Supplemental Digital Content is available in the text.
Keywords: blood platelets, inflammation, interferons, monocytes, thrombosis
Abstract
Objective:
Pulmonary thrombosis is observed in severe acute respiratory syndrome coronavirus 2 pneumonia. Aim was to investigate whether subpopulations of platelets were programmed to procoagulant and inflammatory activities in coronavirus disease 2019 (COVID-19) patients with pneumonia, without comorbidities predisposing to thromboembolism.
Approach and Results:
Overall, 37 patients and 28 healthy subjects were studied. Platelet-leukocyte aggregates, platelet-derived microvesicles, the expression of P-selectin, and active fibrinogen receptor on platelets were quantified by flow cytometry. The profile of 45 cytokines, chemokines, and growth factors released by platelets was defined by immunoassay. The contribution of platelets to coagulation factor activity was selectively measured. Numerous platelet-monocyte (mean±SE, 67.9±4.9%, n=17 versus 19.4±3.0%, n=22; P<0.0001) and platelet-granulocyte conjugates (34.2±4.04% versus 8.6±0.7%; P<0.0001) were detected in patients. Resting patient platelets had similar levels of P-selectin (10.9±2.6%, n=12) to collagen-activated control platelets (8.7±1.5%), which was not further increased by collagen activation on patient platelets (12.4±2.5%, P=nonsignificant). The agonist-stimulated expression of the active fibrinogen receptor was reduced by 60% in patients (P<0.0001 versus controls). Cytokines (IL [interleukin]-1α, IL-1β, IL-1RA, IL-4, IL-10, IL-13, IL, 17, IL-27, IFN [interferon]-α, and IFN-γ), chemokines (MCP-1/CCL2 [monocyte chemoattractant protein 1]), and growth factors (VEGF [vascular endothelial growth factor]-A/D) were released in significantly larger amounts upon stimulation of COVID-19 platelets. Platelets contributed to increased fibrinogen, VWF (von Willebrand factor), and factor XII in COVID-19 patients. Patients (28.5±0.7 s, n=32), unlike controls (31.6±0.5 s, n=28; P<0.001), showed accelerated factor XII–dependent coagulation.
Conclusions:
Platelets in COVID-19 pneumonia are primed to spread proinflammatory and procoagulant activities in systemic circulation.
Highlights.
Circulating platelets in coronavirus disease 2019 (COVID-19) pneumonia demonstrate a specific phenotypic and functional profile that is consistent with that of procoagulant platelets.
Platelets in patients with COVID-19 express constitutively P-selectin and form aggregates with leukocytes, also contributing to inflammation by storing in their granules and releasing cytokines, chemokines, and growth factors.
Circulating platelets contribute to increased coagulability in COVID-19 patient, which is factor XII dependent and tissue factor-factor VII independent.
See accompanying editorial on page 2812
The coronavirus disease 2019 (COVID-19) pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is associated with high mortality rate, attributed to the severity of pneumonia and the development of systemic complications, including target-organ damage and thromboembolic events.1 As disease progresses toward more extensive lung involvement, respiratory failure and acute respiratory distress syndrome are observed in a minority of patients.2 Several reports indicate that thromboembolic events are frequently diagnosed in patients with SARS-CoV-2 infection. Histology and imaging by angiographic computed tomography (CT) revealed microvascular thrombosis or more extensive pulmonary thrombosis, usually not associated with peripheral vein thrombosis.3–6 Pulmonary thrombosis may increase the mismatch between ventilation and perfusion, with severe hypoxemia and precipitating respiratory failure.7 According to CT evidence, ≈25% of COVID-19 patients in medical wards have pulmonary thrombosis, with frequent involvement of segmental and multiple subsegmental pulmonary arteries.4,8
Several observational studies described alterations in hemostasis parameters, reduced platelet count, prolonged prothrombin time (PT), and altered activated partial thromboplastin time (APTT) with decreased plasma fibrinogen in later stages of the disease.6,9–12 However, the clinical picture of disseminated intravascular coagulation is uncommon.13 Hemorrhages, severe depletion of coagulation factors and antithrombin levels, or severe thrombocytopenia are rarely observed.9 The pulmonary inflammatory response to this new coronavirus is characterized by injured endothelial cells, lymphocyte, and granulocyte infiltration.3,14–17 Platelets may trigger the thrombotic process and amplify inflammation through bidirectional signals with leukocytes, the interaction with granulocytes generating neutrophil extracellular traps, the release of bioactive substances and microvesicles along with the generation of procoagulant platelets in addition to canonical aggregation.18 Procoagulant platelets activate coagulation cascade by assembling coagulation factors on their surface and expressing catalytic activities.19,20
In the setting of experimental and human inflammation, recent evidence suggests that thrombosis may be triggered by subpopulations of platelets programmed to procoagulant and proinflammatory activities.18 The aim of the present investigation was to characterize the phenotypic profile of circulating platelets and define their contribution to a prothrombotic and proinflammatory environment, providing evidence of a close intersection of platelets with the inflammatory process.
Materials and Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Subjects and Protocol
All the patients with SARS-CoV-2 pneumonia hospitalized in the Departments of Internal Medicine C and Infectious Diseases of the Verona University Hospital between March 25 and May 3 were considered as potentially eligible for the study except those who were receiving antiplatelet or therapeutic doses of anticoagulation agents for any clinical indication or had comorbidities predisposing to thromboembolism. Clinical and epidemiological variables have been collected at study inclusion. Diagnosis SARS-CoV-2 pneumonia was based on the results of pharyngeal and nose swab demonstrating positivity by means of reverse transcriptase-polymerase chain reaction (Seegene), along with imaging showing ground grass opacities in the lungs by chest roentgenogram or CT. The development of viral pneumonia was in most cases associated with cough, fever, and possibly hypoxia (defined as blood oxygen saturation levels <92% or Pao2/FiO2 <300).10 A radiological pneumonia severity score was used in COVID-19 patients, to obtain a semiquantitative assessment of lung disease in COVID-19, ranking the pulmonary involvement on an 18-point severity scale according to the extent and the characteristics of lung abnormalities.21
Patients were excluded from the study if they had personal history of cardiovascular disease or venous thromboembolism, were experiencing diabetes, were active smokers, had bacterial infections, required mechanical ventilation, or were not able to give their informed consent. Patients were also excluded from the study if plasma d-dimer was above 5000 ng/mL due to suspicion of thromboembolic event, or they had deep vein thrombosis of the lower limbs or pulmonary thromboembolism. Treatment for COVID-19 was permitted, according to local clinical practice. A standard dose of 4000 U enoxaparin was permitted for thromboprophylaxis, with the last dose administered 24 hours before blood sampling. Patients were usually studied during the first week after hospital admission.
Healthy subjects were recruited among the medical staff of the medical departments taking part in the study (mean age, 35 years; range, 27–61; 11 women), provided that they were not treated with antiplatelet or anticoagulation agents and had given their written informed consent. They were considered as reference for the investigational analyses.
The study was designed to have 20 patients for the analysis of platelets and coagulation factors and 20 for the study of platelets and inflammation, to be compared with an equal number of healthy subjects. Coagulation tests were performed in all the studied subjects.
The Ethics Committee for Clinical Research of the Provinces of Verona and Rovigo approved the study (2577CESC clinical data registry and 2622CESC laboratory research). All the patients received an information letter by a physician taking part in the study and gave their informed and witnessed consent to the participation that, for safety reasons, was recorded and signed later by physician and witness, according to the recommendations of the local ethics committee.
