Abstract
Background
The clinical significance of cardiac troponin measurement in patients hospitalised for coronavirus disease 2019 (covid-19) is uncertain. We investigated the prevalence of elevated troponins in these patients and its prognostic value for predicting mortality.
Methods
Studies were identified by searching electronic databases and preprint servers. We included studies of hospitalised covid-19 patients that reported the frequency of troponin elevations above the upper reference limit and/or the association between troponins and mortality. Meta-analyses were performed using random-effects models.
Results
Fifty-one studies were included. Elevated troponins were found in 20.8% (95% confidence interval [CI] 16.8–25.0 %) of patients who received troponin test on hospital admission. Elevated troponins on admission were associated with a higher risk of subsequent death (risk ratio 2.68, 95% CI 2.08–3.46) after adjusting for confounders in multivariable analysis. The pooled sensitivity of elevated admission troponins for predicting death was 0.60 (95% CI 0.54–0.65), and the specificity was 0.83 (0.77–0.88). The post-test probability of death was about 42% for patients with elevated admission troponins and was about 9% for those with non-elevated troponins on admission. There was significant heterogeneity in the analyses, and many included studies were at risk of bias due to the lack of systematic troponin measurement and inadequate follow-up.
Conclusion
Elevated troponins were relatively common in patients hospitalised for covid-19. Troponin measurement on admission might help in risk stratification, especially in identifying patients at high risk of death when troponin levels are elevated. High-quality prospective studies are needed to validate these findings.
Systematic review registration
PROSPERO CRD42020176747
Supplementary Information
The online version contains supplementary material available at 10.1186/s40560-020-00508-6.
Keywords: Covid-19, Meta-analysis, Myocardial injury, Risk prediction, Troponin
Introduction
Coronavirus disease 2019 (covid-19) caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV2) remains a pandemic, with considerable mortality and morbidity exerting pressure on global health-care systems. Patients with covid-19 experience a wide range of disease severity. Prognostic tools that efficiently stratify individual’s risk of experiencing adverse outcomes may facilitate patients and clinicians in the informed decision-making process [1].
Despite being primarily a respiratory infection, covid-19 has important impacts on many vital organs, including the heart [2, 3]. A growing number of reports have documented myocardial injury reflected by elevated circulating cardiac troponin concentrations among infected patients [4–8]. However, elevated troponins frequently occur in patients with conditions other than acute coronary syndromes, and the mechanisms are complex. For this reason, the American College of Cardiology recommended that troponin is ordered for covid-19 patients only when the diagnosis of acute myocardial infraction is being considered on clinical grounds [9]. On the other hand, the UK National Institute for Health and Care Excellence supported troponin testing for wider indications, including patients with non-specific symptoms of possible myocardial injury such as shortness of breath and severe fatigue [10]. Besides, some opinion papers advocated for systematic troponin testing in all covid-19 patients requiring hospital admission for prognostication purpose [11, 12]. These conflicting recommendations regarding the use of troponins in evaluating covid-19 patients reflect major gaps in our understanding of the clinical significance of elevated troponins in this context.
Several recent studies have reported on the relevance of elevated troponins to severity of covid-19 and risk of death, including large retrospective studies from major epicentres such as Wuhan, New York City and some European countries as well as studies with prospective designs [13–16]. We undertook a systematic review and meta-analysis to evaluate the prevalence of elevated troponins in hospitalised covid-19 patients, the performance of elevated troponins in predicting mortality and the quality of currently available evidence.
Methods
This study was conducted following the modified CHARMS (CHecklist for critical Appraisal and data extraction for systematic Reviews of prediction Modelling Studies) for reviews of prognostic factors (CHARMS-PF) guidelines [17] and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (checklist in Table S1) [18]. The study protocol was registered prospectively in PROSPERO (CRD42020176747).
Literature search and eligibility criteria
We searched English and Chinese databases (PubMed, Embase, Chinese National Knowledge Infrastructure and Chinese Biomedical Database) and preprint servers (medRxiv, bioRxiv, ChinaXiv, Research Square and SSRN) for research articles on covid-19 published after 1 December 2019, with no restrictions on language or peer review status. Details of the search strategy are listed in Table S2. The last update of literature search was performed on 15 October 2020. Studies were considered eligible if they were observational or interventional studies that (1) enrolled patients that required hospitalisation for covid-19 pneumonia, (2) measured cardiac-specific troponin T or I concentrations on hospital admission or during hospital stay and (3) reported the prevalence of elevated troponins among patients who received troponin measurement or the association between troponin concentrations and mortality during the follow-up. Elevated troponins were defined as troponin concentrations higher than the upper reference limit value predetermined by the local laboratory. We excluded (1) studies that specifically enrolled patients admitted for cardiovascular reasons, organ transplant recipients or deceased patients; (2) studies that reported myocardial injury but the diagnosis was not based solely on troponin measurements or the upper reference limit of troponin test was not used as the diagnostic criteria; and (3) case reports or case series involving fewer than 10 patients. When more than one study from the same institutions with overlapping time period of recruitment were identified, we chose the one with the largest sample size for inclusion, except when our outcomes of interest were reported only in smaller studies.
Study selection, data extraction and quality assessment
Two researchers (BCZ and WFL) independently screened the identified records, first based on the title and abstract and subsequently based on the full manuscript. The reference lists of relevant manuscripts were searched to identify additional eligible studies. Using predefined forms, both researchers independently extracted data on study design, time of recruitment and follow-up, characteristics of patients and troponin tests, frequency of troponin elevation and, if available, the number of survivors and nonsurvivors among patients with and without elevated troponins. For studies that performed multivariable analysis to assess the association between elevated troponins and mortality, we extracted the adjusted effect estimates with confidence intervals (CI) and the variables in multivariable models. Risk of bias assessment for studies reporting on the prevalence of elevated troponins was conducted using a tool developed for prevalence studies [19]. Key study features assessed were sample selection and measurement quality. The Quality in Prognosis Studies (QUIPS) tool was used to assess the risk of bias of studies on the prognostic performance of troponins [20]. The tool includes the evaluation of 6 domains: study participation, study attrition, prognostic factor measurement, outcome assessment, study confounding, and statistical analysis and reporting. Disagreements between researchers were resolved by consensus, and a third researcher was involved when necessary.
Statistical analysis
Statistical analyses were performed using Stata® version 12.0 (StataCorp, College Station, TX, USA). All meta-analyses were based on random-effects models due to the anticipated high degree of heterogeneity among studies. To estimate the prevalence of elevated troponins in patients admitted for covid-19, we pooled the proportion of patients with elevated troponins among those who received at least one troponin measurement. The Freeman-Tukey double arcsine transformation method was used to account for studies reporting very low prevalence estimates.
To assess the predictive value of troponins for mortality, we pooled the multivariable-adjusted associations between elevated troponins and mortality. For this analysis, studies that did not measure troponins on hospital admission were not included, because troponins are expected to rise in late deterioration of the illness and have less predictive utility at that time. We also excluded studies that modelled troponin as a continuous predictor or did not use the upper reference limit of the troponin test, because the association between troponin concentrations and mortality risk may not be linear and because the use of study-specific optimal cut-off thresholds in meta-analysis may result in overestimation of the prognostic value of a biomarker [21]. We converted adjusted odds ratios reported by included studies to risk ratios (RR) using a published formula [22] and assumed that hazard ratios reasonably approximated RRs. For studies where more than one effect estimates were given for different levels of troponin elevation versus reference, a study-unique effect estimate was generated using a fixed-effects model before being included in the random-effects meta-analysis. We calculated the summary RR with 95% CI using the generic inverse variance method. Considering that dozens of commercial troponin assays with different epitope targets and analytical characteristics are used in clinical practice, we calculated 95% prediction intervals (PI) to inform the distribution of prognostic effects of elevated troponins across different measurement methods in future studies [23].
We evaluated publication bias using the funnel plot and Egger’s test. When significant publication bias was found, we used trim-and-fill method to adjust our results. The magnitude of heterogeneity was assessed by the Higgins I2 statistic. Subgroup analyses in case of substantial heterogeneity (I2 > 50%) were conducted based on the characteristics of troponin assays (troponin T or I, high-sensitivity or contemporary assays) and the geographical location, sample size, risk of bias and peer review status of included studies.
The predictive ability of troponin was further assessed by constructing a hierarchical summary receiver operating characteristic curve using the bivariate model [24]. Based on the model, we determined the overall sensitivity, specificity and positive and negative likelihood ratios of elevated troponins on hospital admission for predicting death. These measures of predictive accuracy reflect the intrinsic performance of troponins and are independent of the mortality of underlying study populations. For practical purposes, we estimated the post-test risk of death using the Fagan nomogram, considering a pre-test probability as the pooled risk of death among the included patients.
Results
Characteristics of selected studies
Of the 6124 unique records identified, 51 studies were selected for this review (Fig. 1, Table 1), including 41 studies that have been peer-reviewed [13–16, 25–61] and 10 published only as a preprint [62–71]. Five were prospective studies [16, 32, 38, 47, 58] and the others were retrospective in design. Patients included in these studies (sample size range, 15–6247; median age, 44–72 years; proportion of men, 43–79%) were admitted to hospitals up to 21 June 2020. Information on methods and findings of troponin measurement in each study were summarised in Table 2.
