Abstract
Pubertal timing is regulated by the complex interplay of genetic, environmental, nutritional and epigenetic factors. Criteria for determining normal pubertal timing, and thus the definition of precocious puberty, have evolved based on published population studies. The significance of the genetic influence on pubertal timing is supported by familial pubertal timing and twin studies. In contrast to the many monogenic causes associated with hypogonadotropic hypogonadism, only four monogenic causes of central precocious puberty (CPP) have been described. Loss-of-function mutations in Makorin Ring Finger Protein 3 (MKRN3), a maternally imprinted gene on chromosome 15 within the Prader-Willi syndrome locus, are the most common identified genetic cause of CPP. More recently, several mutations in a second maternally imprinted gene, Delta-like noncanonical Notch ligand 1 (DLK1), have also been associated with CPP. Polymorphisms in both genes have also been associated with age of menarche in genome-wide association studies. Mutations in the genes encoding kisspeptin (KISS1) and its receptor (KISS1R), potent activators of GnRH secretion, have also been described in association with CPP, but remain rare monogenic causes. CPP has both short- and long-term health implications for children, highlighting the importance of understanding the mechanisms contributing to early puberty. Additionally, given the role of mutations in the imprinted genes MKRN3 and DLK1 in pubertal timing, other imprinted candidate genes should be considered for a role in puberty initiation.
Keywords: central precocious puberty, kisspeptin, GnRH, MKRN3, DLK1, Prader-Willi syndrome, Temple syndrome
Introduction
Puberty is the remarkable period during which the body develops secondary sexual characteristics and becomes capable of reproduction. The mechanisms by which pubertal onset begins remains one of science’s great mysteries.
Physiology of puberty
The hallmark of pubertal onset is reactivation of the hypothalamic-pituitary-gonadal (HPG) axis, which is initially active during the fetal and neonatal periods but then undergoes a period of quiescence in childhood (1). The axis is subsequently reactivated at the onset of puberty with the reemergence of pulsatile hypothalamic gonadotropin-releasing hormone (GnRH) release, likely due to increases in activators such as kisspeptin, the most potent known stimulator of GnRH secretion, and decreases in inhibitors of the axis (1,2). Kisspeptin signals directly to GnRH neurons to control pulsatile GnRH release which leads to release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) from the pituitary, with downstream activation of sex steroid production—testosterone from the testes and estrogen and progesterone from the ovaries—and stimulation of gametogenesis (Figure 1) (1,2).
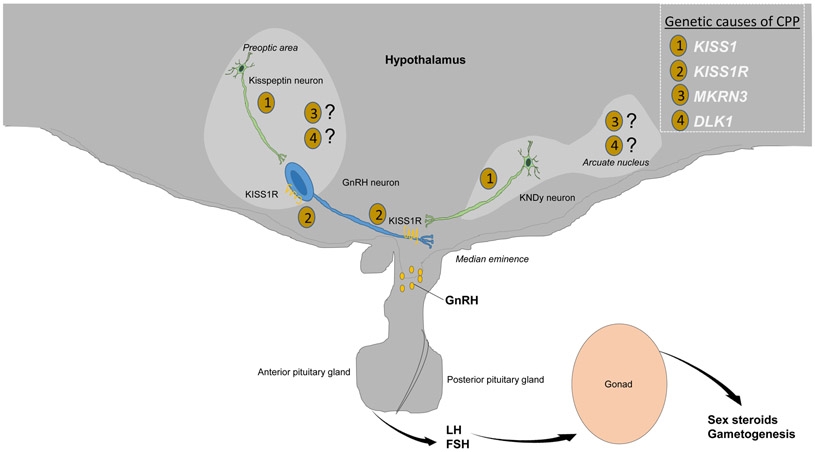
Figure 1.
Activation of the hypothalamic-pituitary-gonadal axis during pubertal onset. The hallmark of pubertal onset is pulsatile hypothalamic gonadotropin-releasing hormone (GnRH) release, likely due to increases in activators such as kisspeptin, the most potent known stimulator of GnRH secretion, produced by kisspeptin neurons in the preoptic area and in the arcuate nucleus (where they co-secrete neurokinin B and dynorphin and are hence known as KNDy neurons). Kisspeptin signals directly via kisspeptin receptors (KISS1R) on GnRH neurons to control pulsatile GnRH release, which in turn leads to release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) from the pituitary, with subsequent downstream activation of sex steroid production—testosterone from the testes and estrogen and progesterone from the ovaries—and gametogenesis. Genetic variants in CPP include mutations in (1) KISS1 and (2) KISS1R, affecting kisspeptin and its receptor, respectively, as well as in (3) MKRN3 and (4) DLK1, for which the mechanisms of action in the hypothalamus are still being fully elucidated.
In girls, activation of the HPG axis leads to the onset of breast development, weight gain, widening of the pelvic bones, increased fat distribution in the hips, thighs and buttocks, and menstruation, the latter of which typically occurs 18 months to 2 years after the onset of breast development (3,4). In boys, puberty results initially in testicular enlargement to a size greater than or equal to 4 mL, followed by phallic lengthening and widening, voice deepening, broadening of the chest and shoulders, appearance of facial and male-pattern distribution hair, and ability to achieve an erection and ejaculation (3,5). In both genders, the pubertal growth spurt is followed by fusion of the epiphyses. A standardized approach to characterizing development of secondary sexual characteristics was established by Tanner in the 1960s to describe pubic hair development in both sexes as well as breast development in girls and genital development in boys (4,5). Tanner stage I in all scales refers to the prepubertal state, while Tanner stage II refers to the first stages of puberty, progressing to final adult development as Tanner stage V (3,4).
Normal pubertal timing
Girls typically enter puberty between ages 8 to 13 years while boys typically enter puberty between 9 to 14 years (4-5). Sex differences are present in the timing of pubertal onset as girls enter puberty approximately one to two years earlier than boys. Interestingly, there are also sex differences in the pathologic variants of pubertal timing as girls are at increased risk for early puberty while boys are increased risk for delayed puberty, yet the mechanisms leading to these differences are unknown (6). The onset of central precocious puberty should be confirmed by an experienced clinician, as isolated thelarche or adrenarche can sometimes be confused for the onset of central puberty (7). The appearance of adult body odor, axillary hair and pubic hair may due to normal age-related activation of the adrenal gland during adrenarche. This typically occurs at the same time as central puberty, but can sometimes occur earlier and is not synonymous with the onset of central puberty (7).
Definitions of normal pubertal timing in girls, including progression of secondary sexual characteristics and pubertal growth spurt, are based on hallmark studies performed by Marshall and Tanner in the late 1960s (4). In the cohort of 192 British girls who were followed, mean Tanner stage II breast development occurred at age 11.5 years (range 8.5-13 years), pubic hair development at 11.69 years, and menarche at 13.47 years (4). However, subsequent studies by Tanner supported the onset of breast development at age 10.0 years and mean age of menarche at 12.9 years (8).
In the United States and Europe, the decline in mean age of menarche with industrialization has essentially plateaued since the 1960s, with further minor declines in the mean age of menarche by 2.5-4 months over the past 25 years, but with a far greater decline observed in age of onset of breast development (9). The Pediatric Research in Office Setting (PROS) performed in the 1990s was the largest study to date, with more than 17,000 participants, evaluated by 65 pediatricians across the United States (10). The authors concluded that on average, Caucasian girls entered puberty by age 10, approximately one year earlier than previously reported, and African-American girls entered puberty between age 8 to 9 years, approximately two years earlier than previously reported (10).
