Abstract
After a centenary fight against malaria, Brazil has seen an opportunity for change with the proposal of the malaria elimination policy set by the Brazilian government, in line with malaria elimination policies in other Latin American countries. Brazilian malaria experts regard eliminating malaria by 2030 to be within reach. Herein we evaluated the likelihood that malaria elimination can be accomplished in Brazil through systematic review of the literature on malaria elimination in Brazil and epidemiological analysis. Fifty-two articles referring to malaria eradication/elimination in Brazil were analyzed to identify challenges and technological breakthroughs for controlling malaria. Monthly deaths (1979–2016) and monthly severe malaria cases (1998–2018) were analyzed according to age groups, geographic region and parasite species. As a result, we observed that the declining malaria burden was mostly attributable to a decline in Plasmodium falciparum-malaria. At the same time, the proportional increase of Plasmodium vivax-malaria in comparison with P. falciparum-malaria was notable. This niche replacement mechanism was discussed in the reviewed literature. In addition, the challenges to P. vivax-malaria elimination outnumbered the available technological breakthroughs. Although accumulated and basic information exists on mosquito vector biology, the lack of specific knowledge about mosquito vector taxonomy and ecology may hamper current attempts at stopping malaria in the country. An impressive reduction in malaria hospitalizations and mortality was seen in Brazil in the past 3 decades. Eliminating malaria deaths in children less than 5 years and P. falciparum severe cases may be achievable goals under the current malaria policy until 2030. However, eliminating P. vivax malaria transmission and morbidity seems unattainable with the available tools. Therefore, complete malaria elimination in Brazil in the near future is unlikely.
INTRODUCTION
Malaria eradication became the Holy Grail after the successful eradication of Anopheles gambiae sensu lato from northeastern Brazil between 1939 and 1941 (Kerr, 1963; Packard and Gadelha, 1997; Killeen et al., 2002; Killeen, 2003). The successes and infrastructure of the Yellow Fever Service deployed for the eradication of Aedes aegypti from huge areas in Brazil (Kerr, 1963) served as the basis for a new Malaria Service created by the Rockefeller Foundation and the Brazilian government. This new entity eradicated An. gambiae s.l. from Brazil with a vector centered approach; insecticides were applied upon all potential larval habitats in 54,000-km2 of wetlands in the Northeastern Brazil (Killeen et al., 2002). The successful An. gambiae s.l. eradication in Brazil became the proof of concept for a wider and more ambitious malaria eradication operation, known as the Global Malaria Eradication Program (GMEP) launched in the 1950s (Packard and Gadelha, 1997). GMEP was premised on the use of chloroquine- based antimalarials and the insecticide dichloro-diphenyl-trichloroethane (DDT) for indoor spraying. GMEP actions resulted in malaria eradication from all developed countries, and a drastic reduction in malaria in tropical Asia and Latin America (Trigg and Kondrachine, 1998). GMEP also helped malaria eradication in the most populated cities and regions in the Atlantic coast of Brazil in 1960–70s (Corrêa and Alves, 1969; Mascarenhas, 1973).
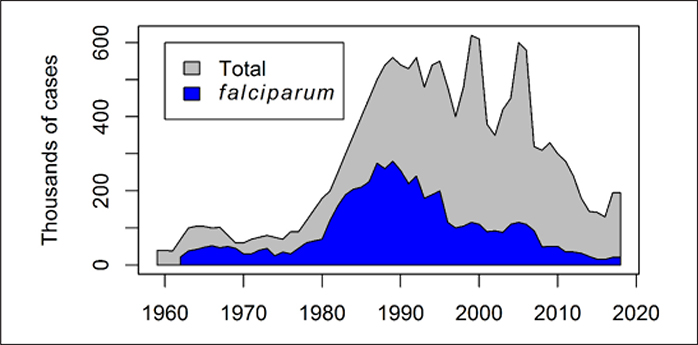
The GMEP successes were hampered by: (1) insecticide resistance, (2) Plasmodium falciparum chloroquine-resistance, (3) anthropological factors (e.g., migration, isolation, lack of education, environmental modification), and (4) operational, administrative and financial difficulties (Palacios Fraire, 1975). After the end of GMEP, malaria re-emerged and malaria burden increased in endemic tropical countries, with the highest transmission levels in sub-Saharan areas of Africa (Trigg and Kondrachine, 1998). In Brazil, malaria was well-controlled during the GMEP. However, after the end of GMEP, malaria emerged in the 1980s, especially in new settlements in Amazonia (Vosti, 1990) (Figure 1).
Figure 1.
Annual number of laboratory-confirmed malaria cases reported in Brazil from 1959 to 2018. The total number of cases, and those due to Plasmodium falciparum, are shown. Data Sources: National Malaria Prevention and Control Programme, Ministry of Health of Brazil; partially adapted from (Ferreira and Castro, 2016).
Eradication means ultimate extermination of a given species on any temporal or spatial scale. However, following multiple failures to eradicate disease, the eradication concept became controversial (Roberts and Enserink, 2007). A critic of disease eradication stated: “It is a moot question which is the more sophisticated: Homo sapiens or Treponema pallidum?” (Kerr, 1963). More recently, a second attempt for eradication, namely malaria global elimination, is proposed (Hommel, 2008). Malaria elimination means that a given species (i.e., P. falciparum) can be removed from humans in a certain location and in a given period under continuous control activities and improvements (World Health Organization, 2015). Today, the global malaria community is even more confident with the recent malaria burden decline and the compliance of the governments of endemic countries with the policy of malaria elimination (Rabinovich et al., 2017). They advocate, for instance, if all endemic countries eliminate malaria globally, the ultimate outcome will be malaria eradication.
The governments of countries in Latin America have complied with the WHO Global Technical Strategy for malaria control and elimination 2016–2030 (World Health Organization, 2015). Because of the research agenda for malaria elimination and eradication (Rabinovich et al., 2017), aligned with the reduction of extreme poverty (United Nations, 2015), Latin American scientists are now more optimistic about the possibility of drastic reductions in P. falciparum malaria mortality (Arevalo-Herrera et al., 2012; Chaccour et al., 2015; Pacheco et al., 2015; van Eer et al., 2018). On the one hand, looking at the malaria trends in the Americas, the incidence of this disease has been reduced drastically in the last decade (Castellanos et al., 2015). On the other hand, malaria elimination has encountered several challenges in Brazil (Silva-Nunes et al., 2012; Ferreira and Castro, 2016) and in Venezuela (Grillet et al., 2019).
Brazil is fully expecting to become recognized as a high-income industrial country (Murphy and Mullis, 2011). In order to join the ranks of high-income industrial countries, neglected tropical diseases associated with poverty, such as malaria, must be controlled (Cashwell et al., 2014; Hotez and Fujiwara, 2014). Herein, we evaluated the probability of malaria elimination in Brazil. We review the literature on malaria interventions and technological breakthroughs contributing to the decline of malaria. In addition, literature on socioeconomic development, historical interventions, and challenges to malaria elimination was reviewed. We counterbalanced the available technological breakthroughs with the challenges for malaria control to evaluate the possible outcomes under the ongoing malaria elimination policy. We validate our interpretations from the systematic review with epidemiological data analysis.
MATERIALS AND METHODS
Study area
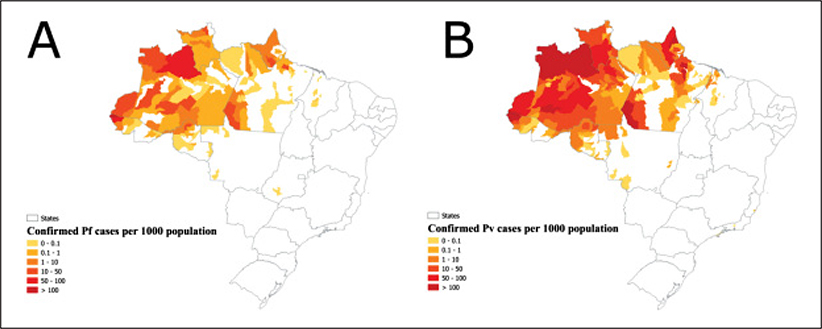
Brazil encompasses the equatorial Amazon rainforest in the Northern Hemisphere to more temperate sub-tropical regions in the Southern Hemisphere, spanning over 35 degrees of latitude in South America. It has a population of 209 million (in 2019), which lives mostly in the municipalities along the Atlantic coast (ibge.gov.br/apps/populacao/projecao//index.html). The 2017 epidemiological profile of malaria transmission is shown (Figure 2).
Figure 2.
Confirmed cases per 1000 population in municipalities, Brazil, 2017. (A) Plasmodium falciparum (Pf). (B) Plasmodium vivax (Pv). Population at risk: 42.5 million people living in transmission zones (20% out of the Brazilian population). Data Source: (World Health Organization, 2018).