Fig. 1.
PRISMA flow chart showing study selection process
Table 1.
Main characteristics of included studies
| Study author | Location | Design | Date of admission | Patients | Comorbidities (HTN/DM/CVD, %) | Disease severitya | Date of last follow-up | Overall mortality, % | Mortality of pts with elevated troponin on admission, % | |
|---|---|---|---|---|---|---|---|---|---|---|
| No. (male, %) | Age, years | |||||||||
| Arcari et al. | Rome, Italy | Retrospective, multi-centre | Mar 15–Apr 30 | 111 (45.9) | 72 (17) | 55.9/18.9/31.5 | NA | May 31 | 20.7 | 30.8 |
| Azoulay et al. | Paris, France | Retrospective, multi-centre | Feb 21–Apr 24 | 376 (76.9) | 62 [53–68] | 49.5/30.3/NA | 100% critical, SOFA score 5 [3, 8] | May 15 | 26.4 | NA |
| Barman et al. | Istanbul, Turkey | Retrospective, multi-centre | Mar 20–Apr 20 | 607 (55.0) | 63 (14) | 43.8/31.6/19.1 | 32.1% critical | Apr 20 | 17.0 | 42.7 |
| Bhatla et al. | Philadelphia, USA | Retrospective, single-centre | Mar 6–May 19 | 700 (44.9) | 50 (18) | 49.6/26.0/1.6 | 11.3% critical | May 24 | 4.3 | NA |
| Bhatraju et al. | Seattle, USA | Retrospective, multi-centre | Feb 24–Mar 9 | 24 (62.5) | 64 (18) | NA/58.3/NA | 100% critical | Mar 23 | 50.0 | NA |
| Buckner et al. | Seattle, USA | Retrospective, multi-centre | Mar 2–Mar 26 | 105 (50.5) | 69 (range 23–97) | 59.0/33.3/38.1 | 48.6% critical | May 8 | 33.3 | NA |
| Cipriani et al. | Padova, Italy | Retrospective, single-centre | Feb 26–Mar 31 | 109 (67.0) | 70 [60–81] | 62.3/24.8/16.5 | 28.4% critical | Apr 1 | 18.3 | 43.9 |
| Du et al. | Wuhan, China | Prospective, single-centre | Dec 25–Feb 7 | 179 (54.2) | 58 (14) | 32.4/18.4/16.2 | NA | Mar 24 | 11.7 | 31.7 |
| Ferguson et al. | North California, USA | Retrospective, multi-centre | Mar 13–Apr 11 | 72 | NA | 36.1/27.8/NA | 29.2% critical | May 2 | 6.9 | NA |
| Franks et al. | St. Louis, USA | Retrospective, single-centre | NA | 182 (56.6) | 64 (range 19–98) | NA | NA | NA | 18.7 | 36.9 |
| Gottlieb et al. | Chicago, USA | Retrospective, single-centre | Mar 4–Jun 21 | 1483 (53.4) | 56 [44–68] | 60.5/42.8/15.4 | 35.6 % critical | Jul 10 | 10.0 | NA |
| Goyal et al. | New York, USA | Retrospective, multi-centre | Mar 3–Mar 27 | 393 (60.6) | 62 [49, 74] | 50.1/25.2/13.7 | NA | Apr 10 | 10.2 | NA |
| Harmouch et al. | Bethlehem, USA | Retrospective, single-centre | Mar 1–Apr 15 | 563 (57.1) | 63 | 50.3/35.2/NA | 24.3 % critical | NA | 14.5 | 36.1 |
| He et al. | Wuhan, China | Retrospective, single-centre | Feb 8–Mar 16 | 94 (57.4) | 69 (10) | 59.6/19.1/12.8 | 100% critical | Mar 16 | 44.7 | NA |
| Heberto et al. | Puebla and Mexico City, Mexico | Prospective, multi-centre | Mar-Apr | 254 (65.7) | 54 (13) | 35.4/31.5/5.5 | NA | To death or discharge | 35.0 | NA |
| Hu et al. | Wuhan, China | Retrospective, single-centre | Jan 8–Feb 20 | 323 (51.4) | 61 (range 23–91) | 32.5/14.6/2.2 | 45.2% severe, 8.0 critical | Mar 10 | 10.8 | NA |
| Huang et al. | Jiangsu, China | Retrospective, multi-centre | Jan 24–Apr 20 | 60 (58.3) | 57 (range 26–97) | 23.3/16.7/5.0 | 100% critical, APACHE II score 14 (5) | Apr 20 | 0 | NA |
| Karbalai et al. | Tehran, Iran | Retrospective, single-centre | Mar-May | 386 (61.1) | 59 (16) | 36.8/34.5/25.1 | 20.5 % critical | To death or discharge | 19.9 | NA |
| Lala et al. | New York, USA | Retrospective, multi-centre | Feb 27–Apr 12 | 2736 (59.6) | 66 | 38.9/26.3/16.6 | CURB-65 score 1.26 (1.10) | Apr 12 | 27.3 | NA |
| Lazzeri et al. | Florence, Italy | Retrospective, single-centre | Mar 1–Mar 31 | 28 (78.6) | 62 (10) | 89.3/39.3/28.6 | 100% critical | Mar 31 | 7.1 | NA |
| Li et al. | Guangzhou, China | Retrospective, single-centre | Jan 24–Feb 25 | 82 (58.5) | 45 (16) | 26.8/21.9/NA | 29.3% severe or critical | Feb 29 | 1.2 | 7.7 |
| Li et al. | Wuhan, China | Retrospective, single-centre | Jan 29–Apr 1 | 2068 (48.6) | 63 [51, 70] | 34.9/14.1/8.8 | 32.0 % critical | Apr 1 | 8.8 | 45.9 |
| Lombardi et al. | Italy | Retrospective, multi-centre | Mar 1–Apr 9 | 614 (70.8) | 67 (13) | 50.5/19.8/15.0 | SOFA score 2 [1, 3] | Apr 23 | 24.1 | 37.4 |
| Lorente-Ros et al. | Madrid, Spain | Retrospective, single-centre | Mar 18–Mar 23 | 707 (62.7) | 67 (16) | 50.5/20.2/10.6 | 52.1% critical | 1 month after admission | 19.8 | 45.2 |
| Lu et al. | Wuhan, China | Retrospective, single-centre | Dec 30–Mar 18 | 50 (58.0) | 66 [58, 73] | 38.0/18.0/18.0 | 100% critical, APACHE II score 12.0 [9.0, 16.3] | To death or discharge | 60.0 | NA |
| Ma et al. | Chongqing, China | Retrospective, single-centre | Jan 21–Mar 2 | 84 (57.1) | 48 [43, 63] | 14.3/11.9/6.0 | 76.2% severe or critical | Mar 2 | 0 | NA |
| Majure et al. | New York, USA | Retrospective, multi-centre | Mar 1–Apr 27 | 6247 (59.9) | 66 [56, 77] | 59.5/36.0/13.3 | 30.2% critical | Apr 30 | 22.4 | 43.5 |
| Mejía-Vilet et al. | Mexico City, Mexico | Prospective, single-centre | Mar 16–May 21 | 569 (66.3) | D, 49 [41, 60]; V, 52 [43, 60] | 28.5/27.8/NA | 35.0% critical | May 24 | 11.1 | NA |
| Nguyen et al. | Chicago, USA | Retrospective, single-centre | Mar 16–Apr 16 | 356 (48.0) | 61 [50, 73] | 69.5/42.1/21.9 | 44% critical | May 25 | 12.6 | 21.4 |
| Nie et al. | Wuhan, China | Retrospective, single-centre | Jan 12–Mar 12 | 311 (61.1) | 63 [54, 70] | NA | 57.9% severe, 9.6% critical | Mar 20 | 35.7 | NA |
| Petrilli et al. | New York, USA | Retrospective, multi-centre | Mar 2–Apr 2 | 1999 (62.6) | 62 [50, 74] | 37.1/25.2/9.9 | 36.1% critical | Apr 7 | 14.6 | NA |
| Price-Haywood et al. | Louisiana, USA | Retrospective, multi-centre | Mar 1–Apr 11 | 1382 (49.0) | 63 (15) | NA | 34.3% critical | May 7 | 23.6 | NA |
| Qi et al. | Chongqing, China | Retrospective, multi-centre | Jan 19–Feb 16 | 267 (55.8) | 48 [35, 65] | 7.5/9.7/4.9 | 18.7% severe or critical | Feb 16 | 1.5 | NA |
| Qin et al. | Hubei, China | Retrospective, multi-centre | Dec 31–Mar 4 | 3219 (47.7) | 57 [45, 66] | 27.8/12.8/6.4 | NA | 28 days after admission | 6.0 | 37.9 |
| Raad et al. | Michigan, USA | Retrospective, multi-centre | Mar 9–Apr 15 | 1020 (49.9) | 63 [52, 73] | 72.7/44.3/12.1 | 50.2% critical | To death or discharge | 17.6 | 32.8 |
| Shah et al. | Albany, USA | Retrospective, multi-centre | Mar 2–Jun 7 | 309 (42.7) | 63 (14) | 84.5/46.3/9.1 | 35.6% critical | To death or discharge | 21.4 | NA |
| Shah et al. | San Francisco, USA | Retrospective, single-centre | Feb 3–Mar 31 | 26 (NA) | NA | NA | 42.3% critical | Apr 25 | 3.8 | NA |
| Shen et al. | Shanghai, China | Retrospective, single-centre | Jan 20–Feb 29 | 325 (51.7) | 51 [36, 64] | 15.6/7.1/3.4 | 3.1% severe, 4.9% critical | Feb 29 | 0.9 | NA |