Based on the PROS study, the Lawson Wilkins Pediatric Endocrine Society published guidelines suggesting that the definition of precocious, or abnormally early, puberty as the onset before age 8 years in females was “outdated” (11). It sparked controversy with its recommendation precocious puberty should be defined as breast development prior to age 7 years in Caucasian girls and prior to age 6 years in African American girls (11). These guidelines suggested an allowance for more flexibility in the extent of evaluation, including whether brain magnetic resonance imaging is necessary, and in the management of affected children between the ages of 6 to 8 years in girls(11).
The guidelines suggested unchanged consideration for evaluation of precocious puberty in boys. Marshall and Tanner in 1970 had suggested pubertal onset after age 9.5 years was normal (5). However, as in girls, the PROS study suggested the onset of puberty was also occurring earlier than previously reported in boys, at a mean age of 10.14 years in Caucasian boys and 9.14 years in African-American boys (12), further supporting ethnic differences in the age of pubertal onset.
These clinical guidelines also raised international awareness about secular trends in pubertal timing and whether the revised age-based definitions were warranted. More recent evaluations in the Copenhagen Puberty Study found earlier age of onset of breast development and slight advancement in age of menarche from children evaluated in the mid-2000s (13). While minor advances in the age of menarche have been observed in the United States and Europe since the 1960s, more dramatic reductions have been observed in countries with improving socioeconomic status such as Indonesia, Brazil, Argentina and China, often in the setting of increased rates of childhood obesity (14-20). Furthermore, increasing recognition of environmental factors and exposures have been described in association with an earlier age of puberty, such as the effects of maternal smoking during pregnancy, of girls being raised without a father, and of some endocrine disrupting chemicals (21-23).
Central precocious puberty
Precocious puberty is traditionally defined as the onset of breast development before age 8 years in girls and testicular enlargement ≥ 4 mL before the age of 9 years in boys (24). Differentiation of precocious puberty from benign variants, such as premature thelarche or premature adrenarche, should be made by an experienced clinician (7).The underlying pathophysiology may be central (GnRH-dependent) or peripheral (GnRH-independent). Central precocious puberty (CPP) is due to premature activation of GnRH secretion. CPP disproportionately affects girls compared to boys; the mechanisms leading to these differences are unknown (24). Understanding more common causes of CPP, including genetic etiologies, is vital given that CPP has important short- and long-term implications for women, including increased risk of psychosocial distress, short stature, obesity, cardiovascular disease and type 2 diabetes in adulthood (25).
The gold standard to establish a biochemical diagnosis is an assessment of gonadotropins, predominantly LH, after administration with exogenous GnRH or GnRH agonists (24). However, basal LH levels are also being utilized in some settings, as improved laboratory methods for LH assays have become available (24,26). In contrast to serum estradiol levels in girls, which are typically not helpful, in boys serum testosterone levels are an excellent marker of precocious puberty, although do not differentiate between central or peripheral etiologies (24). Commonly used imaging studies include bone age x-rays, which typically demonstrate a skeletal age that is advanced by two years or more from chronologic age (24). Pelvic ultrasonography can indicate uterine exposure to estradiol and help identify an etiology of peripheral precocious puberty, but is not part of the diagnostic criteria for precocious puberty (24,26). Brain MRI imaging is an important part of the diagnostic evaluation in order to exclude a hypothalamic hamartoma or other organic abnormalities, especially before the CPP is classified as ‘idiopathic’ (24,26).
In girls, CPP is most commonly deemed idiopathic, for which no identifiable cause is identified (24). However, emerging genetic causes of CPP, especially mutations in Makorin Ring Finger Protein 3 (MKRN3) are increasingly being identified in cases previously deemed idiopathic (27-50). While CPP less commonly affects boys, when it does occur, is more likely to have an identifiable pathologic cause, such as a hypothalamic hamartoma (24). Given its increasing significance, the focus of the remainder of this review will be on the recently identified genetic causes of CPP.
Support for a genetic basis in pubertal timing
The timing of pubertal onset is influenced by a complex interaction of genetic, nutritional, environmental, and epigenetic factors (51). The genetic contribution to pubertal timing is supported by the strong correlation between the ages that children and their parents begin puberty. Twin studies also support a genetic component, demonstrating a higher concordance of the timing of development of secondary sexual characteristics, including menarche in girls, in monozygotic compared to dizygotic twins (52-55).
While variants in more than 50 genes have been described in association with hypogonadotropic hypogonadism, which often presents with delayed puberty, until recently, genetic variants had not been a major identified etiology of CPP (24, 56). To date, mutations in four genes (KISS1, KISS1R, MKRN3, DLK1) have been described as causal variants leading to CPP, with loss-of-function mutations in MKRN3 as the most common genetic etiology identified to date (28,57).
Known genetic variants described in association with CPP
KISS1R
The first identified monogenic cause of CPP, described in 2008, was an activating mutation in G Protein Coupled Receptor 54 (GPR54), now more commonly referred to as KISS1R, encoding a receptor that binds its ligand, kisspeptin (Figure 1) (58). Loss-of-function mutations in KISS1R had been described previously in association with hypogonadotropic hypogonadism (59). A gain-of-function mutation in KISS1R, a substitution of proline for arginine at codon 386, was reported in a girl with CPP. Inheritance could not be assessed as she was adopted. Breast development was reported since birth with progression such that by age 8 years, she had Tanner stage IV breasts and an advanced skeletal age of 11 years.
In vitro studies suggested that this mutation prolongs intracellular KISS1R signaling in response to kisspeptin (58,60). In contrast to other gain-of-function mutations in G protein-coupled receptors, which typically result in constitutive activation of the receptor, the reported gain-of-function mutation in KISS1R did not result in a change in receptor activity under basal conditions or in affinity for the kisspeptin ligand. Rather, data in cellular models support a mechanism involving nonconstitutive receptor activation, in which there is a reduction of the rate of degradation of the kisspeptin receptor, and internalized mutant receptors are recycled back to the plasma membrane rather than being degraded (58,60).
KISS1
Subsequently, a heterozygous missense mutation (p.P74S) in the ligand, kisspeptin, encoded by the gene KISS1, was described in a one-year-old boy and was associated with higher resistance to degradation (61). A second missense mutation in KISS1 was also reported in two girls affected with CPP at age 5.5-6 years; however, it was concluded to be a rare single nucleotide polymorphism, at least in the heterozygous state. Other polymorphisms in KISS1 have been reported but were not found to lead to amino acid changes and therefore were not thought to be causal variants (62). Other investigators have screened cohorts of children with CPP for activating mutations in KISS1 and KISS1R but have not identified mutations, suggesting that these appear to be rare causes of CPP (63-65).
