According to the US Centers for Disease Control and Prevention (CDC), patients with underlying health conditions, including all types of lung and cardiovascular diseases, have an increased risk of developing serious disease when infected by SARS-CoV-2 [1]. Based on prior publications on the effects of acute right heart failure superimposed on systemic infection [2–5], Ryan et al. [6] suggested that right heart failure and concomitant COVID-19 infection may result in increased mortality in pulmonary arterial hypertension (PAH) patients. Surprisingly, the number of hospitalised PAH-COVID-19 patients remained rather low in Italy and the USA so far [7]. In late March 2020, experts from over 32 US PH expert centres answered a query endorsed by the US Pulmonary Hypertension Association. COVID-19 infection was reported in 13 PAH patients, among whom three required intubation and one died. This is consequently raising the question whether and why PAH patients appear to be at lower risk of developing severe COVID-19 [7].
Short abstract
This international survey highlights that a limited number of PAH and CTEPH patients suffered from severe #COVID19 infection https://bit.ly/3jGuBQq
To the Editor:
There is currently limited data available regarding COVID-19 infection in patients with pulmonary hypertension (PH), resulting in poor evidence-based guidance to manage this specific patient population and to reliably predict the clinical course.
According to the US Centers for Disease Control and Prevention (CDC), patients with underlying health conditions, including all types of lung and cardiovascular diseases, have an increased risk of developing serious disease when infected by SARS-CoV-2 [1]. Based on prior publications on the effects of acute right heart failure superimposed on systemic infection [2–5], Ryan et al. [6] suggested that right heart failure and concomitant COVID-19 infection may result in increased mortality in pulmonary arterial hypertension (PAH) patients. Surprisingly, the number of hospitalised PAH-COVID-19 patients remained rather low in Italy and the USA so far [7]. In late March 2020, experts from over 32 US PH expert centres answered a query endorsed by the US Pulmonary Hypertension Association. COVID-19 infection was reported in 13 PAH patients, among whom three required intubation and one died. This is consequently raising the question whether and why PAH patients appear to be at lower risk of developing severe COVID-19 [7].
Considering that more evidence was needed, an international survey was launched from 17 April 2020 to 10 May 2020, in the middle of the pandemic surge in Europe, to evaluate the impact of COVID-19 infection in patients with rare forms of PH, i.e. PAH and chronic thromboembolic pulmonary hypertension (CTEPH).
The aim of the survey was to collect data on the clinical course, treatment, hospitalisations, intensive care unit (ICU) admissions and outcomes of COVID positive patients with PAH and CTEPH. The SurveyMonkey platform was used for data collection. Numbers of available data are listed in brackets.
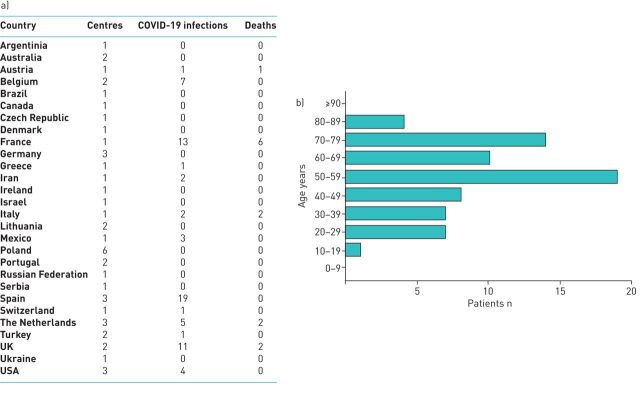
47 PH centres, from 28 countries worldwide, responded to the survey (figure 1a). During the pandemic period, most consultations for patients with PAH and CTEPH occurred remotely (80%), either by tele- (68%) or video-consultation (12%), whereas 9% were followed live, with normal outpatient consultation, 8% exclusively when the patients called and 3% were not followed at all. In total, COVID-19 infections were reported in 70 PAH or CTEPH patients by 19 of the participating centres. 13 of these centres reported more than one case and 28 did not report any case.
FIGURE 1.
COVID-19 in pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension patients. a) Table showing number of participating centres, reported COVID-19 cases and COVID-19 related mortality per country in 47 centres from 28 countries worldwide. b) Age distribution of patients (n=70).
Most patients had idiopathic or heritable PAH (31%), followed by PAH due to connective tissue diseases (23%), CTEPH (20%), PAH associated with congenital heart diseases (10%), porto-pulmonary hypertension (4%), and other causes (11%, including pulmonary veno-occlusive disease, PAH associated with HIV infection or with intake of drugs and toxic oil) (data available for 70 patients). Median age of the cohort was 50–59 years (n=70; figure 1b). Most of the patients were under specific PAH combination therapy (59%), followed by monotherapy (21%) and only a minority were on triple therapy (7% and 13% on oral and parenteral prostanoids, respectively) (n=56). Very few patients were on immunosuppressive therapy (4%). Regarding CTEPH patients, 73% were inoperable or with post-operative residual PH, 18% were waiting for balloon pulmonary angioplasty (BPA) and 9% for pulmonary endarterectomy (PEA); 9% had BPA, but none had PEA delayed, because of the pandemic logistic situation (n=11).
