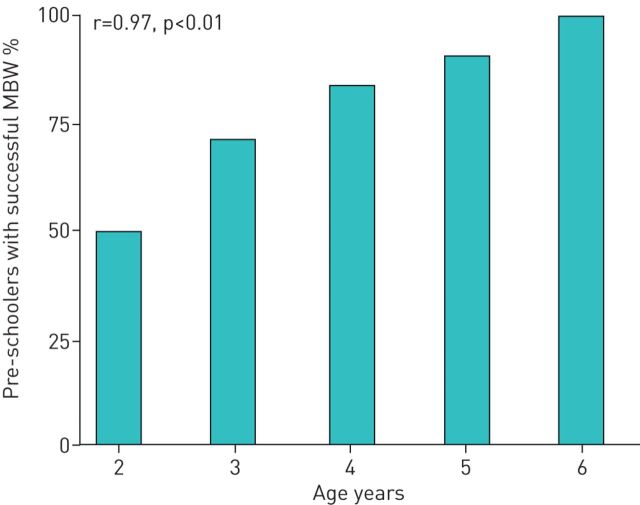Abstract
Background
Multiple-breath washout (MBW)-derived lung clearance index (LCI) detects early cystic fibrosis (CF) lung disease. LCI was used as an end-point in single- and multicentre settings at highly experienced MBW centres in preschool children. However, multicentre feasibility of MBW in children aged 2–6 years, including centres naïve to this technique, has not been determined systematically.
Methods
Following central training, 91 standardised nitrogen MBW investigations were performed in 74 awake preschool children (15 controls, 46 with CF, and 13 with other lung diseases), mean age 4.6±0.9 years at investigation, using a commercially available device across five centres in Germany (three experienced, two naïve to the performance in awake preschool children) with central data analysis. Each MBW investigation consisted of several measurements.
Results
Overall success rate of MBW investigations was 82.4% ranging from 70.6% to 94.1% across study sites. The number of measurements per investigation was significantly different between sites ranging from 3.7 to 6.2 (p<0.01), while the mean number of successful measurements per investigation was comparable with 2.1 (range, 1.9 to 2.5; p=0.46). In children with CF, the LCI was increased (median 8.2, range, 6.7–15.5) compared to controls (median 7.3, range 6.5–8.3; p<0.01), and comparable to children with other lung diseases (median 7.9, range, 6.6–13.9; p=0.95).
Conclusion
This study demonstrates that multicentre MBW in awake preschool children is feasible, even in centres previously naïve, with central coordination to assure standardised training, quality control and supervision. Our results support the use of LCI as multicentre end-point in clinical trials in awake preschoolers with CF.
Short abstract
MBW is feasible in awake preschool children with high success rates in a multicentre setting and LCI detects ventilation inhomogeneity in preschool children with CF. This supports LCI as an end-point in early intervention trials in preschool children with CF. https://bit.ly/3lD4wnj
Introduction
Lung disease remains the leading cause of morbidity and mortality in patients with cystic fibrosis (CF) and starts in the first months of life. Therefore, early diagnosis and therapeutic intervention may be most beneficial [1–6]. To determine efficacy of new therapies that address early CF lung disease in multicentre studies, sensitive, quantitative noninvasive end-points are needed to detect early lung abnormalities and disease progression in preschool children with CF [7]. Recently, we demonstrated that studies of lung morphology and function by lung imaging using magnetic resonance imaging (MRI) and multiple-breath washout (MBW) are feasible in sedated infants and preschool children in a multicentre setting [8, 9]. Both methods were previously shown to detect early abnormalities in CF lung disease such as increased ventilation inhomogeneity as determined by the MBW-derived lung clearance index (LCI) and structural changes and perfusion deficits detected by lung MRI [10–12].
The sensitivity of the LCI to detect response to therapy with a spectrum of interventions including inhaled hypertonic saline, inhaled DNase, CF transmembrane conductance regulator (CFTR) modulators and antibiotic therapy for pulmonary exacerbation, has been demonstrated for several paediatric age groups from infancy to school-age, including multicentre studies with performance of MBW either in sedated infants and preschool children or in awake children 3 years and older at centres highly experienced in this technique in the corresponding age group [6, 10, 13–17]. In addition, observational studies across two MBW-experienced sites of the Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST CF) and an observational study across three MBW-experienced North American CF centres used the LCI as end-point in preschool children [11, 18]. Recently, the success of an approach using standardised training and central quality control on feasibility of multicentre MBW in school-age children including centres previously naïve to the technique in the age group of children 6–11 years old has been demonstrated [19].
However, the multicentre feasibility of MBW in awake preschool children with CF including study centres naïve to the technique in that age group has not been studied systematically. In a previous study in infants and toddlers, we were able to demonstrate feasibility of sulfur hexafluoride (SF6)-MBW in sedated children at three centres following dedicated training of the sites’ staff, and including central reading and supervision [8]. MBW in preschool children can also be performed by inhalation of 100% oxygen to washout nitrogen (N2) from the lungs instead of using SF6 as tracer gas, as alterations of the breathing pattern induced by exposure to pure oxygen found in infants seem to be smaller in this age group [20–22]. However, the age-dependent ability to cooperate is limited in preschool children making the investigations more demanding, which may hamper the feasibility of multicentre trials in this age group.
We hypothesised that our previous approach of central training and quality control [8] would lead to acceptable MBW success rates at centres with and without prior experience in performance of MBW in preschool children. The aim of this study was therefore to implement and validate a standardised multicentre N2-MBW protocol in awake children 2–6 years of age by using an age- and tracer gas-adapted variant of our previous approach [8] and to determine the feasibility of LCI as an outcome measure of early CF lung disease by comparison of results between participating centres and study groups.