MKRN3
Makorin Ring Finger Protein 3 (MKRN3), located within the Prader-Willi syndrome (PWS) region on chromosome 15q11.2 (Figure 2A), was reported in 2013 as the first gene in which loss-of-function mutations were associated with CPP, with variants initially identified by exome sequencing in five families (27). Multiple novel variants, including frameshift, nonsense, and missense mutations, in MKRN3 across various families, ethnicities and geographical regions have now been reported (27-50). Mutations in MKRN3 are now the most common known genetic etiology of CPP and are more common in familial CPP (33-46% of cases) compared to sporadic CPP (3.9% of cases) (28). Affected individuals with CPP due to MKRN3 mutations have a median age of puberty onset of 6.0 years in girls (ranging from 3.0 to 7.5 years) and 8.25 years in boys (ranging from 5.9 to 9.0 years); the earlier onset of puberty in girls suggests that girls may be more severely affected by mutations in MKRN3 than boys. However, based on the pooled analysis of cases of familial CPP, boys with CPP appear to be more likely than girls with CPP to harbor a mutation in MKRN3 (28).
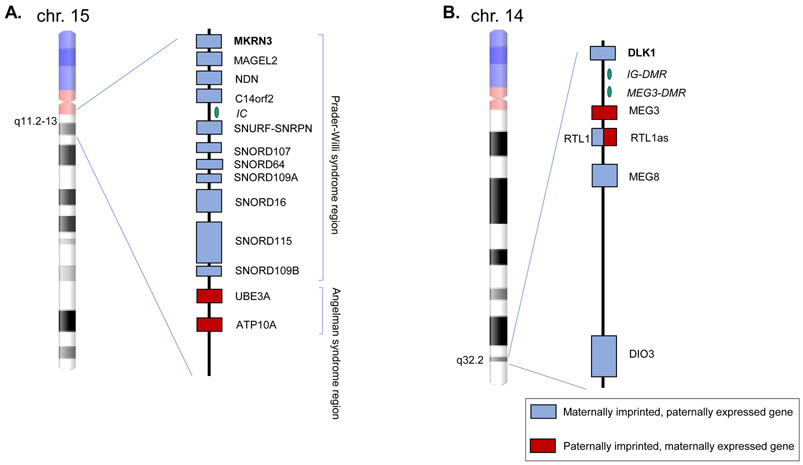
Figure 2.
A. Imprinted genes in the Prader-Willi syndrome and Angelman syndrome regions of chromosome 15q11.2-13. B. Temple syndrome region of chromosome 14q32.2. Not drawn to scale. Maternally imprinted, paternally expressed genes are indicated in blue boxes and paternally imprinted, maternally expressed genes in red boxes. IC: imprinting center, DMR: differentially methylated regions. Adapted from (69, 102).
MKRN3 is maternally imprinted, or silenced; therefore, only the paternal allele is expressed and the MKRN3 protein is expressed in a parent-of-origin specific pattern (66) (Figure 2A and 3). Like other genes in the Prader-Willi locus, MKRN3 is within a conserved cluster, common for imprinted genes, under the control of a bipartite imprinting center, the PWS-IC and AS-IC (Figure 2A) (67). PWS results from a loss of a cluster of genes normally expressed from the paternal allele, while conversely Angelman syndrome results from loss of genes normally expressed from the maternal allele. PWS is characterized by hypotonia, paradoxical early failure to thrive, followed by childhood hyperphagia and obesity, developmental delay, and multiple endocrinopathies including hypogonadism (68). However, CPP has been reported in up to 3.5% of patients with PWS and may be underreported (68). Loss of the paternally inherited genes in the PWS typically occurs via deletion but can also occur by uniparental disomy or epimutations and microdeletions in the imprinting center (69). These mechanisms could also lead to alterations in MKRN3 expression, leading to CPP, but have not yet been reported.
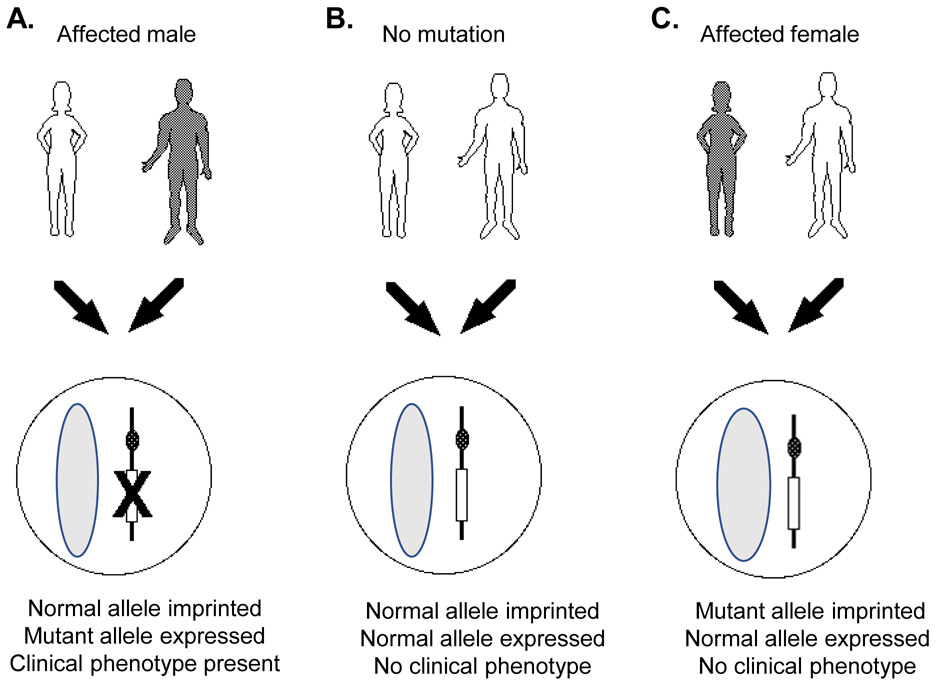
Figure 3.
Imprinting pattern of maternally imprinted, paternally expressed genes MKRN3 and DLK1. A. Maternal allele is imprinted, or silenced; mutant paternal allele is expressed, resulting in phenotype of central precocious puberty. B. Maternal allele is normally imprinted and normal paternal allele is expressed, resulting in normal puberty. C. Maternal allele containing mutation is imprinted; normal paternal allele is expressed, resulting in no pathologic phenotype (i.e., normal puberty). Adapted from (103).
MKRN3, a member of the makorin family, is a zinc finger protein with a characteristic RING (C3HC4) zinc finger motif found in most E3 ubiquitin ligases, as well as an array of C3H domains found in ribonucleoproteins (66). The actions of E3 ubiquitin ligases include mediating the transfer of ubiquitin from an E2 ubiquitin-conjugating enzyme to target protein substrates, which can target the protein substrates for degradation (70). MKRN3 may have multiple protein targets, suggested by a protein-protein interaction study performed in MKRN3 knock-out human pluripotent stem cell lines differentiated into GNRH1-expressing neurons, which identified 81 interacting proteins, including some involved in pubertal timing, insulin signaling, RNA metabolism and cell-cell adhesion (71). Additionally, Mkrn3 was reported to bind to neuronal pentraxin-1 (Nptx1), a secreted protein important in neuronal development and highly expressed in the mouse hypothalamus during pubertal onset (72). Through interaction with an E3 ubiquitin ligase domain in Mkrn3, Nptx1 may be polyubiquitinated by Mkrn3 for degradation (72).
