. Sphingosine-1-phosphate and its potentially paradoxical effects on critical parameters of cutaneous wound healing. J Invest Dermatol 2003;120(4):693–700. [DOI] [PubMed] [Google Scholar]
- Wang W, Yu X, Wu C, Jin H. IL-36gamma inhibits differentiation and induces inflammation of keratinocyte via Wnt signaling pathway in psoriasis. Int J Med Sci 2017;14(10):1002–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Mascarenhas N, Eckmann L, Miyamoto Y, Sun X, Kawakami T, et al. Skin microbiome promotes mast cell maturation by triggering stem cell factor production in keratinocytes. J Allergy Clin Immunol 2017;139(4):1205–16 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkerson BA, Argraves KM. The role of sphingosine-1-phosphate in endothelial barrier function. Biochim Biophys Acta 2014;1841(10):1403–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MR, Nakatsuji T, Gallo RL. Staphylococcus aureus: Master Manipulator of the Skin. Cell Host Microbe 2017;22(5):579–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Fernandes MJ, Turgeon M, Tancrede S, Di Battista J, Poubelle PE, et al. Specific and overlapping sphingosine-1-phosphate receptor functions in human synoviocytes: impact of TNF-alpha. J Lipid Res 2008;49(11):2323–37. [DOI] [PubMed] [Google Scholar]
- Zhou J, Chen J, Yu H. Targeting sphingosine kinase 2 by ABC294640 inhibits human skin squamous cell carcinoma cell growth. Biochem Biophys Res Commun 2018;497(2):535–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.