Methods
Study design
This prospective multicentre validation study was coordinated by the Translational Lung Research Center Heidelberg (TLRC) and conducted across five sites of the clinical trial network within the German Center for Lung Research (DZL) in preparation of an early intervention trial in this age group (ClinicalTrials.gov identifier: NCT03625466) [23]. The study was approved by the Ethics Committee of the University of Heidelberg (coordinating centre) and the local Ethics Committees of all participating sites. Following theoretical and hands-on in-person training at a meeting at the coordinating centre, as well as hands-on in-person training of the members of the different study teams at the different sites by the MBW supervisor (MS), MBW measurements were performed in preschool children following a standardised MBW protocol. The study was conducted under supervision of the central MBW laboratory, providing central data analysis and prompt feedback for troubleshooting. Further details on MBW training can be found in the online supplement.
Study population
Children aged 2–6 years with CF, another lung disease and pulmonary healthy controls were eligible for enrolment in this study. Parents and children obtained detailed information on the study protocol and parents gave their signed informed consent. Details on CFTR genotypes of patients with CF and information on how lung disease was excluded in the healthy control group are available in the online supplement.
Multiple-breath washout test
N2-MBW investigations in preschool children were established at the Airway Research Center North (ARCN), the Biomedical Research in Endstage and Obstructive Lung Disease (BREATH) centre and the TLRC before the start of this study (“experienced centres”), and were established at the Universities of Giessen and Marburg Lung Center (UGMLC) and the CF Center at Charité – Universitätsmedizin Berlin as part of this study (“unexperienced centres”) [10, 24]. Technical MBW procedures were compliant with the American Thoracic Society Technical Statement and were performed in awake, upright sitting children with the commercially available mainstream ultrasonic flowmeter Exhalyzer D and spiroware 3.2.1 as acquisition software (Eco Medics, Duernten, Switzerland) with N2 as tracer gas [10, 11, 22, 25]. Children were distracted by a video while breathing through a mouthpiece with a nose clip in place. The MBW procedure was performed following the same standard operating procedure at all centres. For this study, each MBW attempt is called “measurement” and the collection of all measurements of a patient at one time point is called “investigation”. Therefore, a successful MBW investigation consists of a minimum of two successful measurements. Repeated investigation of the same patient at different time points was allowed. Additional information on MBW testing can be found in the online supplement.
Statistical analysis
Data were analysed using SPSS version 22.0 (SPSS Inc., Chicago, IL, USA) and are shown as mean±sd, median (range) or percentage, if not indicated otherwise. For categorical data, groups were compared with Fisher's exact test and for continuous data with Kruskal–Wallis test followed by the Dunn–Bonferroni approach. Associations between LCI and continuous data were assessed by scatter plot and linear regression with determination of the Pearson correlation coefficient. A linear regression model was used to explain the association between age and success rate of MBW. A p-value of<0.05 was accepted to indicate statistical significance. The upper limit of normal of the LCI was defined as the mean of the control children+1.96×sd of these children, resulting in a value of 8.3.
Results
Demographics of the study population
In total, 74 preschool children (mean age at investigation 4.60±0.91 years; range 2.86–6.99 years), including 46 patients with CF, 13 patients with other lung diseases and 15 healthy controls were prospectively recruited. Other lung diseases included history of recurrent wheeze (n=9), primary ciliary dyskinesia (n=3), and status after oesophageal atresia with tracheo-oesophageal fistula (n=1). Patients with CF and other lung diseases showed comparable demographics to controls (table 1). Overall, anthropometry of all three groups was on average in the normal range.
TABLE 1.
Demographics of study population
| All | Controls | CF | Other lung diseases | |
| Number of children/investigations n/n | 74/91 | 15/15 | 46/63 | 13/13 |
| Experienced centres: | ||||
| ARCN | 17/17 | 3/3 | 4/4 | 10/10 |
| BREATH | 18/21 | 5/5 | 13/16 | 0/0 |
| TLRC | 21/24 | 3/3 | 17/20 | 1/1 |
| Unexperienced centres: | ||||
| UGMLC | 4/12 | 1/1 | 3/11 | 0/0 |
| Berlin | 14/17 | 3/3 | 9/12 | 2/2 |
| Age at investigation years (range) | 4.6±0.9 (2.9–7.0) |
4.7±0.8 (3.0–5.9) |
4.6±0.9 (2.9–7.0) |
4.7±0.9 (3.4–5.8) |
| Sex males/females n/n | 31/43 | 6/9 | 18/28 | 7/6 |
| Weight z-score at investigation | −0.2±0.8 | −0.1±0.7 | −0.3±0.8 | 0.1±0.9 |
| Height z-score at investigation | −0.4±1.4 | 0.1±1.0 | −0.5±1.3 | −0.4±2.0 |
| BMI z-score at investigation | −0.2±1.0 | −0.3±0.8 | −0.2±1.0 | 0.4±1.0 |
| F508del/F508del n (%) | 35 (76.1) | |||
| F508del/other n (%) | 8 (17.4) | |||
| Compound-heterozygous other n (%) | 3 (6.6) | |||
| Pancreatic insufficiency n (%) | 44 (95.7) | |||
Data are presented as mean±sd, unless otherwise stated. Sex, genotype and exocrine pancreatic function are depicted in relation to the individual patient; age, anthropometry and colonisation with Pseudomonas aeruginosa are displayed in relation to the time of the individual investigation. CF: cystic fibrosis; ARCN: CF Center at Airway Research Center North; BREATH: CF Center at Biomedical Research in Endstage and Obstructive Lung Disease Hannover; TLRC: CF Center at Translational Lung Research Center Heidelberg; UGMLC: CF Center at Universities of Giessen and Marburg Lung Center; Berlin: CF Center at Charité - Universitätsmedizin Berlin; BMI: body mass index.
