Abstract
New biologics are being continually developed for paediatric asthma, but it is unclear whether there are sufficient numbers of children in Europe with severe asthma and poor control to recruit to trials needed for registration. To address these questions, the European Respiratory Society funded the Severe Paediatric Asthma Collaborative in Europe (SPACE), a severe asthma registry. We report the first analysis of the SPACE registry, which includes data from 10 paediatric respiratory centres across Europe.
Data from 80 children with a clinical diagnosis of severe asthma who were receiving both high-dose inhaled corticosteroid and long-acting β2-agonist were entered into the registry between January 2019 and January 2020. Suboptimal control was defined by either asthma control test, or Global Initiative for Asthma criteria, or ≥2 severe exacerbations in the previous 12 months, or a combination.
Overall, 62 out of 80 (77%) children had suboptimal asthma control, of whom 29 were not prescribed a biologic. However, in 24 there was an option for starting a licensed biologic. 33 children with suboptimal control were prescribed a biologic (omalizumab (n=24), or mepolizumab (n=7), or dupilumab (n=2)), and for 29 there was an option to switch to a different biologic.
We conclude that the SPACE registry provides data that will support the planning of studies of asthma biologics. Not all children on biologics achieve good asthma control, and there is need for new trial designs addressing biologic switching.
Short abstract
Analysis of the Severe Paediatric Asthma Collaborative in Europe (SPACE) registry reveals that asthma control remains suboptimal for some children on biologics and that the registry has the capacity to facilitate trials of new biologics https://bit.ly/3hPA2fu
Introduction
The development of biologics has greatly expanded the treatment options for children and young people with severe asthma. Indeed, a recent Cochrane review [1] concluded that omalizumab, a monoclonal antibody that binds and inhibits free serum IgE, was effective in both adults and children in reducing asthma exacerbations and hospitalisations, and increased the number of trial subjects who either reduced or stopped inhaled corticosteroids (ICSs). The other licensed biologics for European children target interleukin (IL)-5 (mepolizumab from 6 years of age), IL-4 α receptor, and IL-13 (dupilumab from 12 years of age) [2, 3]. The ongoing or planned clinical trials in children and adolescents of biologics reported in Clinicaltrials.gov include targeting IL-6 (clazakizumab), IL-13 (lebrikizumab and tralokinumab), granulocyte-macrophage colony-stimulating factor (GM-CSF, KB003), and epithelial-cell–derived cytokine thymic stromal lymphopoietin (tezepelumab). These randomised placebo-controlled trials will recruit children from 12 years of age who, despite prescribed pre-defined high-dose treatment regimes, have poorly controlled asthma defined by various combinations of number of exacerbations per year, lung function, total IgE, blood eosinophils and asthma control questionnaire scores. One problem in recruiting to these trials is whether current licensed biologics already address the needs of the European children with severe asthma and poor control. If this is indeed the case, recruitment to new trials that include a placebo arm may be problematic for clinicians.
To obtain more data on children eligible to start a biologic or switch to a different biologic, the European Respiratory Society funded the Severe Paediatric Asthma Collaborative in Europe (SPACE) registry. SPACE is a prospective, noninterventional, pan-European observational registry for severe paediatric asthma where consent is obtained for both longitudinal data entry and to approach families for new studies. The development of the SPACE protocol has previously been reported [4, 5]. From January 2019 to January 2020 data were entered from consented children attending 10 specialised paediatric asthma centres in six European countries. Each centre was asked to enter data for 8–10 children. We now report the first cross-sectional analysis of these data. We aimed to assess whether SPACE has provided rapid information relevant to the planning of new studies in children with severe asthma, and whether the current three licensed biologics in Europe are sufficient to address the clinical needs of children with severe asthma and suboptimal control.
Methods
Registry
Data were entered by the respiratory paediatricians managing children with severe asthma in 10 secondary/ tertiary care centres in six European countries (table 1). The registry was a collaboration with the Health Informatics Centre, University of Dundee (UK). Non anonymised data were available to the local clinician, but only anonymised data were stored in Dundee (UK) using the Health Informatics Centre Safe Haven model. The protocol was approved by the UK Research Ethics Committee (reference 18/LO/0178). Local centres also obtained research ethics committee approval. Informed consent from parents/guardians/young people and assent from children were for both collection of baseline and longitudinal data, and to be approached for future studies. For this first analysis, each centre was asked to recruit and enter data for 8–10 children during routine asthma outpatient clinic appointments.
TABLE 1.