| Stefanini et al. | Milan, Italy | Retrospective, single-centre | Up to Apr 1 | 397 (67.3) | 67 [55, 76] | 56.7/24.6/8.4 | NA | To death or discharge | 23.2 | 45.4 |
| Szekely et al. | Tel Aviv, Israel | Prospective, single-centre | Mar 21–Apr 16 | 100 (63.0) | 66 (17) | 57.0/29.0/16.0 | 29.0% severe, 10.0% critical | NA | NA | NA |
| Tan et al. | Wuhan, China | Retrospective, multi-centre | Feb 8–Apr 6 | 115 (49.6) | 63 [55, 70] | 47.0/25.2/NA | 63.5% severe, 36.5% critical | Apr 6 | 19.1 | 70.0 |
| van den Heuvel et al. | Nijmegen, Netherlands | Retrospective, single-centre | Apr 1–May 12 | 51 (80.4) | 63 [51, 68] | 41.1/17.6/9.8 | 37.2% critical | To death or discharge | 2.0 | NA |
| Wei et al. | Chengdu, China | Retrospective, multi-centre | Jan 16–Mar 10 | 101 (53.5) | 49 [34, 62] | 20.8/13.9/5.0 | 36.6% severe or critical | NA | 3.0 | 18.8 |
| Woo et al. | Philadelphia, USA | Retrospective, multi-centre | Mar 1–Apr 30 | 415 (55.2) | 65 (15) | 69.9/35.0/18.1 | 39.3% critical | 14 days after admission | 21.2 | NA |
| Xu et al. | Guangzhou, China | Retrospective, single-centre | Jan 22–Apr 6 | 15 (60.0) | 44 (13) | 26.7/NA/NA | 53.3% severe, 6.7% critical | Apr 6 | 0 | NA |
| Yang et al. | Wuhan, China | Retrospective, single-centre | Feb 13–Mar 14 | 463 (49.9) | 60 [50, 69] | 38.7/17.3/7.3 | 8.2% severe, 14.3% critical | Mar 28 | 5.8 | 40.0 |
| Yu et al. | Wuhan, China | Prospective, multi-centre | Feb 25–Feb 27 | 226 (61.5) | 64 [57, 70] | 42.5/20.8/9.7 | 100% critical, SOFA score 4 [2, 8] | Apr 9 | 38.5 | NA |
| Zeng et al. | Shenzhen, China | Retrospective, single-centre | Jan 11–Apr 1 | 416 (47.6) | NA | 14.4/5.5/3.1 | 8.4% critical | Apr 1 | 0.7 | NA |
| Zhang et al. | Wuhan, China | Retrospective, single-centre | Feb 1–Mar 15 | 135 (49.6) | 56 [42, 68] | 20.7/11.9/3.0 | 22.2% critical | NA | 13.3 | 35.0 |
| Zhao et al. | Jingzhou, China | Retrospective, single-centre | Jan 16–Feb 10 | 91 (53.8) | 46 | 19.8/3.3/0 | 33.0% severe or critical | Feb 10 | 2.2 | NA |
| Zhou et al. | Wuhan, China | Retrospective, multi-centre | Dec 29–Jan 31 | 191 (62.3) | 56 [46, 67] | 30.4/18.8/7.9 | 34.6% severe, 27.7% critical | Jan 31 | 28.3 | 95.8 |
APACHE Acute Physiology and Chronic Health Evaluation; CVD Cardiovascular disease; CURB-65 Confusion, Urea nitrogen, Respiratory rate, Blood pressure, 65 years of age and older; D Derivation cohort; DM Diabetes mellitus; HTN Hypertension; ICU Intensive care unit; NA Not available; SOFA Sequential Organ Failure Assessment; V Validation cohort
Values were mean (standard deviation), median [interquartile rage] or median (range)
aSeverity of illness was assessed based on covid-19 treatment guidelines (see supplementary Table S7)
Table 2.
Information on troponin measurement in included studies
| Study | Type of Tn assay | Manufacturer | Upper reference limit | Time of measurement | Proportion of patients with Tn measurement, no. measured/no. enrolled (%) | Frequency of Tn elevation, no. elevated/no. measured (%) |
|---|---|---|---|---|---|---|
| Arcari et al. | hs-TnT, hs-TnI | Roche, NA | 14 ng/L, 35 ng/L | Within 24 h of admission | 103/111 (92.8) | 39/103 (37.9) |
| Azoulay et al. | NA | NA | NA | On ICU admission | 343/379 (90.5) | 135/343 (39.4) |
| Barman et al. | hs-TnI | NA | 14 ng/L | On admission | 607/908 (66.9) | 150/607 (24.7) |
| Bhatla et al. | NA | NA | 0.010 ng/mL | On admission | 373/700 (53.3) | 82/373 (22.0) |
| Bhatraju et al. | NA | NA | 0.06 ng/mL | During first 3 days in ICU | 13/24 (54.2) | 2/13 (15.4) |
| Buckner et al. | NA | NA | 0.1 ng/mL | During hospitalisation | 67/105 (63.8) | 13/67 (19.4) |
| Cipriani et al. | hs-TnI | NA | 32 ng/L for men, 16 ng/L for women | On admission and during hospitalisation | 109/136 (80.1) | On admission, 41/109 (37.6); overall, 46/109 (42.2) |
| Du et al. | TnI | NA | 0.05 ng/mL | On admission | 179/179 (100) | 41/179 (22.9) |
| Ferguson et al. | NA | NA | 0.055 ng/mL | Within 24 h of admission | 45/72 (62.5) | 2/45 (4.4) |
| Franks et al. | TnI | Abbott | 0.03 ng/mL | On admission and during hospitalisation | On admission, 128/182 (70.3); overall, 143/182 (78.6) | On admission, 65/128 (50.8); overall, 80/143 (55.9) |
| Gottlieb et al. | NA | NA | 0.9 ng/mL | On admission | 390/1483 (26.3) | 87/390 (22.3) |
| Goyal et al. | NA | NA | 0.5 ng/mL | Within 48 h of admission | 246/393 (62.6) | 11/246 (4.5) |
| Harmouch et al. | TnI | NA | 0.05 ng/mL | On admission | 482/560 (86.1) | 97/482 (20.1) |
| He et al. | hs-TnI | NA | NA | During stay in ICU | 94/94 (100) | 35/94 (37.2) |
| Heberto et al. | hs-TnI | Beckman | 17.5 ng/L | On admission | 254/254 (100) | 64/254 (25.2) |
| Hu et al. | TnI | Siemens | 0.040 ng/mL | On admission | 323/323 (100) | 68/323 (21.1) |
| Huang et al. | TnT | NA | 0.13 ng/mL | On ICU admission | 60/60 (100) | 19/60 (31.7) |
| Karbalai et al. | hs-cTnI | NA | 26 ng/L for men, 11 ng/L for women | During hospitalisation | 386/386 (100) | 115/386 (29.8) |
| Lala et al. | TnI | Abbott | 0.03 ng/mL | Within 24 h of admission | 2736/3047 (89.8) | 1751/2736 (36.0) |
| Lazzeri et al. | TnT | NA | 0.028 ng/mL | On ICU admission | 28/28 (100) | 11/28 (39.3) |
| Li et al. | TnI | Beckman | 0.03 ng/mL | On admission | 82/82 (100) | 13/82 (15.9) |
| Li et al. | hs-TnI | Abbott | 34.2 ng/L | On admission | 2068/2699 (76.6) | 181/2068 (8.8) |
| Lombardi et al. | NA | NA | NA | Within 24 hours of admission | 614/614 (100) | 278/614 (45.3) |
| Lorente-Ros et al. | hs-TnI | NA | 14 ng/L | On admission | 707/707 (100) | 148/707 (20.9) |
| Lu et al. | TnI | NA | 0.4 ng/mL | During stay in ICU | 50/72 (69.4) | 36/50 (72.0) |
| Ma K, et al | TnI | NA | 0.034 ng/mL | During hospitalisation | 84/84 (100) | 9/84 (10.7) |
| Majure et al. | TnI, TnI, TnT, hs-TnT | Siemens, Siemens, Roche, Roche | 0.045 ng/mL, 0.056 ng/mL, 0.01 ng/mL, 19 ng/L | Within 48 hours of admission | 6247/11,159 (56.0) | 1821/6247 (29.1) |
| Mejía-Vilet et al. | TnI | NA | 0.020 ng/mL | On admission | 569/569 (100) | 86/569 (15.1) |
| Nguyen et al. | hs-TnI | NA | 22 ng/L | On admission | 340/356 (95.5) | 140/340 (41.2) |
| Nie et al. | hs-TnI | Abbott | 26.2 ng/L | During hospitalisation | NA | 103/311 (33.1) |
| Petrilli et al. | NA | NA | 0.1 ng/mL | On admission | 2510/2729 (92.0) | NA |
| Price-Haywood et al. | TnI | NA | 0.06 ng/mL | On admission | 1084/1382 (78.4) | 270/1084 (24.9) |
| Qi et al. | hs-TnT | Roche | 14 ng/L | On admission | 76/267 (28.5) | 3/76 (3.9) |
| Qin et al. | hs-TnI, TnI | Various | Various | On admission | 1462/6033 (24.2) | 95/1462 (6.5) |
| Raad et al. | hs-TnI | Beckman | 18 ng/L | On admission | 1020/1044 (97.7) | 390/1020 (38.2) |