In mice, expression of Mkrn3 is high in the hypothalamus in the neonatal and juvenile periods and declines abruptly prior to pubertal onset, supporting its role as an inhibitor of the pathways leading to puberty initiation, and it is hypothesized to act upstream or at the level of kisspeptin and/or GnRH neurons (27). Its inhibitory role is further supported by reports of declining serum MKRN3 levels in girls and boys before pubertal onset (73-75). Serum levels of MKRN3 have also been shown to be lower in girls with CPP compared to age-matched controls and comparable to children matched for pubertal stage (76,77). It would be of interest to validate the MKRN3 assay used in patients expected to have negligible MKRN3 protein synthesis, such as those with frameshift or nonsense mutations, or patients with Prader Willi Syndrome (27, 29).
DLK1
More recently, a mutation in Delta-like noncanonical Notch ligand 1 (DLK1), located on chromosome 14q32.2, was reported as a novel genetic cause of CPP (78) (Figure 2B). Like MKRN3, DLK1 is a maternally imprinted, paternally expressed gene (Figure 3). A family involving four affected sisters was described with the onset of breast development between ages 4.6 to 5.9 years, with biochemically confirmed CPP. The paternal grandmother also had a history of premature menarche, suggesting that she may have also been affected. A complex genetic defect detected by genomic sequencing in DLK1, involving a ~14 kb deletion, encompassing the entirety of exon 1, including the translational start site, and a 269-bp duplication of intron 3 was identified (78). Subsequently, three frameshift mutations of DLK1 were reported in five women from three families with CPP (79). Serum DLK1 concentrations were undetectable in all patients with reported loss-of-function DLK1 mutations (78-79). Interestingly, a metabolic phenotype including increased rates of obesity, early-onset glucose intolerance, type 2 diabetes mellitus, and hyperlipidemia were found to be more prevalent in the patients with DLK1 mutations compared to patients with CPP not identified to have DLK1 mutations. Additionally, two affected sisters also exhibited polycystic ovary syndrome and infertility (79). The emerging clinical phenotype suggests that DLK1 may represent a novel link between reproduction and metabolism.
DLK1, also known as Preadipocyte factor 1 (Pref-1) or Fetal antigen 1 (FA1), encodes an EGF-like membrane-bound protein, is likely involved in the Notch signaling pathway, and is a modulator of adipogenesis (80). The DLK1 imprinting pattern is under the control of two differentially methylated regions (DMRs), the intergenic differentially methylated region (IG-DMR) and the post-fertilization-derived secondary MEG3-DMR (Figure 2B) (80). Loss of DLK1 in conjunction with two other genes from the paternally inherited chromosome, RTL1 and DIO3, leads to Temple syndrome (82). Mechanistically, the loss of the paternal alleles is typically acquired through maternal uniparental disomy, deletion, or less commonly epigenetic loss of methylation or deletion of the DMRs (83). Temple syndrome is characterized by pre- and post-natal growth retardation, hypotonia, motor delay, and small hands. Conversely, loss of the maternally inherited alleles, Maternally Expressed 3 (MEG3), Maternally Expressed 8 (MEG8) and Retrotransposon Like 1 antisense (RTL1as), leads to Kagami syndrome, which is characterized by dysmorphic facies, bell-shaped thorax with coat-hanger appearance of the ribs, abdominal wall defects, pulmonary hypoplasia and intellectual disability (69). Interestingly, CPP is described in 86% of affected individuals in Temple syndrome, with a mean age of menarche at age 10 years and 2 months, which may contribute to the syndrome’s phenotype of short stature in adulthood (82).
The causative gene of this reproductive phenotype in Temple syndrome was not previously identified. However, the role of DLK1 in pubertal timing had been implicated by a genome-wide association study (GWAS) showing that single nucleotide polymorphisms (SNPs) near the paternally inherited DLK1 were associated with earlier age of menarche (84). Subsequently monogenic mutations in DLK1 in association with CPP were reported (78-79). Interesting, this is in contrast to loss-of-function mutations in MKRN3, which were first described in association with CPP and subsequently SNPs near MKRN3 were identified by GWAS in association with earlier age of menarche (27,83).
Polymorphisms in other candidate genes such as LIN28B and the paternally imprinted gene KCNK9 have also been described in association with the age of menarche and remain candidates as monogenic causes of CPP (84). Lin28 Homolog B (LIN28B), located on chromosome 6, a post-transcriptional repressor of microRNAs of the let7 family, has been implicated in pubertal timing by genome-wide association studies in which a genomic sequence variant near LIN28B was strongly associated with earlier age of menarche and short stature (84,85). Patients with CPP have been screened for causative variants in LIN28B, but none have been identified to date (86).
Role of imprinted genes in puberty
Two of the four described monogenic causes of CPP are imprinted genes, raising the possibility that imprinting plays an important role in the regulation of puberty and that other imprinted genes might also be involved in pubertal timing (87). There are several hundred genes predicted to be imprinted in the human genome and approximately 50 human imprinted genes have been described to date (88). Genomic imprinting allows a gene to be expressed in a parent-of-origin specific manner through epigenetic modification which silences, or imprints, one allele without modifying the DNA sequence (88). Thus, in contrast to most autosomal genes which have biallelic expression, imprinted genes typically exhibit monoallelic, or mostly monoallelic, expression (Figure 3). Well known epigenetic modifications include changes in methylation of DNA CpG (cytosine-guanine) dinucleotides or histone protein modifications (89). However, gene expression can also vary based on tissue-specific and developmental-specific patterns of epigenetic modifications as well as environmental influences.
Imprinted genes are important in growth and development, as evident by alterations in growth observed in disorders such as Beckwith-Wiedemann and Russell-Silver syndromes (90). The kinship, or parental conflict, theory proposes that imprinting may have evolved because of competition between the maternal and paternal genomes, resulting in maternal alleles that are silenced for genes that may increase demands on the mother (88,91-93). This theory posits that the gene silencing ensures the mother’s survival, allows equal distribution of nutrients among offspring, and that the most important relationship is the interaction between child and mother (94). Models of imprinting disorders in rodents and humans suggest that paternally expressed genes tend to promote growth while maternally expressed genes tend to restrict growth (95). The correlation of paternally inherited variants in MKRN3 and DLK1 in GWAS studies as well as monogenetic mutations in these genes in CPP, has been proposed to be due to differential effects from the mother versus father in terms of the influence of timing of sexual maturation and its contribution to overall fitness (87).
Epigenetic modifications have an increasingly recognized role in pubertal timing (51). Methylome profiling of girls with precocious and normally-timed pubertal onset before and after puberty revealed a widespread pattern of differential DNA hypermethylation. The majority of differentially methylated regions were hypermethylated in the post-pubertal group, compared to the prepubertal group, suggesting that pubertal onset is associated with specific changes in the regulatory control of epigenetic modification (96).
Treatment
The mainstay of treatment for CPP is GnRH agonist therapy, which is typically administered by intramuscular injection or a subcutaneous implant (97). The capacity of endogenous GnRH to stimulate LH synthesis and release can be interrupted by continuous delivery of a GnRH agonist, leading to desensitization and downregulation of GnRH signaling (98-99). Downstream effects include decreased release of pituitary gonadotropins and, subsequently, decreased release of gonadal sex steroids. Use of GnRH antagonists has potential therapeutic potential. However, use has been limited by availability, modes of administration, cost and lack of approved indication. Newer kisspeptin and neurokinin B antagonists are also being developed and may be alternative therapeutic options in the future (100-101).