Summary of Severe Paediatric Asthma Collaborative in Europe participating centres and the number of children with severe asthma recruited from each centre
| Hospital | City, country | Number of children recruited |
| Anna Meyer Children's University Hospital | Florence, Italy | 10 |
| Emma Children's Hospital AMC | Amsterdam, The Netherlands | 9 |
| Erasmus MC-Sophia Children's Hospital | Rotterdam, The Netherlands | 11 |
| Marien Hospital | Wesel, Germany | 11 |
| Marmara University Faculty of Medicine Pendik Hospital | Pendik, Turkey | 2 |
| Oslo University Hospital | Oslo, Norway | 7 |
| Sapienza University of Rome | Rome, Italy | 5 |
| Royal Brompton Hospital | London, UK | 10 |
| Royal Hospital for Sick Children | Edinburgh, UK | 6 |
| Royal London Hospital | London, UK | 9 |
Eligibility
In developing the eligibility criteria for SPACE, members reviewed the current literature on definitions of severe paediatric asthma, as previously reported [4, 5]. This included reviewing the ERS/American Thoracic Society (ATS) guidelines [6]. Inclusion criteria for children with “severe asthma” for the SPACE registry were: 1) age 6 to 17 years, 2) clinical and spirometry evidence of asthma, and 3) requiring high-dose ICSs and a long-acting β-agonist (LABA) for ≥6 months, and/or systemic corticosteroids for ≥25% of the previous year. The SPACE definition of high-dose ICS for all manufactures and inhaler types has previously been reported [4], and includes >800 μg·day−1 beclomethasone or equivalent by dry powder inhaler or CFC-metered dose inhaler, or >380 μg·day−1 beclomethasone or equivalent by hydrofluroalkane metered dose inhaler. Children with conditions that mimicked asthma symptoms were excluded [4]. The registry was developed with the review and feedback from the patient advisory group supported by the European Lung Foundation ((ELF) www.europeanlung.org). Members of the ELF and patient advisory group are members of the steering committee of the SPACE registry.
Suboptimal asthma control
Suboptimal control in children with severe asthma was defined as either an asthma control test (ACT) score of ≤19 [7], or meeting the criteria for “partly controlled” or “uncontrolled” asthma defined by the Global Initiative for Asthma (GINA) assessment questions [8], or with ≥2 severe exacerbations during the previous 12 months, or any combination. A severe exacerbation was defined as an episode requiring either systemic corticosteroids for ≥3 days for acute asthma, or an unplanned hospital admission, or any combination.
Allergic asthma
Allergic asthma was defined as a positive skin-prick test or blood specific IgE [6] to perennial aeroallergen and/or food allergen of >0.35 kU·L−1 [9]. A baseline blood eosinophils level of ≥150 cells·μL−1 was defined as “high” [10]. A clinically high fractional exhaled nitric oxide (FeNO) was defined as >35 ppb [11].
Lung function
Predicted spirometry values of forced expiratory volume in 1 s (FEV1) and forced vital capacity were calculated from raw data, using the Global Lung Initiative equations [12], with bronchodilator reversibility defined as ≥12% change in FEV1 [13].
Adherence
Adherence of children to prescribed therapy was assessed as part of normal clinical management. Entry into the database did not require “objective” assessment of adherence by, for example, Smart Inhaler. There was however an optional section of the registry that included questions on objective assessment of adherence by prescription refill rate, electronic monitoring and serum drug levels.
Option to start or switch biologic
In children with severe asthma and suboptimal control, the option for starting or switching a biologic was assessed by mapping SPACE data to the current EU licensed biologics (table 2).
TABLE 2.
Eligibility criteria for the three currently licensed biologics, based on manufacturers’ recommendations
| Biologics | Licensed eligibility criteria |
| Omalizumab | 1. Age ≥6 years |
| 2. Uncontrolled asthma with frequent symptoms and multiple documented severe asthma exacerbations despite daily high-dose inhaled corticosteroids, plus a long-acting inhaled β-agonist# | |
| 3. IgE mediated asthma (positive skin-prick test and/or raised specific IgE). | |
| 4. For age ≥12 years, reduced lung function (FEV1<80%) | |
| 5. Total IgE over 30 and up to 700 or 1300 IU·mL−1 according to age | |
| Mepolizumab | 1. Age ≥6 years |
| 2. Baseline blood eosinophils ≥150 cells·μL−1 | |
| 3. ≥2 exacerbations a year (systemic corticosteroids use, unplanned medical visits/hospital admissions) | |
| Dupilumab | 1. Aged ≥12 years |
| 2. Eosinophilic/type 2 asthma | |
| 3. Or, oral corticosteroid dependent or with comorbid moderate-to-severe atopic dermatitis |
#: We defined uncontrolled asthma as an asthma control test score of ≤19, or “partly controlled/uncontrolled” using the Global Initiative for Asthma assessment questions, or having ≥2 exacerbations a year, or a combination. FEV1: forced expiratory volume in 1 s.