| Shah et al. | TnI | NA | 0.05 ng/mL | During hospitalisation | 309/635 (48.7) | 116/309 (37.5) |
| Shah et al. | TnI | NA | 0.05 ng/mL | During hospitalisation | 14/26 (53.8) | 5/14 (35.7) |
| Shen et al. | TnI | Siemens | 0.040 ng/mL | On admission | 325/325 (100) | 80/325 (24.6) |
| Stefanini et al. | hs-TnI | Beckman | 19.6 ng/L | On admission | 397/397 (100) | 130/397 (32.7) |
| Szekely et al. | TnI | Abbott | 28 ng/L | On admission | 100/100 (100) | 20/100 (20) |
| Tan et al. | hs-TnI | Abbott | 26.2 ng/L | On admission | 115/115 (100) | 20/115 (17.4) |
| van den Heuvel et al. | hs-TnT | Roche | 14 ng/L | During hospitalisation | 47/51 (92.2) | 24/47 (51.1) |
| Wei et al. | hs-TnT | Roche | 14 ng/L | On admission | 101/103 (98.1) | 16/101 (15.8) |
| Woo et al. | hs-TnT, TnI | Roche, Siemens | 19 ng/L, 0.040 ng/mL | On admission | NA | NA |
| Xu et al. | TnI | NA | NA | On admission | 15/15 (100) | 1/15 (6.7) |
| Yang et al. | TnI | Siemens | 0.040 ng/mL | On admission | 463/463 (100) | 45/463 (9.7) |
| Yu et al. | hs-TnI, TnI | Abbott, NA | 28 ng/L, 0.3 ng/mL | During stay in ICU | 226/226 (100) | 61/226 (27.0) |
| Zeng et al. | TnI | NA | 0.026 ng/mL | On admission | 345/416 (82.9) | 29/345 (8.4) |
| Zhang et al. | hs-TnT | Roche | 14 ng/L | On admission | 135/135 (100) | 40/135 (29.6) |
| Zhao et al. | TnI | NA | 0.01 ng/mL | On admission | 88/91 (96.7) | 3/88 (3.4) |
| Zhou et al. | hs-TnI | Abbott | 28 ng/L | On admission | 145/191 (75.9) | 24/145 (16.6) |
Hs High-sensitivity, ICU Intensive care unit, NA Not available, Tn Troponin
Prevalence of elevated troponins
In total, 49 studies reported or allowed calculation of prevalence estimates for elevated troponins above the upper reference limit in patients hospitalised for covid-19. The risks of bias of these studies in estimating prevalence are summarised in Table S3. The studies differed in patient population (patients admitted to hospital or patients admitted in ICU) and timing of troponin measurement (on admission or during hospital stay). We decided to conduct separate analyses for studies using different methodologies because these may have significant influence on the observed prevalence of elevated troponins.
In 35 studies (22,473 patients) where the prevalence of elevated troponins at the time of hospital admission could be extracted [13–16, 25, 27, 28, 31–39, 43–47, 50, 51, 54, 55, 57, 59–61, 65–67, 69–71], the pooled estimate was 20.8% (95% CI 16.8–25.0 %) with substantial heterogeneity (I2 = 98.0%) (Figure S1). No publication bias was suggested by the funnel plot (Figure S2) or the Egger’s test (P = 0.292). When we exclude studies that measured troponin in less than 90% of consecutively admitted patients, the remaining 19 studies (5930 patients) deemed at low risk of selection bias yielded a pooled prevalence of 22.9% (95% CI 17.6–28.6 %).
In 9 studies (1470 patients) [30, 31, 34, 41, 48, 52, 53, 56, 64], the prevalence of elevated troponins during the course of hospital stay was reported; the pooled estimate was 34.2% (95% CI 26.2–42.6 %) (Figure S3). Seven studies (814 patients) enrolled only patients admitted to ICU [26, 29, 40, 42, 58, 62, 63], and the pooled prevalence of elevated troponins was 38.0% (95% CI 28.2–48.3%) (Figure S4).
Elevated troponins and mortality
In 28 studies [13–15, 25–27, 31, 32, 34, 37, 38, 41, 43–46, 48, 49, 51, 52, 55, 57, 61, 65, 67, 68, 70, 71], data on the relationship between troponins (on admission or during hospital stay) and mortality of patients with covid-19 could be extracted. The risks of bias of these studies in assessing the prognostic value of troponins are summarised in Table S4. Many of the studies were at high risk of selection bias due to the lack of systematic troponin measurement and incomplete in-hospital follow-up. Besides, 10 studies did not adjust for relevant confounders (e.g. age, cardiovascular comorbidities). The results of studies that conducted multivariable analysis and the confounders adjusted for were listed in Table S5. In the following analyses, we focused only on studies that measured troponins on hospital admission, because troponin tests at this point of time might be useful for early risk stratification, whereas tests ordered during hospitalisation may have been a response to patients’ deteriorating conditions and thus would have more diagnostic but less predictive values.
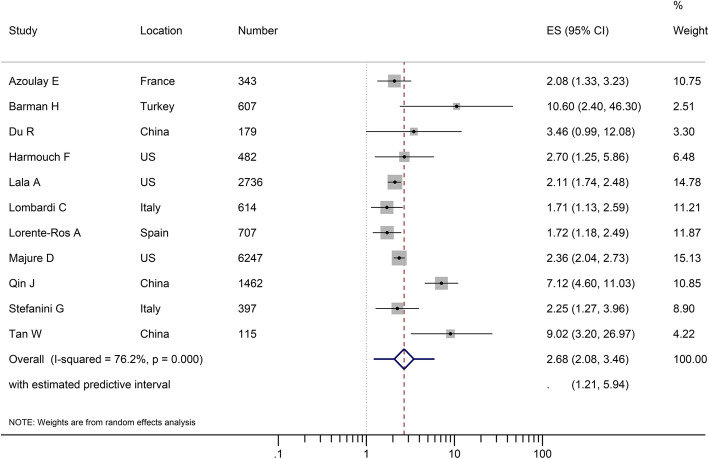
We conducted a meta-analysis of 11 studies (13,889 patients) that reported multivariable-adjusted associations between admission troponins above the upper reference limit and mortality [13–15, 26, 27, 32, 37, 45, 46, 55, 67]. Elevated troponins on admission were associated with an increased risk of death (RR 2.68, 95% CI 2.08–3.46). There was substantial heterogeneity (I2 = 76.2%); the 95% prediction interval was wide (1.12–5.94) but did not include 1 (Fig. 2). Possible publication bias was found by the funnel plot (Figure S5a) but not confirmed by the Egger’s test (P = 0.203). We conducted a sensitivity analysis using the trim-and-fill method, which confirmed the stability of the association (RR 2.59, 95% CI 2.01–3.35) (Figure S5b). When we excluded 5 studies judged as having high risk of bias from meta-analysis, elevated troponins on admission remained a significant risk factor for subsequent death (RR 2.08, 95% CI 1.81–2.40), and the heterogeneity was eliminated (I2 = 0%). The results of subgroup analyses were shown in Table S6.
Fig. 2.
Forest plot showing the confounder-adjusted association between elevated troponins on hospital admission and mortality, quantified as risk ratio (RR) of death in patients with elevated troponins relative to those with non-elevated troponins. CI, confidence interval
To further characterise the predictive performance of troponins, we included 20 studies (15,488 patients) that reported the number of deaths in those admitted with elevated and non-elevated troponins in a bivariate meta-analysis [14, 15, 25, 27, 31, 32, 34, 37, 43–46, 51, 55, 57, 61, 65, 67, 70, 71]. The overall sensitivity of elevated troponins on admission for predicting death was 0.60 (95% CI 0.54–0.65); the specificity was 0.83 (0.77–0.88) (Fig. 3). The positive and negative likelihood ratios were 3.61 (2.74–4.70) and 0.48 (0.43–0.54), respectively. Considering a pre-test probability of death of 17% (the pooled estimate from the 20 included studies), the post-test probability of death for patients with elevated troponins on admission was approximately 42%, while that of patients with non-elevated troponins on admission was approximately 9% (Fig. 4).
Fig. 3.