Chest Radiograph and CT Perfusion Angiography
Standard chest radiograph was obtained in all patients. A radiograph severity score, specifically designed for COVID-19 pneumonia, was applied.22 To calculate the score, lungs are divided into 6 zones on frontal chest projection. The scores of the 6 lung zones are then added to obtain an overall CXR SCORE ranging from 0 to 18, where score 0 indicates no lung abnormalities, score 1, interstitial infiltrates, score 2, interstitial and alveolar infiltrates (interstitial predominance), and score 3 indicates interstitial and alveolar infiltrates (alveolar predominance).22 Severity score was calculated by experienced radiologists blinded to the characteristics of studied patients and laboratory data. CT perfusion angiography scan was performed, when indicated, with a 64-row multiple detector CT scanner (Brilliance 64; Philips). Scan timing was determined using a bolus-tracking technique, focusing on the pulmonary trunk.
Blood Sampling and Cell Isolation
All the blood samples used in the present study were collected by straight needle venipuncture, directly into evacuated blood tubes containing 0.105 mmol/L buffered sodium citrate or citric acid, citrate, dextrose when indicated.
Peripheral Blood Sample for Platelet Analysis
Plasma was separated by centrifugation at 1300×g for 15 minutes at room temperature. Platelet-rich plasma (PRP) was obtained by centrifugation of blood at 180g at room temperature for 15 minutes and was used in all the functional platelet tests performed. Platelet-free plasma, for measurement of circulating platelet microparticles, was obtained further centrifuging PRP at 1600g for 10 minutes at room temperature and subsequently at 13 000g for 2 minutes. Washed platelets were obtained by further centrifugation of blood collected in tubes containing citric acid, citrate, dextrose, first at 180 for 15 minutes and then centrifuged at 700×g for 10 minutes. Platelet pellet was washed twice and resuspended in HEPES buffer. According to the safety procedures related to COVID-19 epidemic, plasma and platelet preparations were treated with para formaldehyde 4%, when instrumentation located outside dedicated areas had to be used, as indicated in the text.
Blood Cell Count and Morphology
Instrumentation was Sysmex XN-9000 (Sysmex, Kobe, Japan) equipped with The Sysmex DI-60 system (DI-60; Sysmex)—an automated digital cell imaging analyzer.23 May-Grunwald-Giemsa staining was used.
Platelet-Leukocyte Aggregates
The presence of platelet-leukocyte aggregates was determined as described previously.24
Briefly, 20 µL of citrated whole blood was gentle mixed with a cocktail containing platelet- and leukocyte-specific antibodies as anti-CD41, anti-CD14, anti-CD66b, and anti-CD45. After 15 minutes of incubation, samples were fixed and lysed using fix/lyse solution (Cal-Lyse Lysing Solution) for another 10 minutes at room temperature to allow the red cell lysis.
Monocytes and neutrophils were defined to the CD45-positive population, based on CD14 and CD66b expression, respectively. Leukocyte-platelet aggregates were detected by the positivity of monocytes or neutrophils at platelet marker CD41. Fluorescence Minus One was used as a negative control.
Platelet Phenotype
To evaluate the difference in platelet activation markers, PRP from citrate blood sample from healthy subjects or COVID-19 patients was diluted to 50 000 platelets/µL in HEPES buffer. Fluorescein isothiocyanate–labeled anti-CD62P (cluster of differentiation 62P, P-selectin) and the fluorescein isothiocyanate–labeled monoclonal antibody PAC-1, which specifically binds the active form of integrin αIIbβ3 complex, were added to the platelet suspension with or without 10 µg/mL of collagen, for 15 minutes at room temperature. Samples were fixed with HEPES buffer containing 4% of platelet function analyzer. Flow cytometry analysis was performed using BD FACS CANTO II (BD Biosciences), and data were analyzed using FlowJo software.
Platelet-Derived Microvesicles
The number of circulating platelet-derived microvesicles (PMV) was quantified as described by Giacomazzi et al.25 Briefly PRP from citrate blood patients and healthy control samples was centrifuged at 1600g for 20 minutes at room temperature followed by subsequent centrifugation at 13000g for 2 minutes at room temperature to obtain plasma-free platelets. Plasma-free platelet was marked with phycoerythrin-labeled anti-CD41 for 30 minutes at room temperature. Samples were fixed with a 4% of platelet function analyzer solution. Quantification of microparticles (µL) was assessed using Trucount tube (BD Bioscience).
Platelet Degranulation
Washed platelets (1×108) from or citric acid, citrate, dextrose blood patients and healthy subjects’ samples were stimulated with thrombin (0.1 U/mL), used to induce degranulation of washed platelet, and immediately centrifuged at 15 000×g for 3 minutes. Supernatants were collected and fixed for the assay of released cytokines, chemokines, and growth factors.
Assay of Cytokines, Chemokines, and Growth Factors
A panel of 45 cytokines (Tables 3 and 4), chemokines, and growth factors was analyzed in the releasate of degranulated platelets and in plasma treated with para formaldehyde 4% obtained from the same citrate and or citric acid, citrate, dextrose blood sample (for plasma and degranulated platelet, respectively) of COVID-19 patients and healthy subjects. Immunoassay was performed using Human ProcartaPlex Panel 1 multiplex (ThermoFisher Scientific, Waltham, MA) following the manufacturer’s instructions.
Table 3.