Analysis
Data are summarised as either mean±sem, or as median (interquartile range, IQR).
Results
Data on 80 children with severe asthma were entered into the SPACE registry (table 3). Objective assessment of adherence was reported in 24 (30%) children using prescription refill rate (n=20), electronic monitoring (n=9), and drug level (n=1). Allergic asthma was defined in 58/80 (73%) children. High FeNO was reported in 28 of the 55 where FeNO was recorded (table 3).
TABLE 3.
Summary demographics and findings of all recruited children. Demographics, background, investigation results, asthma control scores and respiratory treatments for all participants
| Registry variables | Number of patients |
| Age | |
| 6–11 years; mean±sem | 26 (32%); 9.37±0.31 years |
| ≥12 years; mean±sem | 54 (68%); 14.70±0.22 years |
| Overall mean±sem | 12.96±0.33 years |
| Sex male/female | 49 (61%)/ 31 (39%) |
| Ethnicity | Caucasians: 60 (75%); South-east Asians: 8 (10%); Black: 8 (10%); Mixed: 4 (5%) |
| Parental history | Asthma: 38; Atopic eczema: 17; Allergic rhinitis: 33 |
| Smoking history | Personal smoking: 2; Paternal smoking: 41; Maternal smoking: 12 |
| Allergic asthma | |
| Skin prick tests | Positive for perennial aeroallergen: 31; Positive for food allergen: 14 |
| Specific IgE | Positive for perennial aeroallergen: 42; Positive for food allergen: 16 |
| Highest blood eosinophils (cells·µL-1) in the 6 months prior to enrolment | ≥150: 41; <150: 11; Not recently assessed: 28 |
| Highest blood IgE (IU·mL−1) in the 6 months prior to enrolment | ≥700: 21; 30–699: 26; <30: 5; Not recently assessed: 28 |
| Highest FeNO (ppb) in the 6 months prior to enrolment | >35: 28; 20–35: 11; <20: 16; Not recently assessed: 25 |
| Asthma control | |
| ACT score | |
| Median (IQR) ACT | 20 (16–23) |
| Control by ACT | Poor control (<20): 26; Good control (≥20): 31; Not assessed: 23 |
| GINA asthma control | Uncontrolled: 25; Partly controlled: 23; Well controlled: 32 |
| ≥2 exacerbations per year | Total:49; With good ACT/GINA control: 12 |
| Suboptimal control by SPACE definition | 62 |
| Best FEV1 (% predicted) in the 6 months prior to enrolment | <70%: 7; 70–79%: 10; 80–89%: 16; ≥90%: 46; Not recently assessed: 1 |
Data are presented as n (%) or n, unless otherwise stated. FeNO: exhaled nitric oxide fraction; ACT: asthma control test; IQR: interquartile range; GINA: Global Initiative for Asthma; SPACE: Severe Paediatric Asthma Collaborative in Europe; FEV1: forced expiratory volume in 1 s.
Overall, 62/80 (77%) had suboptimal asthma control (table 3). Clinically low lung function (FEV1 of <80% predicted) in children with suboptimal control was reported in 17 children, with a FEV1 of <70% predicted in seven (table 3).
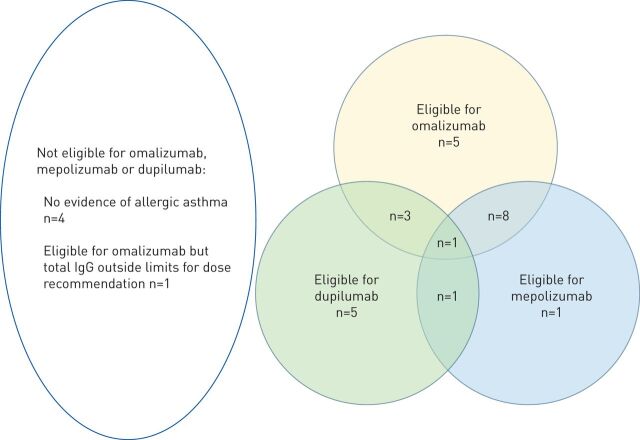
All children were receiving high-dose ICSs, as defined by the SPACE protocol [4]; 15 children were on ICS and LABA alone (table 4). 29 out of 38 children not prescribed a biologic, had suboptimal asthma control. Of those with suboptimal control, 17 were eligible for omalizumab, 11 was eligible for mepolizumab, and 10 was eligible for dupilumab. Overlap in eligibility is shown in figure 1. Overall, five children with suboptimal control were ineligible to start a licensed biologic (n=4 no allergic asthma, n=1 IgE outside the licensed range, fig. 1).
