Neutrophils are central to the pathophysiology of COPD, yet there are no licensed therapies directly targeting neutrophil-mediated inflammation [1]. Neutrophils are recruited to the COPD airway via chemoattractants such as interleukin-8 (CXCL-8) and are activated by pro-inflammatory cytokines abundant in inflamed airways [2]. Following activation, neutrophils can degranulate, releasing damaging proteases and mediators, or can form neutrophil extracellular traps (NETs), which consist of neutrophil DNA, granule products and antimicrobial proteins that trap microbes and prevent bacterial dissemination [3]. However, excessive NET formation (or NETosis) may cause host damage and has been implicated in multiple diseases [4–6], including COPD [4, 7–9].
Short abstract
The implications of these findings are significant for development of CXCR2 antagonists and other mechanisms targeting neutrophil activation or NETosis, suggesting that IL-8-dependent mechanisms will only work in a subset of COPD patients https://bit.ly/32SeisO
To the Editor:
Neutrophils are central to the pathophysiology of COPD, yet there are no licensed therapies directly targeting neutrophil-mediated inflammation [1]. Neutrophils are recruited to the COPD airway via chemoattractants such as interleukin-8 (CXCL-8) and are activated by pro-inflammatory cytokines abundant in inflamed airways [2]. Following activation, neutrophils can degranulate, releasing damaging proteases and mediators, or can form neutrophil extracellular traps (NETs), which consist of neutrophil DNA, granule products and antimicrobial proteins that trap microbes and prevent bacterial dissemination [3]. However, excessive NET formation (or NETosis) may cause host damage and has been implicated in multiple diseases [4–6], including COPD [4, 7–9].
Multiple triggers of NETosis in vivo and in vitro have been reported, including host-derived cytokines such as CXCL-8 [10], the effects of which are exerted via CXCR1 and CXCR2 receptors. A recent study showed that sputum from individuals with COPD induced NETosis in peripheral blood neutrophils from healthy donors and that this could be prevented through CXCR2 blockade, suggesting that in COPD, CXCR2 agonists such as CXCL-8 are key mediators of both neutrophil activation and NET release [7].
Danirixin, a competitive and specific CXCR2 antagonist, has been shown to block CXCR2-mediated neutrophil activation in animal and human in vitro studies without blocking the CXCR1-mediated oxidative burst [11, 12]. We therefore performed an in vivo and in vitro study to determine whether danirixin treatment resulted in reduced NETosis and neutrophil activation in individuals with COPD.
Using a 3:1 allocation, participants with COPD were randomised to receive either danirixin (hydrochloride salt) 35 mg twice daily or placebo for 14 days in a double-blind, single-centre study (GSK Study 207551, NCT03250689). Participants were aged between 50 and 75 years with a clinical diagnosis of COPD (forced expiratory volume in 1 s (FEV1)/forced vital capacity ratio <0.7 and FEV1 ≥40% predicted at screening), had elevated sputum NETs on screening assay for histone–elastase complexes of >0.5 units·mL−1 sputum [4], and were current or former cigarette smokers (≥10-pack-year history). Patients with other lung diseases or recent pneumonia were excluded.
Key assessments included spirometry, induced sputum and blood samples at screening, and on days 1, 7 and 14. Sputum measurements included NETs (immunoassays for histone–elastase and DNA–elastase complexes) and sputum neutrophils using methods described previously [4].
The primary endpoint was change from baseline in sputum NETs as measured via histone–elastase immunoassay, and although this was a pilot study where sample size was based on feasibility, the study was powered for a 70% probability of detecting a true reduction of 30% reduction in NETs. A mixed-effects model with repeated measures was used to estimate the mean percent change from baseline for the sputum NETs quantified by histone–elastase with adjustment for treatment group, logged baseline NETs and treatment group by day interaction. The mean and corresponding 95% credible intervals were calculated for the danirixin and placebo treatment groups for day 7 and 14. Statistical analyses were carried out on SAS Studio 3.6 (Basic Edition).
All study participants provided written, informed consent. Ethical approval was provided by East of Scotland Research Ethics Service 1 (ref. 17/SS/0111). The research was carried out in accordance with the principles of the Declaration of Helsinki.