Perspectives
The more recent recognition of MKRN3 and DLK1 as important influences in pubertal timing allows for opportunities for innovation in the diagnosis and treatment of CPP and for increased likelihood of identifying the etiology of ‘idiopathic’ CPP. GnRH agonist stimulation tests are the current gold standard for the diagnosis of CPP but are time-consuming, invasive and expensive. Biochemical measurement of serum levels of MKRN3 or DLK1 proteins could be innovative, less labor-intensive diagnostic tools for earlier detection of CPP (24). Additionally, assessment of serum levels of MKRN3 and DLK1 may serve as a way to identify unrecognized mutations in these genes, prompting genetic analysis which is not currently standard in clinical practice (76-79). The manipulation of MKRN3 and DLK1 activity also has therapeutic implications, as antagonizing these proteins or their respective targets could offer new strategies for the treatment of CPP.
Conclusion
In contrast to the many genetic causes identified in hypogonadotropic hypogonadism, to date only four monogenetic causes of CPP have been identified, including mutations in KISS1, KISS1R, MKRN3 and DLK1 (28,58,61,77,78). CPP that was previously classified as ‘idiopathic’ is increasingly being explained by the identification of genetic variants, especially in MKRN3 (78,79). Eliciting a detailed family history of pubertal timing is important as this may raise suspicion for genetic etiologies and should be interpreted within the context of a possible imprinting inheritance pattern (24,28). Novel genetic variants are likely to continue to be identified and reported, and consideration of imprinted genes, which appear to have an important role in the control of puberty, should continue to be explored (78, 87) . While presently this is an exciting time for the field as new genes involved in precocious puberty are identified, ultimately these discoveries advance our understanding of the fundamental biology of pubertal onset and provide opportunities for innovation in the diagnosis and treatment of pubertal disorders.
Acknowledgements
The authors would like to thank Soukayna Chouman for her assistance with the figures.
Funding
This work was supported by the National Institutes of Health 5K12HD051959 and Loan Repayment Program, Pediatric Endocrine Society Clinical Scholar Award and Boston Children’s Office of Faculty Development Award supporting Dr. Roberts and by National Institutes of Health R01HD082314 awarded to Dr. Kaiser.
Footnotes
Declaration of Interest
There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- 1.Palmert MR, Boepple PA. Variation in the timing of puberty: clinical spectrum and genetic investigation. J Clin Endocrinol Metab 2001. 86 2364–2368. [DOI] [PubMed] [Google Scholar]
- 2.Fergani C, Navarro V. Expanding the Role of Tachykinins in the Neuroendocrine Control of Reproduction. Reproduction 2016. 153 R1–R15. [DOI] [PubMed] [Google Scholar]
- 3.Wheeler M Physical changes of puberty. Endocrinol Metab Clin North Am 1991. 20 1–14. [PubMed] [Google Scholar]
- 4.Marshall W, Tanner J. Variations in pattern of pubertal changes in girls. Arch Dis Child 1969. 44 291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marshall W, Tanner J. Variations in the pattern of pubertal changes in boys. Arch Dis Child 1970. 45 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bianco S A potential mechanism for the sexual dimorphism in the onset of puberty and incidence of idiopathic central precocious puberty in children: sex-specific kisspeptin as an integrator of puberty signals. Frontiers in Endocrinology 2012. 3 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaplowitz P, Bloch C. Evaluation and referral of children with signs of early puberty. Pediatrics 2016. 137 e20153732. [DOI] [PubMed] [Google Scholar]
- 8.Tanner J, Davies P. Clinical longitudinal standards for height and height velocity for North American children. J Pediatr 1985. 107 317–329. [DOI] [PubMed] [Google Scholar]
- 9.Parent A, Teilmann G, Juul A, Niels S, Toppari J, Bourguignon J. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends and changes after migration. Endocrine reviews 2003. 24 668–693. [DOI] [PubMed] [Google Scholar]
- 10.Herman-Giddens ME, Slora EJ, Wasserman RC, Bourdony CJ, Bhapkar MV, Koch GG, Hasemeier CM. Secondary sexual characteristics and menses in young girls seen in office practice: a study from the Pediatric Research in Office Settings network. Pediatrics 1997. 99 505–512. [DOI] [PubMed] [Google Scholar]
- 11.Kaplowitz P, Oberfield S. Reexamination of the age limit for defining when puberty is precocious in girls in the United States: implications for evaluation and treatment. Drug and Therapeutics and Executive Committees of the Lawson Wilkins Pediatric Endocrine Society. Pediatrics 1999. 104 936–941. [DOI] [PubMed] [Google Scholar]
- 12.Herman-Giddens ME, Steffes J, Harris D, Slora E, Hussey M, Dowshen SA, Wasserman R, Serwint J, Smitherman L, Reiter E. Secondary sexual characteristics in boys: data from the Pediatric Research in Office Settings Network. Pediatrics 2012. 130 1058–1068. [DOI] [PubMed] [Google Scholar]
- 13.Asglaede L, Sorensen K, Petersen JH, Skakkebaek NE, Juul A. Recent decline in age of breast development: the Copenhagen Puberty Study. Pediatrics 2009. 123 e932–9. [DOI] [PubMed] [Google Scholar]
- 14.Brix N, Ernst A, Lauridsen LLB, Parner E, Stovring H, Olsen J, Henriksen TB, Ramlau-Hansen CH. Timing of puberty in boys and girls: a population based study. Paediatr Perinatal Epidemiol 2019. 33 70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papadimitriou A, Pantsiotou S, Douros K, Papadimitriou DT, Nicolaidou P, Fretzayas A. Timing of pubertal onset in girls: evidence for non-Gaussian distribution. J Clin Endocrinol Metabolism 2008. 93 4422–5. [DOI] [PubMed] [Google Scholar]
- 16.Semiz S, Kurt F, Kurt DT, Zencir M, Sevinc O. Pubertal development of Turkish children. J Pediatr Endocrinol Metab 2008. 21 951–61. [DOI] [PubMed] [Google Scholar]
- 17.Wahab A, Wilopo SA, Hakimi M, Ismail D. Declining age of menarche in Indonesia: a systematic review and meta-analysis. Int J Adolesc Med Health 2018. Epub. [DOI] [PubMed] [Google Scholar]
- 18.Tc Feibelmann, Sliva AP, Resende DC, Resende EA, Scatena LM, Borges Mde F. Puberty in a sample of Brazilian schoolgirls: timing and anthropometric characteristics. Arch Endocrinol Metab 2015. 59 105–11. [DOI] [PubMed] [Google Scholar]
- 19.Chen FF, Wang YF, Mi J. Timing and secular trend of pubertal development in Beijing girls. World J Pediatr 2014. 10 74–79. [DOI] [PubMed] [Google Scholar]