Overall feasibility of multicentre MBW in preschool children
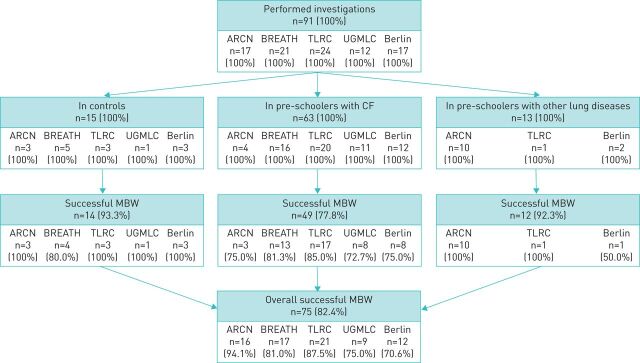
We performed 91 N2-MBW investigations in these awake preschool children across five study sites to determine the feasibility of multicentre preschool MBW (table 1). Each site investigated at least one control child with an overall success rate of 93.3% (figure 1). The success rate in children with CF was 77.8%. The success rate in children with other lung disease was comparable to that in controls (92.3%). The trend towards a lower success rate in children with CF compared to the other groups was not significant (p=0.218). For the complete study population, the success rate was 82.4% (figure 1). Comparison of the total number of MBW measurements across study sites revealed no differences except a higher number of attempts at UGMLC compared to BREATH and TLRC (Supplemental Table E2). Combined analysis of the average number of measurements per MBW investigation of the unexperienced sites (UGMLC, Berlin) versus the experienced sites (ARCN, BREATH, TLRC) revealed a higher number of attempts to achieve a comparable number of successful MBW measurements (p=0.91) and number of successful investigations (p=0.08) at the unexperienced sites (table 2). There was no difference between controls, children with CF or other lung diseases concerning the number of performed and successful measurements (table 2).
FIGURE 1.
Success rates of multicentre MBW investigations in awake preschool children without lung disease (control), with CF and with other lung diseases. Summary of success rates for technically acceptable MBW investigations for the total study population and for individual study sites. MBW: multiple-breath washout; CF: cystic fibrosis; ARCN: CF Center at Airway Research Center North; BREATH: CF Center at Biomedical Research in Endstage and Obstructive Lung Disease Hannover; TLRC: CF Center at Translational Lung Research Center Heidelberg; UGMLC: CF Center at Universities of Giessen and Marburg Lung Center; Berlin: CF Center at Charité - Universitätsmedizin Berlin.
TABLE 2.
Success rates of MBW investigations in awake preschool children
| Cohort | Centres | Number of performed investigations n | Number of performed measurements mean±sd | Number of successful measurements mean±sd | At least 1 successful measurement n (%) | At least 2 successful measurements n (%) |
| All | Experienced | 62 | 3.8±1.6** | 2.1±0.8 | 58 (93.5) | 54 (87.1) |
| Unexperienced | 29 | 5.2±2.5 | 2.1±1.3 | 24 (82.8) | 21 (72.4) | |
| Controls | Experienced | 11 | 4.2±2.1 | 2.1±0.5* | 11 (100.0) | 10 (90.9) |
| Unexperienced | 4 | 5.5±1.9 | 3.0±0.8 | 4 (100.0) | 4 (100.0) | |
| CF | Experienced | 40 | 3.7±1.7* | 1.9±0.9 | 36 (90.0) | 33 (82.5) |
| Unexperienced | 23 | 5.3±2.6 | 2.0±1.2 | 19 (82.6) | 16 (69.6) | |
| Other lung diseases | Experienced+ | 11 | 3.8±0.8 | 2.7±0.5 | 11 (100.0) | 11 (100.0) |
| Unexperienced§ | 2 | 3.0±1.4 | 1.5±2.1 | 1 (50.0) | 1 (50.0) |
Experienced centres are the CF Center at Airway Research Center North, the CF Center at Biomedical Research in Endstage and Obstructive Lung Disease Hannover, and the CF Center at Translational Lung Research Center Heidelberg. Unexperienced centres are the CF Center at Universities of Giessen and Marburg Lung Center and the CF Center at Charité - Universitätsmedizin Berlin. MBW: multiple-breath washout; CF: cystic fibrosis. *: p<0.05 and **: p<0.01 versus unexperienced centres; +: limited to the CF Center at Airway Research Center North and the CF Center at Translational Lung Research Center Heidelberg; §: limited to the CF Center at Charité - Universitätsmedizin Berlin.
Feasibility of the first MBW investigation in preschool children
To control for a possible learning effect in eight study participants with repeated investigations (three patients with two, each two patients with three and four and one patient with five investigation time points), we performed the same analysis taking into account the first MBW investigation of each participant in this study only (table 3, Supplemental Table E3). Again, the number of MBW measurements performed per investigation was higher at the unexperienced sites compared to the experienced sites to achieve a comparable number of successful measurements (p=0.88) and successful investigations (p=0.12). As for all investigations, the average number of performed and successful measurements was comparable between controls, children with CF or other lung diseases when analysis was based on the first MBW investigation of each participant in this study (table 3, supplemental table E3).
TABLE 3.
Success rates of only the first MBW investigation in this study in awake preschool children
| Cohort | Centre | Number of performed investigations n | Number of performed measurements mean±sd | Number of successful measurements mean±sd | Number of successful investigations n (%) |
| All | Experienced | 56 | 3.9±1.7* | 2.1±0.8 | 49 (87.5) |
| Unexperienced | 18 | 5.4±2.9 | 2.1±1.3 | 13 (72.2) | |
| Controls | Experienced | 11 | 4.2±2.1 | 2.1±0.5* | 10 (90.9) |
| Unexperienced | 4 | 5.5±1.9 | 3.0±0.8 | 4 (100.0) | |
| CF | Experienced | 34 | 3.8±1.8 | 1.9±0.9 | 28 (82.4) |
| Unexperienced | 12 | 5.8±3.3 | 1.8±1.3 | 8 (66.7) | |
| Other lung diseases | Experienced¶ | 11 | 3.8±0.8 | 2.7±0.5 | 11 (100.0) |
| Unexperienced+ | 2 | 3.0±1.4 | 1.5±2.1 | 1 (50.0) |
Experienced centres are the CF Center at Airway Research Center North, the CF Center at Biomedical Research in Endstage and Obstructive Lung Disease Hannover, and the CF Center at Translational Lung Research Center Heidelberg. Unexperienced centres are the CF Center at Universities of Giessen and Marburg Lung Center and the CF Center at Charité - Universitätsmedizin Berlin. MBW: multiple-breath washout; CF: cystic fibrosis. *: p<0.05 versus unexperienced centres; ¶: limited to the CF Center at Airway Research Center North and the CF Center at Translational Lung Research Center Heidelberg; +: Limited to the CF Center at Charité - Universitätsmedizin Berlin.