To further characterise the effects of danirixin on neutrophil activation, a series of in vitro investigations were performed in collaboration with the University of Dundee (Dundee, UK). Peripheral blood neutrophils were isolated from healthy donors by Percoll gradient centrifugation as previously described [13]. Neutrophils were pre-treated with either 10 µM danirixin (hydrochloride salt) at 37°C for 10 min or vehicle control (dimethylsulfoxide). Cells were then treated with 10 ng·mL−1 CXCL-8 (R&D systems), which binds to both CXCR1 and CXCR2; 1 µM Gro-α (CXCL-1) (Peprotech), which only binds to CXCR2; or 10% soluble sputum from patients with COPD. Soluble sputum was obtained by 8× volume dilution in PBS followed by centrifugation at 3000g for 15 min. Concentration of soluble sputum was determined by preliminary experiments to establish a concentration that caused consistent neutrophil activation measured through CD11b upregulation without loss of viability. Cells were incubated with activators at 37°C for 30 min, followed by addition of 1% bovine serum albumin. Samples were labelled with a phycoerythrin-labelled CD11b antibody (BD Biosciences) for 30 min at 4°C and then fixed in 4% paraformaldehyde followed by fluorescence-activated cell sorting analysis. CXCL-8 sputum concentration was measured by ELISA (R&D Systems). Changes in mean receptor expression were analysed using paired t-tests.
43 participants were screened, with 19 randomised (14 to danirixin and five to placebo) out of a planned 24; there were no withdrawals. The study was terminated early due to cessation of the danirixin development programme. There were no clinically significant differences between the danirixin and placebo treatment groups at baseline in terms of age (mean±sd 65±7 versus 62±6 years for danirixin versus placebo, respectively), sex (43% versus 40% were male), current smoking status (20% versus 43%), FEV1 (69.5±18.4% versus 79.2±7.5% predicted) or body mass index (27.1±4.7 versus 30.9±6.5 kg·m−2).
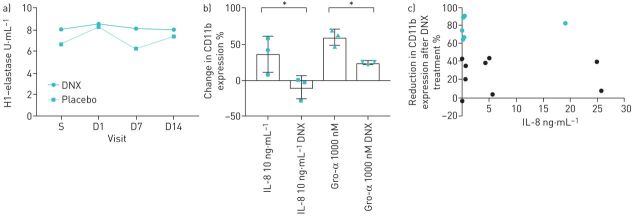
There was no significant difference in per cent change from baseline in sputum NETs between the danirixin and placebo groups (figure 1a), as measured by histone–elastase complexes (mean±se change +3.2±22.3% versus −24.7±16.9%) and DNA–elastase complexes (+33.9±56.7% versus +29.3±3.7%, respectively). There was no significant difference in percent change from baseline in sputum neutrophil count between danirixin (−14.9±17.34%) and placebo (−7.6±5.33%). A high degree of variability in sputum mediators measured was observed.
FIGURE 1.
a) Randomised controlled trial of danirixin (DNX) (n=14) versus placebo (n=5). Data are presented as median histone (H1)–elastase complexes (primary outcome) across the four trial visits. Note that this figure is generated from a post hoc analysis; the primary analysis was from the mixed model. b) Purified neutrophils from healthy donors (n=3) were stimulated with interleukin (IL)-8 (10 ng·mL−1) or Gro-α (1000 nM) in the presence or absence of DNX (10 μM). % change in CD11b expression was determined by fluorescence-activated cell sorting analysis of mean fluorescent intensity (IL-8 paired t-test, p=0.0165; Gro-α paired t-test, p=0.0187). c) Neutrophil activation is blocked in response to DNX in some, but not all, COPD samples, independent of IL-8 sputum concentration; % reduction in CD11b expression after exposure of healthy volunteer neutrophils to 10% soluble sputum from patients with COPD in the presence of DNX (10 μM) against sputum IL-8 concentration (correlation, r=0.26, p=0.35). S: start of study; D1: day 1; D7: day 7; D14: day 14. *: p<0.05.
Adverse events were reported in 60% (three participants) in the placebo group and 43% (six participants) in the danirixin group. No adverse event occurred in more than one participant and on the whole, they were mild in intensity. One participant each in the placebo (malaise, myalgia, dry mouth, night sweats and decreased appetite) and danirixin (sputum increased and dyspnoea) groups that reported adverse events that were considered to be drug related. No fatalities or serious adverse events were reported during the study.