Systematic review
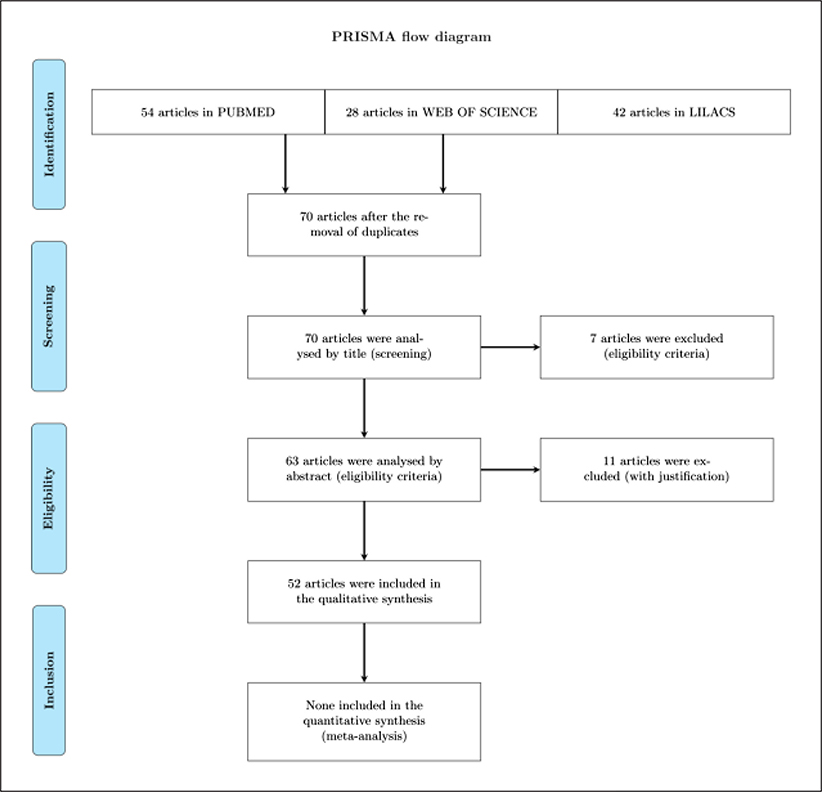
A systematic review was conducted of all available articles regarding malaria eradication or malaria elimination in Brazil. Potentially relevant articles in English, Portuguese, French or Spanish were accessed in March 14th 2018 from PUBMED (ncbi.nlm.nih.gov/pubmed/), LILACS (lilacs.bvsalud.org/en/) and WEB OF SCIENCE (apps-webofknowledge.ez329.periodicos.capes.gov.br) to review their full texts. We applied text search strategy using the combination of Health Sciences Descriptors (decs.bvs.br/I/homepagei.htm). Terms and keywords utilized were as follows “Brazil AND malaria AND disease eradication” or “Brazil AND malaria AND disease elimination”. We selected the following fields in text search strategy ALL FIELDS PUBMED, TOPIC/ALL YEARS WEB OF SCIENCE and ALL INDEXES LILACS. Only original and non- duplicated research studies were included. Eligibility for inclusion of articles was based on explicit relation to either technological breakthroughs for malaria control or challenges regarding malaria (re)emergence or both. After eliminating duplicates, we identified relevant papers by screening titles and abstracts. Two independent investigators (MAOP, RTAB) performed identification and screening of articles, while a third investigator (GZL) reviewed screening and performed eligibility of the articles included herein (Figure 3). Full articles reading were achieved by applying a strategy based on PICOs (P, patient, population or problem; I, intervention, C, comparison; O, outcomes; s, type of study) (Methley et al., 2014).
Figure 3.
The PRISMA Flow Diagram. Summary of the process of systematic literature review and synthesis.
Epidemiological analysis
We performed the following analyses: (1) malaria burden in Brazil, (2) geographical differences, (3) age-dependent outcomes, and (4) P falciparum vs. P vivax.
We define malaria burden as the incidence rate of mortality or hospitalization attributable to malaria. Mortality data was obtained from the Ministry of Health, 19792016. Hospitalization data was obtained from the Unified Health System, 1998–2018. Malaria hospitalization and mortality records were extracted using the code 084 in the International Classification of Diseases (ICD) 9 for data before 1995 and the codes B50- B54 in the ICD-10 for data from 1995 onwards. Data were organized in a 4-D matrix containing the following dimensions: (1) TIME, monthly malaria mortality rate (per 100,000 ppl.) from Jan 1979 to Dec 2016 (mortality) or monthly malaria hospitalization rate (per 100,000 ppl.) from Jan 1998 to Dec 2018; (2) GEOGRAPHIC, Brazilian states (26) and a Federal District; (3) AGE, age groups (less than 1 year, 0–4, 5–9, …, 80+); (4) PARASITE, malaria parasite species. Population data were interpolated in monthly series (epipoi.info/popweaver/). All analyses were performed using the EPIPOI software (epipoi.info/).
Ethical issues
Utilized data are of public domain according to the Brazilian Law of Information Access (12.527/2011). Patient information is not available.
RESULTS
Systematic review
Fifty-two articles in total were included in the qualitative synthesis (Figure 2). The overall number of articles referring to ‘malaria elimination’ (n = 39) was 3-fold higher than those about ‘malaria eradication’ (n = 13). The use of ‘malaria eradication’ referring to the Global Malaria Eradication Program 1955–1969 was frequently used before 2010. Out of 13 articles with the ‘malaria eradication’ term, 12 were published in-between 1963–2010. Starting in 2011 the term ‘malaria elimination’ appearance increased exponentially in the literature. Thirty-seven out of 39 articles with reference to the ‘malaria elimination’ term were published from 2011 to 2018.
Malaria eradication era
We identified 4 technological breakthroughs for controlling malaria under the policy of malaria eradication in Brazil: (1) larval control, (2) Global Malaria Eradication Program (GMEP), (3) income and literacy increase, and (4) Global Malaria Control Strategy (GMCS). In contrast, we identified 6 challenges that prevented malaria eradication from Brazil: (1) socioeconomic deficit, (2) insecticide resistance, (3) chloroquine-resistant P falciparum, (4) logistic issues, (5) simian malaria, and (6) eradication failure in specific contexts (Table 1).
Table 1.
Technological breakthroughs and challenges for controlling malaria in the ‘eradication era’ in Brazil
| Technological breakthroughs | Challenges | ||
|---|---|---|---|
| Types | References | Types | References |
| 1. Larval control | (Packard and Gadelha, 1997; Killeen et al., 2002; Killeen, 2003) | 1. Socioeconomic deficit | (Palacios Fraire, 1975; Vosti, 1990; Packard and Gadelha, 1997) |
| 2. Global Malaria Eradication Program | (Corrêa and Alves, 1969; Mascarenhas, 1973; Barata, 1997; Packard and Gadelha, 1997; Trigg and Kondrachine, 1998; Bleakley, 2010; Silva and Paiva, 2015) | 2. Insecticide resistance | (Palacios Fraire, 1975) |
| 3. Income and literacy increase | (Bleakley, 2010) | 3. Plasmodium falciparum resistance to chloroquine | (Palacios Fraire, 1975) |
| 4. Global Malaria Control Strategy | (Trigg and Kondrachine, 1998) | 4. Logistic issues | (Palacios Fraire, 1975) |
| 5. Simian malaria | (Deane, 1969; Palacios Fraire, 1975) | ||
| 6. Eradication is not always possible | (Kerr, 1963) | ||
The Larval control (Table 1) employed by the Rockefeller Foundation and the Brazilian government, under the leadership of Fred Soper, was an outstanding success through a vertically integrated programme that relied overwhelmingly on larval control. The success behind the Malaria Service of Northeast Brazil, where An. gambiae s.l. had invaded, was the organization of its activities. A cartographic unit mapped infested areas using aerial photographs. A field laboratory and epidemiological section allowed training, surveillance and decision-making. The entire infested area was divided into zones. All potential larval habitats were treated with Paris Green (a larvicide with established toxicity) in each zone during one week. This approach was soon repeated and suppressed malaria in Upper Egypt and Zambia, Africa, in the 1940s. These successful approaches were neglected after the advent of DDT. The policy for mosquito eradication under the Global Malaria Eradication Program (Table 1) shifted toward domestic adulticide methods.
A former Brazilian president Juscelino Kubitschek (1956–1961) established development policies for Brazil in the beginning of the Global Malaria Eradication Program. Brazil committed with the World Health Organization to convert its malaria control programs into eradication programs. The Malaria Eradication Group was set up in the federal government and all states complied with the new policy for malaria eradication in Brazil. Vector control was based on three annual spraying cycles of DDT in endemic regions. Malaria cases in humans were diagnosed by microscopy and treated with chloroquine. As a result, the lowest number of malaria cases in the history of Brazil was seen in 1961 when ~37 thousand cases were laboratory-confirmed. The populated cities in the Atlantic coast of Brazil became malaria-free. As seen in former endemic countries, such as the United States, people born after eradication have higher income as adults than the preceding generation. The Income and literacy increase (Table 1) after eradication was an important driver of development in the Atlantic coast region of Brazil. The Sao Paulo State Malaria Eradication Program eradicated malaria, among others such as Chagas disease, in the cities and rural areas. This state is now the most developed and richest in Brazil.
Failures to eradicate malaria in Brazil
Notwithstanding, the Global Malaria Eradication Program poorly accomplished its goals in certain regions in Latin America and Brazil, specifically in Amazonia. The phenomenon of Insecticide resistance (Table 1) was recognized. Particularly, endophagy and exophily (biting indoors and resting outdoors) of mosquito vectors (e.g., Anopheles darlingi) were recognized as challenges. Mosquito vectors did not rest on DDT-sprayed surfaces when indoors and usually rested in unsprayed areas such as clothing. The use of chloroquine in a mass treatment program involved massive and frequent exposure of the parasite to the drugs. This led to the selection of resistance to the 4-aminoquinolines and then Plasmodium falciparum resistance to chloroquine (Table 1). People mobility helped the dispersion of chloroquine-resistant strains of P. falciparum.