Cytokines, chemokines, and growth factors in plasma
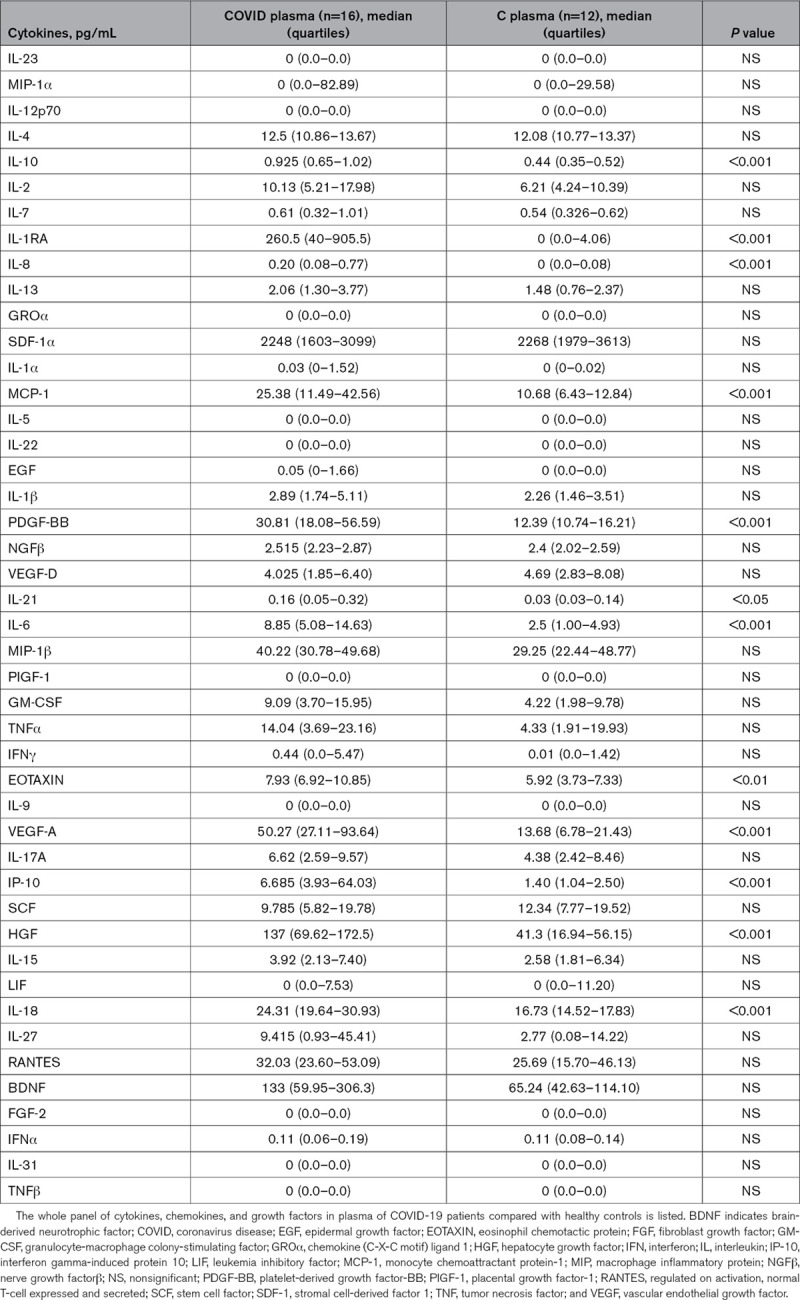
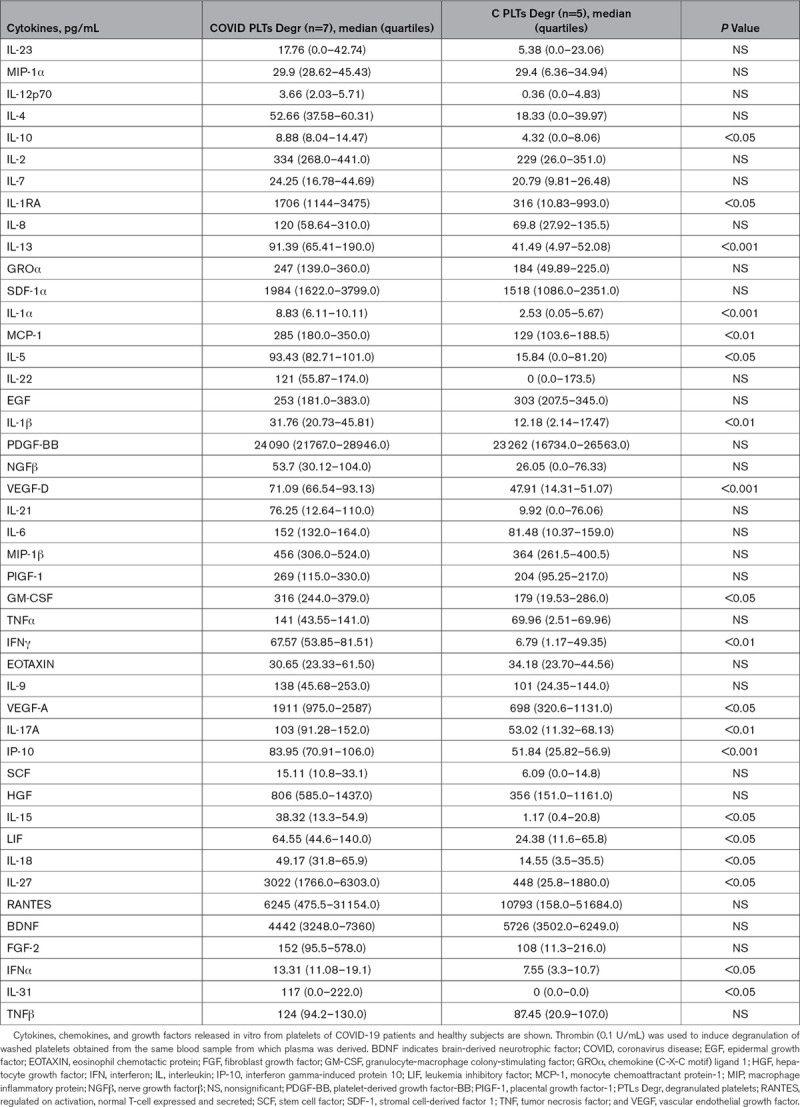
Table 4.
Cytokines, chemokines, and growth factors released from platelets
Coagulation and Coagulation Factors Assays for Evaluation of Procoagulant Platelets
Coagulation assays encompassing APTT, PT, as well as fibrinogen, the coagulation factors XII, VIII, and VII in plasma, and PRP were analyzed with the Instrumentation Laboratory ACL TOP 700 analyzer (Instrumentation Laboratory, Bedford, MA). The assays for VWF (von Willebrand factor) antigen, VWF collagen binding (CB), and VWF ristocetin cofactor assays were performed on an ACL AcuStar instrument (Instrumentation Laboratory, Bedford, MA).
To explore the contribution of platelets to blood coagulation and coagulation factor activity, the standard protocol of coagulation assays was modified using PRP instead of plasma. To further assess the contribution of platelets to the activity of coagulation factors, in a subset of COVID-19 patients, washed platelets were suspended in the corresponding volume of plasma obtained from pooled plasma samples of healthy controls and tested in the assays of coagulation factors. References were the activities of coagulation factors measured in plasma and PRP of healthy subjects.
Biochemical Profile and Other Tests
The PFA-100 platelet function analyzer (Siemens Healthcare Diagnostics, Milano, Italy), equipped with collagen-ADP and collagen-epinephrine cartridges was used to test platelet aggregation-dependent clot formation in whole blood under high-flow conditions.
Statistical Analysis
Individual data and mean are shown in figures. Data are presented as mean and SD or median and quartiles in the tables. Mean of differences and 95% CIs for each comparison between two independent groups are shown in the main text. The study was designed to explore mechanisms of disease; therefore, sample dimension was not calculated a priori. One-way ANOVA, followed by Newman-Keuls or Bonferroni test, and Student t test were used. The Mann-Whitney U test was performed when normal distribution of the data could not be assumed (Kolmogorow-Smirnov and D’Agostino and Pearson tests). The Pearson correlation coefficient r was calculated to explore significant associations. A statistical significance (P) value <0.05 was set.
Results
Clinical Case Exemplifying Inflammation-Related Thrombosis in the Lung Vasculature
CT perfusion angiography, performed in a patient (not included in the analyzed series) with SARS-CoV-2 pneumonia causing severe respiratory failure and associated high d-dimer values (<10 000 µg/L), showed filling defects of segmental and subsegmental branches of a pulmonary artery and the corresponding next venous plexus branches representing a local generation of the thrombi (Figure 1).
Figure 1.
