Abstract
Background and Purpose
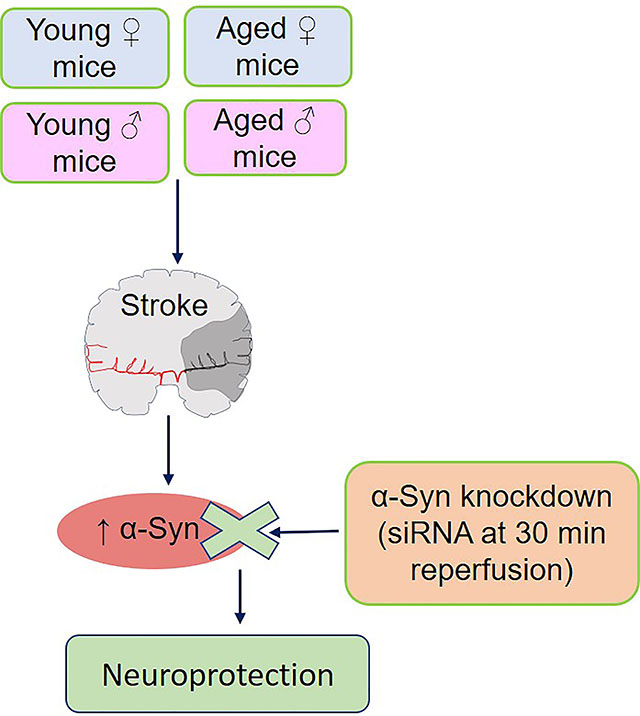
Increased expression of α-Synuclein (α-Syn) is known to mediate secondary brain damage after stroke. We presently studied if α-Syn siRNA can protect ischemic brain irrespective of sex and age.
Methods
Adult and aged male and female mice were subjected to transient middle cerebral artery occlusion. α-Syn siRNA was administered intravenous at 30 min or 3h reperfusion. Post-stroke motor deficits were evaluated between day 1 and 7 and infarct volume was measured at day 7 of reperfusion.
Results
α-Syn knockdown significantly decreased post-stroke brain damage and improved post-stroke motor function recovery in adult and aged mice of both sexes. However, the window of therapeutic opportunity for α-Syn siRNA is very limited.
Conclusions
α-Syn plays a critical role in ischemic brain damage and preventing α-Syn protein expression early after stroke minimizes post-stroke brain damage leading to better functional outcomes irrespective of age and sex.
Keywords: Focal ischemia, neuroprotection, motor function
Graphical Abstract
INTRODUCTION
α-Synuclein (α-Syn) is the major Parkinson’s Disease pathology associated protein.1 We recently showed that focal ischemia increases α-Syn expression and aggregation in the brains of young and aged rodents of both sexes.2 We also previously showed that siRNA-mediated knockdown of α-Syn decreases infarction and promotes better neurological recovery after focal ischemia in adult male rodents.2, 3 Stroke Treatment Academic Industry Roundtable stipulates that preclinical stroke therapies should be tested in both sexes, and in aged animals.4, 5 Hence, we currently tested the efficacy of α-Syn siRNA in adult and aged male and female mice subjected to focal ischemia. We further tested the window of opportunity for α-Syn siRNA treatment.
METHODS
The data that support the findings of this study are available from the first author (Bharath Chelluboina; chelluboina@wisc.edu) upon reasonable request. Animal procedures were approved by the University of Wisconsin Research Animal Resources and Care Committee. Animals were cared in compliance with the Guide for the Care and Use of Laboratory Animals [U.S. Department of Health and Human Services publication no. 86–23 (revised)]. Experiments were conducted in compliance with the “Animal Research: Reporting of In Vivo Experiments” guidelines. Animals were randomly assigned to experimental groups.
Focal ischemia
Adult (10–12 weeks) and aged (65–67 weeks) C57BL/6 mice (Jackson Laboratories USA) of both sexes were subjected to middle cerebral artery occlusion (MCAO; 60 min for adult and 35 min for aged) under isoflurane anesthesia using a silicone-coated nylon monofilament (Doccol USA) followed by 7 days of reperfusion.2, 3 Sham-operated animals served as control. 120 mice were used for the study. Regional cerebral blood flow and physiological parameters (pH, PaO2, PaCO2, hemoglobin and glucose) were monitored and rectal temperature was maintained at 37.0° ± 0.5°C during surgery. Mice with no noticeable neurological deficits or infarction and those that showed a hemorrhage were excluded.
siRNA treatment
Mice were injected via retroorbital route with 50 nmol (100 µl) of Ambion® silencer select in vivo grade (Thermo-Fisher USA) α-Syn siRNA or control siRNA cocktails mixed with 60 µl transfection enhancer of polyethylene glycol-liposome in vivo transfection reagent (cat# 5041, Altogen Biosystems USA). Sequences (5’->3’) of the α-Syn siRNAs used as a cocktail are (cat# 4457308; siRNA IDs# s74072-s74074) sense: GGAAGGAGUGGUUCAUGGAtt, CCAAAGAGCAAGUGACAAAtt and CUCUAUGUAGGUUCCAAAAtt, and antisense: UCCAUGAACCACUCCUUCCtt, UUUGUCACUUGCUCUUUGGtc and UUUUGGAACCUACAUAGAGga. A nontargeting siRNA cocktail (cat# 4404020; siRNA ID#:s813) was used as control. The siRNA cocktails were injected either at 30 min or 3h of reperfusion.