The Socioeconomic deficit (Table 1) unduly delayed eradication operations and proved the malaria eradication program as unattainable in Amazonia. Human ecology was as challenging as vector ecology. Poorly built houses and shelters were found in areas where malaria transmission persisted. Demographic characteristics (e.g., human migrations or isolations) were closely related to variations in malaria transmission. Extreme examples of demographic complexity were jungle people living in nomadic and isolated societies, engaged in production of rubber, wood, nuts, gold, diamonds, and other raw materials. Such circumstances often required specific approaches, generally with an in-depth anthropological operation to define the possibilities of malaria control.
Operational, administrative and financial difficulties were Logistic issues (Table 1). The funds allocated to malaria eradication programs were not adequate to offset salary increases, inflation, growing needs imposed by technical problems and the tremendously increased prices of DDT (75% in 3-years) purchased by the Brazilian government in the 1970s. The three annual spraying cycles of DDT adopted in the endemic regions in the South and Eastern Brazil was impractical in many hard-to-reach communities in the Amazonia.
A further aspect that highlighted the difficulty in achieving malaria eradication was Simian malaria (Table 1). Monkey plasmodia added, theoretically, a new challenge to the malaria eradication campaigns. They could infect susceptible humans living in close relationship with forests harbored by mosquito vectors.
The failures from the malaria eradication campaigns in Amazonia were analyzed and the synthetic explanation for unsuccessful outcomes was Eradication is not always possible (Table 1). The malaria eradication campaigns were time-limited, highly prescriptive and centralized. After the end of the Global Malaria Eradication Program, malaria re-emerged in places where improvements in the malaria situation were partial. The Ministerial Conference on Malaria Control in 1992 and the World Health Assembly in 1993 created the Global Malaria Control Strategy (Table 1). This strategy differed from the approach used in the eradication era. It was rooted in the primary health care approach and called for flexible, decentralized programmes, based on disease rather than parasite control. The Global Malaria Control Strategy represented the beginning of the malaria elimination era.
Malaria elimination era
In the malaria elimination era, we identified 12 technological breakthroughs. One fraction is represented by tools that have already helped controlling malaria. Health care diagnostic-and-treatment integration and artemisinin combination therapy were cited as responsible for decreasing P. falciparum or severe malaria (Table 2). Others are promising tools, such as novel modeling approaches, antimalarial drugs, immunization strategies, vector control intervention, or molecular tools. Notwithstanding, the number of challenges for malaria elimination were 2-fold greater than the number of technological breakthroughs, summing up to 24 in total. Out of these, at least half was related to P. vivax prevalence and one-third, to socioenvironmental factors in Brazil (Table 2).
Table 2.
Technological breakthroughs and challenges for eliminating malaria in Brazil
Decreasing Plasmodium falciparum and severe cases (Table 2) were accomplished by the malaria control policies set in Brazil: (1) PCMAM, Amazon Basin Malaria Control Programme, 1989; (2) PIACM, Intensification Plan of Malaria Control Activities in the Legal Amazon, 2000; and (3) NMPCP, National Malaria Prevention and Control Programme, 2003. The current first line treatment for P falciparum is artemether-lumefantrine (artemisin combination therapy) plus a gametocidal dose of primaquine. A short-term challenge, however, is Evolution of Plasmodium falciparum resistance (Table 2); resistance to artemisin in P falciparum has arisen in Guyana, on the border of Brazil. The resistant strains evolved independently from those previously found in Southeast Asia. The mechanism of resistance is in the propeller domain of the kelch13 gene.
The P. vivax issue in the malaria elimination era
The Decreasing Plasmodium falciparum (Table 2) trend was followed by the decline of P vivax. However, the P. vivax decline was not achieved with the same magnitude of success. Different from P falciparum, asexual forms of P. vivax are sequestered in the liver as hypnozoites. This mechanism leads to Plasmodium vivax relapse (Table 2), which is a form of recurrent malaria in a previously treated person. Chloroquine alone cannot kill hypnozoites in the liver and is further related to Plasmodium vivax resistance to chloroquine (Table 2). Chloroquine combined with primaquine is recommendable for a radical cure of P. vivax malaria. However, the use of primaquine is hampered by the G6PDd issue (Table 2). Primaquine can induce haemolysis in glucose-6-phosphate dehydrogenase deficient (G6PDd) individuals. The prevalence of G6PDd is 10% of the Brazilian population and the frequency of haemolysis in G6PDd patients is 90%.
The use of primaquine is also not indicated in pregnant women. Malaria in pregnancy (Table 2) causes health problems in newborns such as iron deficiency anemia. Pregnant women are reservoirs of P. vivax and can infect mosquito vectors and maintain parasite transmission. Similarly, Asymptomatic and Submicroscopic infections (Table 2) represent a fraction of infections that remain undetected in a population and maintain low residual transmission. They can also lead to Tïansfusion-transmitted malaria (Table 2).
Human mobility (Table 2) of asymptomatic carriers of P. vivax living under specific Socioeconomic aspects (Table 2) can promote emergence of malaria associated with Environmental changes (Table 2). These carriers have strong epidemiologic characteristics (travelers, immigrants) and can be drivers for Plasmodium vivax high genetic diversity (Table 2). Examples of such a scenario are places with Gold mining, Country border malaria, Indigenous malaria or Urban malaria (Table 2). Detection of new foci of infection (Table 2) can be a challenge when a new outbreak of malaria starts with the presence of asymptomatic carriers. This situation requires Integrate surveillance (Table 2).
P vivax causes a spectrum of clinical symptoms in patients, ranging from asymptomatic to severe cases. Variant immunological responses (Table 2) are associated with variants of toll-like receptors (TLRs) and influence an individual’s susceptibility to P. vivax malaria and parasitemia. Complicated Plasmodium vivax malaria (Table 2) is becoming more frequent due to unknown reasons. Another related phenomenon is chloroquine- induced pruritus (Table 2) as an adverse event in patients being treated with chloroquine for P. vivax malaria.
Vector control in the malaria elimination era
Large-scale vector control strategies in Amazonia are unfeasible due to local environment conditions coupled with vector ecology and behavior. Malaria vector control commodities (e.g., insecticide residual spraying or insecticide treated nets) are distributed to only 5% of the Amazonian populations.
Simian malaria in the malaria elimination era
Simian malaria represents a challenge to malaria elimination. If monkey plasmodia can infect humans and cause disease, malaria elimination goals based on eliminating malaria disease can become even harder to achieve. This issue is not well understood in Amazonia. In the Atlantic forest, however, Reservoir-monkeys (Table 2) can play an important role as reservoirs of monkey and human plasmodia that can infect humans. Some of these infections become symptomatic and are reported by the health authorities. They recognize these autochthonous cases as belonging to the transmission cycle known as Bromeliad- malaria (Table 2). This term refers to the main malarial vector (Anopheles cruzii) in which larvae breeds in the leaves of bromeliads in Atlantic forest.
Tools for controlling P. vivax in the malaria elimination era
Because P vivax malaria is spatially and temporally clustered, sensitive tools for better stratifying the risk of infection and targeting control interventions are needed. Genomic analysis and molecular tools (Table 2) and Modeling (Table 2) methods are being utilized toward Hotspot identification (Table 2). Molecular epidemiology investigations are currently being performed by the network of the International Centers of Excellence for Malaria Research (Table 2; ICEMR). The prevalence of multi-clone infections of P vivax, which indicate high genetic diversity, is estimated by parasite genotyping methods. Declines in this prevalence are coupled with declining parasite transmission. Modeling (Table 2) methods are applied for predictions under hypothetical scenarios in simulated interventions. Agent-based-models can reproduce the spatiotemporal variations of malaria transmission in an Amazonian environment dominated by river floodings. The invasion of P. vivax in an Atlantic forest island is modeled in circumstances of low or higher biodiversity. Housing interventions (Table 2) are being proposed in indigenous populations suffering from highly endemic malaria transmission.
Specific tools for parasite suppression are also on development. Plasmodium vivax drugs (Table 2) were tested in an open label randomized clinical trial. Artemisin- based combinations or chloroquine in combination with a short course of primaquine were effective and safe for P vivax control. The main drawbacks were the adverse events; a new drug, Tafenoquine, is promising in its potential for radical cure of P. vivax. Experimental assays for antimalarial drugs (Table 2) with Wistar rats and Plasmodium berghei was developed as an experimental model. This model can be used for testing new antimalarial drugs at preclinical stages. Naturally acquired immunity vaccine candidate (Table 2) is a promising strategy based on immune responses specific to P vivax antigens generated in natural infections. Whether their immunogenic potential can be applied to vaccine candidates is yet to be determined in field trials under natural conditions of parasite exposure. Plasmodium vivax vaccine (Table 2) that is considered the most promising candidate is the circumsporozoite protein. This candidate can, in theory, prevent the entry of sporozoites into hepatocytes or inhibit the liver stage development of hypnozoites. A P vivax circumsporozoite protein recombinant chimera (PvRMC-CSP) was proposed as a promising vaccine candidate for further development. Interestingly, the PvRMC-CSP was recognized by naturally acquired antibodies from individuals living in areas where malaria is endemic.
New tools for vector control are also needed. Ivermectin vector control (Table 2) is an additional vector control tool that is receiving increased attention from the malaria elimination community. Ivermectin is an endectocide drug; ivermectin treated- cattle can be bitten by mosquito vectors, which will die afterwards. The increased importance of ivermectin relies on its application in outdoor/residual malaria transmission and in places where insecticide resistance is prevalent.