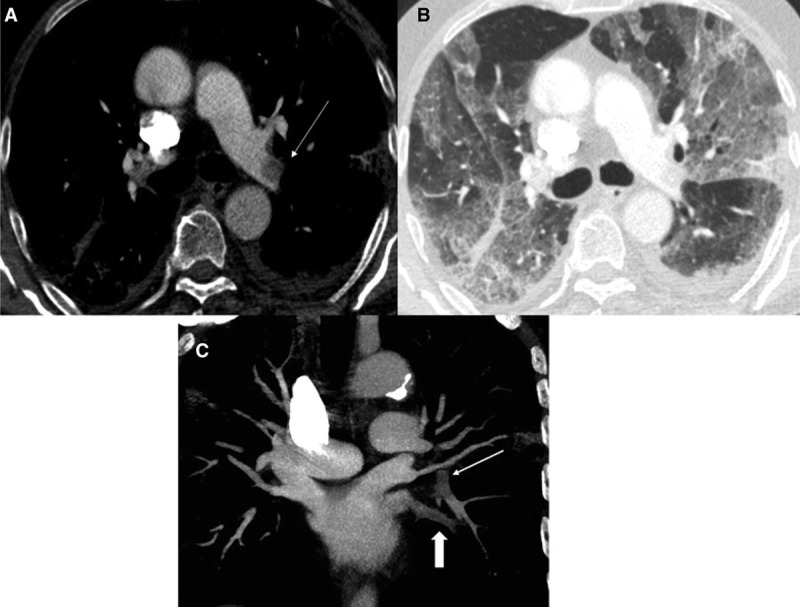
Computed tomography perfusion angiography (CTPA) scans. Axial CTPA images with mediastinal (A) and lung (B) window showing filling defects involving the proximal tract of left pulmonary artery (arrow). Diffuse ground glass opacifications together with diffuse thickening of interlobular septa are visible (B). MIP (maximum intensity projection) reformatted images on coronal plane (C) show filling defects both in some segmental and subsegmental branches of left pulmonary artery (small arrow) and in corresponding next venous branches (large arrow).
Patients’ Characteristics
The final study population consisted in 37 patients with SARS-CoV-2 pneumonia and 28 healthy controls. The assays of blood coagulation were performed in 32 patients. Due to technical reasons, specific tests could not be performed in some cases, as indicated in figure legends and tables. Some experiments that were intended as confirmatory of the hypothesis and were performed in a limited number of cases.
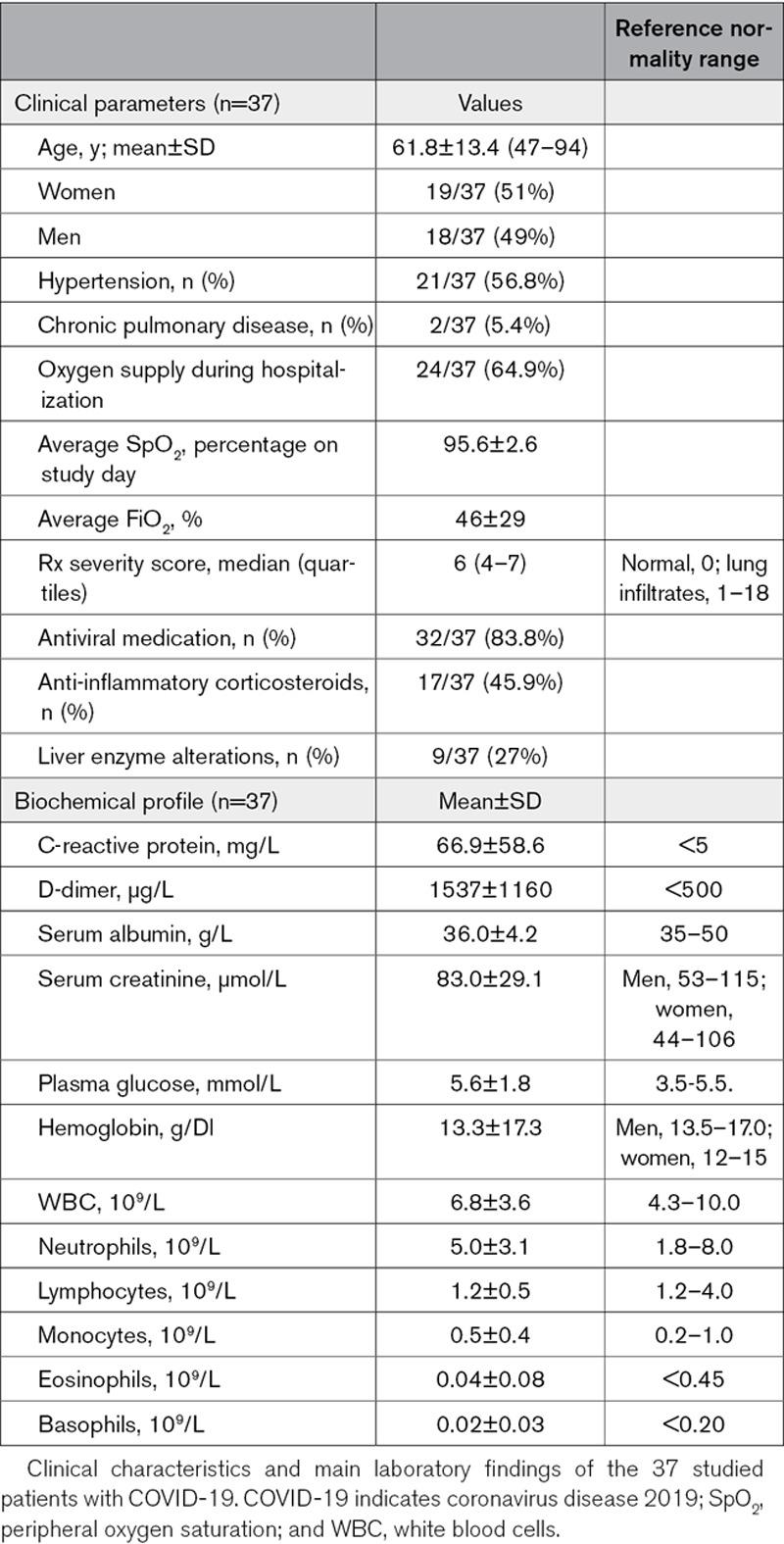
The clinical characteristics of COVID-19 patients are shown in Table 1. Their medical history was ordinary apart from hypertension (54.8%). According to the strict inclusion criteria, none of the patients had diabetes, renal failure, cardiovascular disease, and heart failure. Two patients were smokers in the past; none of them was an active smoker. More than half of the patients required oxygen supplementation during hospitalization. Peripheral oxygen saturation was normal at the time of blood sampling, whether or not oxygen was administered. Rx severity score ranged between 1 and 16 in the studied patients (Table 1). d-dimer was elevated ranging between 427 and 2144 µg/L (Table 1). According to the study protocol and clinical practice, if patients received prophylactic doses of enoxaparin (4000 units/day), blood sampling was performed immediately before dosing (24 hours after previous dose was administered) to avoid any residual drug activity. This was testified by electronic recoding of drug administration and by undetectable heparin in plasma (anti factor X assay was randomly performed in 5 plasma samples from studied patients).
Table 1.
Clinical characteristics and biochemical profile of COVID-19 patients
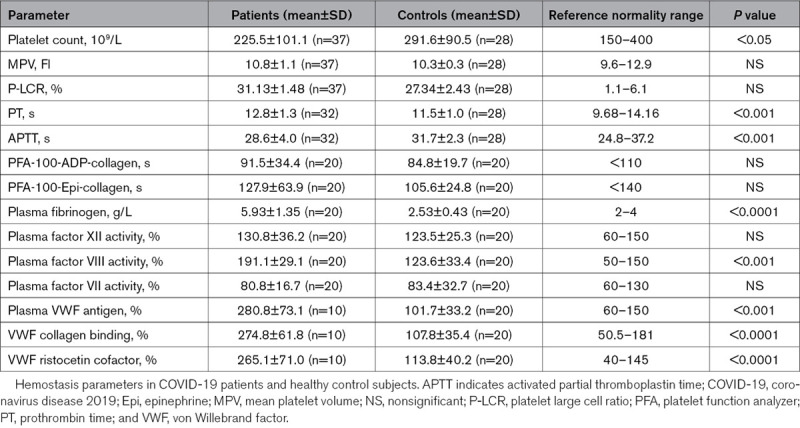
Platelet Number, Dimension, and Hemostasis Tests
Platelet count was lower in COVID-19 patients compared with controls. Thrombocytopenia (platelet count below 150×109/L) was observed in 6 patients (3 mild and 3 moderate, ie, <150 000/mmc and <100 000/mmc, respectively). In routine hemostasis tests, APTT was significantly shorter, whereas PT was significantly increased in patients compared with healthy controls (Table 2). Coagulation factors involved in the crucial steps of the coagulation cascade were determined. Factor VIII and fibrinogen activity, as well as VWF antigen, CB, and ristocetin cofactor, were significantly higher in the plasma of COVID-19 patients than in controls. The results of platelet aggregation tests in whole blood were similar in patients and controls (Table 2).