Age dependency of feasibility of MBW in preschool children
Across all centres, feasibility of MBW was 50% in 2-year-old children (2 of 4 investigations) and the percentage of successful MBW investigations increased continuously to 90.6% (29 of 32 investigations) at the age of 5 years (figure 2). The percentage of preschool children with a successful MBW investigation significantly correlated with age at investigation (r=0.97, p<0.01). The regression coefficient indicated an increase in the success rate of 11.9±1.6% with every year of life.
FIGURE 2.
Multiple-breath washout (MBW) success rates according to age. MBW investigations are grouped according to the age of the children at time of investigation, where “2” stands for the age group of children from 2.0 to 2.9 years of age and so on. The Pearson correlation coefficient r and p-values are provided.
Reasons for unsuccessful MBW measurements
Overall, 91 MBW investigations were performed in this study. Of these, 20 (22.0%) MBW investigations consisted of measurements that were all acceptable. Fifty-five (60.4%) MBW investigations consisted of at least two MBW measurements fulfilling quality criteria and were thus rated as successful MBW investigation, but further MBW measurements of the same investigation did not meet acceptability criteria. The remaining 16 (17.6%) MBW investigations were rated as unsuccessful because they had less than two MBW measurements fulfilling quality criteria (figure 1). The main reasons for unsuccessful MBW measurements were leaks, not meeting end of test criteria and an irregular breathing pattern. Further reasons for unsuccessful measurements are listed in table 4.
TABLE 4.
Reasons and frequencies of unsuccessful MBW measurements
| Finding in individual measurements | Unsuccessful MBW investigations, n=16 | Successful MBW investigations with at least 1 unacceptable measurement, n=55 |
| Leak | 5 (31.3) | 27 (49.1) |
| End of test criteria not met | 2 (12.5) | 10 (18.2) |
| Irregular breathing pattern | 2 (12.5) | 5 (9.1) |
| End of test criteria not met/irregular breathing pattern | 2 (12.5) | 4 (7.3) |
| Leak/end of test criteria not met | 1 (6.3) | 3 (5.5) |
| Leak/end of test criteria not met / irregular breathing pattern | 2 (12.5) | 1 (1.8) |
| End of test criteria not met/no washout started | 0 (0.0) | 2 (3.6) |
| Leak/irregular breathing pattern | 1 (6.3) | 0 (0.0) |
| Leak/swallow at critical time point | 0 (0.0) | 1 (1.8) |
| Leak/no washout started | 0 (0.0) | 1 (1.8) |
| No washout started | 0 (0.0) | 1 (1.8) |
| Use of wrong DSR set | 1 (6.3) | 0 (0.0) |
Data are presented as n (%). MBW: multiple-breath washout; DSR: dead space reducer.
Multicentre MBW in preschool children with CF, other lung diseases and controls
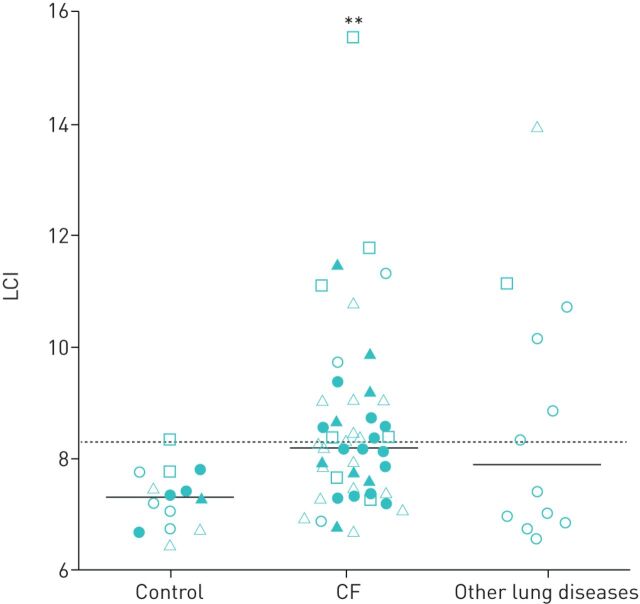
In control preschoolers, the median LCI was 7.3 (table 5, supplemental table E4, figure 3) without significant differences in LCI between controls from different sites (p=0.38), in the coefficient of variation (CV) of the LCI between different sites (p=0.56), or in the frequency of children with an abnormal LCI (p=0.41). Preschool children with CF showed a significantly higher median LCI than controls (8.2; p<0.01) (table 5, supplemental table E4, figure 3) and significantly more children with CF had an abnormal LCI compared to controls (table 5, supplemental table E4; p<0.05). There were no significant differences in LCI between children with CF from different sites (p=0.11), in CV of the LCI between sites (p=0.65) and in the frequency of CF patients with abnormal LCI at different sites (p=0.76). The median LCI of preschool children with other lung diseases (7.9) was comparable to children with CF (p=0.95) and tended to be increased compared to controls, but this trend did not reach statistical significance (p=0.057) (table 5, figure 3). Of note, the two preschool children with other lung diseases with the highest LCI values were those patients with primary ciliary dyskinesia (figure 3).
TABLE 5.