- 20.Martin MA, Valeggia C. Timing of pubertal growth and menarche in indigenous Qom girls of Argentina. Ann Hum Biol 2018. 45 321–329. [DOI] [PubMed] [Google Scholar]
- 21.Brix N, Ernst A, Lauridsen LLB, Parner ET, Olsen J, Henriksen TB, Ramlau-Hansen CH. Maternal smoking during pregnancy and timing of puberty in sons and daughters: a population-based cohort study. Am J Epidemiol 2019. 188 47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaydosh L, Belsky DW, Domingue BW, Boardman JD, Harris KM. Father absence and accelerated reproductive development in non-Hispanic white women in the United States. Demography 2018. 55 1245–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolff MS, Pajak A, Pinney SM, Windham GC, Galvez M, Rybak M, Silva MJ, Ye X, Calafat AM, Kushi LH, et al. Association of urinary phthalate and phenol biomarkers with menarche in a multiethnic cohort of young girls. Reprod Toxicol 2017. 67 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Latronico A, Brito V, Carel J. Causes, diagnosis and treatment of central precocious puberty. Lancet Diabetes Endocrinol 2016. 4 265–274. [DOI] [PubMed] [Google Scholar]
- 25.Yoo J Effects of early menarche on physical and psychosocial health problems in adolescent girls and adult women. Korean J Pediatr 2016. 59 355–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bangalore Krishna K, Fuqua JS, Rogol AD, Klein KO, Popovic J, Houk CP, Charmandari E, Lee PA, Freire AV, Ropelato MG, et al. Use of gonadotropin-releasing hormone analogs in children: update by an international consortium. Horm Res Pediatr 2019. 91 357–372. [DOI] [PubMed] [Google Scholar]
- 27.Abreu A, Dauber A, Macedo D, Noel S, Brito V, Gill J, Cukier P, Thompson I, Navarro V, Gagliardi P et al. Central precocious puberty causes by mutations in the imprinted gene MKRN3. N Engl J Med 2013. 368 2467–2475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valadares LP, Meireles CG, De Toledo IP, Santarem de Oliveira R, Goncalves de Castro LP, Abreu AP, Carroll RS, Latronico AC, Kaiser UB, Guerra ENS, et al. MKRN3 mutations in central precocious puberty: a systematic review and meta-analysis. J Endocr Soc 2019. 3 979–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aycan Z, Savaş-Erdeve Ş, Çetinkaya S, Kurnaz E, Keskin M, Şahin NM, Bayramoğlu E, Ceylaner G. Investigation of MKRN3 mutation in patients with familial central precocious puberty. J Clin Res Pediatr Endocrinol 2018. 10 223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bessa DS, Macedo DB, Brito VN, França MM, Montenegro LR, Cunha-Silva M, Silveira LG, Hummel T, Bergadá I, Braslavsky D. High frequency of MKRN3 mutations in male central precocious puberty previously classified as idiopathic. Neuroendocrinology 2016. 105 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fanis P, Skordis N, Toumba M, Papaioannou N, Makris A, Kyriakou A, Neocleous V, Phylactou LA. Central precocious puberty cause by novel mutations in the promoter and 5’-UTR region of the imprinted MKRN3 gene. Front Endocrinol (Lausanne) 2019. 10 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Christoforidis A, Skordis N, Fanis P, Dimitriadou M, Sevastidou M, Phelan MM, Neocleous V, Phylactou LA. A novel MKRN3 nonsense mutation causing familial central precocious puberty. Endocrine 2017. 56 446–449. [DOI] [PubMed] [Google Scholar]
- 33.Dimitrova-Mladenova MS, Stefanova EM, Glushkova M, Todorova AP, Todorov T, Konstantinova MM, Kazakova K, Tincheva RS. Males with paternally inherited MKRN3 mutations may be asymptomatic. J Pediatr 2016. 179 263–265. [DOI] [PubMed] [Google Scholar]
- 34.Grandone A, Cantelmi G, Cirillo G, Marzuillo P, Luongo C, Giudice EMD, Perrone L. A case of familial central precocious puberty caused by a novel mutation in the Makorin RING Finger Protein 3 gene. BMC Endocr Disord 2015. 15 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeong HR, Lee HS, Hwang JS. Makorin Ring Finger 3 gene analysis in Koreans with familial precocious puberty. J Pediatr Endoccrinol Metab 2017. 30 1197–1201. [DOI] [PubMed] [Google Scholar]
- 36.Känsäkoski J, Raivio T, Juul A, Tommiska J. A missense mutation in MKRN3 in a Danish girl with central precocious puberty and her brother with early puberty. Pediatr Res 2015. 78 709–711. [DOI] [PubMed] [Google Scholar]
- 37.Lee H, Jin H-S, Shim Y, Jeong H, Kwon E, Choi V, Kim M-C, Chung I-S, Jeong S-Y, Hwang J. Low frequency of MKRN3 mutations in central precocious puberty among Korean girls. Horm Metab Res 2015. 48 118–122. [DOI] [PubMed] [Google Scholar]
- 38.Lin W-D, Wang C-H, Tsai F-J. Genetic screening of the Makorin Ring Finger 3 gene in girls with idiopathic central precocious puberty. Clin Chem Lab Med 2016. 54 e93–6. [DOI] [PubMed] [Google Scholar]
- 39.Macedo DB, Abreu AP, Reis ACS, Montenegro LR, Dauber A, Beneduzzi D, Cukier P, Silveira LFG, Teles MG, Carroll RS, et al. Central precocious puberty that appears to be sporadic caused by paternally inherited mutations in the imprinted gene Makorin Ring Finger 3 J Clin Endocrinol Metab 2014. 99 E1097–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Macedo D, França MM, Montenegro L, Cunha-Silva M, Bessa DS, Abreu AP, Kaiser UB, Mendonca BB, Jorge AA, Brito VN, et al. Central precocious puberty caused by a heterozygous deletion in the MKRN3 promoter region. Neuroendocrinology 2018. 107 127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neocleous V, Shammas C, Phelan MM, Nicolaou S, Phylactou LA, Skordis N. In silico analysis of a novel MKRN3 missense mutation in familial central precocious puberty. Clin Endocrinol (Oxf) 2016. 84 80–84. [DOI] [PubMed] [Google Scholar]
- 42.Nishioka J, Shima H, Fukami M, Yatsuga S, Matsumoto T, Ushijima K, Kitamura M, Koga Y. The first Japanese case of central precocious puberty with a novel MKRN3 mutation. Hum Genome Var 2017. 4 17017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ortiz-Cabrera NV, Riveiro-Álvarez R, López-Martínez MÁ, Pérez-Segura P, Aragón-Gómez I, Trujillo-Tiebas MJ, Soriano-Guillén L. Clinical exome sequencing reveals MKRN3 pathogenic variants in familial and nonfamilial idiopathic central precocious puberty. Horm Res Paediatr 2016. 87 88–94. [DOI] [PubMed] [Google Scholar]
- 44.Schreiner F, Gohlke B, Hamm M, Korsch E, Woelfle J. MKRN3 mutations in familial central precocious puberty. Horm Res Paediatr 2014. 82 122–126. [DOI] [PubMed] [Google Scholar]