Epidemiological analysis integrated with the systematic review
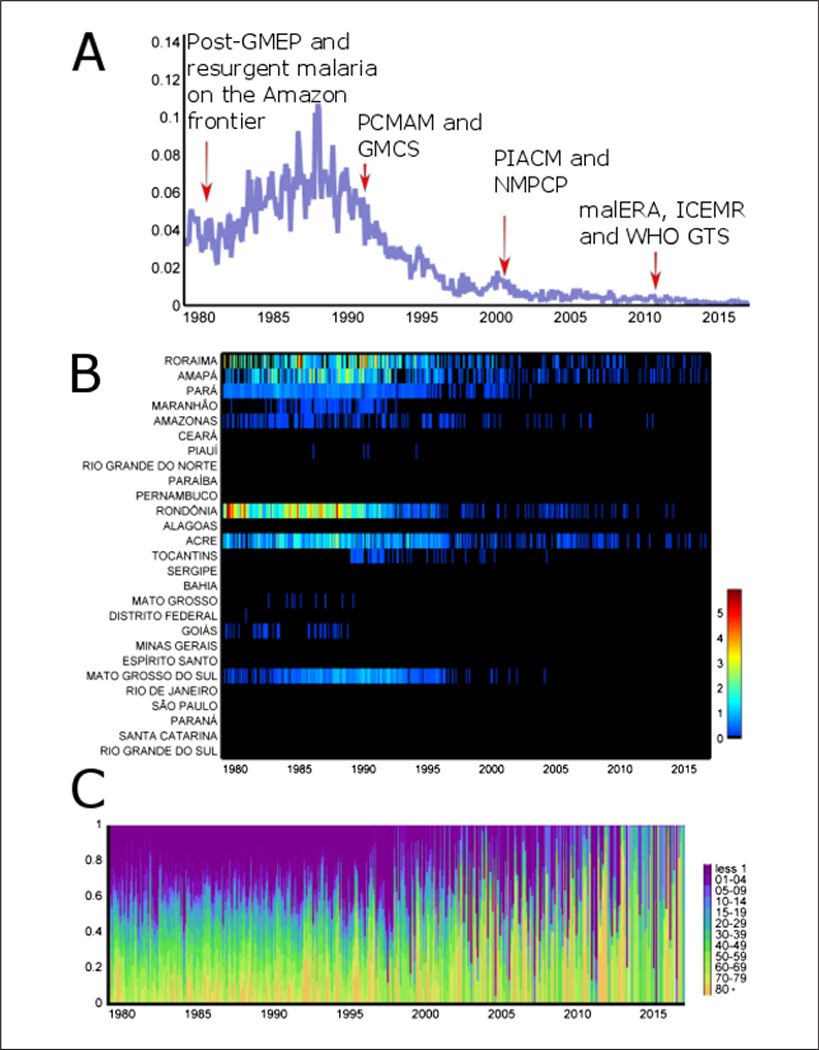
Resurgent malaria on the frontier of Amazonia was observed after national integration starting from 1970 (Figure 4A). Waves of migrants from the malaria-free (South and East) regions of Brazil settled in the Amazonian states of Para, Rondonia, Amazonas, Mato Grosso, Amapa, Acre, and Roraima to engage in new economic activities (mining, logging, cattle ranching, farming). This massive human influx caused local deforestation, increases in vector (An. darlingi) abundance and a dramatic increase in malaria incidence in the 1980s. The monthly malaria mortality rate of 0.035 (per 100,000 ppl.; an average of 50 malaria deaths per month) in 1979 increased by 300%, and peaked to 0.11 (per 100,000 ppl.) with 150 malaria deaths per month in 1988 (Figure 4A).
Figure 4.
(A) Malaria monthly mortality rate (per 100,000 ppl.), 1979–2016, in Brazil. Red arrows show a given period of malaria policy depicted from the systematic review. GMEP, Global Malaria Eradication Program; PCMAM, Amazon Basin Malaria Control Programme; GMCS, Global Malaria Control Strategy; PIACM, Intensification Plan of Malaria Control Activities in the Legal Amazon; NMPCP, National Malaria Prevention and Control Programme; malERA, Malaria Eradication Research Agenda; ICEMR, International Centers of Excellence for Malaria Reseach; WHO GTS, World Health Organization Global Technical Strategy. (B) Malaria monthly mortality rate (per 100,000 ppl.), 1979–2016, per 26 states and a federal district. (C) Malaria monthly mortality rate (proportion, 0 – 1) per age-groups (less 1 year, —, 80 and plus). Data source: Ministry of Health.
All states in the Amazon Region and two in the Brazilian Savannah (‘Cerrado’) Region (Mato Grosso do Sul and Goias) concentrated the majority of malaria deaths. Specifically, one-third of monthly malaria deaths in Brazil occurred in Rondonia state in 1985 (Figure 4B).
In 1989, the Ministry of Health Amazon Basin Malaria Control Programme (PCMAM) started earlier treatment of malaria cases to reduce transmission and mortality. The network of malaria diagnosis and treatment outposts was greatly expanded throughout Amazonia. The overall malaria incidence decreased by 60% from 1989 to 1996. The proportion of P falciparum infections decreased from 47 to 29% in the same period. As a result, the total number of deaths from malaria was 39 in 2016 and the monthly malaria mortality rate approaches 0 (Figure 4A).
Deaths due to malaria were proportionally more frequent in children (< 5 years), particularly in those less than 1 year of age in the 1980s and 1990s. Starting in the 2000s, there was a decrease in the proportional mortality in children under five years of age, and a concomitant increase in proportional mortality in older adults (Figure 4C).
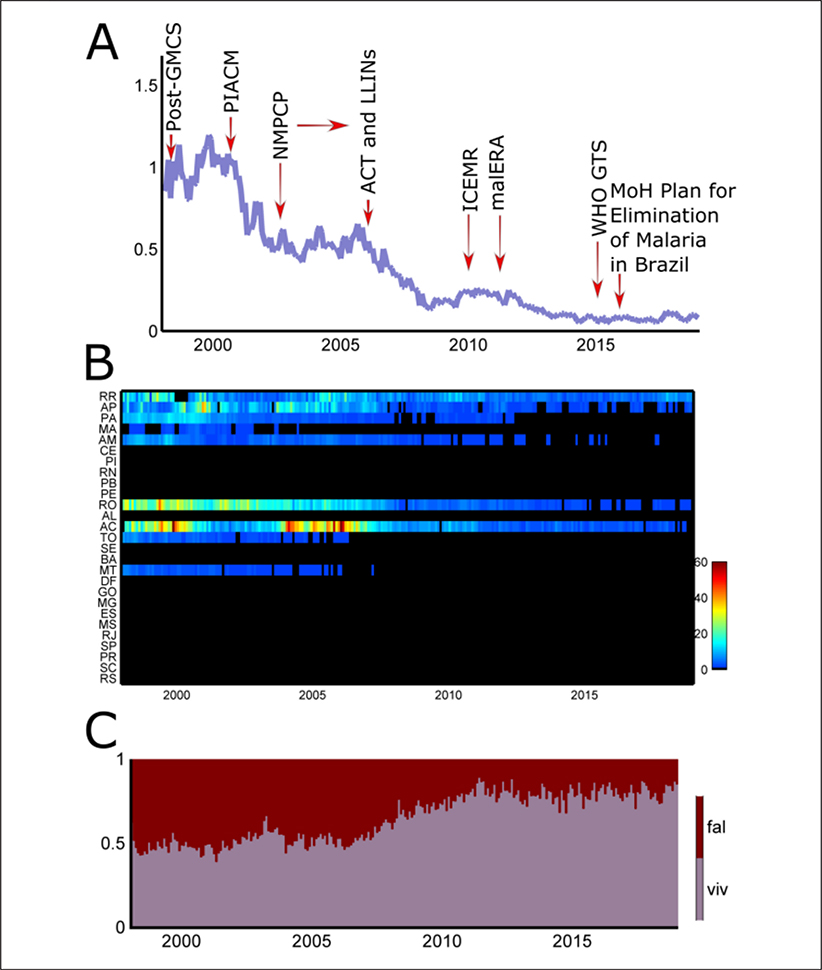
The gains associated with PCMAM, however, were lost during the last quarter of the 1990s. More than 600 thousand microscopically confirmed malaria cases were recorded in 1999. This culminated with 2,500 monthly cases of severe malaria, and a monthly malaria hospitalization rate of ~1.1 per100.000 ppl. in the late 1990s (Figure 5A).
Figure 5.
(A) Malaria monthly hospitalization rate (per 100,000 ppl.), 1998–2018, in Brazil. Red arrows show a given period of malaria policy depicted from the systematic review. GMCS, Global Malaria Control Strategy; PIACM, Intensification Plan of Malaria Control Activities in the Legal Amazon; NMPCP, National Malaria Prevention and Control Programme; ACT, Artemisin Combination Therapy; LLINs, long-lasting insecticide-treated bed nets; ICEMR, International Centers of Excellence for Malaria Reseach; malERA, Malaria Eradication Research Agenda; WHO GTS, World Health Organization Global Technical Strategy; MoH, Ministry of Health of Brazil. (B) Malaria monthly hospitalization rate (per 100,000 ppl.), 1998–2018, per 26 states and a federal district. RR, Roraima; AP, Amapá; PA, Pará; MA, Maranhäo; AM, Amazonas; CE, Ceará; PI, Piauí; RN, Rio Grande do Norte; PB, Paraíba; PE, Pernambuco; RO, Rondônia; AL, Alagoas; AC, Acre; TO, Tocantins; SE, Sergipe; BA, Bahia; MT, Mato Grosso; DF, Distrito Federal; GO, Goiás; MG, Minas Gerais; ES, Espirito Santo; MS, Mato Grosso do Sul; RJ, Rio de Janeiro; SP, Säo Paulo; PR, Paraná; SC, Santa Catarina; RS, Rio Grande do Sul. (C) Malaria monthly hospitalization rate (proportion, 0 – 1) per parasite species (fal = Plasmodium falciparum, viv = P vivax). Data source: Unified Health System.