TABLE 4.
Therapies in addition to high-dose inhaled corticosteroids (ICSs) and long-acting β2 agonist (LABA).
| Nonbiologic addition | Biologic addition | Number of children |
| Montelukast | 15 | |
| Tiotropium | 4 | |
| Ipratropium | 1 | |
| Triamcinolone | 1 | |
| Montelukast, Tiotropium | 1 | |
| Montelukast, Tiotropium Ipratropium | 1 | |
| Omalizumab | 10 | |
| Montelukast | Omalizumab | 15 |
| Ipratropium | Omalizumab | 1 |
| Montelukast, Tiotropium | Omalizumab | 4 |
| Montelukast, Ipratropium | Omalizumab | 1 |
| Mepolizumab | 5 | |
| Montelukast | Mepolizumab | 2 |
| Tiotropium | Mepolizumab | 1 |
| Ipratropium | Mepolizumab | 1 |
| Montelukast | Dupilumab | 1 |
| Tiotropium | Dupilumab | 1 |
All children were receiving a high dose of inhaled corticosteroids (ICSs), as defined by the SPACE protocol [4]. 15 children were on ICS and LABA alone.
FIGURE 1.
The number of children who were not prescribed a biologic and who had suboptimal asthma control based on the Severe Paediatric Asthma Collaborative in Europe criteria, eligible for omalizumab, mepolizumab and dupilumab.
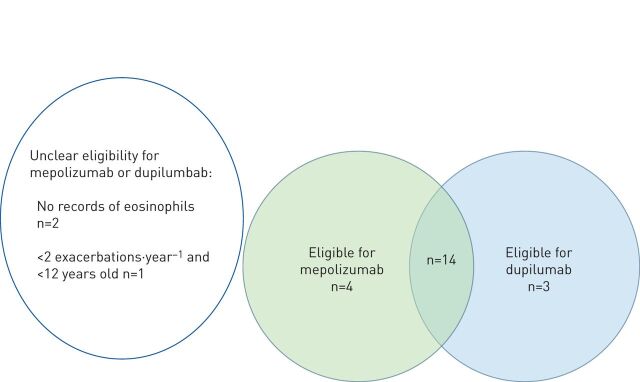
Thirty-three children of 42 who were prescribed a biologic had suboptimal control. Of the 24 children prescribed omalizumab, 18 were eligible to switch to mepolizumab based on either current (within 6 months) or a previous blood eosinophil count, and 17 were eligible to switch to dupilumab (fig. 2). An option of switching from omalizumab to mepolizumab could not be determined in two children due to no recorded eosinophil count, and one child was ineligible for mepolizumab because of fewer than two exacerbations a year and being <12 years.
FIGURE 2.
The number of children who were prescribed omalizumab with suboptimal asthma control based on the Severe Paediatric Asthma Collaborative in Europe criteria who were eligible to switch to a different biologic. For two children, eligibility to switch was unclear due to lack of data on eosinophil count. One child with no option to switch had <2 exacerbations (therefore ineligible for mepolizumab) and was <12 years old (therefore ineligible for dupilumab).
Seven children on mepolizumab had suboptimal asthma control, and five of these were eligible to switch to dupilumab. One child on mepolizumab was eligible to switch to omalizumab. No switch option was available for one child on mepolizumab due to a history of a failed clinical response to omalizumab, as well as aged less that 12 years. Two children on dupilumab had suboptimal control, and both children were eligible to switch to mepolizumab.