Distribution of LCI between study sites in preschool children
| Diagnosis | Total | Experienced centres | Unexperienced centres | |
| Control | Number of children | 14 | 10 | 4 |
| LCI, median (range) | 7.3 (6.5–8.3) | 7.2 (6.5–7.8) | 7.5 (7.2–8.3) | |
| LCICV, median (range), % | 2.8 (0.0–10.7) | 4.2 (0.0–10.7) | 2.2 (2.0–7.1) | |
| Investigations with abnormal LCI, n (%) | 1 (7.1) | 0 (0.0) | 1 (25.0) | |
| CF | Number of children | 49 | 33 | 16 |
| LCI, median (range) | 8.2 (6.7–15.5)** | 8.2 (6.7–11.3)** | 8.4 (6.8–15.5) | |
| LCICV, median (range), % | 4.2 (0.3–17.1) | 4.0 (0.3–8.9) | 4.2 (0.7–17.1) | |
| Investigations with abnormal LCI, n (%) | 23 (46.9)** | 14 (42.2)* | 9 (56.3) | |
| Other lung diseases | Number of children | 12 | 11 | 1 |
| LCI, median (range)+ | 7.9 (6.6–13.9) | 7.4 (6.6–13.9) | 11.1 | |
| LCICV, median (range), %+ | 2.6 (0.6–8.4) | 2.3 (0.6–8.4) | 5.2 | |
| Investigations with abnormal LCI, n (%) | 6 (50.0)** | 5 (45.5) | 1 (100.0) |
Experienced centres are the CF Center at Airway Research Center North, the CF Center at Biomedical Research in Endstage and Obstructive Lung Disease Hannover, and the CF Center at Translational Lung Research Center Heidelberg. Unexperienced centres are the CF Center at Universities of Giessen and Marburg Lung Center and the CF Center at Charité - Universitätsmedizin Berlin. LCI: lung clearance index; CF: cystic fibrosis; CV: coefficient of variation. *: p<0.05 and **: p<0.01 versus control; +: in case of a single investigation, the result of this investigation is reported without range.
FIGURE 3.
Summary of lung clearance index (LCI) in preschool children without lung disease (control), with cystic fibrosis (CF) and with other lung diseases in the multicentre setting. Dotted line represents upper limit of normal of 8.3. Open circles (CF Center at Airway Research Center North), closed circles (CF Center at Biomedical Research in Endstage and Obstructive Lung Disease Hannover), open triangles (CF Center at Translational Lung Research Center Heidelberg), closed triangles (CF Center at Universities of Giessen and Marburg Lung Center) and open squares (CF Center at Charité - Universitätsmedizin Berlin) represent data from individual children from the respective study site. Solid horizontal lines represent median values for each group. **: p<0.01 versus control group.
Discussion
This study demonstrates that multicentre N2-MBW is feasible in awake preschool children with CF and other lung diseases with high success rates using a mouthpiece as interface. In addition, we show that the frequency and range of early ventilation inhomogeneity detected by multicentre MBW is comparable to previous single- and multicentre studies in preschool children with CF [10, 11]. Further, this study demonstrates that multicentre MBW can be established successfully in a clinical trial network including study sites without prior experience in MBW in this challenging age group. Our results support LCI as a promising noninvasive quantitative outcome measure for monitoring of early abnormalities in lung function in multicentre surveillance studies, as well as early intervention clinical trials in preschool children with CF.
High rates of technically acceptable MBW investigations across study groups (77–93%) and study sites (70–94%) with an overall success rate of 82% (figure 1; table 2) could be achieved using the previously established approach of central training, implementation of standard operating procedures and harmonised MBW protocols in combination with continuous supervision and central MBW analysis that has been used for SF6-MBW in infants and preschool children [8]. Interestingly, the success rate tended to be lower in children with CF (77.8%) than in those with other lung diseases (92.3%) or controls (93.3%; figure 1). However, this trend did not reach statistical significance (p=0.218). This finding may be related to the circumstances that children with CF were in some cases undergoing several other tests at their appointments, limiting the time for MBW measurements and/or attention span of preschool children, whereas the majority of children with other lung diseases and all control children attended the centres for MBW investigations only. The two main reasons for unsuccessful MBW measurements in our study were leaks and irregular breathing patterns. There was no difference in MBW feasibility if either all MBW investigations including repeat studies in the same children were included, or if the analysis was based on the first MBW investigation in each child only. This may be explained by the fact that at the experienced sites that performed N2-MBW in this age group for diagnostics before start of this study, the first MBW investigation taking place as part of this study in a participant may not have been the overall first MBW investigation in this child. Therefore, some children may have been more advanced on their learning curve on MBW performance, as have been the MBW operators at these sites. Further, the majority of repeated investigations (64.7%) was performed at the two centres that were previously naïve to the performance of N2-MBW in this age group. The number of investigations at these sites may in part have been driven by the MBW operators for training purposes rather than the ability of the child to perform the test, leading to a consistently higher number of MBW measurements that were performed per MBW investigation, regardless whether this was the first investigation in a child or the third. However, it is encouraging that with more experience, a lower number of MBW measurements per investigation was needed to achieve a successful investigation.
Compared to the MBW success rate of our study, a recent single centre study using SF6-MBW in un-sedated preschool children with and without various lung diseases demonstrated that success rates of MBW investigations were highly age-dependent and very low (0–33%) in un-sedated children under the age of 3 years [26]. As reported in this study [26], we found a strong correlation between age at investigation and success rates of MBW investigations in preschool children, however, the rate of successful MBW investigations in 2-year-old children was substantially higher in our study (50%). In all 2-year-old children included in our study, MBW in this study was their first MBW investigation. While the number of children in this age group was limited in our study (n=4), it is promising that two of these young preschoolers were already able to perform successful MBW investigations. We speculate that this higher success rate may be attributable to the research setting in our study (with probably more time per MBW investigation) compared to the routine outpatient setting in the study by Downing and colleagues [26]. The success rates in the older preschool age groups of our study is comparable to the high success rate of >70% in the previous study using SF6-MBW (figure 2) [26]. This is of special interest as the typical age range for paediatric investigation plans for clinical trials in children is 2–5 years of age [27]. Therefore, it seems possible to include at least some 2-year-old children in trials assessing safety and efficacy across the age range of 2–5 years.