As a consequence, the Ministry of Health implemented a comprehensive plan to reduce overall malaria, severe morbidity and mortality, to eliminate malaria transmission in the urban area of state capitals in Amazonia, and to prevent resurgence in malaria-free areas. In 2000, the Intensification Plan of Malaria Control Activities in the Legal Amazon (PIACM) targeted 254 municipalities (32.1% of the total number of municipalities in the Amazon), which gathered 93.6% of the malaria cases. Control measures were tailored to each specific epidemiological setting. A strong reduction in morbidity (40%) was seen in the following years (Figure 5A).
Notwithstanding, all states in Northern Brazil (e.g., Mato Grosso and those in the Amazon region) continued contributing to hospitalizations from malaria after 2001 (Figure 5B). Specifically, states in Western Amazonia (Rondonia and Acre) had high rates of hospitalizations (up to 60 per month per 100,000 ppl.) in the beginning of the 2000s (Figure 5B).
In 2003, the Ministry of Health launched the National Malaria Prevention and Control Programme (NMPCP) to reinforce the goals established in the PIACM. Following the decentralization of the health system during the 1990s each municipality could adopt different control strategies. Mass distribution of long lasting insecticide-treated nets (LLINs) started in Acre state in 2006, and was later expanded to all high-risk areas in Amazonia, from 2008 on. The network of malaria outposts increased to nearly 3,500 with ~ 4,500 microscopists and 7,600 health agents up to 2013. Malaria hospitalization rates approached 0, with a total of 500 severe malaria cases in 2013 (Figure 5A).
During the period of 1990–2008, severe cases were mostly due to P falciparum (80–100%; Figure 5C). The successful use of artemisin combination therapy (artemether- lumefantrine) in the aforementioned network of malaria outposts reduced the P falciparum malaria burden. In recent years, however, the monthly proportion of severe cases due to P vivax has increased to 50% (Figure 5C). This is related to The P. vivax issue in the malaria elimination era.
Based on the achievements on the decreasing burden of malaria in Brazil (Figures 4A, 5A) and worldwide, many globally based initiatives were launched for malaria elimination and eradication, such as the International Centers of Excellence for Malaria Reseach (ICEMR) and the Malaria Eradication Research Agenda (malERA), starting in 2010. In 2015, Brazil was awarded by the Pan American Health Organization (PAHO) the Malaria Champions of the Americas Award. In the same year the NMPCP of the Ministry of Health launched the Plan for Elimination of Malaria in Brazil. The plan complies with the policies recommended by the World Health Organization Global Technical Strategy. The Brazilian plan focuses on eliminating P. falciparum malaria by 2030.
In the years 2017–2018, the number of total malaria cases (Figure 1) and severe cases (Figure 5A) have increased. The 2017 epidemiological profile of P. falciparum (Figure 2A) and P. vivax (Figure 2B) in Brazil shows widespread hotspots of malaria transmission in the Amazonian states of Amazonas, Acre, Rondonia, Pará, Amapá and Roraima. These hotspots (Figures 2A, 2B) of active and residual malaria transmission are obstacles to the plan of malaria elimination in Brazil.
DISCUSSION
The malaria elimination policy in Brazil is herein evaluated through analysis of results from systematic review and epidemiological analysis. Literature review showed (1) increasing importance of P. vivax-malaria in Brazil and (2) decreasing deaths and severe cases from P. falciparum-malaria. In concordance, epidemiological analysis indicated (1) a new burden from P. vivax- attributable hospitalizations and morbidity and (2) a diminished burden from P. falciparum-malaria.
The ‘eradication era’ was responsible for important achievements, such as the overall decline of malaria transmission in Brazil in late 1960 (Correa and Alves, 1969; Mascarenhas, 1973; Barata, 1997; Trigg and Kondrachine, 1998; Bleakley, 2010; Silva and Paiva, 2015). After the end of the Global Malaria Eradication Program (Palacios Fraire, 1975), malaria burden increased, particularly in the Amazon Region (Vosti, 1990). The 1992 Global Malaria Control Strategy was proposed as a flexible and cost- effective program (Trigg and Kondrachine, 1998). This was the basis for several national programs for controlling malaria globally in the 1990s and 2000s. For instance, the National Malaria Prevention and Control Program (NMPCP) was launched by the Brazilian Ministry of Health in 2003 (Ferreira and Castro, 2016). By means of the NMPCP, malaria deaths and severe cases decreased significantly in Brazil after 2010 on.
The Malaria Elimination Research Agenda (malERA) was proposed in 2011. The malERA’s main goals are reducing the burden and eliminating malaria in the 91 countries and territories with ongoing malaria transmission in the world. Its ultimate goal is to eradicate malaria globally (Rabinovich et al., 2017). Countries and territories are working under the global technical strategy for eliminating malaria. On the one hand, there are countries that are moving towards malaria elimination. Decreased endemic malaria in Suriname in South America was related to the mass- distribution of free insecticide-impregnated bednets and improved access to malaria services (van Eer et al., 2018). On the other hand, there are other countries that are not succeeding; malaria elimination in Haiti in Central America seems unachievable (Boncy et al., 2015). In Brazil, the general perception from the scientific community is that elimination is not on the horizon (Ferreira and Castro, 2016; Siqueira et al., 2016; Recht et al., 2017). Two malaria experts were cautious regarding the 2030 goal for malaria elimination (Ferreira and Castro, 2016). Since 2016, malaria cases have increased in Brazil (World Health Organization, 2018).
Parallel with the malERA and the World Health Organization advocacy for malaria elimination (Rabinovich et al., 2017), the National Institutes of Health International Centers of Excellence for Malaria Research (ICEMR) constitute research centers in malaria-endemic regions (Rao, 2012). ICEMR’s main goal is to help build local capacity for malaria control and elimination. Its applied research has led to new tools, i.e., a malaria molecular epidemiology toolbox for genotyping parasites (Pacheco et al., 2015). In Brazil, this toolbox was applied to endemic malaria hotspots in Western Amazonia and the authors found high genetic diversity of P. vivax (Barbosa et al., 2014; Fontoura et al., 2016). This suggests multiple sources of infection that maintain ongoing malaria transmission. This further complicates current malaria elimination efforts.
There are challenges to fully achieve the goals of the malaria elimination policy in Brazil. One assumption is that clinical symptoms can be prevented by efficacious antimalarial treatments. However, current treatments lead to asymptomatic hosts who can infect mosquito vectors, i.e., An. darlingi (Sallum et al., 2019); this may further cause clinical infections in other susceptible humans in another region. A well-known prospective cohort study in a rural settlement showed that P. falciparum disappearance and P. vivax decline are transient (Vitor-Silva et al., 2016). The interruption of the active case detection strategy was followed by a resurgence of both parasites in the same locality 3-years later (Vitor-Silva et al., 2016). Another prospective cohort study ended transmission of P. falciparum, but did not eliminate P. vivax prevalence (Barbosa et al., 2014). After the interruption of this study, malaria rebounded in the locality. In addition, P. vivax-malaria poses multiple challenges: (1) asymptomatic malaria, (2) submicroscopic malaria, (3) relapsing malaria, (4) malaria in pregnancy, (5) G6PD issue, (7) transfusion-transmitted malaria, and so on (Lacerda et al., 2012; Monteiro et al., 2014b; Alho et al., 2017; Costa et al., 2017; Recht et al., 2017).
Decreasing malaria burden in the epidemiological analysis 1979–2018 may lead to the logical fallacy that malaria elimination is within reach. The epidemiological scenario is truly dynamic and trends can change directions suddenly. The current World Malaria Report reported an increase in malaria in Brazil and Venezuela (World Health Organization, 2018). Interestingly, the promising eradication of malaria in Venezuela was certified by the Pan American Health Organization in 1961 (Kerr, 1963). About 60 sixty years later Venezuela is the hub of a humanitarian crisis with possible spillover of vector-borne diseases including malaria to neighbor countries, including Brazil (Grillet et al., 2019).
Socio-environmental factors maintain malaria residual transmission in Brazil. The gold mining scenario studied in the 1990s (Vosti, 1990) is still active in Amazonia (Silva-Nunes et al., 2012). Furthermore, urban malaria (Recht et al., 2017), indigenous malaria (Leandro-Reguillo et al., 2015) and country border malaria (Chuquiyauri et al., 2012) are examples of other scenarios that maintain active malaria transmission in Brazil.