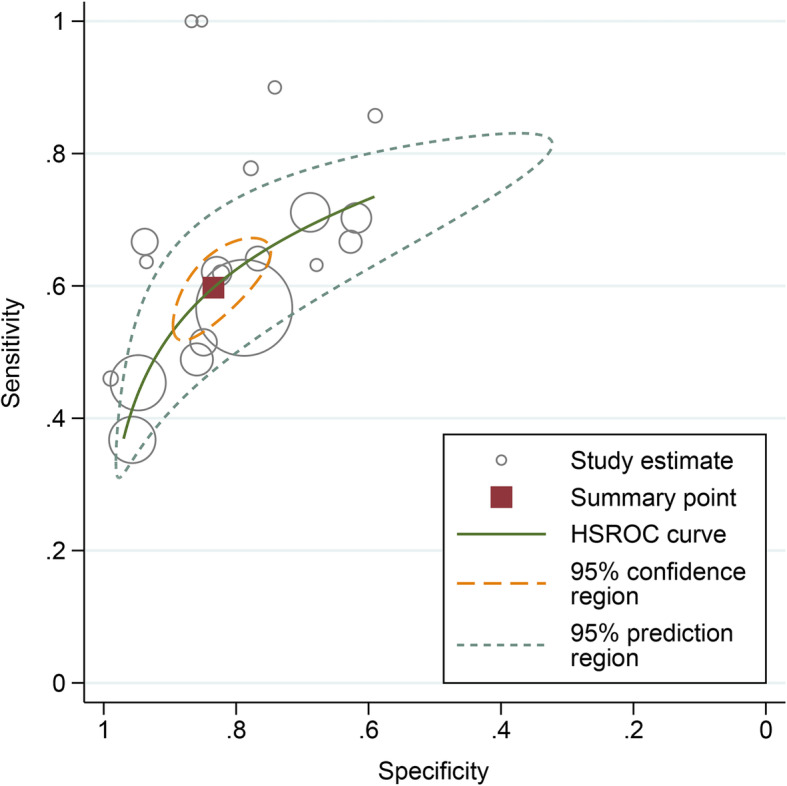
Performance of troponins on admission for predicting mortality. The brown square represents the summary operating point of the curve that summarises the prognostic performance of troponins (sensitivity 0.60, 95% CI 0.54–0.65; specificity 0.83, 95% CI 0.77–0.88; positive likelihood ratio 3.61, 95% CI 2.74–4.70; negative likelihood ratio 0.48, 95% CI 0.43–0.54). The area under the hierarchical summary receiver operating characteristic curve was 0.74, 95% CI 0.70–0.78.
Fig. 4.
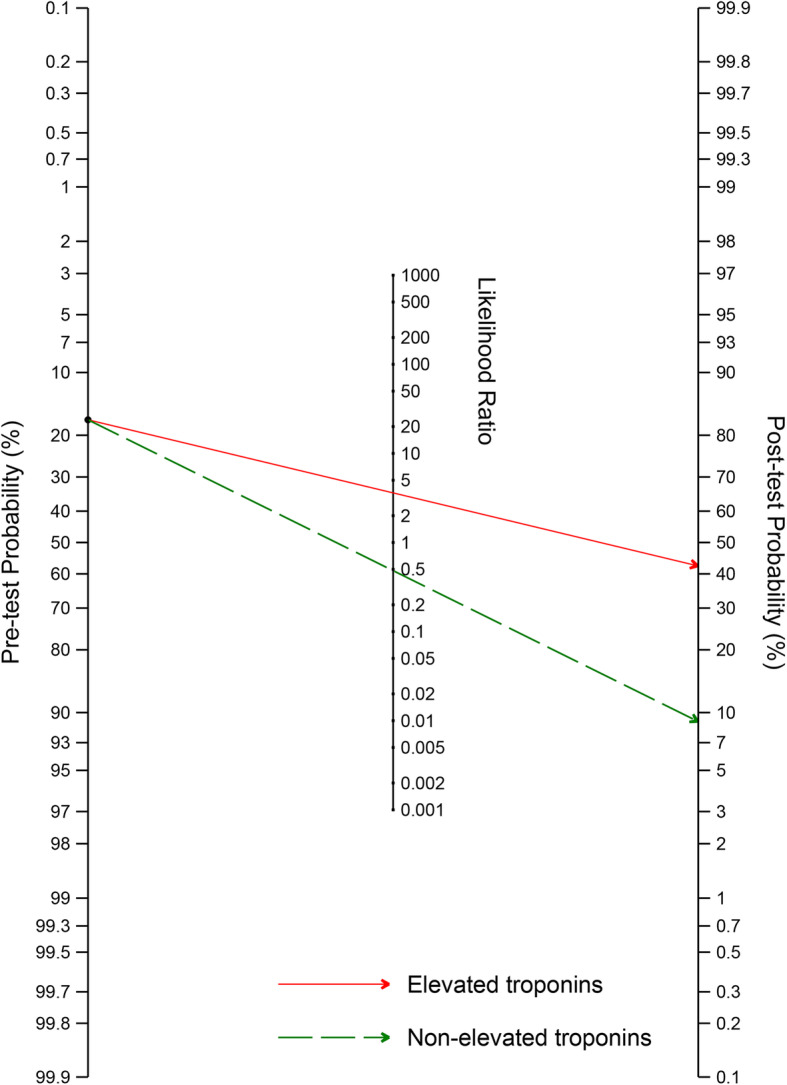
Fagan nomogram for calculation of post-test probability of death based on elevated (red) or non-elevated (green) troponins on admission. The Fagan nomogram is based on a pre-test probability of 17%, which is the pooled mortality estimate in 20 included studies, a positive likelihood ratio of 3.61, and a negative likelihood ratio of 0.48
Discussion
The findings of this systematic review and meta-analysis demonstrated that elevated troponins are relatively common in patients hospitalised for covid-19 and appear to be independently associated with death.
Elevated troponins had a pooled prevalence of 20.8% in patients with covid-19 on hospital admission. The estimate appeared higher when the troponin concentrations during hospital stay was considered and in patients admitted to ICU. Our findings are consistent with those of previous studies showing that myocardial injury occurs frequently in patients with severe respiratory infections caused by other viruses or bacteria [72–74]. Common mechanisms of myocardial injury in these conditions may include oxygen supply-demand mismatch, systemic hyperinflammation, and microvascular dysfunction and thrombosis. Besides, recent pathological and imaging studies indicated that acute atherosclerotic plaque rupture, stress cardiomyopathy, direct cardiomyocytes damage by the SARS-CoV-2 virus and myocarditis may also be the causes of troponin elevation in some patients with covid-19 [75–78]. We observed significant heterogeneity in the prevalence of elevated troponins across individual studies, which is likely attributable to several factors. The demographics and burden of comorbidities of patient populations from different countries may be different. Besides, there are no universal criteria for hospital admission for covid-19 patients; the criteria may vary across different places and different phases of the disease spread. These resulted in considerable heterogeneity among study cohorts in baseline characteristics such as age, cardiovascular diseases and the severity of respiratory infection, which would produce widely varying frequencies of elevated troponins. Moreover, multiple troponin assays with different analytical sensitivities were used for assessing myocardial injury. Studies that used high-sensitivity assays may find higher prevalence of elevated troponins than those using less sensitive earlier-generation troponin assays. Despite the heterogeneity, our pooled estimate with a relatively narrow confidence interval represents a substantial minority of patients with elevated troponins on hospital admission, indicating an important involvement of the heart in severe forms of covid-19.
Similar to findings in patients with other severe respiratory illnesses, elevated troponins appeared to have prognostic implications for patients admitted for covid-19. Some recent systematic reviews and meta-analyses have reported on the associations between elevated troponins and adverse outcomes of covid-19 [5–8, 79, 80]. However, there are several limitations to these analyses, including not accounting for the time point of troponin measurements and the cut-off threshold for troponin elevation, the variability in outcome definitions, the lack of adjustment for confounders and the inclusion of overlapping cohorts in analysis. In this study, we addressed the predictive value of troponin by focusing on troponin measured on hospital admission and on the hard outcome of death. We found that admission troponin concentrations higher than the upper reference limit were associated with a more than twofold risk of death in multivariable analyses. The stability of this association was supported by various sensitivity and subgroup analyses. Thus, troponin testing may provide prognostic information independent of other routinely assessed demographic and clinical factors for covid-19 patients early on patient admission, so that it might help clinicians in triage decision-making. Our results suggested that measurement of troponin levels at the time of hospital admission for covid-19 might be included in the diagnostic workup to identify patients at increased risk of worse outcome and those who may require higher level of surveillance and more intensive treatment.
In addition, our bivariate analysis suggested that troponin may be especially helpful to identify patients at high mortality risk when it is elevated. However, the prognosis of patients with normal troponin levels was still worrisome (mortality of ~ 9%). This is not surprising because injuries in other organ systems during the course of disease are also important factors of death [81]. Thus, the detection of a normal troponin level at hospital admission may not be considered as a sign of very low mortality risk or criteria for early discharge. The combination of troponin with other clinical information might achieve more accurate prognostication than either alone [45].
While higher mortality rates of patients with elevated troponins were consistently reported by the included studies, only two studies ascertained the mechanisms of death of patients [14, 15]. Interestingly, there was no significant difference in causes of death between patients with or without elevated troponin who deceased. It might be hypothesised that elevated troponins reflect the severity of involvement of different organs and tissues in covid-19 patients and may predict higher mortality of both cardiovascular and non-cardiovascular causes.