Western blotting
Protein samples (40 μg) were electrophoresed, transferred to nitrocellulose membranes and probed with monoclonal α-Syn antibody (Cell Signaling Technology) followed by horseradish peroxidase-conjugated secondary antibody as described earlier.2 Blots were stripped and reprobed with monoclonal GAPDH antibody (Santa Cruz Biotechnology). Blots were developed using enhanced chemiluminescence (Life Technologies USA) and quantified with Image Studio software (LI-COR Biotechnology USA).
Outcome analysis
Infarct volume and motor function analysis were carried out in a blinded manner. Motor function was evaluated by rotarod test (4 min on a cylinder rotating at 8 rpm) and beam walk test (number of foot faults while crossing a 120-cm long beam) from day 1 to 7 of reperfusion.2, 3 Mice were trained for 3 days before MCAO. On day 7, mice were euthanized by transcardiac 4% phosphate-buffered paraformaldehyde perfusion, brains were postfixed, cryoprotected, sectioned (coronal; 40 μm), stained with cresyl violet and scanned using NIH ImageJ software. Infarct volume was estimated by numeric integration of data from 5 serial sections with respect to the sectional interval and corrected to account for edema and differential shrinkage.2
RESULTS
In adult mice subjected to transient MCAO, administration of α-Syn siRNA at 30 min of reperfusion decreased the infarct volume (by 29% in males and by 57% in females) measured at 7 days of reperfusion compared with sex-matched control siRNA treated cohorts (Fig. 1A & C; p<0.05). α-Syn siRNA also significantly curtailed the post-ischemic motor dysfunction compared to the control siRNA treated cohorts between days 5 to 7 of reperfusion in adult mice of both sexes (Fig. 1B & D). When α-Syn siRNA was injected at at 3h of reperfusion, neither males nor females showed any significant reduction in infarct volume or improvement in post-stroke motor function (Fig. 1A to D). We previously showed a significant knockdown of α-Syn protein when mice were treated with α-Syn siRNA at 30 min of reperfusion.3 However, when mice were treated with α-Syn siRNA at 3h of reperfusion, there was no significant knockdown of α-Syn protein compared to the control siRNA treated cohort (Supplementary Fig. I).
Fig. 1: Early, but not delayed treatment with α-Syn siRNA is neuroprotective after focal ischemia.
(A and C) Cresyl violet-stained brain sections from representative adult male (A) and female (C) mice treated with control siRNA, α-Syn siRNA at 30 min or 3h of reperfusion euthanized at 7 days of reperfusion. Bars in the histogram show infarct volume. (B and D) Motor function (rotarod test and beam walk test) in the male (B) and female (D) mice between day 1 and 7 of reperfusion. Data is mean ± SD (n = 8–12/group). *P<0.05 compared with control siRNA group by one-way ANOVA followed by Dunn’s multiple comparison test (infarct volume) and by two-way repeated-measures ANOVA followed by Sidak’s multiple comparisons posttest (motor function).
α-Syn siRNA treatment decreased the infarct volume and improved the motor function recovery at 7 days of reperfusion in aged mice of both sexes compared to the sex-matched control siRNA treated cohorts (by 46% in males and 21% in females; p<0.05) (Fig. 2A to D).
Fig. 2: α-Syn siRNA treatment protected aged male and female mice subjected to transient MCAO.
(A and C) Cresyl violet-stained brain sections from the representative aged male (A) and female (C) mice treated with control siRNA and α-Syn siRNA euthanized on day 7 of reperfusion. Bars in the histogram show infarct volume. (B and D) Motor function (rotarod test and beam walk test) in the male (B) and female (D) mice between day 1 and 7 of reperfusion. Data is mean ± SD (n = 5 to 6/group). *P<0.05 compared to control siRNA group by Mann-Whitney U test (infarct volume) and by two-way repeated-measures ANOVA followed by Sidak’s multiple comparisons posttest (motor function).
DISCUSSION
We recently showed that stroke upregulates α-Syn protein expression, aggregation, and nuclear translocation in both rodents and humans.2, 3 We also showed that both knockout and knockdown of α-Syn protects the post-stroke rodent brain leading to better neurological recovery.2, 3 This gave an impetus to test α-Syn siRNA following focal ischemia in both sexes as a function of age as stipulated by Stroke Treatment Academic Industry Roundtable.
α-Syn protein aggregation and development of Parkinson’s Disease pathogenesis were reported to be higher in males than females.6 In addition, estrogens dose-dependently inhibit α-Syn aggregation in females.7 Furthermore, α-Syn is a marker of Parkinson’s Disease progression only in males.8 However, both male and female rodents show increased α-Syn protein expression after stroke and preventing it protected brain in a sex-independent manner.