Pneumonia was the most frequent presentation at diagnosis (56%), followed by only fever (28%), then upper respiratory tract infection (13%), other symptoms including myalgia, dyspnoea or cough (13%), PH exacerbation and/or right heart failure (5%); anosmia and/or ageusia was observed in 3% of the patients and 3% were asymptomatic. Venous thromboembolism or haemoptysis were not reported at diagnosis (n=61). Nasopharyngeal swabs (74%) and a few low-dose computed tomography (CT) scans (15%) were used to confirm COVID-19 diagnosis, irrespective of the geographic location (n=66). The diagnosis was clinically suspected but unproven in 11% and no patient received a bronchoalveolar lavage. Most of the patients displayed typical COVID-19 CT (54%), 7% harboured atypical CT and 39% did not undergo a CT scan at diagnosis; normal CT was not reported (n=46). The majority of the patients were hospitalised on a general ward (46%), 17% needed ICU admission, 6% were in other healthcare facilities (including nursing homes, intermediate care unit and social-sanitary centre) and 30% were treated at home (n=63). The median duration of symptoms was 6.0 days (range: 1–36 days). The median duration of hospitalisation was 3.4 days (range: 2–32 days) and patients were mostly hospitalised in the hospital of the responding centre (47%), 31% in a local hospital and 22% in another expert centre. Most of the patients needed respiratory support, 57% received oxygen, 12% required high-flow nasal cannula, 2% received continuous positive airway pressure or bilevel positive airway pressure, 11% required invasive mechanical ventilation and no patient was reported on extracorporal membrane oxygenation (ECMO) (n=65). COVID-19 treatment consisted in most of the cases of antibiotics (41%, including 20% azithromycin) followed by hydroxychloroquine or chloroquine (31%), lopinavir or ritonavir (14%), itraconazole (1%) and other treatments including tocilizumab, methylprednisolone, mycophenolate, favipiravir and rituximab (14%); none of them received remdesivir/GS-5734 (n=70). For all cases (n=70), mortality was 19%, 20% for PAH and 14% for CTEPH patients.
The results of this international survey show that few PAH and CTEPH patients required ICU admission or invasive ventilation and none were treated with ECMO, in contrast to expectations for patients with underlying respiratory or cardiovascular diseases [1]. The observed case-fatality rate, estimated as the number of deaths per 100 reported cases, was 19%, which is high in comparison with the rate observed in the general population (5.9% to 16.3%, in the USA and Belgium, respectively; data extracted on 25 May 2020 [8]), and with hospital mortality in the large series from New York City (9.7% [9]). However, comparing mortality to a general population is difficult; there was an important variability between countries (figure 1a), partially explained by age distribution (Pearson correlation between the number of deaths and the number of patients older than 60 years of age: r=0.93, p=0.02), and by disrupted systematic follow-up (correlation between the number of deaths and the proportion of patients without remote or live consultation: r=0.98, p=0.02), suggesting underestimation of benign cases.
Shielding at home could, in part, explain the low number of cases reported in this survey since the PAH and CTEPH population is medically educated, thereby better prepared to respect social distancing instructions, and sometimes socially isolated by the disease and its consequences. Horn et al. [7] also suggested a potential protective effect of PAH medication by different mechanisms involving pulmonary endothelial cells. In the current international survey, most patients actually benefited from combination therapy of endothelin receptor antagonists, phosphodiesterase 5 inhibitors and/or prostanoids (62%), showing a net progress in comparison with most recent registries (i.e. 41% in COMPERA) [10], in agreement with the most recent European Society of Cardiology/European Respiratory Society recommendations [11]. The use of anticoagulants was not queried in this survey; however, no case of venous thromboembolism was reported despite the increased prevalence of pulmonary embolism/thrombosis [12–15] and the presence of microthrombi within pulmonary capillaries [14, 15] reported in COVID-19 patients.
In summary, this survey, although incomplete, highlights that a limited number of PAH and CTEPH patients suffered from COVID-19, whereas the case-fatality rate related to COVID-19 was rather high in comparison with the general population. Further comprehensive investigation would be required to elucidate 1) whether underreporting of benign cases can explain the heterogeneity of outcome among different countries, and 2) whether tight adherence to social distancing is the main explanation or other physiopathological factors may prevent COVID-19 infection in severe PH.