- 45.Settas N, Dacou-Voutetakis C, Karantza M, Kanaka-Gantenbein C, Chrousos GP, Voutetakis A. Central precocious puberty in a girl and early puberty in her brother caused by a novel mutation in the MKRN3 gene. J Clin Endocrinol Metab 2014. 99 E647–651. [DOI] [PubMed] [Google Scholar]
- 46.Simon D, Ba I, Mekhail N, Ecosse E, Paulsen A, Zenaty D, Houang M, Jesuran-Perelroizan M, Filippo GD, Salerno M, Simonin G, et al. Mutations in the maternally imprinted gene MKRN3 are common in familial central precocious puberty. Eur J Endocrinol 2016. 174 1–8. [DOI] [PubMed] [Google Scholar]
- 47.Simsek E, Demiral M, Ceylaner S, Kırel B. Two frameshift mutations in MKRN3 in Turkish patients with familial central precocious puberty. Horm Res Paediatr 2016. 87 405–411. [DOI] [PubMed] [Google Scholar]
- 48.Stecchini MF, Macedo DB, Reis ACS, Abreu AP, Moreira AC, Castro M, Kaiser UB, Latronico AC, Antonini SR. Time course of central precocious puberty development caused by an MKRN3 gene mutation: a prismatic case. Horm Res Paediatr 2016. 86 126–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vries LD, Gat-Yablonski G, Dror N, Singer A, Phillip M. A novel MKRN3 missense mutation causing familial precocious puberty. Hum Reprod 2014. 29 2838–2843. [DOI] [PubMed] [Google Scholar]
- 50.Lu W, Wang J, Li C, Sun M, Hu R, Wang W. A novel mutation in 5’-UTR of Makorin Ring Finger 3 gene association with the familial precocious puberty. Acta Biochim Biophys Sin (Shanghai) 2018. 50 1291–1293. [DOI] [PubMed] [Google Scholar]
- 51.Leka-Emiri S, Chrousos G, Kanaka-Gatenbein C. The mystery of puberty initiation: genetics and epigenetics of idiopathic central precocious puberty (ICPP). J Endocrinol Invest 2017. 40 789–802. [DOI] [PubMed] [Google Scholar]
- 52.Morris D, Jones M, Schoemaker M, Ashworth A, Swerdlow A. Familial concordance for age at menarche: analyses from the Breakthrough Generations Study. Paediatr Perinat Epidemiol 2011. 25 306–311. [DOI] [PubMed] [Google Scholar]
- 53.Van den Berg S, Setiawan A, Bartels M, Polderman T, van der Vaart A, Boomsma D. Individual differences in puberty onset in girls: Bayesian estimation of heritabilities and genetic correlations. Behav Genet 2006. 36 261–270. [DOI] [PubMed] [Google Scholar]
- 54.Sharma J The genetic contribution to pubertal growth and development studied by longitudinal growth data on twins. Ann Hum Biol 1983. 10 163–171. [DOI] [PubMed] [Google Scholar]
- 55.Treloar S, Martin N. Age at menarche as a fitness trait: nonadditive genetic variance detected in a large twin sample. Am J Hum Genet 1990. 47 137–148. [PMC free article] [PubMed] [Google Scholar]
- 56.Topaloglu AK. Update on the genetics of idiopathic hypogonadotropic hypogonadism. J Clin Res Pediatr Endocrinol 2017. 9 113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grandone A, Capristo C, Cirillo G, Sasso M, Umano GR, Mariani M, Miraglia Del Giudice E, Perrone L. Molecular screening of MKRN3, DLK1, and KCNK9 genes in girls with idiopathic central precocious puberty. Horm Res Paediatr 2017. 88 194–200. [DOI] [PubMed] [Google Scholar]
- 58.Teles M, Bianco S, Brito V, Trarbach E, Kuohung W, Xu S, Seminara S, Mendonca B, Kaiser U, Latronico A. GPR54-activating mutation in a patient with central precocious puberty. N Engl J Med 2008. 358 709–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seminara S, Messager S, Chatzidaki E, Thresher R, Acierno J Jr, Shagoury J, Bo-Abbas Y, Kuohung W, Schwinof K, Hendrick A et al. The GPR54 gene as a regulator of puberty. N Engl J Med 2003. 349 1614–1627. [DOI] [PubMed] [Google Scholar]
- 60.Bianco S, Vandepas L, Correa-Medina M, Gereben B, Mukherjee A, Kuohung W, Carroll R, Teles MG, Latronico Ana Claudia, Kaiser UB. Kiss1r intracellular trafficking and degradation: effect of the Arg386Pro disease-associated mutation. Endocrinology 2011. 152 1616–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Silveira L, Noel S, Silveira-Neto A, Abreu A, Brito V, Santos M, Bianco S, Kuohung W, Xu S, Gryngarten M et al. Mutations of the KISS1 gene in disorders of puberty. J Clin Endocrinol Metab 2010. 95 2276–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ko J, Lee H, Hwang J. KISS1 gene analysis in Korean girls with central precocious puberty: a polymorphism, p.P110T suggested to exert a protective effect. Endocr J 2010. 57 701–709. [DOI] [PubMed] [Google Scholar]
- 63.Krstevska-Konstantinova M, Jovanovska J, Tasic V, Montenegro L, Beneduzzi D, Silveira L, Gucev Z. Mutational analysis of KISS1 and KISS1R in idiopathic central precocious puberty. J Pediatr Endocrinol Metab 2014. 27 199–201. [DOI] [PubMed] [Google Scholar]
- 64.Tommiska J, Sorensen K, Aksglaede L, Koivu R, Puhakka L, Juul A, Raivio T. LIN28B, LIN28A, KISS1 and KISS1R in idiopathic central precocious puberty. BMC Res Notes 2011. 22 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leka-Emiri S, Louizou E, Kambouris M, Chrousos G, De Roux N, Kanaka-Gantenbein C. Absence of GPR54 and TACR3 mutations in sporadic cases of idiopathic central precocious puberty. Horm Res Paediatr 2014. 81 177–181. [DOI] [PubMed] [Google Scholar]
- 66.Jong M, Carey A, Caldwell K, Lau M, Handel M, Driscoll D, Stewart C, Rinchik E, Nicholls R. Imprinting of a RING zinc-finger encoding gene in the mouse chromosome region homologous to the Prader-Willi syndrome genetic region. Hum Mol Genet 1999. 8 795–803. [DOI] [PubMed] [Google Scholar]
- 67.Perk J, Makedonski K, Lande L, Cedar H, Razin A, Shemer R. The imprinting mechanism of the Prader-Willi/Angelman regional control center. EMBO J 2002. 21 5807–5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Crinò A, Schiaffini R, Ciampalini P, Spera S, Beccaria L, Benzi F, Bosio L, Corrias A, Gargantini L, Salvatoni A et al. Hypogonadism and pubertal development in Prader-Willi syndrome. Eur J Pediatr 2003. 162 327–333. [DOI] [PubMed] [Google Scholar]
- 69.Hoffman K, Heller R. Uniparental disomies 7 and 14. Best Pract Res Clin Endocrinol Metab 2011. 25 77–100. [DOI] [PubMed] [Google Scholar]
- 70.Deshaies RJ, Joazeiro CA. RING domain E3 ubiquitin ligases. Annu Rev Biochem 2009. 78 399–434. [DOI] [PubMed] [Google Scholar]
- 71.Yellapragada V, Liu X, Lund C, Känsäkoski J, Pulli K, Vuoristo S, Lundin K, Tuuri T, Variosalo M, Raivio Tl. MKRN3 Interacts With Several Proteins Implicated in Puberty Timing but Does Not Influence GNRH1 Expression. Front Endocrinol (Lausanne) 2019. 10 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu J, Kong X, Chen F. Mkrn3 functions as a novel ubiquitin E3 ligase to inhibit Nptx1 during puberty initiation. Oncotarget 2017. 8 85102–85109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Busch A, Hagen C, Almstrup K, Juul A. Circulating MKRN3 levels decline during puberty in healthy boys. J Clin Endocrinol Metab 2016. 101 2588–2593. [DOI] [PubMed] [Google Scholar]