We acknowledge several limitations to our meta-analysis. There is significant heterogeneity in the prevalence of elevated troponins on admission and the strength of their association with mortality, presumably reflecting differing patient background, troponin assays and therapeutic practices among study centres. In addition, many of the included studies did not measure troponin systematically for all patients on their admission, and the indications for troponin measurement were poorly reported. Selective troponin sampling may have resulted in a systematic overestimation of the prevalence of elevated troponins in these studies. However, our pooled analysis of 19 studies at low risk of bias (studies that measured troponin concentrations on admission in > 90% of patients) yielded similar prevalence estimate. On the other hand, patients that did not received troponin measurement tended to have milder illness, lower prevalence of myocardial injury and lower risks of subsequent death [49]. Thus, their exclusion might have biassed the observed strength of association between elevated troponins and death toward a smaller magnitude. Overall, while we cannot be certain about the accuracy of estimates for the prevalence of elevated troponins and the associated risk of death, we believe that important inferences can still be made. Across a heterogeneous group of patients hospitalised for covid-19, myocardial injury reflected by troponin concentrations above the upper reference limit was relatively common and did consistently correlate with excess risk of death.
In this study, despite that we evaluated the association between elevated troponins and death based on multivariable analyses, our result may still be subject to residual confounding. Baseline comorbidities such as cardiovascular and kidney diseases are both strongly associated with higher troponin concentrations and are independent risk factors for mortality of covid-19 [82, 83], but they were not fully adjusted for in some of the included studies. However, dissecting the relative contributions of entwined conditions of pre-existing chronic myocardial injury, new-onset acute myocardial injury and reduced troponin clearance caused by renal impairment may be impossible, even if individual patient data from original studies were available. Thus, we could not address the question whether covid-19 infection-related myocardial injury directly influences the survival of patients. However, this limitation should not detract from the potential value of elevated troponins as a marker for early identification of covid-19 patients at high risk of death.
Other limitations of this study included the fact that we could not evaluate the utility of serial troponin testing during the first few days after admission in the identification of and risk assessment for patients with myocardial injury, because single troponin measurements on admission were reported in most studies. In addition, we were not able to evaluate the long-term impact of elevated troponins on admission or during hospital stay on the cardiovascular health of covid-19 survivors. Given the limitations of available evidence, the justification of measuring troponin as a prognostic tool for patients hospitalised for covid-19 warrants further investigation. Ongoing registries that systematically collect cardiovascular data in covid-19 patients, such as the CAPACITY-COVID [84], will hopefully contribute to a better understanding of the implications of troponin measurements in patients with covid-19.
Conclusion
The present meta-analysis suggests that among patients hospitalised for covid-19, admission troponin concentrations above the upper reference limit are common and are predictive for subsequent death. Clinically, the presence of elevated troponins on admission may facilitate risk stratification by enabling early identification of patients at high mortality risk. Large prospective studies with systematic troponin sampling and adequate follow-up are needed to validate the prognostic implications of elevated troponins for patients admitted for covid-19.
Supplementary Information
Additional file 1: Table S1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist. Table S2. Literature search strategy (PubMed as example). Table S3. Risk of bias assessment for studies on the prevalence of elevated troponin in patients hospitalised for covid-19. Table S4. Quality in Prognostic Studies (QUIPS) risk of bias assessment for studies on the association between elevated troponin and mortality. Table S5. Multivariable-adjusted association between elevated troponin and mortality in patients hospitalised for covid-19. Table S6. Subgroup analyses on the prognostic value of elevated troponins on admission for predicting death. Table S7. Covid-19 severity of illness classification. Figure S1. Pooled prevalence of elevated troponins on hospital admission. Figure S2. Funnel plot for assessing publication bias in the prevalence of elevated troponins on hospital admission. Figure S3. Pooled prevalence of elevated troponins during hospital stay. Figure S4. Pooled prevalence of elevated troponins in patients admitted to intensive care unit. Figure S5. (a) Funnel plot for assessing publication bias in the association between elevated admission troponins and mortality risk. (b) Funnel plot after applying the trim-and-fill method.
Acknowledgements
None.
Abbreviations
- Covid-19
Coronavirus disease 2019
- SARS-CoV2
Severe acute respiratory syndrome coronavirus-2
- CHARMS
CHecklist for critical Appraisal and data extraction for systematic Reviews of prediction Modelling Studies
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- CI
Confidence interval
- QUIPS
Quality in Prognosis Studies
- RR
Risk ratio
- PI
Prediction interval
Authors’ contributions
BCZ, WFL and SHL conceived the study, conducted literature search, extracted data, performed statistical analysis and drafted the manuscript. XY, TYH and QWD extracted data, performed statistical analysis and helped in interpreting the data and drafting the manuscript. BWZ, MX, CL and KXL helped in data interpretation and critically revised the manuscript. BCZ, WFL and SHL contributed equally to the work. The author(s) read and approved the final manuscript.
Funding
This work was supported by the Guangdong Basic and Applied Basic Research Foundation [2019A1515110655 to Bing-Cheng Zhao] and the Science and Technology Project of Guangdong Province [2014A020212660 to Miao Xu].
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wynants L, Van Calster B, Collins GS, Riley RD, Heinze G, Schuit E, et al. Prediction models for diagnosis and prognosis of covid-19 infection: systematic review and critical appraisal. BMJ. 2020;369:m1328. doi: 10.1136/bmj.m1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu PP, Blet A, Smyth D, Li H. The science underlying COVID-19: implications for the cardiovascular system. Circulation. 2020;142(1):68–78. doi: 10.1161/CIRCULATIONAHA.120.047549. [DOI] [PubMed] [Google Scholar]
- 3.Guzik TJ, Mohiddin SA, Dimarco A, Patel V, Savvatis K, Marelli-Berg FM, et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020;116(10):1666–1687. doi: 10.1093/cvr/cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bavishi C, Bonow RO, Trivedi V, Abbott JD, Messerli FH, Bhatt DL. Acute myocardial injury in patients hospitalized with COVID-19 infection: A review. Prog Cardiovasc Dis. 2020.. [DOI] [PMC free article] [PubMed]
- 5.Li JW, Han TW, Woodward M, Anderson CS, Zhou H, Chen YD, et al. The impact of 2019 novel coronavirus on heart injury: a systematic review and meta-analysis. Prog Cardiovasc Dis. 2020;63(4):518–524. doi: 10.1016/j.pcad.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X, Guan B, Su T, Liu W, Chen M, Bin Waleed K, et al. Impact of cardiovascular disease and cardiac injury on in-hospital mortality in patients with COVID-19: a systematic review and meta-analysis. Heart. 2020;106(15):1142–1147. doi: 10.1136/heartjnl-2020-317062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parohan M, Yaghoubi S, Seraji A. Cardiac injury is associated with severe outcome and death in patients with Coronavirus disease 2019 (COVID-19) infection: a systematic review and meta-analysis of observational studies. Eur Heart J Acute Cardiovasc Care. 2020;9(6):665–77. doi: 10.1177/2048872620937165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santoso A, Pranata R, Wibowo A, Al-Farabi MJ, Huang I, Antariksa B. Cardiac injury is associated with mortality and critically ill pneumonia in COVID-19: A meta-analysis. Am J Emerg Med. 2020. [DOI] [PMC free article] [PubMed]
- 9.Januzzi JL. Troponin and BNP use in COVID-19. American College of Cardiology. https://www.acc.org/latest-in-cardiology/articles/2020/03/18/15/25/troponin-and-bnp-use-in-covid19. Accessed 21 Oct 2020.