Discussion
There are two major outcomes of the first-year analysis of data entered into the SPACE registry. First, we have demonstrated that it is feasible to enter data on children with severe asthma that is both compliant with European Union General Data Protection Regulation and approved by research ethics committees across Europe. The rapidly searchable capacity of the anonymised SPACE database is thus a resource for assessing the feasibility of both pharmacokinetic studies and randomised controlled trials of new biologics in children with severe asthma. Furthermore, as a registry, children and parents/guardians have provided assent and consent to be approached for future asthma studies, and for longitudinal data entry. The SPACE registry in its current format will therefore be able to support recruitment into pan-European clinical trials of new asthma therapies in children, and to provide post-marketing data on biologics.
Second, for children with severe asthma not currently prescribed a biologic, we found that, for most with suboptimal asthma control, there was an option of starting one of the three biologics licensed by the European Medicines Agency, subject to national restrictions. However, for five children of the 29 children with suboptimal control, there was no option to start a biologic, suggesting that there remains a need to develop biologics with less restrictive eligibility criteria. For those children prescribed a biologic, we also identified a group with suboptimal asthma control. Although the reasons for poor control in these children could not be identified from the SPACE database, one potential explanation is that the current biologic is not fully supressing asthmatic airway inflammation. However, for the majority of children on a biologic with suboptimal asthma control, there was an option to switch to a different licensed biologic. These data suggest that trials of new therapies at the point where clinicians would consider switching to a different biologic is feasible in Europe. However, designing trials at this switch point, as recently advocated by Pilette et al. [14] may need to be pragmatic, since switching children with severe asthma and suboptimal control from an active biologic to a placebo is problematic.
There are limitations to this study. First, limited time of clinicians meant that not all eligible children with severe asthma could be approached for consent, and our data may not be generalisable to all children with severe asthma. But over the next years we intend to expand the number of participating centres, and record data on the total number of children managed with severe asthma, and the number of parents and children approached for consent for the registry. Second, standardised “objective” assessment of adherence was not a pre-requisite for inclusion to SPACE, and it is unclear to what extent adherence contributed to suboptimal control. However, a biologic may be initiated by a paediatrician even if a child has suboptimal adherence in order to reduce the risk of a fatal attack. Thus, trials of biologics where good adherence must be first demonstrated may not reflect the full complexity of paediatric practice in real-life.
In summary, we have demonstrated the feasibility of recruiting children with severe asthma across Europe into a prospective registry, and the capacity to generate data in real-time to support the planning of studies of new asthma therapies. This first analysis of the SPACE database suggests that there remain gaps that need to be covered by new biologics, and that there is a need to innovative pragmatic trial design for children currently prescribed a biologic.
Acknowledgements
The authors thank James Chalmers, who facilitated the liaison with the Health Informatics Centre at the University of Dundee (Dundee, UK); and Julie Westwood (asthma nurse specialist at the Royal Hospital for Sick Children, Edinburgh, UK), Simone Hashimoto (Emma Children's Hospital, Amsterdam, The Netherlands) and Dominika Ambrozej, who facilitated local recruitment and data entry. The authors also thank James Paton who is the European Respiratory Society Clinical Research Collaboration Link Person for the SPACE registry. SPACE acknowledges the contribution from the ELF and the patient representatives from the patient advisory group, who are members of the SPACE steering committee.
Footnotes
Data availability: Anonymised individual data are available on request to recognised academic institutions, health service organisations or commercial research organisations with experience in medical research.
Author contributions: J. Grigg and F. Rusconi are joint chief investigators of the SPACE Clinical Research Collaboration. B. Karadag, M.W.H Pijnenburg and F. Rusconi performed the literature review for registry entry criteria. N.M. Liu analysed the data. All other authors contributed equally to the registry design and implementation, and data entry. All authors contributed to writing the final manuscript.
Conflict of interest: N.M. Liu has nothing to disclose.
Conflict of interest: K.C.L. Carlsen has nothing to disclose.
Conflict of interest: S. Cunningham has nothing to disclose.
Conflict of interest: G. Fenu reports advisory board fees from GSK, clinical trial sponsorship from Sanofi, expenses for congresses and meetings from Novartis, personal fees for teaching a course from Chiesi, and expenses for congresses and meetings from Lusofarmaco, outside the submitted work.
Conflict of interest: L.J. Fleming reports grants from Asthma UK, and fees for expert consultation and speakers fees from AstraZeneca, GSK, Novartis, Teva, Bohringer Ingelheim, Respiri and Sanofi paid direct to her institution and outside of the submitted work
Conflict of interest: M. Gappa reports that she has received honoraria for lectures and/or consultancy from Boehringer Ingelheim, GSK, Novartis and Sanofi. She believes that this has not influenced any of the submitted work.