Aging leads to increased α-Syn phosphorylation and oligomerization.9, 10 A study with PTEN-induced kinase 1 knockout rats showed an age-dependent spontaneous accumulation of α-Syn in the brain.11 The present study showed that α-Syn siRNA therapy is protective in aged mice subjected to focal ischemia, but the efficacy to promote neurological recovery is not as robust as in young/adult animals. Moreover, α-Syn siRNA is efficacious only if given at 30 min of reperfusion and a delay up to 3h is not an option. We recently demonstrated that microRNA miR-7 represses α-Syn protein expression and genetic deletion of α-Syn ablates miR-7–mediated neuroprotection.2 Furthermore, we identified a significant reduction in brain damage by suppressing post-ischemic induction of α-Syn expression with a miR-7 mimic administered at 30 min, but not at 2h, of reperfusion. This suggests that targeting α-Syn as early as possible after stroke is necessary to reduce the toxic accumulation of α-Syn and the downstream pathogenesis. The mcroRNA and siRNA have similarities in both nucleotide number/length and they both regulate mRNA translation. Thus, it is highly likely that the present outcome observed at 3h might be due to early translation than a late transcription of α-Syn, resulting in the limited efficacy by siRNA knockdown. Studies also suggest that once induced, α-Syn could initiate mechanisms including inflammation and apoptosis to promote neuronal degeneration, although the exact mechanism is being actively explored.3, 12 Our data indicate that the therapeutic window of α-Syn targeting lies within 3 hours after stroke. However, it will be interesting to examine the α-Syn modulating therapies further to prevent post-stroke brain damage.
Supplementary Material
Funding:
Supported in part by NIH RO1 grant NS101960.
Abbreviations
- α-Syn
α-Synuclein
- MCAO
Middle cerebral artery occlusion
Footnotes
Conflicts of interest: Authors declare no conflicts.
REFERENCES
- 1.Meade RM, Fairlie DP, Mason JM. Alpha-synuclein structure and parkinson’s disease – lessons and emerging principles. Molecular Neurodegeneration. 2019;14:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim T, Mehta SL, Morris-Blanco KC, Chokkalla AK, Chelluboina B, Lopez M, et al. The microrna mir-7a-5p ameliorates ischemic brain damage by repressing alpha-synuclein. Sci Signal. 2018;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim T, Mehta SL, Kaimal B, Lyons K, Dempsey RJ, Vemuganti R. Poststroke induction of alpha-synuclein mediates ischemic brain damage. J Neurosci. 2016;36:7055–7065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albers GW, Goldstein LB, Hess DC, Wechsler LR, Furie KL, Gorelick PB, et al. Stroke treatment academic industry roundtable (stair) recommendations for maximizing the use of intravenous thrombolytics and expanding treatment options with intra-arterial and neuroprotective therapies. Stroke. 2011;42:2645–2650 [DOI] [PubMed] [Google Scholar]
- 5.Kim T, Chelluboina B, Chokkalla AK, Vemuganti R. Age and sex differences in the pathophysiology of acute cns injury. Neurochem Int. 2019;127:22–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mason DM, Wang Y, Bhatia TN, Miner KM, Trbojevic SA, Stolz JF, et al. The center of olfactory bulb-seeded alpha-synucleinopathy is the limbic system and the ensuing pathology is higher in male than in female mice. Brain Pathol. 2019;29:741–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirohata M, Ono K, Morinaga A, Ikeda T, Yamada M. Anti-aggregation and fibril-destabilizing effects of sex hormones on α-synuclein fibrils in vitro. Experimental neurology. 2009;217:434–439 [DOI] [PubMed] [Google Scholar]
- 8.Caranci G, Piscopo P, Rivabene R, Traficante A, Riozzi B, Castellano AE, et al. Gender differences in parkinson’s disease: Focus on plasma alpha-synuclein. Journal of Neural Transmission. 2013;120:1209–1215 [DOI] [PubMed] [Google Scholar]
- 9.Chen M, Wang T, Yue F, Li X, Wang P, Li Y, et al. Tea polyphenols alleviate motor impairments, dopaminergic neuronal injury, and cerebral α-synuclein aggregation in mptp-intoxicated parkinsonian monkeys. Neuroscience. 2015;286:383–392 [DOI] [PubMed] [Google Scholar]
- 10.Chen M, Yang W, Li X, Li X, Wang P, Yue F, et al. Age- and brain region-dependent α-synuclein oligomerization is attributed to alterations in intrinsic enzymes regulating α-synuclein phosphorylation in aging monkey brains. Oncotarget. 2016;7:8466–8480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Creed RB, Goldberg MS. Analysis of α-synuclein pathology in pink1 knockout rat brains. Frontiers in Neuroscience. 2019;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Creed RB, Goldberg MS. Analysis of alpha-synuclein pathology in pink1 knockout rat brains. Front Neurosci. 2018;12:1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