Table 2.
Hemostasis parameters
Platelet Morphology on Blood Smear
On microscopic examination, platelet anisopoichilocytosis was observed, with discoid or star-shaped elements (dormant platelets) and giant platelets with pseudopods (dendritic activated platelets; Figure 2A through 2D). Additionally, the blood smear unveiled the presence of neutrophilic granulocytes and monocytes with attached platelets (platelet satellitism; Figure 2E through 2H) and apparent platelet engulfment by atypical lymphocytes of reactive appearance and by large granulated lymphocytes (Figure 2E and 2F).
Figure 2.
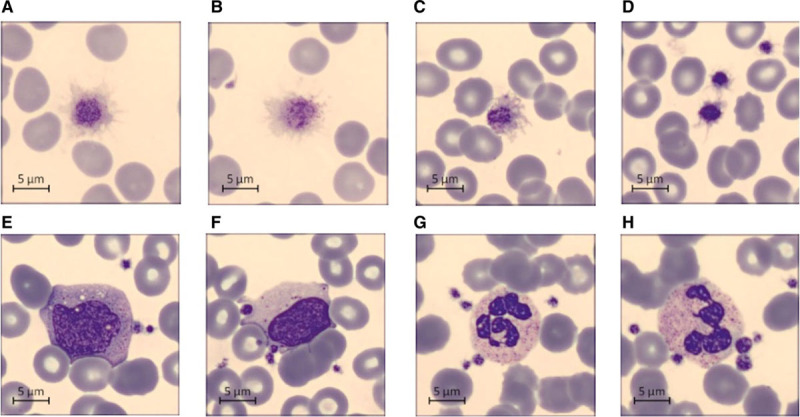
Representative blood smear from patients with severe acute respiratory syndrome coronavirus 2 pneumonia showing platelet anisopoichilocytosis (A–D), platelet satellitism (E–H), and platelet engulfment by lymphocytes (F).
Platelet-Leukocyte Aggregates
We quantified the observed platelet satellitism by flow cytometry. The analysis of monocyte-platelet and neutrophil-platelet aggregates demonstrated a significant increase in both aggregates among COVID-19 patients in respect to healthy controls (Figure 3A and 3B), represented as the percentage of double positivity of total recorded events with single positivity for platelet αIIb integrin (Figure I in the Data Supplement). The difference between COVID-19 and healthy subjects in monocyte-platelet aggregates was +48.4% (95% CI, +37.9 to +59.4) and +25.6% (95% CI, +17.6 to +33.6) in neutrophil-platelet aggregates.
Figure 3.
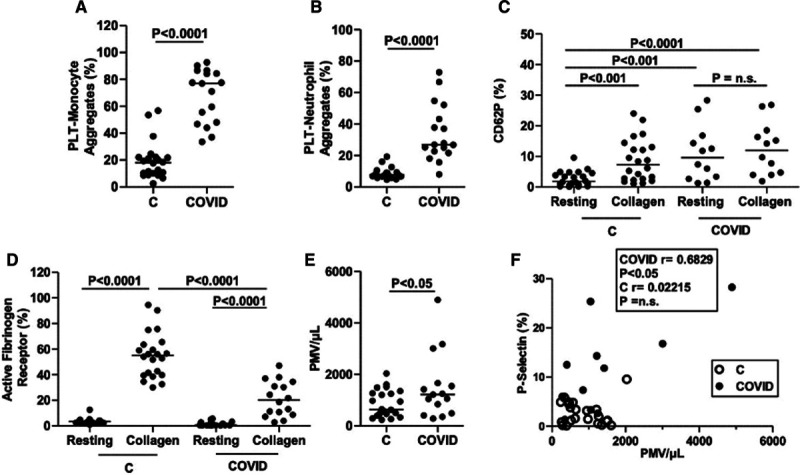
Platelet phenotype. Whole-blood analysis of monocytes and neutrophil-platelet aggregates shows higher percentage of plateletmonocyte aggregates (A) and platelet-neutrophil aggregates (B) in citrated whole blood from coronavirus disease 2019 (COVID-19) patients (n=17) than healthy controls (n=22). The percentage of resting platelets expressing P-selectin in COVID-19 patients (n=12) is similar to that observed in platelets from healthy controls (n=22) stimulated with collagen (C). P-selectin expression does not further increase when platelets are stimulated with collagen (C). The expression of the active form of fibrinogen receptor αIIbβ3, as detected by the monoclonal antibody PAC-1, is similar under resting conditions in patients and healthy controls and lower in patients (n=16) in platelets stimulated with collagen (D). The number of platelet-derived microvesicles (PMV) is slightly higher in patients (n=15) than in controls (n=22; E) and correlates with the surface expression of P-selectin in COVID-19 patients (F). CD62P (P-selectin) indicates cluster of differentiation 62P; and PTL, platelets.
Platelet Phenotype and In Vitro Platelet Activation
We observed significant differences in the expression of P-selectin (CD62P)—a marker of α-granule secretion—in COVID-19 resting platelets compared with healthy controls (+8.2% [95% CI, +4.2 to +8.4]; Figure 3C). No further increase was observed in the P-selectin surface expression of patients when platelets were stimulated with 10 µg/mL collagen, while P-selectin expression after collagen stimulation increased ≈4× in healthy controls (+6.0% [95% CI, +3.5 to +8.4]; Figure 3C).
The expression of the active fibrinogen receptor αIIbβ3 was <4 % in resting platelets both from healthy controls and COVID-19 patients (Figure 3D). The increase in the expression of active form (PAC-1 binding) of the fibrinogen receptor following stimulation with 10 µg/mL collagen was found to be lower in platelets from COVID-19 patients compared with healthy controls (−33.7% [95% CI, 23.0%–44.4%]; Figure 3D).
Platelet-derived microvesicles in peripheral blood were assessed by analyzing CD41−(αIIb) positive events in the size range of 100 to 1000 nm (Figure 3E). The number of platelet-derived microvesicles was slightly higher in patients than in controls (+648.2 events [95% CI, +35.9 to +1261]), and the number of circulating microvescicles positively correlated with the surface expression of P-selectin in resting platelets collected from COVID-19 patients (Figure 3F).
Platelet Content in Cytokines, Chemokines, and Growth Factors
We examined the content of platelet granules by assessing the amount of cytokines, chemokines, and growth factors releasable in ex vivo–stimulated, washed platelets, from which leukocytes had been carefully removed (undetectable by flow cytometry and cell count). We found statistically significant increases in the amount of cytokines (IL-1α, IL-1β, IL-1RA, IL-4, IL-10, IL-13, IL, 17, IL-27, IFN [interferon]-α, and IFN-γ), chemokines (MCP-1/CCL2), and growth factors (VEGF [vascular endothelial growth factor]-A/D) that were expressed in patients compared with controls in plasma and in platelet releasate. Tables 3 and 4 show the comparison of data referring to pair-matched number of platelets as indicated in Methods. RANTES (regulated on activation, normal T-cell expressed and secreted) and PDGF-BB (platelet-derived growth factor-BB) were highly expressed in platelets of both patients and controls. The protein profile in the plasma of the same subjects was different, and some cytokines were found only in platelet releasate. Moreover, some cytokines not detectable in plasma were contained in platelets of COVID-19 platelets but not healthy controls, namely IL-5, IL-13, IL-22, and IL-31. Tables 3 and 4 show the whole panel of assayed cytokines, chemokines, and growth factors.