Compared to our study, the SHIP study being the largest interventional study that used the change in LCI as primary end-point in preschool children with CF to date reported a slightly higher overall success rate of 89% [17]. This may be explained by several differences compared to our study including: 1) up to six repeated investigations per patient in the SHIP study, which may have led to a learning effect in the children; 2) the use of a face mask as interface, which may be easier for children to perform MBW; and 3) inclusion of children from the age of 3 years instead of 2 years as in our study [17].
Another recent study investigated the feasibility of N2-MBW in preschool children comparing two interfaces, mouthpiece and face mask [28]. In that study, the success rate in children under the age of 4 years using a mouthpiece was <30% [28]. In comparison, the success rate in children under 4 years of age was 68.0% (17 of 24 investigations) and therefore substantially higher in our study (figure 2). As the settings for MBW investigation using the mouthpiece were rather comparable in our study and the study by Robinson and colleagues [28] with an adequate period for each child, we speculate that the number of MBW measurements per investigation may have been higher in our study, while it was not reported in the previous study [28]. If a face mask was used in the former study, the success rate in children under the age of 4 years was 85% and reached rates of 90–100% in older preschool children that were comparable to the results of our study in these age groups using a mouthpiece (figure 2) [28]. While there are advantages and disadvantages of both interfaces in the preschool age range that have been discussed elsewhere [22], the reason for using the mouthpiece in our study on feasibility of multicentre MBW was the preparation of a randomised-controlled trial with repeated MBW investigations over a trial duration of 2 years in preschool children with CF aged 2–5 years at screening (ClinicalTrials.gov identifier: NCT03625466). Therefore, we decided to avoid the possible influence of a changing interface dead space following growth and development of the facial features over time when using a facemask on MBW outcomes, and used a mouthpiece with nose clip instead. Our approach may require a longer training phase of the children to ensure breathing stability and leak-free sealing around the mouthpiece, but these efforts will pay off in observational studies over several years where comparable MBW settings are a pre-requisite for longitudinal analysis of lung function.
Besides the role of LCI in surveillance of lung disease and its use to investigate response to therapy in patients with CF, MBW gains increasing attention for monitoring of lung function in other childhood lung disease such as asthma or PCD [29, 30]. In this context, the high success rate of MBW measurements in preschool children with other lung diseases (figure 1) and detection of substantially elevated LCI values in the two children with PCD participating in our study is encouraging and provides initial support of LCI as promising noninvasive quantitative outcome measure in other early-onset lung diseases in children (figure 3).
A limitation of this study is the unknown transferability of the high MBW success rates obtained in our study conducted in the well-equipped translational research network of the DZL with experienced clinical research personnel and lung function laboratories at each study site. Feasibility and transferability of our results to a broader multicentre clinical trial setting may be strongly influenced by the established infrastructure. Recent studies on the effect of inhaled hypertonic saline and CFTR modulators in preschool children at sites highly experienced in preschool MBW using LCI as an end-point support transferability of our results to multicentre clinical trials [17, 31]. However, replication studies in larger networks, such as the ECFS-Clinical Trial Network or the CF Therapeutics Development Network, will be necessary to determine whether our findings can be replicated in other trial networks before LCI can be used as a widespread outcome measure in multicentre clinical trials in preschool children.
In summary, our results demonstrate that MBW for assessment of lung function is feasible with sufficient success rates in awake preschool children with CF, other lung diseases and controls using a commercially available mainstream ultrasonic flowmeter. In addition, this study shows that following initial training and under continuous supervision by a specialised paediatric MBW core laboratory, the LCI is sensitive to detect abnormalities of lung function in preschool children with CF in a multicentre setting. Our study supports the use of LCI as diagnostic tool for surveillance of early lung disease, and as a potential quantitative end-point in multicentre clinical trials testing early interventions in preschool children with CF and possibly other early-onset lung diseases.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00408-2020.SUPPLEMENT (162.5KB, pdf)
Acknowledgments
The authors thank the children and their families for their participation in the study.
Footnotes
This article has supplementary material available from openres.ersjournals.com.
Author contributions: M. Stahl and M.A. Mall conceived and designed the study. M. Stahl, C. Joachim, I. Kirsch, T. Uselmann, Y. Yu, N. Alfeis, C. Berger, R. Minso, I. Rudolf, C. Stolpe, X. Bovermann, L. Liboschik, A. Steinmetz, D. Tennhardt, F. Dörfler, J. Röhmel, K. Unorji-Frank, C. Rückes-Nilges, B. von Stoutz, L. Naehrlich, M.V. Kopp, A-M. Dittrich, O. Sommerburg and M.A. Mall acquired, analysed and interpreted the data. M. Stahl, C. Joachim, I. Kirsch, T. Uselmann, Y. Yu, N. Alfeis, C. Berger, R. Minso, I. Rudolf, C. Stolpe, X. Bovermann, L. Liboschik, A. Steinmetz, D. Tennhardt, F. Dörfler, J. Röhmel, K. Unorji-Frank, C. Rückes-Nilges, B. von Stoutz, L. Naehrlich, M.V. Kopp, A-M. Dittrich, O. Sommerburg and M.A. Mall wrote the manuscript or revised it critically for important intellectual content.
Conflict of interest: M. Stahl reports grants from Mukoviszidose eV, personal fees from Vertex Pharmaceuticals and grants from Christiane Herzog Foundation, during the conduct of the study.
Conflict of interest: C. Joachim has nothing to disclose.
Conflict of interest: I. Kirsch has nothing to disclose.