Acknowledgements
This survey is a common initiative of European Respiratory Society (ERS) Assembly 13 on Pulmonary Vascular Diseases, ERS Clinical Research Collaboration PHAROS and European Reference Network for Rare Lung Diseases (ERN-lung), and is endorsed by patient association PHA Europe. We thank Valerija Arsovski from the ERS office in Lausanne (Switzerland) for logistic support and the survey participants: Yochai Adir (Carmel Medical Center, Haifa, Israel), Joan Barbera (Hospital Clínic, Barcelona, Spain), Roberto J. Bernardo (Stanford University, CA, USA), Laurent Bertoletti (University Hospital, Saint-Etienne, France), Aleksandar Bokan (Institute for pulmonary diseases of Vojvodina, Sremska Kamenica, Serbia), Karin Boomars (Erasmus Medical Center, Rotterdam, The Netherlands), Jørn Carlsen (Rigshospitalet, University of Copenhagen, Copenhagen, Denmark), Michele D'Alto (Monaldi Hospital, University “L. Vanvitelli”, Naples, Italy), Anna Doboszynska (Pulmonary Department, Warmia-Mazury University, Olsztyn, Poland), Aleksandra Furdyna (Department of Internal Medicine, Medical University of Warsaw, Warshaw, Poland), Sean Gaine (Mater Dublin, Dublin, Ireland), Henning Gall (Lung Center, University of Giessen, Giessen, Germany), Pitsiou Georgai (Aristotle University Thessaloniki, G.H.G.Papanikolaou, Thessaloniki, Greece), Pavel Jansa (General University Hospital, Prague, Czech Republic), Wojciech Jacheć (Department of Cardiology Zabrze, Medical University of Silesia, Katowice, Poland), Carlos Jardim (Heart Institute, University of Sao Paulo, Sao Paulo, Brazil), Elena Jurevičienė (University Hospital Santaros Klinikos, Vilnius, Lithuania), Dominic Keating (Alfred Hospital, Melbourne, Australia), Derya Kocakaya (Marmara University Hospital, Istambul, Turkey), Lisa Kohlbacher (Medical University, Vienna, Austria), Yaroslav Kondratsky (Regional Clinical Hospital, Lviv, Ukraine), Beata Kusmierczyk (National Institute of Cardiology, Warsaw, Poland), Irene Lang (Medical University, Vienna, Austria), Tobias Lange (University Medical Center, Regensburg, Germany), Skaidrius Miliauskas (Hospital of Lithuanian university of health sciences, Kaunas, Lithuania), Karen Olsson (Medical School, Hannover, Germany), Gul Öngen (Cerrahpaşa Medical Faculty, Istambul, Turkey), Caneva Jorge Osvaldo (Fundacion Favaloro, Buenos Aires, Argentina), Małgorzata Peregud-Pogorzelska (Department of Cardiology, Pomeranian Medical University, Szczecin, Poland), Georgai Pitsiou (Aristotle University Thessaloniki, G.H.G.Papanikolaou, Thessaloniki, Greece), Rui Plácido (Academic Medical Centre, Lisbon, Portugal), Tomas Pulido (National Heart Institute, Mexico City, Mexico), Farid Rashidi (University of Medical Sciences, Tabriz, Iran), Abilio Reis (Centro Hospitalar Universitário, Porto, Portugal), Olivier Sitbon (Kremlin-Bicêtre Hospital, Paris, France), Jesús Ribas Sola (Hospital Universitari de Bellvitge, Barcelona, Spain), John Ryan (University of Utah, Salt Lake City, UT, USA), Sandeep Sahay (Houston Methodist Hospital, Houston, TX, USA), Silvia Ulrich (University Hospitals, Zurich, Switzerland), Jolanda van Haren-Willems (Radboud University Medical Centre, Nijmegen, The Netherla,ds), Alexander Volkov (Institute of Rheumatology, Moscow, Russian Federation), Anton Vonk Noordegraaf (University Medical Center Amsterdam, The Netherlands), Jason Weatherald (University of Calgary, Calgary, Canada), Helen Whitford (The Alfred Hospital, Melbourne, Australia) and Katarzyna Widejko (Copper Medical Centre, Legnica, Poland), for having kindly answered the survey.
Footnotes
Conflict of interest: C. Belge reports personal fees from Actelion/Janssen and MSD/Bayer outside the submitted work.
Conflict of interest: R. Quarck has nothing to disclose.
Conflict of interest: L. Godinas reports personal fees from Actelion outside the submitted work.
Conflict of interest: D. Montani reports grants and personal fees from Actelion and Bayer, personal fees from GSK and Pfizer, grants, personal fees and nonfinancial support from MSD, personal fees from Chiesi and Boerhinger, and nonfinancial support from Acceleron, outside the submitted work.