Analysis of Procoagulant Platelets and Relations With Coagulation Tests
We explored the intrinsic and extrinsic pathways of blood coagulation to assess the procoagulant activity of platelets from COVD-19 patients. Using PRP instead of plasma, we could evaluate the contribution of platelets functionally apt to contribute effectively to coagulation cascade (Figure 4A). We found that in 23 out of 32 COVID-19 patients, a shortened APTT (below the lower interquartile of controls), either when plasma or PRP were used (Figure 4A).
Figure 4.
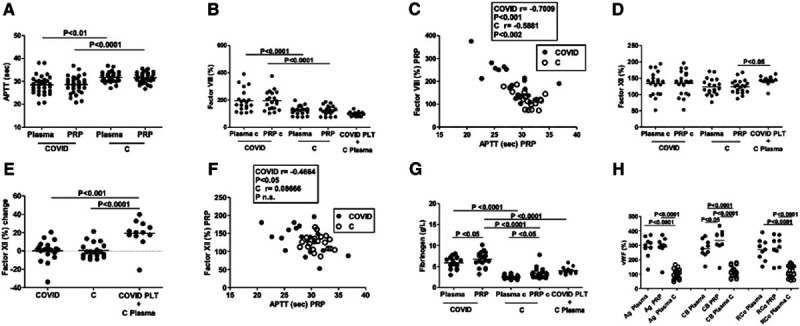
Coagulation and coagulation factors assays. Activated partial thromboplastin time (APTT) was tested using plasma and platelet-rich plasma (PRP) from coronavirus disease 2019 (COVID-19) patients (n=32) and healthy controls (n=28; A). The activity of the coagulation factor VIII is similarly higher in plasma and PRP in COVID-19 patients, correlates with APTT (B and C), and is not stored in platelets, as demonstrated by the effects of platelets from patients added to control plasma (B). Factor XII activity does not differ in patients (n=20) and controls (n=20; D) but correlates with APTT only in patients (F) and increases when platelets from patients were suspended in control plasma (n=12; D and E). Plasma VWF (von Willebrand factor) antigen (Ag), collagen binding (CB), and ristocetin cofactor (RCo) is increased in COVID-19 patients (n=9) compared with controls (n=20; G). Fibrinogen activity is higher in plasma and PRP from patients (n=20) than controls (n=20; H). PTL indicates platelets.
To define which factors contributed to the accelerated coagulation through the intrinsic pathways, we measured factor XII, factor VIII, and factor VII using plasma and PRP in a group of 20 patients and 18 controls. We also measured fibrinogen, VWF antigen, CB, and ristocetin cofactor both in plasma and PRP. Factor VIII activity was similarly higher in plasma (+72.3% [95% CI, +32.4 to +112.2]) and PRP (+71.2% [95% CI, +35.7 to +106.6]) from patients than in controls (Figure 4B). A highly significant negative correlation was observed between factor VIII and APTT in all the conditions both in patients and controls (Figure 4C; Figure IIIA in the Data Supplement). No statistically significant differences were observed in factor XII activity between patients and controls when either PRP or plasma was tested (Figure 4D). We further analyzed the contribution of platelet to the activity of coagulation factors in COVID-19 patients suspending washed platelets in control plasma. While no difference was observed as for factor VIII (Figure 4B), increase in factor XII activity was observed when washed platelets from patients were suspended in control plasma (+18.3% [95% CI, +4.3% to +32.3%]) versus PRP of controls (Figure 4D and 4E). A statistically significant correlation between factor XII and APTT was found either testing PRP or plasma from COVID-19 patients but not from healthy controls (Figure 4F; Figure IIIB in the Data Supplement).
Highly significant difference in VWF antigen, CB, and ristocetin cofactor was observed between patients and controls, as well as between plasma and PRP concerning CB in the group of patients (+56.1% [95% CI, +31.8 to 80.3]; Figure 4G). Similarly, fibrinogen activity was higher when PRP or washed platelets from patients were tested, as compared with plasma (Figure 3H). Fibrinogen activity increased in PRP of healthy subjects compared with plasma. Fibrinogen and VWF measured in PRP and plasma of patients and controls were unrelated to APTT (Figure IIIC and IIID in the Data Supplement).
We also found that PT was prolonged in patients compared with healthy subjects (plasma, +1.2 s [95% CI, +0.6 to +1.8]; PRP, +1.2 s [95% CI, +0.7 to +0.8]; Figure IVA in the Data Supplement). No differences were observed between patients and controls as for factor VII activity, either when plasma, PRP, or mixed COVID-19 platelets and control plasma were assayed (Figure IVB in the Data Supplement). Factor VII correlated with PT in all tested conditions (Figure IVC and IVD in the Data Supplement).
Relations Between Coagulation Abnormalities and Severity of SARS-CoV-2 Pneumonia
We found that the main clinical readout of pneumonia severity, that is, the requirement of oxygen supplementation for respiratory failure, grouped patients who had altered coagulation test (Figure VA in the Data Supplement) with a significantly shorter APTT in patients requiring oxygen (−4.0% [95% CI, −5.9 to −2.2]). Also the radiograph severity score of pneumonia allows to dissect patients with mild pneumonia, who have normal APTT from patients with moderate-to-severe pneumonia (with score 4–16 and requiring oxygen supplementation), who have frequently accelerated APTT (−3.7 s [95% CI, −5.5 to −1.8]; Figure VB in the Data Supplement).