An important byproduct of such socioenvironmental factors includes human mobility, which leads to the exportation of malaria from Amazonia to southern Brazilian counties (Tauil, 2011). Parasites imported from Amazonia in this fashion fuel bromeliad- malaria on the Atlantic coast (Lorenz et al., 2015). Bromeliad-malaria is the transmission of Plasmodium spp. by a bromeliad mosquito species (Anopheles cruzii) to humans or monkeys in the Atlantic forest. Generally P. vivax or P. malariae are transmitted to humans, and P. brasilianum or P. simium are transmitted to monkeys. However, Plasmodium simium caused an outbreak of malaria cases in inhabitants in Rio de Janeiro in 2015–16 (Brasil et al., 2017). The simian malaria issue is not novel; back in the 1960s, Deane described this phenomenon that challenged the eradication paradigm (Deane, 1969; Palacios Fraire, 1975).
Proposals for new technological breakthroughs are needed. Perhaps, it is the time to go beyond the available malaria commodities and to reconcile with the practical malariology adopted in the first half of the twentieth century (Baird, 2017). Practical malariologists conceived precise modifications to natural or man-made environments aimed at making those less hospitable to specific anopheline mosquito vector species (Baird, 2017). Practical malariology achieved very significant reductions in burdens of morbidity and mortality (Killeen et al., 2002; Killeen, 2003). Adult vector control is not effective with the current tools. The insecticide-impregnated bednets can have long-term drawbacks, such as the changing behavior of mosquito vectors’ biting peaks (Ferreira et al., 2017). A promising tool for adult vector control is the endectocide drug ivermectin (Chaccour et al., 2015). The experimental effect of this drug was recently tested with good prospects for use against P vivax transmission in Amazonia, by suppressing the numbers of adult mosquito vectors (Pinilla et al., 2018). However, basic knowledge about mosquito vector species in transmission areas is still lacking (Bourke et al., 2018). A recent study showed that An. darlingi (now this species is identified as Nyssorhynchus darlingi) has high vectorial capacity (up to 1.5 infected bites per person/day), which is responsible for the high P vivax propagation in Amazonian rural communities (Sallum et al., 2019). These authors compared such epidemiological metrics to those observed for P falciparum in sub-Saharan Africa regions. The malaria community is now becoming aware of the lack of comprehensive understanding of this important element (mosquito vectors) of the malaria elimination endeavor (Baia-da-Silva et al., 2019).
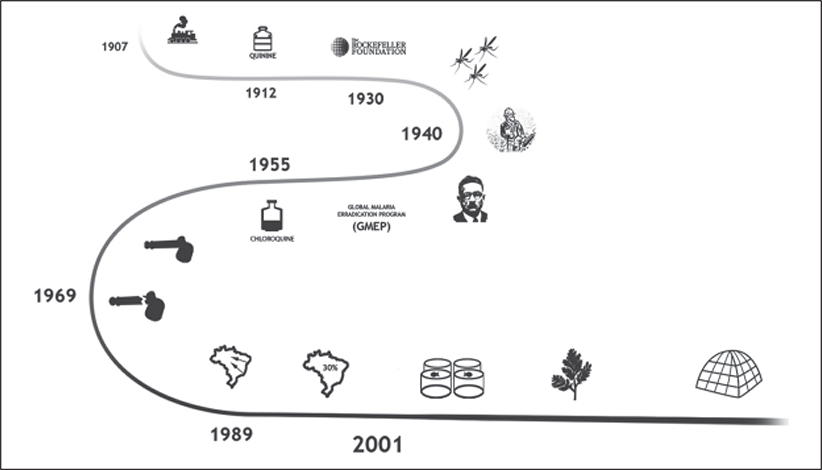
The Centenary Brazilian fight against malaria has been benefitted from national programs of malaria control and technological innovations (Figure 6). Focus, on the other hand, should be on the changing cost-benefit perception of society and environment disturbances caused by new economic enterprises. Malaria elimination in Brazil in the next decade will require a strategy that combines the lessons from the past and a capability of adapting to an evolving epidemiological, socio-economic, and technological setting. As Brazil continues its path to eliminate malaria, it will be crucial that attention does not wane as the situation continues to improve.
Figure 6.
Infographic of the history of malaria emergence and elimination in Brazil. Brazil. (1907) The Devil’s Railroad: the Madeira-Mamore epidemic. (1912) Introduction of quinine as therapeutic and/or prophylactic measure. (1930) The role of Rockefeller Foundation to the eradication of an African malarial vector in Brazil: a successful study case for malaria eradication. (1940) The American epidemiologist Fred Soper headed the campaign responsible for eliminating Anopheles arabiensis in Brazil, and used this example as the main reason for the eminent success of the Global Malaria Eradication Program in 1950’s. (1955) Chloroquine, a synthetic lab-made quinine drug, was a technological breakthrough in this period because it could be produced in large scales in an industrial approach. (1969) Initial successful outcomes of the GMEP with malaria elimination in some parts of the world were achieved. However, the 1968 magnum opus Rachel Carson’s Silent Spring buried attempts of widespread use of DDT due to its bioaccumulation in top predators in the food chains. This culminated with the end of the GMEP in 1969. (1989) Period of migratory influx and alterations of natural habitats in the Amazon during 1970–1980, concurrently with the new economic model named as the Free Economic Zone, settled in Manaus, which attracted multinational entrepreneurs, triggering migratory flow from other states of Brazil to Amazon. (2001) a new economic cycle began in the Amazon, aiming at empowering the overall economy, involving agroforestry activities, particularly fish farming, causing the development of permanent larval habitats for malarial mosquitoes. Treatment of P. falciparum with Artemisinin-based combination therapy (ACT) started free for all ages in public sector. Insecticide Treated bed Nets (ITNs) and Long Lasting Insecticide-treated Nets (LLINs) are distributed free of charge to all age groups.
CONCLUSIONS
Brazil has seen an impressive reduction in malaria hospitalizations and mortality in the past 3 decades, but complete malaria elimination in Brazil in the near future remains unlikely. On the one hand, eliminating malaria deaths in children less than 5 years and P falciparum severe cases by 2030 are achievable goals under the current malaria policy. On the other hand, eliminating P. vivax malaria transmission and morbidity seems unattainable with the available technological breakthroughs. Overall, this evidence suggests that malaria elimination might not be generally achieved in Brazil in the next decade.
Complete malaria elimination in the near future in Brazil will require better tools for the challenges posed by P. vivax transmission and morbidity. Specifically, new drugs that can cure relapsing patients with P. vivax and/or block transmission to competent malaria vectors certainly are needed.
Acknowledgements
JOM and MAOP were supported by the SESACRE – UFAC – FMABC agreement process n. 007/2015. GZL was supported by the Sâo Paulo Research Foundation (FAPESP) and Biota-FAPESP Program, process n. 2014/09774-1.
Footnotes
Competing interests
None declared.
REFERENCES
- Alho RM, Machado KVA, Val FFA, Fraiji NA, Alexandre MAA, Melo GC, Recht J, Siqueira AM, Monteiro WM & Lacerda MVG (2017). Alternative transmission routes in the malaria elimination era: an overview of transfusion-transmitted malaria in the Americas. Malaria Journal 16: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alencar FEC, Malafronte RS, Cerutti C, Fernandes LN, Buery JC, Fux B, Rezende HR & Miranda AE (2017). Reassessment of asymptomatic carriers of Plasmodium spp. in an endemic area with a very low incidence of malaria in extra-Amazonian Brazil. Malaria Journal 16: 452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarenga DAM, Pina-Costa A, Bianco C, Moreira SB, Brasil P, Pissinatti A, Daniel-Ribeiro CT & Brito CFA (2017). New potential Plasmodium brasilianum hosts: tamarin and marmoset monkeys (Family Calli- trichidae). Malaria Journal 16: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo MS, Messias MR, Figueiró MR, Gil LHS, Probst CM, Vidal NM, Katsuragawa TH, Krieger MA, Pereira da Silva LH & Ozaki LS (2013). Natural Plasmodium infection in monkeys in the state of Rondonia (Brazilian Western Amazon). Malaria Journal 12: 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arevalo-Herrera M, Quiñones ML, Guerra C, Céspedes N, Giron S, Ahumada M, Piñeros JG, Padilla N, Terrientes Z, Rosas Á, Padilla JC, Escalante AA, Beier JC & Herrera S (2012). Malaria in selected non-Amazonian countries of Latin America. Acta Tropica 121: 303314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baia-da-Silva DC, Brito-Sousa JD, Rodovalho SR, Peterka C, Moresco G, Lapouble OMM, Melo G.C.de, Sampaio VS, Alecrim MGC, Pimenta P, Lima JBP, Lacerda MVG & Monteiro WM (2019). Current vector control challenges in the fight against malaria in Brazil. Revista da Sociedade Brasileira de Medicina Tropical 52: e20180542. [DOI] [PubMed] [Google Scholar]
- Baird JK (2017). Malaria control by commodities without practical malario- logy. BMC Public Health 17: 590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballut PC, Siqueira AM, Orlando AC, Alexandre MA, Alecrim MGC & Lacerda MV (2013). Prevalence and risk factors associated to pruritus in Plasmodium vivax patients using chloroquine in the Brazilian Amazon. Acta Tropica 128: 504–508. [DOI] [PubMed] [Google Scholar]
- Barata RB (1997). Organización tecnológica del control de la malaria en Sao Paulo, Brasil, de 1930 a 1990. Revista Panamericana de Salud Publica 1: 335343. [PubMed] [Google Scholar]