Having observed no apparent beneficial effects of danirixin on markers of neutrophil activation in this patient population, we hypothesised that heterogeneity in the expression of inflammatory mediators between patients with COPD may impact individual responses to CXCR2 antagonism [7]. For example, in a previous cohort of 99 patients with COPD, CXCL-8 concentrations ranged from 0.3 to 123 ng·mL−1 [4]. We therefore hypothesised that in keeping with our in vivo results, we would observe heterogeneity in response to danirixin. Purified neutrophils from healthy donors were stimulated with CXCL-8 (10 ng·mL−1) or CXCL-1 (1000 nM); as previously reported [11], showing a significant increase in CD11b expression (figure 1b). Pre-treatment with danirixin (10 µM) significantly reduced CD11b activation by a mean±sd of 46.4±15.8%) in CXCL-8-treated cells (paired t-test, p=0.0165) and 35.6±2.52% in CXCL-1-treated cells (paired t-test, p=0.0187). In contrast, when 15 COPD sputa were tested as the stimulus for neutrophils from healthy volunteers, highly variable inhibition of neutrophil activation was observed (figure 1c). Six samples (shown in green) showed a <50% inhibition of neutrophil activation; however, this did not correlate with sputum CXCL-8 concentration, suggesting that CXCR2 blockade prevented neutrophil activation in some samples but not all.
Despite previous studies suggesting in vitro that NETosis could be reduced through CXCR2 inhibition [7], we saw no evidence of a reduction in NETosis in the danirixin group in vivo.
Although our study was limited by a small sample size and the early termination of the danirixin development programme, we saw no consistent signal of reductions in NETosis, suggesting even had the study achieved its original sample size target, it is unlikely a significant reduction in NETs would have been observed. A high degree of variability in individual patient responses was seen.
Danirixin has previously been shown to successfully block CXCL-1-induced CD11b upregulation as a marker of neutrophil activation [11, 12, 14], and so to understand the negative clinical results with danirixin, we replicated this in vitro. CXCL-8 is an inflammatory cytokine that has been previously shown to activate neutrophils and stimulate NET formation through activation of CXCR1 and, predominantly, CXCR2. Pre-treatment with 10 µM danirixin, a dose that has been previously shown to block CXCR2 fully, reduced CXCL-8-mediated CD11b expression by an average of 46.4%. Similar results were obtained with CXCL-1, a specific CXCR2 agonist. This suggests that danirixin inhibits neutrophil activation in response to CXCR2 agonists but at the doses used, some residual activation was observed. Since danirixin is a CXCR2 antagonist, it would be expected that any potential reduction in neutrophil activation or NET formation would be dependent of the concentration of CXCR2 agonists, with prior work suggesting CXCL-8 would be the key mediator [7]. We, however, observed inconsistent results with different COPD sputa when using these as the stimulus for neutrophil activation. Measurement of CXCL-8 concentration in the 15 sputum samples showed no correlation with reduction in neutrophil activation. Our results demonstrate that there is a subset of individuals with COPD in whom neutrophil activation is independent of CXCR2, which could also explain why we failed to see a reduction in NET formation in our study.
The implications of our findings are significant for development of CXCR2 antagonists and other mechanisms targeting NETosis, suggesting that this mechanism will only work in a subset of COPD patients, perhaps explaining previous inconsistent efficacy results with this mechanism of action [14, 15]. Our results indicate the complexity of targeting neutrophilic inflammation using a single activation pathway, suggesting that additional research is required in order to develop a personalised medicine approach.
Acknowledgements
The authors would like to thank Sarah Waite, Attiya Idrees, Nakash Shetty, Caron Moss and other members of the GSK and University of Dundee study team during the conduct of the study.
Footnotes
Support statement: This study (GSK study 207551) was funded by GSK. Funding information for this article has been deposited with the Crossref Funder Registry.
Conflict of interest: H.R. Keir has nothing to disclose.
Conflict of interest: H. Richardson has nothing to disclose.
Conflict of interest: C. Fillimore is an employee of and shareholder in GSK.
Conflict of interest: A. Shoemark has nothing to disclose.
Conflict of interest: A.L. Lazaar is an employee of and shareholder in GSK.
Conflict of interest: B.E. Miller is an employee of and shareholder in GSK.
Conflict of interest: R. Tal-Singer is a former employee of and current shareholder in GSK, and reports personal fees from Immunomet outside the submitted work.