Discussion
The clinical finding of high prevalence of pulmonary thrombosis in COVID-19 patients,8,21 recently confirmed by autopsy identification of microvascular thrombosis in the areas of inflamed lungs,5 prompted us to explore the contribution of platelets to this process. We considered the hypothesis that platelets could have a central role in the process of thromboinflammation in COVID-19, advanced on the basis of experimental models of septic and aseptic inflammation, as well as in human viral pneumonia.18,26
To this aim, we carefully selected patients with confirmed COVID-19 pneumonia without other conditions that might predispose to thromboembolism, to obtain an unbiased evaluation of their platelet profile. Coagulation tests are altered, with slight reduction in APTT, associated with slightly prolonged PT. This finding is in agreement with the clinical characteristics of our patients, who had mild-to-moderate SARS-CoV-2 pneumonia. Consistently with previous observations, shortened APTT has been previously observed in patients who survived to SARS-CoV-2 pneumonia, while prolonged APTT was typically observed in those who did not survive, indicating sepsis-induced coagulopathy.9,27 Platelet count in patients is lower with normal dimensional distribution in comparison to healthy subjects (Table 2), although potentially masked by accelerated turnover. The results of platelet function tests with PFA-100 were normal, indicating normality in thrombus formation induced by platelet aggregation under high shear stress, suggesting that no major alterations in the platelet-dependent hemostatic process occur in these patients, at least as assessed using an instrument designed to investigate platelet functional defects. However, single or small aggregates of platelets adherent to monocytes and neutrophils are frequently observed on blood smear, along with large platelets presenting multiple pseudopods. Microscopy revealed similarly activated platelets in influenza virus infection.28
Quantitative analysis using flow cytometry showed high percentage of platelet-monocyte and platelet-granulocyte conjugates in peripheral blood. This is a critical step in cross-activation allowing the amplifications of platelet activation, with changes in their functionality and leukocyte recruitment.22 Our findings extend to moderate disease the evidence that platelet-neutrophil aggregates are increased in patients with severe COVID-19 pneumonia.17 The P-selectin and integrin αIIb/β3 were shown to play major roles in platelet-monocyte interaction and platelet-mediated reprogramming of monocyte responses in patients with severe COVID-1913. We previously demonstrated that monocytes and neutrophils from COVID-19 patients have a constitutive active STAT3 (signal transducer and activator of transcription 3) signaling pathway (pSTATY705), which contribute to the increased expression of several proinflammatory cytokines, including IL-6, IL-8, and TNF-α (tumor necrosis factor-alpha). In this scenario, we can envision a situation in which the interaction between αIIb/β3 on the platelets and other integrins present on the surface of inflammatory monocytes promote or sustain the expression of activated pSTAT3 inside the monocytes, resulting in IL-6 release, that in turn can act by sustaining the inflammatory process.29
Similarly, increased numbers of platelet-leukocyte conjugates have been observed in peripheral blood in influenza and dengue virus infection.28,30
Our finding that P-selectin is constitutively expressed in COVID-19 patients to a magnitude similar to that observed in control subjects, only after stimulation with a strong platelet agonist, indicates that α-granule secretion has occurred in vivo and that P-selectin is abundantly available for interaction with PSGL-1 (P-selectin glycoprotein ligand-1) present on leukocyte cell membrane. Additional mechanisms could be involved in platelet-leukocyte adhesion.31 Neutrophils recruited at the site of inflammation determine lung pathology through the release of extracellular traps (neutrophil extracellular traps)32 and extracellular histones lead to platelet activation and pulmonary microvascular thrombosis, as observed in several experimental models including influenza pneumonia and in COVID-19 human pneumonia.17,33,34
In addition, there is a well-established modulation of monocyte cytokine responses by activated platelets through P-selectin binding.35
We focused on the content and potential release of cytokines, chemokines, and growth factors from activated platelets. Thrombin-induced degranulation of washed platelets reveals their potential role in the local and systemic inflammation and immune modulation. In fact, comparing plasma and platelet releasate from patients and controls, a specific contribution of platelet to inflammation and host defence can be defined. Interestingly, a number of cytokines are related to the skewing of TH2 and TH17 lymphocytes (ie, IL-13, IL-31, IL-17A, and IL-21). The cytokine profile also highlights a contribution to tissue remodeling through angiogenesis and fibrogenesis. Some cytokines and chemokines are released from platelets but are not found in plasma, indicating that platelets may represent a reservoir for local delivery.
Whether releasable cytokines, chemokines, and growth factors are taken up by circulating platelets or synthesized by megakaryocytes and platelets20,36 has to be defined by further investigation. The finding that platelets release proteins that are stored in the α-granules upon in vitro stimulation, while P-selectin is already expressed on platelets’ surface in COVID-19 patients, further supports previous evidence about compartmentation in platelet granules and selectivity for platelet response to stimuli.33 Showing that circulating platelets are only partly degranulated, we can infer that the variety of high and low molecular weight compounds that are stored in platelet granules become strong contributors to the amplification of inflammation and platelet-centered thrombosis at the site of platelet adhesion and activation.16,26 Concerning the Sars-CoV-2 infection, an inappropriate immune response to the infection, which is reflected systemically by changes in plasma levels of cytokines and chemokines, like IL (interleukin)-1β, IL-2, IL-17, IFN-γ, IL-6, IL-10, TNF-α, and VEGF, has been described.37,38 While the increment of some cytokine levels is proportional to the disease severity, for example, IL-10 and IL-6, for others, like IL-1β, the levels generally rise particularly during the severe stage contributing to hypercoagulability and disseminated intravascular coagulation.39 In the present study, we describe the increment of molecules, like IL-10, IL-6, and MCP-1, but not IL-1β and TNF-α and IFN-γ in the serum of our COVID-19 cohort, which reflects the mild disease severity of the patients.
Our results indicate that platelets with procoagulant phenotype are generated in COVID-19 patients, either in the peripheral circulation or from inflammation-programmed megakaryocytes.36,40 Given the limitations due to safety protocols, requiring inactivation of the SARS-CoV-2 virus in biological samples, we could not obtain a more extensive characterization of procoagulant profile by measuring binding of annexin V to platelets expressing phosphatidylserine.19 Previous studies indicate that P-selectin expression can be enhanced in vitro by strong agonists and through the engagement of integrins, and this is associated to a procoagulant phenotype and the release of microvesicles.19
Consistent with the evidence that fibrinogen is stored in α-granule subpopulations and secreted differently from P-selectin,40 platelets from COVID-19 patients contribute to the amount of bioactive fibrinogen and VWF. Platelets may carry on their surface the secreted fibrinogen and be covered with fibrin.20 Similarly to human influenza and streptococcus pneumonia,30 we also found that platelets from patients with SARS-CoV-2 pneumonia show a blunted αIIββ3 activation in response to strong platelet agonists.17 This could be related to a secondary inactivation of the receptor by either fibrinogen or fibrin binding and negative feedback mechanisms.41,42
We sought to demonstrate that platelets promote coagulation exploring separately the intrinsic and the extrinsic pathways of coagulation. This approach has the potential to identify the contribution of specific coagulation factors and platelets.43 Accelerated APTT in COVID-19 patients is closely related to the amount of bioactive factor VIII and factor XII. Factor VIII, which is abundant in plasma of COVID-19 patients, is not stored in platelets. Factor XII, which is also higher in washed platelets, PRP, and plasma from patients who have the shortest APTT, has been identified as a major determinant of the thrombus formation but is not critically involved in hemostasis.44,45
In the setting of inflammation, factor XII is activated by several factors including collagen, DNA, and histones.18 Activated platelets expressing polyphosphates, which are stored in dense granules under resting conditions, activate circulating factor XII.46–48 This is consistent with our observation that factor XII activity does not correlate with APTT when either plasma or PRP of healthy controls were tested. While our results concerning coagulation factors are consistent with our hypothesis that fits with the general model that is being defining in thromboinflammation, we obtained confirmation that further research is required to identify sensitive functional assays for the assessment of the procoagulant activity of platelets.
Platelets may contribute to a prothrombotic phenotype and inflammation in COVID-19 patients also by releasing microvesicles, as suggested by the slight increase in the number of circulating platelet-derived microvesicles and their potential bioactivity.22
We found that PT is prolonged in COVID-19 patients. Given the close correlation with PT, one could argue that reduced bioavailability of factor VII is causally involved. Nonetheless, inhibitory activities are also plausible.6,15 This finding further supports the hypothesis that the tissue factor–factor VII pathway has a minor role in the prothrombotic condition associated with COVID-19.
We hypothesize that platelet priming occurs in the lung where platelet interaction in the inflammatory environment and platelet generation from resident megakaryocytes take place.49 Megakaryocytes are a rich source of cytokines and growth factors that could potentially influence inflammatory/fibrotic lung diseases, as revealed by RNA analysis showing skewing toward a function in the innate immunity.49 Numerous megakaryocytes were found in the inflamed areas of the lung in patients with COVID-19.6 Circulating platelets may, therefore, reflect parent megakaryocytes in their phenotype and function as platform allowing the effective generation of fibrin, favored by increased release of coagulation factors from endothelium and liver. Platelets interact with activated or injured endothelium and are guided by conjugated leukocyte to the site of inflammation and jointly contribute to this process.50,51 This could be considered part of the host defence in response to infection by a variety of different viruses, including HIV, coxsackie B3 virus, dengue virus, and ebola virus,52 leading to thrombus formation within the lung vasculature but also extending to the systemic circulation.