Conflict of interest: T. Uselmann has nothing to disclose.
Conflict of interest: Y. Yu has nothing to disclose.
Conflict of interest: N. Alfeis has nothing to disclose.
Conflict of interest: C. Berger has nothing to disclose.
Conflict of interest: R. Minso has nothing to disclose.
Conflict of interest: I. Rudolf has nothing to disclose.
Conflict of interest: C. Stolpe has nothing to disclose.
Conflict of interest: X. Bovermann has nothing to disclose.
Conflict of interest: L. Liboschik has nothing to disclose.
Conflict of interest: A. Steinmetz has nothing to disclose.
Conflict of interest: D. Tennhardt has nothing to disclose.
Conflict of interest: F. Dörfler has nothing to disclose.
Conflict of interest: J. Röhmel has nothing to disclose.
Conflict of interest: K. Unorji-Frank has nothing to disclose.
Conflict of interest: C. Rückes-Nilges has nothing to disclose.
Conflict of interest: B. von Stoutz has nothing to disclose.
Conflict of interest: L. Naehrlich reports that he has received institutional fees for site participation in clinical trials from Vertex Pharmaceuticals.
Conflict of interest: M.V. Kopp has nothing to disclose.
Conflict of interest: A-M. Dittrich has nothing to disclose.
Conflict of interest: O. Sommerburg has nothing to disclose.
Conflict of interest: M.A. Mall reports grants from the German Federal Ministry of Education and Research and the Einstein Foundation Berlin during the conduct of the study; advisory board, consultancy, lecture and clinical trial fees from Boehringer Ingelheim, advisory board and consultancy fees from Arrowhead Pharmaceuticals, advisory board, consultancy, lecture and clinical trial fees from Vertex Pharmaceuticals, advisory board and consultancy fees from Santhera, consultancy fees from Galapagos and Sterna Biologicals, advisory board and consultancy fees from Enterprise Therapeutics, and consultancy fees from Antabio, outside the submitted work.
Support statement: This work was supported in part by the German Ministry for Education and Research (grants 82DZL00401, 82DZL0040A1, 82DZL10106 and 82DZL10501), the Dietmar Hopp Foundation and the Mukoviszidose Förderverein Giessen e.V. M. Stahl was supported by the German Cystic Fibrosis Association Mukoviszidose e.V. (grant 15/01) and the Christiane Herzog Foundation (C-H-P 1803). M.A. Mall was supported by the Einstein Foundation Berlin (EP-2017-393). The funders had no role in study design, data collection or analysis, the decision to publish or preparation of the manuscript. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Bell SC, Mall MA, Gutierrez H, et al. . The future of cystic fibrosis care: a global perspective. Lancet Respir Med 2020; 8: 65–124. doi: 10.1016/S2213-2600(19)30337-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grasemann H, Ratjen F. Early lung disease in cystic fibrosis. Lancet Respir Med 2013; 1: 148–157. doi: 10.1016/S2213-2600(13)70026-2 [DOI] [PubMed] [Google Scholar]
- 3.Mall MA, Mayer-Hamblett N, Rowe SM. Cystic fibrosis: emergence of highly effective targeted therapeutics and potential clinical implications. Am J Respir Crit Care Med 2020; 201: 1193–1208. doi: 10.1164/rccm.201910-1943SO [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mall MA, Graeber SY, Stahl M, et al. . Early cystic fibrosis lung disease: role of airway surface dehydration and lessons from preventive rehydration therapies in mice. Int J Biochem Cell Biol 2014; 52: 174–179. doi: 10.1016/j.biocel.2014.02.006 [DOI] [PubMed] [Google Scholar]
- 5.Mall MA, Stahl M, Graeber SY, et al. . Early detection and sensitive monitoring of CF lung disease: prospects of improved and safer imaging. Pediatr Pulmonol 2016; 51: S49–S60. doi: 10.1002/ppul.23537 [DOI] [PubMed] [Google Scholar]
- 6.Stahl M, Wielputz MO, Ricklefs I, et al. . Preventive inhalation of hypertonic saline in infants with cystic fibrosis (PRESIS). A randomized, double-blind, controlled study. Am J Respir Crit Care Med 2019; 199: 1238–1248. doi: 10.1164/rccm.201807-1203OC [DOI] [PubMed] [Google Scholar]
- 7.Ramsey BW, Banks-Schlegel S, Accurso FJ, et al. . Future directions in early cystic fibrosis lung disease research: an NHLBI workshop report. Am J Respir Crit Care Med 2012; 185: 887–892. doi: 10.1164/rccm.201111-2068WS [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stahl M, Graeber SY, Joachim C, et al. . Three-center feasibility of lung clearance index in infants and preschool children with cystic fibrosis and other lung diseases. J Cyst Fibros 2018; 17: 249–255. doi: 10.1016/j.jcf.2017.08.001 [DOI] [PubMed] [Google Scholar]
- 9.Wielputz MO, von Stackelberg O, Stahl M, et al. . Multicentre standardisation of chest MRI as radiation-free outcome measure of lung disease in young children with cystic fibrosis. J Cyst Fibros 2018; 17: 518–527. doi: 10.1016/j.jcf.2018.05.003 [DOI] [PubMed] [Google Scholar]
- 10.Stahl M, Wielpütz MO, Graeber SY, et al. . Comparison of lung clearance index and magnetic resonance imaging for assessment of lung disease in children with cystic fibrosis. Am J Respir Crit Care Med 2017; 195: 349–359. [DOI] [PubMed] [Google Scholar]