Conflict of interest: J.D. Chalmers reports grants from GSK during the conduct of the study; and grants and personal fees from AstraZeneca and Boehringer Ingelheim, grants from Gilead Sciences, grants and personal fees from Grifols and Insmed, and personal fees from GSK, Chiesi, Napp, Novartis and Zambon, outside the submitted work.
Conflict of interest: D. Mohan is an employee of and shareholder in GSK.
References
- 1.Cowburn AS, Condliffe AM, Farahi N, et al. Advances in neutrophil biology: clinical implications. Chest 2008; 134: 606–612. doi: 10.1378/chest.08-0422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung KF. Cytokines in chronic obstructive pulmonary disease. Eur Respir J 2001; 18: Suppl. 34, 50s–59s. doi: 10.1183/09031936.01.00229701 [DOI] [PubMed] [Google Scholar]
- 3.Porto BN, Stein RT. Neutrophil extracellular traps in pulmonary diseases: too much of a good thing? Front Immunol 2016; 7: 311. doi: 10.3389/fimmu.2016.00311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dicker AJ, Crichton ML, Pumphrey EG, et al. Neutrophil extracellular traps are associated with disease severity and microbiota diversity in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol 2018; 141: 117–127. doi: 10.1016/j.jaci.2017.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mozzini C, Garbin U, Fratta Pasini AM, et al. An exploratory look at NETosis in atherosclerosis. Intern Emerg Med 2017; 12: 13–22. doi: 10.1007/s11739-016-1543-2 [DOI] [PubMed] [Google Scholar]
- 6.Liu T, Wang FP, Wang G, et al. Role of neutrophil extracellular traps in asthma and chronic obstructive pulmonary disease. Chin Med J 2017; 130: 730–736. doi: 10.4103/0366-6999.201608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pedersen F, Waschki B, Marwitz S, et al. Neutrophil extracellular trap formation is regulated by CXCR2 in COPD neutrophils. Eur Respir J 2018; 51: 1700970. doi: 10.1183/13993003.00970-2017 [DOI] [PubMed] [Google Scholar]
- 8.Wright TK, Gibson PG, Simpson JL, et al. Neutrophil extracellular traps are associated with inflammation in chronic airway disease. Respirology 2016; 21: 467–475. doi: 10.1111/resp.12730 [DOI] [PubMed] [Google Scholar]
- 9.Grabcanovic-Musija F, Obermayer A, Stoiber W, et al. Neutrophil extracellular trap (NET) formation characterises stable and exacerbated COPD and correlates with airflow limitation. Respir Res 2015; 16: 59. doi: 10.1186/s12931-015-0221-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skopelja-Gardner S, Jones JD, Rigby WFC. “NETtling” the host: breaking of tolerance in chronic inflammation and chronic infection. J Autoimmun 2018; 88: 1–10. doi: 10.1016/j.jaut.2017.10.008 [DOI] [PubMed] [Google Scholar]
- 11.Busch-Petersen J, Carpenter DC, Burman M, et al. Danirixin: a reversible and selective antagonist of the CXC chemokine receptor 2. J Pharmacol Exp Ther 2017; 362: 338–346. doi: 10.1124/jpet.117.240705 [DOI] [PubMed] [Google Scholar]
- 12.Miller BE, Mistry S, Smart K, et al. The pharmacokinetics and pharmacodynamics of danirixin (GSK1325756) –a selective CXCR2 antagonist – in healthy adult subjects. BMC Pharmacol Toxicol 2015; 16: 18. doi: 10.1186/s40360-015-0017-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haslett C, Guthrie LA, Kopaniak MM, et al. Modulation of multiple neutrophil functions by preparative methods or trace concentrations of bacterial lipopolysaccharide. Am J Pathol 1985; 119: 101–110. [PMC free article] [PubMed] [Google Scholar]
- 14.Lazaar AL, Miller BE, Tabberer M, et al. Effect of the CXCR2 antagonist danirixin on symptoms and health status in COPD. Eur Respir J 2018; 52: 1801020. doi: 10.1183/13993003.01020-2018 [DOI] [PubMed] [Google Scholar]
- 15.Rennard SI, Dale DC, Donohue JF, et al. CXCR2 antagonist MK-7123. A phase 2 proof-of-concept trial for chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2015; 191: 1001–1011. doi: 10.1164/rccm.201405-0992OC [DOI] [PubMed] [Google Scholar]