- Barbosa S, Gozze AB, Lima NF, Batista CL, Bastos M.da S., Nicolete VC, Fontoura PS, Gonçalves RM, Viana SAS, Menezes MJ, Scopel KKG, Cavasini CE, Malafronte RS, Silva- Nunes M, Vinetz JM, Castro MC & Ferreira MU (2014). Epidemiology of disappearing Plasmodium vivax malaria: a case study in rural Amazonia. PLoS Neglected Tropical Diseases 8: e3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardají A, Martínez-Espinosa FE, Arévalo- Herrera M, Padilla N, Kochar S, Ome-Kaius M, Bôtto-Menezes C, Castellanos ME, Kochar DK, Kochar SK, Betuela I, Mueller I, Rogerson S, Chitnis C, Hans D, Menegon M, Severini C, del Portillo H, Dobaño C, Mayor A, Ordi J, Piqueras M, Sanz S, Wahlgren M, Slutsker L, Desai M, Menéndez C & on behalf of the PregVax Study Group (2017). Burden and impact of Plasmodium vivax in pregnancy: A multi-centre prospective observational study. PLoS Neglected Tropical Diseases 11: e0005606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleakley H (2010). Malaria eradication in the Americas: a retrospective analysis of childhood exposure. American Economic Journal: Applied Economics 2: 1–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boncy PJ, Adrien P, Lemoine JF, Existe A, Henry PJ, Raccurt C, Brasseur P, Fenelon N, Dame JB, Okech BA, Kaljee L, Baxa D, Prieur E, El Badry MA, Tagliamonte MS, Mulligan CJ, Carter TE, Beau de Rochars VM, Lutz C, Parke DM & Zervos MJ (2015). Malaria elimination in Haiti by the year 2020: an achievable goal? Malaria Journal 14: 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bôtto-Menezes C, Bardají A, dos Santos Campos G, Fernandes S, Hanson K, Martínez-Espinosa FE, Menéndez C & Sicuri E (2016). Costs associated with malaria in pregnancy in the Brazilian Amazon, a low endemic area where Plasmodium vivax predominates. PLoS Neglected Tropical Diseases 10: e0004494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke BP, Conn JE, de Oliveira TMP, Chaves LSM, Bergo ES, Laporta GZ & Sallum MAM (2018). Exploring malaria vector diversity on the Amazon Frontier. Malaria Journal 17: 342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasil P, Zalis MG, de Pina-Costa A, Siqueira AM, Júnior CB, Silva S, Areas ALL, Pelajo-Machado M, de Alvarenga DAM, da Silva Santelli ACF, Albuquerque HG, Cravo P, Santos de Abreu FV, Peterka CL, Zanini GM, Suárez Mutis MC, Pissinatti A, Lourengo-de-Oliveira R, de Brito CFA, Ferreira-da-Cruz MF, Culleton R & Daniel-Ribeiro CT (2017). Outbreak of human malaria caused by Plasmodium simium in the Atlantic Forest in Rio de Janeiro: a molecular epidemiological investigation. Lancet Global Health 5: e1038–e1046. [DOI] [PubMed] [Google Scholar]
- Cabrera-Mora M, Fonseca JA, Singh B, Oliveira-Ferreira J, Lima-Junior JC, Calvo-Calle JM & Moreno A (2015). Induction of multifunctional broadly reactive T cell responses by a Plasmodium vivax circumsporozoite protein recombinant chimera. Infection and Immunity 83: 3749–3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashwell A, Tantri A, Schmidt A, Simon G & Mistry N (2014). BRICS in the response to neglected tropical diseases. Bulletin of the World Health Organization 92: 461–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos LG, Carter KH, Escalada RP, Ade MP, Singh P, Espinal MA & Mujica OJ (2015). Malaria in the Americas: Trends from 1959 to 2011. American Journal of Tropical Medicine and Hygiene 92: 302–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaccour CJ, Rabinovich NR, Slater H, Canavati SE, Bousema T, Lacerda M, ter Kuile F, Drakeley C, Bassat Q, Foy BD & Kobylinski K (2015). Establishment of the Ivermectin Research for Malaria Elimination Network: updating the research agenda. Malaria Journal 14: 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuquiyauri R, Paredes M, Peñataro P, Torres S, Marin S, Tenorio A, Brouwer KC, Abeles S, Llanos-Cuentas A, Gilman RH, Kosek M & Vinetz JM (2012). Socio-demographics and the development of malaria elimination strategies in the low transmission setting. Acta Tropica 121: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrêa R. de R. & Alves UP (1969). Informes sôbre o programa de erradicaçâo da malária do Estado de Sâo Paulo. Revista de Saúde Pública 3: 93–104. [PubMed] [Google Scholar]
- Costa AG, Ramasawmy R, Ibiapina HNS, Sampaio VS, Xábregas LA, Brasil LW, Tarragô AM, Almeida ACG, Kuehn A, Vitor-Silva S, Melo GC, Siqueira AM, Monteiro WM, Lacerda MVG & Malheiro A (2017). Association of TLR variants with susceptibility to Plasmodium vivax malaria and parasitemia in the Amazon region of Brazil. PLoS ONE 12: e0183840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daher A, Pereira D, Lacerda MVG, Alexandre MAA, Nascimento CT, Alves de Lima e silva JC, Tada M, Ruffato R, Maia I, dos Santos TC, Marchesini P, Santelli AC & Lalloo DG (2018). Efficacy and safety of artemisinin-based combination therapy and chloroquine with concomitant primaquine to treat Plasmodium vivax malaria in Brazil: an open label randomized clinical trial. Malaria Journal 17: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel-Ribeiro CT, Lacerda MVG & Oliveira-Ferreira J (2008). Paludisme dû à Plasmodium vivax en Amazonie brésilienne: quelques aspects de son épidémiologie, de ses manifestations cliniques et des réactions immunitaires naturellement acquises. Bulletin de la Société de Pathologie Exotique 101: 243248. [PubMed] [Google Scholar]
- Deane LM (1969). Plasmodia of monkeys and malaria eradication in Brazil. Revista Latinoamericana de Microbiología y Parasitologia 11: 69–73. [PubMed] [Google Scholar]
- Ferreira MU & Castro MC (2016). Challenges for malaria elimination in Brazil. Malaria Journal 15: 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira CP, Lyra SP, Azevedo F, Greenhalgh D & Massad E (2017). Modelling the impact of the long-term use of insecticide-treated bed nets on Anopheles mosquito biting time. Malaria Journal 16: 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo MAP, Di Santi SM, Manrique WG, André MR & Machado RZ (2017). Identification of Plasmodium spp. in Neotropical primates of Maranhense Amazon in Northeast Brazil. PLoS ONE 12: e0182905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontoura PS, Finco BF, Lima NF, de Carvalho JF, Vinetz JM, Castro MC & Ferreira MU (2016). Reactive case detection for Plasmodium vivax malaria elimination in rural Amazonia. PLoS Neglected Tropical Diseases 10: e0005221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillet ME, Hernandez-Villena JV, Llewellyn MS, Paniz-Mondolfi AE, Tami A, Vincenti-Gonzalez MF, Marquez M, Mogollon-Mendoza AC, Hernandez-Pereira CE, Plaza-Morr JD, Blohm G, Grijalva MJ, Costales JA, Ferguson HM, Schwabl P, Hernandez-Castro LE, Lamberton PHL, Streicker DG, Haydon DT, Miles MA, Acosta-Serrano A, Acquattela H, Basanez MG, Benaim G, Colmenares LA, Conn JE, Espinoza R, Freilij H, Graterol-Gil MC, Hotez PJ, Kato H, Lednicky JA, Martinez CE, Mas-Coma S, Morris JG, Navarro JC, Ramirez JL, Rodriguez M, Urbina JA, Villegas L, Segovia MJ, Carrasco HJ, Crainey JL, Luz SLB, Moreno JD, Gonzalez OON, Ramirez JD & Alarcôn-de Noya B (2019). Venezuela’s humanitarian crisis, resurgence of vector-borne diseases, and implications for spillover in the region. The Lancet. Infectious Diseases 19: e149–e161. [DOI] [PubMed] [Google Scholar]
- Hommel M (2008). Towards a research agenda for global malaria elimination. Malaria Journal 7(Suppl 1): S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotez PJ. & Fujiwara RT (2014). Brazil’s neglected tropical diseases: an overview and a report card. Microbes and Infection 16: 601–606. [DOI] [PubMed] [Google Scholar]
- Kerr JA (1963). Lessons to be learned from failures to eradicate. American Journal of Public Health and the Nation’s Health 53: 27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killeen GF (2003). Following in Soper’s footsteps: northeast Brazil 63 years after eradication of Anopheles gambiae. The Lancet. Infectious Diseases 3: 663–666. [DOI] [PubMed] [Google Scholar]
- Killeen GF, Fillinger U, Kiche I, Gouagna LC & Knols BGJ (2002). Eradication of Anopheles gambiae from Brazil: lessons for malaria control in Africa? The Lancet Infectious Diseases 2: 618–627. [DOI] [PubMed] [Google Scholar]