- 10.NICE guideline [NG171]. Covid-19 rapid guideline: acute myocardial injury. National Institute for Health and Care Excellence. https://www.nice.org.uk/guidance/NG171. Accessed 21 Oct 2020. [PubMed]
- 11.Chapman AR, Bularga A, Mills NL. High-sensitivity cardiac troponin can be an ally in the fight against COVID-19. Circulation. 2020;141(22):1733–1735. doi: 10.1161/CIRCULATIONAHA.120.047008. [DOI] [PubMed] [Google Scholar]
- 12.Loungani RS, Rehorn MR, Newby LK, Katz JN, Klem I, Mentz RJ, et al. A care pathway for the cardiovascular complications of COVID-19: insights from an institutional response. Am Heart J. 2020;225:3–9. doi: 10.1016/j.ahj.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lala A, Johnson KW, Januzzi JL, Russak AJ, Paranjpe I, Richter F, et al. Prevalence and impact of myocardial injury in patients hospitalized with covid-19 infection. J Am Coll Cardiol. 2020;76(5):533–546. doi: 10.1016/j.jacc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lombardi CM, Carubelli V, Iorio A, Inciardi RM, Bellasi A, Canale C, et al. Association of troponin levels with mortality in Italian patients hospitalized with coronavirus disease 2019: results of a multicenter study. JAMA Cardiol. 2020;5(11):1274–1280. doi: 10.1001/jamacardio.2020.3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qin JJ, Cheng X, Zhou F, Lei F, Akolkar G, Cai J, et al. Redefining cardiac biomarkers in predicting mortality of inpatients with COVID-19. Hypertension. 2020;76(4):1104–1112. doi: 10.1161/HYPERTENSIONAHA.120.15528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szekely Y, Lichter Y, Taieb P, Banai A, Hochstadt A, Merdler I, et al. Spectrum of cardiac manifestations in COVID-19: a systematic echocardiographic study. Circulation. 2020;142(4):342–353. doi: 10.1161/CIRCULATIONAHA.120.047971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riley RD, Moons KGM, Snell KIE, Ensor J, Hooft L, Altman DG, et al. A guide to systematic review and meta-analysis of prognostic factor studies. BMJ. 2019;364:k4597. doi: 10.1136/bmj.k4597. [DOI] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoy D, Brooks P, Woolf A, Blyth F, March L, Bain C, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65(9):934–939. doi: 10.1016/j.jclinepi.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 20.Hayden JA, van der Windt DA, Cartwright JL, Cote P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158(4):280–286. doi: 10.7326/0003-4819-158-4-201302190-00009. [DOI] [PubMed] [Google Scholar]
- 21.Potgieter D, Simmers D, Ryan L, Biccard BM, Lurati-Buse GA, Cardinale DM, et al. N-terminal pro-B-type natriuretic peptides’ prognostic utility is overestimated in meta-analyses using study-specific optimal diagnostic thresholds. Anesthesiology. 2015;123(2):264–271. doi: 10.1097/ALN.0000000000000728. [DOI] [PubMed] [Google Scholar]
- 22.Grant RL. Converting an odds ratio to a range of plausible relative risks for better communication of research findings. BMJ. 2014;348:f7450. doi: 10.1136/bmj.f7450. [DOI] [PubMed] [Google Scholar]
- 23.Riley RD, Elia EG, Malin G, Hemming K, Price MP. Multivariate meta-analysis of prognostic factor studies with multiple cut-points and/or methods of measurement. Stat Med. 2015;34(17):2481–2496. doi: 10.1002/sim.6493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee J, Kim KW, Choi SH, Huh J, Park SH. Systematic review and meta-analysis of studies evaluating diagnostic test accuracy: A practical review for clinical researchers-Part II. Statistical methods of meta-analysis. Korean J Radiol. 2015;16(6):1188–1196. doi: 10.3348/kjr.2015.16.6.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arcari L, Luciani M, Cacciotti L, Musumeci MB, Spuntarelli V, Pistella E, et al. Incidence and determinants of high-sensitivity troponin and natriuretic peptides elevation at admission in hospitalized COVID-19 pneumonia patients. Intern Emerg Med. 2020;15(8):1467–1476. doi: 10.1007/s11739-020-02498-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Azoulay E, Fartoukh M, Darmon M, Geri G, Voiriot G, Dupont T, et al. Increased mortality in patients with severe SARS-CoV-2 infection admitted within seven days of disease onset. Intensive Care Med. 2020;46(9):1714–1722. doi: 10.1007/s00134-020-06202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barman HA, Atici A, Sahin I, Alici G, Aktas Tekin E, Baycan OF, et al. Prognostic significance of cardiac injury in COVID-19 patients with and without coronary artery disease. Coron Artery Dis. 2020. [DOI] [PMC free article] [PubMed]
- 28.Bhatla A, Mayer MM, Adusumalli S, Hyman MC, Oh E, Tierney A, et al. COVID-19 and cardiac arrhythmias. Heart Rhythm. 2020;17(9):1439–1444. doi: 10.1016/j.hrthm.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhatraju PK, Ghassemieh BJ, Nichols M, Kim R, Jerome KR, Nalla AK, et al. Covid-19 in critically ill patients in the Seattle region — case series. N Engl J Med. 2020;382(21):2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buckner FS, McCulloch DJ, Atluri V, Blain M, McGuffin SA, Nalla AK, et al. Clinical features and outcomes of 105 hospitalized patients with COVID-19 in Seattle, Washington. Clin Infect Dis. 2020;71(16):2167–2173. doi: 10.1093/cid/ciaa632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cipriani A, Capone F, Donato F, Molinari L, Ceccato D, Saller A, et al. Cardiac injury and mortality in patients with Coronavirus disease 2019 (COVID-19): insights from a mediation analysis. Intern Emerg Med. 2020. [DOI] [PMC free article] [PubMed]
- 32.Du RH, Liang LR, Yang CQ, Wang W, Cao TZ, Li M, et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020;55(5):2000524. doi: 10.1183/13993003.00524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferguson J, Rosser JI, Quintero O, Scott J, Subramanian A, Gumma M, et al. Characteristics and outcomes of coronavirus disease patients under nonsurge conditions, Northern California, USA, March-April 2020. Emerg Infect Dis. 2020;26(8):1679–1685. doi: 10.3201/eid2608.201776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Franks CE, Scott MG, Farnsworth CW. Elevated cardiac troponin I is associated with poor outcomes in COVID-19 patients at an academic medical center in midwestern USA. J Appl Lab Med. 2020;5(5):1137–1139. doi: 10.1093/jalm/jfaa092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gottlieb M, Sansom S, Frankenberger C, Ward E, Hota B. Clinical course and factors associated with hospitalization and critical illness among COVID-19 patients in Chicago, Illinois. Acad Emerg Med. 2020;27(10):963–973. doi: 10.1111/acem.14104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, et al. Clinical characteristics of Covid-19 in New York City. N Engl J Med. 2020;382(24):2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harmouch F, Shah K, Hippen JT, Kumar A, Goel H. Is it all in the heart? Myocardial injury as major predictor of mortality among hospitalized COVID-19 patients. J Med Virol. 2020. [DOI] [PubMed]
- 38.Heberto AB, Carlos PCJ, Antonio CRJ, Patricia PP, Enrique TR, Danira MPJ, et al. Implications of myocardial injury in Mexican hospitalized patients with coronavirus disease 2019 (COVID-19) Int J Cardiol Heart Vasc. 2020;30:100638. doi: 10.1016/j.ijcha.2020.100638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu L, Chen S, Fu Y, Gao Z, Long H, Wang JM, et al. Risk factors associated with clinical outcomes in 323 COVID-19 hospitalized patients in Wuhan, China. Clin Infect Dis. 2020;71(16):2089. doi: 10.1093/cid/ciaa539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang M, Yang Y, Shang F, Zheng Y, Zhao W, Luo L, et al. Clinical characteristics and predictors of disease progression in severe patients with COVID-19 infection in Jiangsu province, China: a descriptive study. Am J Med Sci. 2020;360(2):120–128. doi: 10.1016/j.amjms.2020.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karbalai Saleh S, Oraii A, Soleimani A, Hadadi A, Shajari Z, Montazeri M, et al. The association between cardiac injury and outcomes in hospitalized patients with COVID-19. Intern Emerg Med. 2020;15(8):1415–1424. doi: 10.1007/s11739-020-02466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lazzeri C, Bonizzoli M, Batacchi S, Cianchi G, Franci A, Fulceri GE, et al. Cardiac involvment in COVID-19-related acute respiratory distress syndrome. Am J Cardiol. 2020;132:147–149. doi: 10.1016/j.amjcard.2020.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li C, Li M, Gan L, Zhang Y, Lei CL. Heart and liver damage in coronavirus disease-2019. Guang Dong Yi Xue. 2020;41:874–877. [Google Scholar]
- 44.Li C, Jiang J, Wang F, Zhou N, Veronese G, Moslehi JJ, et al. Longitudinal correlation of biomarkers of cardiac injury, inflammation, and coagulation to outcome in hospitalized COVID-19 patients. J Mol Cell Cardiol. 2020;147:74–87. doi: 10.1016/j.yjmcc.2020.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lorente-Ros A, Monteagudo Ruiz JM, Rincon LM, Ortega Perez R, Rivas S, Martinez-Moya R, et al. Myocardial injury determination improves risk stratification and predicts mortality in COVID-19 patients. Cardiol J. 2020;27(5):489–496. doi: 10.5603/CJ.a2020.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Majure DT, Gruberg L, Saba SG, Kvasnovsky C, Hirsch JS, Jauhar R. Usefulness of elevated troponin to predict death in patients with COVID-19 and myocardial injury. Int J Cardiol. 2020;S0002-9149(20):31097–31093. doi: 10.1016/j.amjcard.2020.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mejía-Vilet JM, Cordova-Sanchez BM, Fernandez-Camargo DA, Mendez-Perez RA, Morales-Buenrostro LE, Hernandez-Gilsoul T. A risk score to predict admission to the intensive care unit in patients with Covid-19: the ABC-GOALS score. Salud Publica Mex. 2020. [DOI] [PubMed]