- 74.Hagen C, Sorensen, Mieritz M, Johannsen T, Almstrup, Juul A. Circulating MKRN3 levels decline prior to pubertal onset and through puberty: a longitudinal study of healthy girls. J Clin Endocrinol Metab 2015. 100 1920–1926. [DOI] [PubMed] [Google Scholar]
- 75.Varimo T, Dunkel L, Vaaralahti K, Miettinien P, Hero M, Raivio T. Circulating makorin ring finger protein 3 levels decline in boys before the clinical onset of puberty. Eur J Endocrinol 2016. 174 785–790. [DOI] [PubMed] [Google Scholar]
- 76.Grandone A, Cirillo G, Sasso M, Capristo C, Tornese G, Marzuillo P, Luongo C, Rosaria Umano G, Festa A, Coppola R et al. MKRN3 levels in girls with central precocious puberty and correlation with sexual hormone levels: a pilot study. Endocrine 2018. 59 203–208. [DOI] [PubMed] [Google Scholar]
- 77.Jeong HR, Lee HJ, Shim YS, Kang MJ, Yang S, Hwang IT. Serum Makorin ring finger protein 3 values for predicting central precocious puberty in girls. Gynecol Endocrinol 2019. 35 732–736. [DOI] [PubMed] [Google Scholar]
- 78.Dauber A, Cunha-Silva M, Macedo D, Brito V, Abreu A, Roberts S, Montenegro L, Andrew M, Kirby A, Weirauch M, et al. Paternally inherited DLK1 deletion associated with familial central precocious puberty. J Clin Endocrinol Metab 2017. 102 1557–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gomes LG, Cunha-Silva M, Crespo RP, Ramos CO, Montengero LR, Canton A, Lees M, Spoudeas H, Dauber A, Macedo DB, et al. Dlk1 is a novel link between reproduction and metabolism. J Clin Endocrinol Metab 2019. 104 2112–2120. [DOI] [PubMed] [Google Scholar]
- 80.Falix F, Aronson D, Lamers W, Gaemers I. Possible roles of DLK1 in the Notch pathway during development and disease. Biochim Biophys Acta 2012. 1822 988–995. [DOI] [PubMed] [Google Scholar]
- 81.Kagami M, O’Sullivan M, Green A, Watabe Y, Arisaka O, Masawa N, Matsuoka K, Fukami M, Matsubara K, Kato F et al. The IG-DMR and MEG3-DMR at human chromosome 14q32.2: hierarchical interaction and distinct functional properties as imprinting control centers. PLoS Genet 2010. 6 e1000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ioannides Y, Lokulo-Sodipe K, Mackay D, Davies J, Temple I. Temple syndrome: improving the recognition of an underdiagnosed chromosome 14 imprinting disorder: an analysis of 51 published cases. J Med Genet 2014. 51 495–501. [DOI] [PubMed] [Google Scholar]
- 83.Ogata T, Kagami M, Ferguson-Smith Ac. Molecular mechanisms regulating phenotypic outcome in paternal and maternal uniparental disomy for chromosome 14. Epigenetics 2008. 3 181–187. [DOI] [PubMed] [Google Scholar]
- 84.Perry JR, Day F, Elks CE, Sulem P, Thompson DJ, Ferreira T, He C, Chasman DI, Esko T, Thorleifsson G et al. Parent-of-origin specific allelic associations among 106 genomic loci for age at menarche. Nature 2014. 514 92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ong K, Elks CE, Li S, Zhao JH, Luan J, Andersen LB, Bingham SA, Brage S, Smith GD, Ekelund U et al. Genetic variation in LIN28B is associated with the timing of puberty. Nat Genet 2009. 41 729–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Silveira-Neto A, Leal L, Emerman A, Henderson K, Piskounova E, Henderson B, Gregory R, Silveira L, Hirschhorn J, Nguyen T et al. Absence of functional LIN28B mutations in a large cohort of patients with idiopathic central precocious puberty. Horm Res Paediatr 2012. 78 144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kotler J, Haig D. The tempo of human childhood: a maternal foot on the accelerator, a paternal foot on the brake. Evol Anthropol 2018. 27 80–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ishida M, Moore G. The role of imprinted genes in humans. Mol Aspects Med 2013. 34 826–840. [DOI] [PubMed] [Google Scholar]
- 89.Elhamamsy A Role of DNA methylation in imprinting disorders: an updated review. J Assist Reprod Genet 2017. 34 549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jirtle R, Sander M, Barrett. Genomic imprinting and environmental disease susceptibility. Environ Health Perspect 2000. 108 271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Giabicani E, Brioude F, Le Bouc Y, Netchine I. Imprinted disorders and growth. Ann Endocrinol (Paris) 2017. 78 112–113. [DOI] [PubMed] [Google Scholar]
- 92.Haig D Genomic imprinting and kinship: how good is the evidence? Annu Rev Genet 2004. 38 553–585. [DOI] [PubMed] [Google Scholar]
- 93.Haig D The kinship theory of genomic imprinting. Annual Review of Ecology and Systematics 2000. 31 9–32. [Google Scholar]
- 94.Wilkins JF, Haig D. Parental modifiers, antisense transcripts and loss of imprinting. Proc Biol Sci 2002. 269 1841–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Haig D, Wharton R. Prader-Willi syndrome and the evolution of human childhood. Am J Hum Biol 2003. 15 320–329. [DOI] [PubMed] [Google Scholar]
- 96.Moore T, Reik W. Genetic conflict in early development: parental imprinting in normal and abnormal growth. Rev Reprod 1996. 1 73–77 [DOI] [PubMed] [Google Scholar]
- 97.Bessa DS, Maschietto M, Aylwin CF, Canton APM, Brito VN, Macedo DB, Cunha-Silva M, Palhares HMC, de Resende EAMR, Borgets MF et al. Methylome profiling of healthy and central precocious puberty girls. Clic Epigenetics 2018. 10 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Willemsen R, Elleri D, Williams R, Ong K, Dunger D. Pros and cons of GnRHa treatment for early puberty in girls. Nat Rev Endocrinol 2014. 10 352–363. [DOI] [PubMed] [Google Scholar]
- 99.Ortmann O, Weiss J, Diedrich K. Gonadotropin-releasing hormone (GnRH) and GnRH agonists: mechanism of action. Reprod Biomed Online 2002. 5 1–7. [DOI] [PubMed] [Google Scholar]
- 100.Blumenfeld Z. Investigational and experimental GnRH analogs and associated neurotransmitters. Expert Opin Investig Drugs 2017. 26 661–667. [DOI] [PubMed] [Google Scholar]
- 101.Newton C, Anderson R, Millar R. Therapeutic neuroendocrine agonist and antagonist analogs of hypothalamic neuropeptides as modulators of the hypothalamic-pituitary-gonadal axis. Endocr Dev 2016. 30 106–29. [DOI] [PubMed] [Google Scholar]
- 102.Cheon CK. Genetics of Prader-Willi syndrome and Prader-Willi-Like syndrome. Ann Pediatr Endocrinol Metab. 2016. 21 126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McGowan R, Martin CC. Genomic Imprinting [Internet]. 1998. [cited 2020]. Available from: http://people.ucalgary.ca/~browder/imprinting.html [Google Scholar]