Conflict of interest: P. Escribano Subias reports personal fees and grants from Janssen, and personal fees from MSD, GlaxoSmithKline and Ferrer, outside the submitted work.
Conflict of interest: J-L. Vachiery reports grants from Actelion J&J, and other support from Bayer HealthCare, Bial Portela, PhaseBio, Respira Therapeutics and United Therapeutics, outside the submitted work.
Conflict of interest: H. Nashat reports grants from Actelion Pharmaceuticals outside the submitted work.
Conflict of interest: J. Pepke-Zaba reports grants, personal fees and nonfinancial support from Actelion, grants and personal fees from Merck, and personal fees from Bayer, outside the submitted work.
Conflict of interest: M. Humbert reports grants and personal fees from Actelion, and personal fees from GSK, Merck and Acceleron, outside the submitted work.
Conflict of interest: M. Delcroix reports grants and other support from Actelion/J&J, and other support from Bayer, MSD, Reata, Bellarophon and Acceleron, outside the submitted work.
References
- 1.CDC People at increased risk and other people who need to take extra precautions. www.cdc.gov/coronavirus/2019-ncov/specific-groups/high-risk-complications.html Date last updated: 11 September 2020. Date last accessed: 25 May 2020.
- 2.Padang R, Chandrashekar N, Indrabhinduwat M, et al. . Aetiology and outcomes of severe right ventricular dysfunction. Eur Heart J 2020; 41: 1273–1282. doi: 10.1093/eurheartj/ehaa037 [DOI] [PubMed] [Google Scholar]
- 3.Campo A, Mathai SC, Le PJ, et al. . Outcomes of hospitalisation for right heart failure in pulmonary arterial hypertension. Eur Respir J 2011; 38: 359–367. doi: 10.1183/09031936.00148310 [DOI] [PubMed] [Google Scholar]
- 4.Sztrymf B, Souza R, Bertoletti L, et al. . Prognostic factors of acute heart failure in patients with pulmonary arterial hypertension. Eur Respir J 2010; 35: 1286–1293. doi: 10.1183/09031936.00070209 [DOI] [PubMed] [Google Scholar]
- 5.Haddad F, Peterson T, Fuh E, et al. . Characteristics and outcome after hospitalization for acute right heart failure in patients with pulmonary arterial hypertension. Circ Heart Fail 2011; 4: 692–699. doi: 10.1161/CIRCHEARTFAILURE.110.949933 [DOI] [PubMed] [Google Scholar]
- 6.Ryan JJ, Melendres-Groves L, Zamanian RT, et al. . Care of patients with pulmonary arterial hypertension during the coronavirus (COVID-19) pandemic. Pulm Circ 2020; 10: 2045894020920153. doi: 10.1177/2045894020920153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horn EM, Chakinala M, Oudiz R, et al. . Could pulmonary arterial hypertension patients be at a lower risk from severe COVID-19? Pulm Circ 2020; 10: 2045894020922799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johns Hopkins Coronavirus Resource Center (CRC) Mortality analysis. https://coronavirus.jhu.edu/data/mortality Date last updated: 30 September 2020. Date last accessed: 25 May 2020.
- 9.Richardson S, Hirsch JS, Narasimhan M, et al. . Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020; 323: 2052–2059. doi: 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoeper MM, Huscher D, Ghofrani HA, et al. . Elderly patients diagnosed with idiopathic pulmonary arterial hypertension: results from the COMPERA registry. Int J Cardiol 2013; 168: 871–880. doi: 10.1016/j.ijcard.2012.10.026 [DOI] [PubMed] [Google Scholar]
- 11.Galie N, Humbert M, Vachiery JL, et al. . 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015; 46: 903–975. doi: 10.1183/13993003.01032-2015 [DOI] [PubMed] [Google Scholar]
- 12.Klok FA, Kruip MJHA, van der Meer NJM, et al. . Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 2020; 191: 145–147. doi: 10.1016/j.thromres.2020.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bompard F, Monnier H, Saab I, et al. . Pulmonary embolism in patients with Covid-19 pneumonia. Eur Respir J 2020; 12: 2001365. doi: 10.1183/13993003.01365-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huertas A, Montani D, Savale L, et al. . Endothelial cell dysfunction: a major player in SARS-CoV-2 infection (COVID-19). Eur Respir J 2020; 56: 2001634. doi: 10.1183/13993003.01634-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ackermann M, Verleden SE, Kuehnel M, et al. . Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med 2020; 383: 120–128. doi: 10.1056/NEJMoa2015432 [DOI] [PMC free article] [PubMed] [Google Scholar]