- 11.Stanojevic S, Davis SD, Retsch-Bogart G, et al. . Progression of lung disease in preschool patients with cystic fibrosis. Am J Respir Crit Care Med 2017; 195: 1216–1225. doi: 10.1164/rccm.201610-2158OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wielpütz MO, Puderbach M, Kopp-Schneider A, et al. . Magnetic resonance imaging detects changes in structure and perfusion, and response to therapy in early cystic fibrosis lung disease. Am J Respir Crit Care Med 2014; 189: 956–965. doi: 10.1164/rccm.201309-1659OC [DOI] [PubMed] [Google Scholar]
- 13.Amin R, Subbarao P, Jabar A, et al. . Hypertonic saline improves the LCI in paediatric patients with CF with normal lung function. Thorax 2010; 65: 379–383. doi: 10.1136/thx.2009.125831 [DOI] [PubMed] [Google Scholar]
- 14.Amin R, Subbarao P, Lou W, et al. . The effect of dornase alfa on ventilation inhomogeneity in patients with cystic fibrosis. Eur Respir J 2011; 37: 806–812. doi: 10.1183/09031936.00072510 [DOI] [PubMed] [Google Scholar]
- 15.Davies J, Sheridan H, Bell N, et al. . Assessment of clinical response to ivacaftor with lung clearance index in cystic fibrosis patients with a G551D-CFTR mutation and preserved spirometry: a randomised controlled trial. Lancet Respir Med 2013; 1: 630–638. doi: 10.1016/S2213-2600(13)70182-6 [DOI] [PubMed] [Google Scholar]
- 16.Milla CE, Ratjen F, Marigowda G, et al. . Lumacaftor/ivacaftor in patients aged 6-11 years with cystic fibrosis and homozygous for F508del-CFTR. Am J Respir Crit Care Med 2017; 195: 912–920. doi: 10.1164/rccm.201608-1754OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ratjen F, Davis SD, Stanojevic S, et al. . Inhaled hypertonic saline in preschool children with cystic fibrosis (SHIP): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet Respir Med 2019; 7: 802–809. doi: 10.1016/S2213-2600(19)30187-0 [DOI] [PubMed] [Google Scholar]
- 18.Ramsey KA, Rosenow T, Turkovic L, et al. . Lung clearance index and structural lung disease on computed tomography in early cystic fibrosis. Am J Respir Crit Care Med 2016; 193: 60–67. doi: 10.1164/rccm.201507-1409OC [DOI] [PubMed] [Google Scholar]
- 19.Saunders C, Jensen R, Robinson PD, et al. . Integrating the multiple breath washout test into international multicentre trials. J Cyst Fibros 2020; 19: 602–607. doi: 10.1016/j.jcf.2019.11.006 [DOI] [PubMed] [Google Scholar]
- 20.Simpson SJ, Ranganathan S, Park J, et al. . Progressive ventilation inhomogeneity in infants with cystic fibrosis after pulmonary infection. Eur Respir J 2015; 46: 1680–1690. doi: 10.1183/13993003.00622-2015 [DOI] [PubMed] [Google Scholar]
- 21.Jensen R, Stanojevic S, Klingel M, et al. . A Systematic approach to multiple breath nitrogen washout test quality. PLoS ONE 2016; 11: e0157523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robinson PD, Latzin P, Ramsey KA, et al. . Preschool multiple-breath washout testing. An official American Thoracic Society technical statement. Am J Respir Crit Care Med 2018; 197: e1–e19. doi: 10.1164/rccm.201801-0074ST [DOI] [PubMed] [Google Scholar]
- 23.Seeger W, Welte T, Eickelberg O, et al. . [The German centre for lung research - translational research for the prevention, diagnosis and treatment of respiratory diseases]. Pneumologie 2012; 66: 464–469. doi: 10.1055/s-0032-1310086 [DOI] [PubMed] [Google Scholar]
- 24.Stahl M, Joachim C, Wielputz MO, et al. . Comparison of lung clearance index determined by washout of N2 and SF6 in infants and preschool children with cystic fibrosis. J Cyst Fibros 2019; 18: 399–406. doi: 10.1016/j.jcf.2018.11.001 [DOI] [PubMed] [Google Scholar]
- 25.Anagnostopoulou P, Latzin P, Jensen R, et al. . Normative data for multiple breath washout outcomes in school-aged Caucasian children. Eur Respir J 2020; 55: 1901302. doi: 10.1183/13993003.01302-2019 [DOI] [PubMed] [Google Scholar]
- 26.Downing B, Irving S, Bingham Y, et al. . Feasibility of lung clearance index in a clinical setting in pre-school children. Eur Respir J 2016; 48: 1074–1080. doi: 10.1183/13993003.00374-2016 [DOI] [PubMed] [Google Scholar]
- 27.European Medicines Agency. Paediatric Investigation Plans. www.ema.europa.eu/en/human-regulatory/research-development/paediatric-medicines/paediatric-investigation-plans Date last accessed: 17 May, 2020.
- 28.Robinson PD, Lum S, Moore C, et al. . Comparison of facemask and mouthpiece interfaces for multiple breath washout measurements. J Cyst Fibros 2018; 17: 511–517. doi: 10.1016/j.jcf.2018.01.003 [DOI] [PubMed] [Google Scholar]
- 29.Kowalik K, Dai R, Safavi S, et al. . Persistent ventilation inhomogeneity after an acute exacerbation in preschool children with recurrent wheezing. Pediatr Allergy Immunol 2020; 31: 608–615. doi: 10.1111/pai.13245 [DOI] [PubMed] [Google Scholar]
- 30.Kinghorn B, McNamara S, Genatossio A, et al. . Comparison of multiple breath washout and spirometry in children with primary ciliary dyskinesia and cystic fibrosis and healthy controls. Ann Am Thorac Soc 2020; 17: 1085–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McNamara JJ, McColley SA, Marigowda G, et al. . Safety, pharmacokinetics, and pharmacodynamics of lumacaftor and ivacaftor combination therapy in children aged 2–5 years with cystic fibrosis homozygous for F508del-CFTR: an open-label phase 3 study. Lancet Respir Med 2019; 7: 325–335. doi: 10.1016/S2213-2600(18)30460-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00408-2020.SUPPLEMENT (162.5KB, pdf)