Conflict of interest: B. Karadag reports for the multicentre, phase III dupilumab study EFC14153 from Sanofi, outside the submitted work.
Conflict of interest: F. Midulla has nothing to disclose.
Conflict of interest: L. Petrarca has nothing to disclose.
Conflict of interest: M.W.H. Pijnenburg reports a consultancy fee paid to her department by Sanofi Genzyme during the conduct of the study.
Conflict of interest: T. Reier-Nilsen has nothing to disclose.
Conflict of interest: N.W. Rutjes has nothing to disclose.
Conflict of interest: F. Rusconi has nothing to disclose.
Conflict of interest: J. Grigg reports personal fees for asthma advisory boards from AstraZeneca and GSK, personal fees for a continuing professional development lecture from Novartis, and personal fees for an asthma advisory board from Vifor Pharma, outside the submitted work.
Support statement: This study was supported by the European Respiratory Society (grant SPACE CRC). Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Normansell R, Walker S, Milan SJ, et al. . Omalizumab for asthma in adults and children. Cochrane Database Syst Rev 2014: CD003559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed H, Turner S. Severe asthma in children-a review of definitions, epidemiology, and treatment options in 2019. Pediatr Pulmonol 2019; 54: 778–787. doi: 10.1002/ppul.24317 [DOI] [PubMed] [Google Scholar]
- 3.Delimpoura V, Bostantzoglou C, Liu N, et al. . Novel therapies for severe asthma in children and adults. Breathe (Sheff) 2018; 14: 59–62. doi: 10.1183/20734735.018917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu NM, van Aalderen W, Carlsen KCL, et al. . Severe Paediatric Asthma Collaborative in Europe (SPACE): protocol for a European registry. Breathe (Sheff) 2018; 14: 93–98. doi: 10.1183/20734735.002018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rusconi F, Fernandes RM, Pijnenburg MWH, et al. . The Severe Paediatric Asthma Collaborative in Europe (SPACE) ERS Clinical Research Collaboration: enhancing participation of children with asthma in therapeutic trials of new biologics and receptor blockers. Eur Respir J 2018; 52: 1801665. doi: 10.1183/13993003.01665-2018 [DOI] [PubMed] [Google Scholar]
- 6.Chung KF, Wenzel SE, Brozek JL, et al. . International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014; 43: 343–373. doi: 10.1183/09031936.00202013 [DOI] [PubMed] [Google Scholar]
- 7.Schatz M, Sorkness CA, Li JT, et al. . Asthma control test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol 2006; 117: 549–556. doi: 10.1016/j.jaci.2006.01.011 [DOI] [PubMed] [Google Scholar]
- 8.Global Initiative for Asthma. Pocket guide for asthma management and prevention (for adults and children older than 5 years). A pocket guide for health professionals. https://ginasthma.org/wp-content/uploads/2018/03/wms-GINA-main-pocket-guide_2018-v1.0.pdf Date last updated: 2018.
- 9.Pastorello EA, Incorvaia C, Ortolani C, et al. . Studies on the relationship between the level of specific IgE antibodies and the clinical expression of allergy: I. Definition of levels distinguishing patients with symptomatic from patients with asymptomatic allergy to common aeroallergens. J Allergy Clin Immunol 1995; 96(5 Pt 1): 580–587. doi: 10.1016/S0091-6749(95)70255-5 [DOI] [PubMed] [Google Scholar]
- 10.Katz LE, Gleich GJ, Hartley BF, et al. . Blood eosinophil count is a useful biomarker to identify patients with severe eosinophilic asthma. Ann Am Thorac Soc 2014; 11: 531–536. doi: 10.1513/AnnalsATS.201310-354OC [DOI] [PubMed] [Google Scholar]
- 11.Dweik RA, Boggs PB, Erzurum SC, et al. . An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FeNO) for clinical applications. Am J Respir Crit Care Med 2011; 184: 602–615. doi: 10.1164/rccm.9120-11ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quanjer PH, Stanojevic S, Cole TJ, et al. . Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J 2012; 40: 1324–1343. doi: 10.1183/09031936.00080312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller MR, Hankinson J, Brusasco V, et al. . Standardisation of spirometry. Eur Respir J 2005; 26: 319–338. doi: 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 14.Pilette C, Brightling C, Lacombe D, et al. . Urgent need for pragmatic trial platforms in severe asthma. Lancet Respir Med 2018; 6: 581–583. doi: 10.1016/S2213-2600(18)30291-1 [DOI] [PubMed] [Google Scholar]