- 48.Nie SF, Yu M, Xie T, Yang F, Wang HB, Wang ZH, et al. Cardiac troponin I is an independent predictor for mortality in hospitalized patients with COVID-19. Circulation. 2020;142(6):608–610. doi: 10.1161/CIRCULATIONAHA.120.048789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petrilli CM, Jones SA, Yang J, Rajagopalan H, O'Donnell L, Chernyak Y, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among black patients and white patients with covid-19. N Engl J Med. 2020;382(26):2534–2543. doi: 10.1056/NEJMsa2011686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raad M, Dabbagh M, Gorgis S, Yan J, Chehab O, Dagher C, et al. Cardiac injury patterns and inpatient outcomes among patients admitted with COVID-19. Am J Cardiol. 2020;133:154–161. doi: 10.1016/j.amjcard.2020.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shah P, Doshi R, Chenna A, Owens R, Cobb A, Ivey H, et al. Prognostic value of elevated cardiac troponin i in hospitalized covid-19 patients. Am J Cardiol. 2020;135:150–153. doi: 10.1016/j.amjcard.2020.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shah SJ, Barish PN, Prasad PA, Kistler A, Neff N, Kamm J, et al. Clinical features, diagnostics, and outcomes of patients presenting with acute respiratory illness: a retrospective cohort study of patients with and without COVID-19. EClinicalMedicine. 2020;27:100518. doi: 10.1016/j.eclinm.2020.100518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shen Y, Zheng F, Sun D, Ling Y, Chen J, Li F, et al. Epidemiology and clinical course of COVID-19 in Shanghai, China. Emerg Microbes Infect. 2020;9(1):1537–1545. doi: 10.1080/22221751.2020.1787103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stefanini GG, Chiarito M, Ferrante G, Cannata F, Azzolini E, Viggiani G, et al. Early detection of elevated cardiac biomarkers to optimise risk stratification in patients with COVID-19. Heart. 2020;106(19):1512–1518. doi: 10.1136/heartjnl-2020-317322. [DOI] [PubMed] [Google Scholar]
- 56.van den Heuvel FMA, Vos JL, Koop Y, van Dijk APJ, Duijnhouwer AL, de Mast Q, et al. Cardiac function in relation to myocardial injury in hospitalised patients with COVID-19. Neth Heart J. 2020;28(7-8):410–417. doi: 10.1007/s12471-020-01458-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wei JF, Huang FY, Xiong TY, Liu Q, Chen H, Wang H, et al. Acute myocardial injury is common in patients with COVID-19 and impairs their prognosis. Heart. 2020;106(15):1154–1159. doi: 10.1136/heartjnl-2020-317007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu Y, Xu D, Fu S, Zhang J, Yang X, Xu L, et al. Patients with COVID-19 in 19 ICUs in Wuhan, China: a cross-sectional study. Crit Care. 2020;24(1):219. doi: 10.1186/s13054-020-02939-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zeng JH, Wu WB, Qu JX, Wang Y, Dong CF, Luo YF, et al. Cardiac manifestations of COVID-19 in Shenzhen, China. Infection. 2020;48(6):861–870. doi: 10.1007/s15010-020-01473-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao XY, Xu XX, Yin HS, Hu QM, Xiong T, Tang YY, et al. Clinical characteristics of patients with 2019 coronavirus disease in a non-Wuhan area of Hubei Province, China: a retrospective study. BMC Infect Dis. 2020;20(1):311. doi: 10.1186/s12879-020-05010-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.He H, Jin Z, Ren Y, Wang J, Wen W, Miao Y, et al. Clinical course and features of critical patients with COVID-19: a single-center, retrospective study from Wuhan Huoshenshan Hospital. Res Square. 2020; [Preprint].
- 63.Lu S, He H, Ra L, Wu Y, Deng L, Wang P, et al. The Cardiac Injury in Hospitalized Patients with Severe COVID-19 in Wuhan, China. Res Square. 2020; [Preprint].
- 64.Ma K-L, Liu Z-H, Cao C-F, Liu M-K, Liao J, Zou J-B, et al. COVID-19 myocarditis and severity factors: an adult cohort study. medRxiv. 2020; [Preprint].
- 65.Nguyen AB, Upadhyay GA, Chung B, Smith B, Besser SA, Johnson JA, et al. Outcomes and Cardiovascular Comorbidities in a Predominantly African-American Population with COVID-19. medRxiv. 2020; [Preprint].
- 66.Qi D, Yan X, Tang X, Peng J, Yu Q, Feng L, et al. Epidemiological and clinical features of 2019-nCoV acute respiratory disease cases in Chongqing municipality, China: a retrospective, descriptive, multiple-center study. medRxiv. 2020; [Preprint].
- 67.Tan W, Zhu Y, Yi H, Lin Y, Liu Y, Wu Z, et al. Development a Nomogram to Predict Prognosis in Severe and Critically Ill Patients with COVID-19. Res Square. 2020; [Preprint].
- 68.Woo S, Rios-Diaz A, Kubey A, Cheney-Peters D, Ackermann L, Chalikonda D, et al. Development and validation of a web-based severe COVID-19 risk prediction model. medRxiv. 2020; [Preprint]. [DOI] [PMC free article] [PubMed]
- 69.Xu W, Luo Q, Chen D, Lei Z, Chen Y, Wang J, et al. Clinical features in mild type and severe type of COVID-19 patients. Res Square. 2020; [Preprint].
- 70.Yang S, Ma L, Wang Y-L, Wang Q, Tong Q, Chen M, et al. Risk factors for critical-ill events of patients with COVID-19 in Wuhan, China: a retrospective cohort study. medRxiv. 2020; [Preprint].
- 71.Zhang J, Ding D, Cao C, Zhang J, Huang X, Fu P, et al. Myocardial characteristics as the prognosis for COVID-19 patients. medRxiv. 2020; [Preprint].
- 72.Gao C, Wang Y, Gu X, Shen X, Zhou D, Zhou S, et al. Association between cardiac injury and mortality in hospitalized patients infected with avian influenza A (H7N9) virus. Crit Care Med. 2020;48(4):451–458. doi: 10.1097/CCM.0000000000004207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pizzini A, Burkert F, Theurl I, Weiss G, Bellmann-Weiler R. Prognostic impact of high sensitive troponin T in patients with influenza virus infection: A retrospective analysis. Heart Lung. 2020;49(1):105–109. doi: 10.1016/j.hrtlng.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 74.Vestjens SMT, Spoorenberg SMC, Rijkers GT, Grutters JC, Ten Berg JM, Noordzij PG, et al. High-sensitivity cardiac troponin T predicts mortality after hospitalization for community-acquired pneumonia. Respirology. 2017;22(5):1000–1006. doi: 10.1111/resp.12996. [DOI] [PubMed] [Google Scholar]
- 75.Imazio M, Klingel K, Kindermann I, Brucato A, De Rosa FG, Adler Y, et al. COVID-19 pandemic and troponin: indirect myocardial injury, myocardial inflammation or myocarditis? Heart. 2020;106(15):1127–1131. doi: 10.1136/heartjnl-2020-317186. [DOI] [PubMed] [Google Scholar]
- 76.Tersalvi G, Vicenzi M, Calabretta D, Biasco L, Pedrazzini G, Winterton D. Elevated troponin in patients with coronavirus disease 2019: possible mechanisms. J Card Fail. 2020;26(6):470–475. doi: 10.1016/j.cardfail.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lindner D, Fitzek A, Brauninger H, Aleshcheva G, Edler C, Meissner K, et al. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol. 2020;5(11):1281–1285. doi: 10.1001/jamacardio.2020.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(11):1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aikawa T, Takagi H, Ishikawa K, Kuno T. Myocardial injury characterized by elevated cardiac troponin and in-hospital mortality of COVID-19: An insight from a meta-analysis. J Med Virol. 2020. [DOI] [PMC free article] [PubMed]
- 80.Vrsalovic M, Vrsalovic PA. Cardiac troponins predict mortality in patients with COVID-19: A meta-analysis of adjusted risk estimates. J Infect. 2020;81(3):e99–e100. doi: 10.1016/j.jinf.2020.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Potere N, Valeriani E, Candeloro M, Tana M, Porreca E, Abbate A, et al. Acute complications and mortality in hospitalized patients with coronavirus disease 2019: a systematic review and meta-analysis. Crit Care. 2020;24(1):389. doi: 10.1186/s13054-020-03022-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen R, Liang W, Jiang M, Guan W, Zhan C, Wang T, et al. Risk factors of fatal outcome in hospitalized subjects with coronavirus disease 2019 from a nationwide analysis in China. Chest. 2020;158(1):97–105. doi: 10.1016/j.chest.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Linschoten M, Asselbergs FW. CAPACITY-COVID: a European Registry to determine the role of cardiovascular disease in the COVID-19 pandemic. Eur Heart J. 2020;41(19):1795–1796. doi: 10.1093/eurheartj/ehaa280. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist. Table S2. Literature search strategy (PubMed as example). Table S3. Risk of bias assessment for studies on the prevalence of elevated troponin in patients hospitalised for covid-19. Table S4. Quality in Prognostic Studies (QUIPS) risk of bias assessment for studies on the association between elevated troponin and mortality. Table S5. Multivariable-adjusted association between elevated troponin and mortality in patients hospitalised for covid-19. Table S6. Subgroup analyses on the prognostic value of elevated troponins on admission for predicting death. Table S7. Covid-19 severity of illness classification. Figure S1. Pooled prevalence of elevated troponins on hospital admission. Figure S2. Funnel plot for assessing publication bias in the prevalence of elevated troponins on hospital admission. Figure S3. Pooled prevalence of elevated troponins during hospital stay. Figure S4. Pooled prevalence of elevated troponins in patients admitted to intensive care unit. Figure S5. (a) Funnel plot for assessing publication bias in the association between elevated admission troponins and mortality risk. (b) Funnel plot after applying the trim-and-fill method.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.