- Lacerda MV, Mourao MP, Alexandre MA, Siqueira AM, Magalhaes BM, Martinez-Espinosa FE, Santana Filho FS, Brasil P, Ventura AM, Tada MS, Couto VS, Silva AR, Silva RS & Alecrim MG (2012). Understanding the clinical spectrum of complicated Plasmodium vivax malaria: a systematic review on the contributions of the Brazilian literature. Malaria Journal 11: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laporta GZ, Prado P.I.K.L. de, Kraenkel RA, Coutinho RM & Sallum MAM (2013). Biodiversity can help prevent malaria outbreaks in tropical forests. PLoS Neglected Tropical Diseases 7: e2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leandro-Reguillo P, Thomson-Luque R, Monteiro WM & de Lacerda MVG (2015). Urban and architectural risk factors for malaria in indigenous Amazonian settlements in Brazil: a typological analysis. Malaria Journal 14: 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz C, Virginio F, Aguiar BS, Suesdek L & Chiaravalloti-Neto F (2015). Spatial and temporal epidemiology of malaria in extra-Amazonian regions of Brazil. Malaria Journal 14: 408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascarenhas RS (1973). Historia da saúde pública no Estado de Sao Paulo. Revista de Saúde Pública 7: 433–446. [DOI] [PubMed] [Google Scholar]
- Methley AM, Campbell S, Chew-Graham C, McNally R & Cheraghi-Sohi S (2014). PICO, PICOS and SPIDER: a comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Services Research 14: 579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro W, Val FFA, Siqueira AM, Franca GP, Sampaio VS, Melo GC, Almeida ACG, Brito MAM, Peixoto HM, Fuller D, Bassat Q, Romero GAS, Maria Regina F O & Lacerda MV (2014a). G6PD deficiency in Latin America: systematic review on prevalence and variants. Memorias do Instituto Oswaldo Cruz 109: 553–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro WM, Franca GP, Melo GC, Queiroz AL, Brito M, Peixoto HM, Oliveira MRF, Romero GA, Bassat Q & Lacerda MV (2014b). Clinical complications of G6PD deficiency in Latin American and Caribbean populations: systematic review and implications for malaria elimination programmes. Malaria Journal 13: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy F & Mullis M (2011). Financial integration in the Americas, changing geopolitics and Brazilian foreign policy. Journal of Globalization, Competitiveness & Governability 5: 16–29. [Google Scholar]
- Oliveira-Ferreira J, Lacerda MV, Brasil P, Ladislau JL, Tauil PL & Daniel-Ribeiro CT (2010). Malaria in Brazil: an overview. Malaria Journal 9: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco MA, van Eijk AM, Barry AE, Gamboa D, Volkman SK, Escalante AA, Vallejo AF, Felger I, Pradhan K, Carlton JM, Krogstad DJ, Cui L, Ferreira MU, Herrera S, Greenhouse B, Mueller I & Vinetz JM (2015). Malaria molecular epidemiology: lessons from the International Centers of Excellence for Malaria Research network. American Journal of Tropical Medicine and Hygiene 93: 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard RM & Gadelha P (1997). A land filled with mosquitoes: Fred L. Soper, the Rockefeller foundation, and the Anopheles gambiae Invasion of Brazil. Medical Anthropology 17: 215–238. [DOI] [PubMed] [Google Scholar]
- Palacios Fraire S (1975). Analysis of the principal problems impeding normal development of malaria eradication programs. Bulletin of the Pan American Health Organization 9: 283–294. [PubMed] [Google Scholar]
- Pearson RD, Amato R, Auburn S, Miotto O, Almagro-Garcia J, Amaratunga C, Suon S, Mao S, Noviyanti R, Trimar- santo H, Marfurt J, Anstey NM, William T, Boni MF, Dolecek C, Tran HT, White NJ, Michon P, Siba P, Tavul L, Harrison G, Barry A, Mueller I, Ferreira MU, Karunaweera N, Randrianarivelojosia M, Gao Q, Hubbart C, Hart L, Jeffery B, Drury E, Mead D, Kekre M, Campino S, Manske M, Cornelius VJ, MacInnis B, Rockett KA, Miles A, Rayner JC, Fairhurst RM, Nosten F, Price RN & Kwiatkowski DP (2016). Genomic analysis of local variation and recent evolution in Plasmodium vivax. Nature Genetics 48: 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedroni HC, Bettoni CC, Spalding SM & Dalla Costa T (2006). Plasmodium berghei: Development of an irreversible experimental malaria model in Wistar rats. Experimental Parasitology 113: 193–196. [DOI] [PubMed] [Google Scholar]
- Pinilla YT, Lopes SCP, Sampaio VS, Andrade FS, Melo GC, Orfano AS, Secundino NFC, Guerra MGVB, Lacerda MVG, Kobylinski KC, Escobedo-Vargas KS, Lopez-Sifuentes VM, Stoops CA, Baldeviano GC, Tarning J, Vasquez GM, Pimenta PFP & Monteiro WM (2018). Promising approach to reducing Malaria transmission by ivermectin: sporontocidal effect against Plasmodium vivax in the South American vectors Anopheles aquasalis and Anopheles darlingi. PLoS Neglected Tropical Diseases 12: e0006221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzitutti F, Pan W, Barbieri A, Miranda JJ, Feingold B, Guedes GR, Alarcon- Valenzuela J & Mena CF (2015). A validated agent-based model to study the spatial and temporal heterogeneities of malaria incidence in the rainforest environment. Malaria Journal 14: 514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovich RN, Drakeley C, Djimde AA, Hall BF, Hay SI, Hemingway J, Kaslow DC, Noor A, Okumu F, Steketee R, Tanner M, Wells TNC, Whittaker MA, Winzeler EA, Wirth DF, Whitfield K & Alonso PL (2017). malERA: An updated research agenda for malaria elimination and eradication. PLoS Medicine 14: e1002456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao M (2012). The International Centers of Excellence for Malaria Research. Acta Tropica 121: 157. [DOI] [PubMed] [Google Scholar]
- Recht J, Siqueira AM, Monteiro WM, Herrera SM, Herrera S & Lacerda MVG (2017). Malaria in Brazil, Colombia, Peru and Venezuela: current challenges in malaria control and elimination. Malaria Journal 16: 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts L & Enserink M (2007). Malaria. Did they really say …eradication? Science (New York, N.Y.) 318: 1544–1545. [DOI] [PubMed] [Google Scholar]
- Rosas-Aguirre A, Speybroeck N, Llanos- Cuentas A, Rosanas-Urgell A, Carrasco-Escobar G, Rodriguez H, Gamboa D, Contreras-Mancilla J, Alava F, Soares IS, Remarque E, D’Alessandro U & Erhart A (2015). Hotspots of malaria transmission in the Peruvian Amazon: rapid assessment through a parasitological and serological survey. PLoS ONE 10: e0137458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallum MAM, Conn JE, Bergo ES, Laporta GZ, Chaves LSM, Bicker- smith SA, de Oliveira TMP, Figueira EAG, Moresco G, Oliver L, Struchiner CJ, Yakob L & Massad E (2019). Vector competence, vectorial capacity of Nyssorhynchus darlingi and the basic reproduction number of Plasmodium vivax in agricultural settlements in the Amazonian Region of Brazil. Malaria Journal 18: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepúlveda N, Stresman G, White MT & Drakeley CJ (2015). Current mathematical models for analyzing anti-malarial antibody data with an eye to malaria elimination and eradication. Journal of Immunology Research 2015: 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva R & Paiva CHA (2015). The Juscelino Kubitschek government and the Brazilian Malaria Control and Eradication Working Group: collaboration and conflicts in Brazilian and international health agenda, 1958–1961. Historia, Ciencias, Saude - Manguinhos 22: 95–114. [DOI] [PubMed] [Google Scholar]
- Silva-Nunes M, Moreno M, Conn JE, Gamboa D, Abeles S, Vinetz JM & Ferreira MU (2012). Amazonian malaria: Asymptomatic human reservoirs, diagnostic challenges, environmentally driven changes in mosquito vector populations, and the mandate for sustainable control strategies. Acta Tropica 121: 281–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siqueira AM, Mesones-Lapouble O, Marchesini P, Sampaio V.de S., Brasil P, Tauil PL, Fontes CJ, Costa FTM, Daniel-Ribeiro CT, Lacerda MVG, Damasceno CP & Santelli ACS (2016). Plasmodium vivax Landscape in Brazil: Scenario and Challenges. American Journal of Tropical Me dicine and Hygiene 95: 87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauil PL (2011). The prospect of eliminating malaria transmission in some regions of Brazil. Memorias do Instituto Oswaldo Cruz 106 Suppl 1: 105–106. [DOI] [PubMed] [Google Scholar]
- Trigg PI. & Kondrachine AV (1998). Commentary: malaria control in the 1990s. Bulletin of the World Health Organization 76: 11–16. [PMC free article] [PubMed] [Google Scholar]
- United Nations (2015). Transforming our world: the 2030 Agenda for Sustainable Development. UN: New York, pp.1–41. [Google Scholar]
- van Eer ED, Bretas G & Hiwat H (2018). Decreased endemic malaria in Suriname: moving towards elimination. Malaria Journal 17: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitor-Silva S, Siqueira AM, de Souza Sampaio V, Guinovart C, Reyes-Lecca RC, de Melo GC, Monteiro WM, del Portillo HA, Alonso P, Bassat Q & Lacerda MVG (2016). Declining malaria transmission in rural Amazon: changing epidemiology and challenges to achieve elimination. Malaria Journal 15: 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosti SA (1990). Malaria among gold miners in southern Para, Brazil: estimates of determinants and individual costs. Social Science Medicine 30: 1097–1105. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2015). Global technical strategy for malaria 2016–2030. WHO: Geneva, pp.1–35. [Google Scholar]
- World Health Organization (2018). World Malaria Report 2018. WHO: Geneva, pp.1–210. [Google Scholar]