The present investigation was not designed as a case-control study; we studied healthy subjects to obtain reference values for the assays exploring the contribution of platelets to coagulation and coagulation factors, as well the investigation on the proinflammatory activity of platelets. The finding of shortened APTT recapitulates the platelet abnormalities and relates to clinical characteristic of the patients. In fact, in almost all the patients without severe respiratory failure, that is, not requiring O2 supplementation because SO2 was above 92%, or having a low radiological score, platelet-conditioned APTT was similar to that observed in healthy controls. Further investigation on the contribution of age and comorbidities to the procoagulant and proinflammatory activities of platelets is warranted.
In the present investigation, we did not explore the mechanism generating a specific platelet profile. We propose a general model derived from the analysis of circulating platelets, in which leukocyte interaction and proinflammatory, prothrombotic activities of inflammation-programmed platelets are central, closely resembling the events previously described in human and experimental viral pneumonia, with similarities with atherothrombosis.20,45 Translating the present information to the pathophysiology and the clinical setting of SARS-CoV-2 pneumonia, we can infer that microvascular thrombosis may extend upstream to larger arteries and downstream to pulmonary veins in the severely inflamed tissues. This is exemplified by the images of angiographic CT performed in a patient with COVID-19 pneumonia with severe lung failure, showing filling defects representing the local generation of the thrombi (Figure 1).
The potential role of platelets in thromboinflammation raises questions on the optimal target for pharmacological intervention.18 Preventing cytokine activity has been advocated as an adjunctive therapy in SARS-CoV-2 pneumonia. Targeting GP (glycoprotein) Ib, P-selectin, PAR-1, and αIIbβ3, or the immune-like receptors GP VI and CLEC-2 (C-type lectin receptor 2), prevents thrombosis and inflammation, although this may increase the risk of hemorrhage.18 Recent evidence referring to A/N1H1 influenza pneumonia indicates that the P2Y12 inhibitor clopidogrel in association with antiviral agents improves survival to lethal amounts of influenza virus in mouse models31; furthermore, ticagrelor improves ventilation indices and reduces inflammation in humans.53 This may apply to SARS-CoV-2 pneumonia and may diminish lung inflammation and the prothrombotic and proinflammatory activities in additional target organs. Activation of coagulation cascade might be targeted by inhibitors of the Factor XII pathway.54 Properly designed experimental and clinical trials are warranted to test the hypothesis.
Sources of Funding
This study was supported by Fondazione Cariverona (V. Bronte and E. Tacconelli), Fondazione TIM (V. Bronte), and MIUR (Ministero Istruzione Università e Ricerca; P. Minuz).
Disclosures
None.
Supplementary Material
Footnotes
Nonstandard Abbreviations and Acronyms
- APTT
- activated partial thromboplastin time
- CB
- collagen binding
- CLEC-2
- C-type lectin receptor 2
- COVID-19
- coronavirus disease 2019
- CT
- computed tomography
- GP
- glycoprotein
- IFN
- interferon
- IL
- interleukin
- PMV
- platelet-derived microvesicle
- PRP
- platelet-rich plasma
- PSGL-1
- P-selectin glycoprotein ligand-1
- PT
- prothrombin time
- SARS-CoV-2
- severe acute respiratory syndrome coronavirus 2
- TNF-α
- tumor necrosis factor-alpha
- VEGF
- vascular endothelial growth factor
- VWF
- von Willebrand factor
These authors contributed equally to this article.
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/ATVBAHA.120.315175.
For Sources of Funding and Disclosures, see page 2987.
Contributor Information
Francesco Taus, Email: francesco.dima@aovr.veneto.it.
Gianluca Salvagno, Email: gianluca.salvagno@univr.it.
Stefania Canè, Email: stefania.cane@univr.it.
Cristiano Fava, Email: cristiano.fava@univr.it.
Fulvia Mazzaferri, Email: fulvia.mazzaferri@univr.it.
Elena Carrara, Email: elena.carrara@univr.it.
Varvara Petrova, Email: varvara.petrova@univr.it.
Roza Maria Barouni, Email: rozamaria.barouni@univr.it.
Francesco Dima, Email: francesco.dima@aovr.veneto.it.
Andrea Dalbeni, Email: andrea.dalbeni@univr.it.
Simone Romano, Email: simone.denitto@libero.it.
Giovanni Poli, Email: giovanni.poli@aovr.veneto.it.
Marco Benati, Email: marco.benati@univr.it.
Simone De Nitto, Email: simone.denitto@libero.it.
Giancarlo Mansueto, Email: giancarlo.mansueto@univr.it.
Manuela Iezzi, Email: m.iezzi@unich.it.
Evelina Tacconelli, Email: evelina.tacconelli@univr.it.
Giuseppe Lippi, Email: giuseppe.lippi@univr.it.
Vincenzo Bronte, Email: vincenzo.bronte@univr.it.
References
- 1.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatraju PK, Ghassemieh BJ, Nichols M, Kim R, Jerome KR, Nalla AK, Greninger AL, Pipavath S, Wurfel MM, Evans L, et al. COVID-19 in critically ill patients in the Seattle Region — case series. N Engl J Med. 2020;382:2012–2022. doi: 10.1056/nejmoa2004500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wichmann D, Sperhake J-P, Lütgehetmann M, Steurer S, Edler C, Heinemann A, Heinrich F, Mushumba H, Kniep I, Schröder AS, et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med. 2020;173:268–277. doi: 10.7326/M20-2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cattaneo M, Bertinato EM, Birocchi S, Brizio C, Malavolta D, Manzoni M, Muscarella G, Orlandi M. Pulmonary embolism or pulmonary thrombosis in COVID-19? Is the recommendation to use high-dose heparin for thromboprophylaxis justified? Thromb Haemost. 2020;120:1230–1232. doi: 10.1055/s-0040-1712097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ackermann M, Verleden S, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nunes Duarte-Neto A, de Almeida Monteiro RA, da Silva LFF, Malheiros DMAC, de Oliveira EP, Theodoro Filho J, Pinho JRR, Soares Gomes-Gouvêa M, Salles APM, de Oliveira IRS, et al. Pulmonary and systemic involvement of COVID-19 assessed by ultrasound-guided minimally invasive autopsy [published online May 22, 2020]. Histopathology. doi: 10.1111/his.14160 [Google Scholar]
- 7.Thachil J, Srivastava A. SARS-2 coronavirus-associated hemostatic lung abnormality in COVID-19: is it pulmonary thrombosis or pulmonary embolism? [published online May 12, 2020] Semin Thromb Hemost. doi: 10.1055/s-0040-1712155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bompard F, Monnier H, Saab I, Tordjman M, Abdoul H, Fournier L, Sanchez O, Lorut C, Chassagnon G, Revel M. Pulmonary embolism in patients with COVID-19 pneumonia. Eur Respir J. 2020;18:1233–1234. doi: 10.1017/CBO9781107415324.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–2040. doi: 10.1182/blood.2020006000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;365:497–506. doi: 10.1016/S0140-6736:30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pavoni V, Gianesello L, Pazzi M, Stera C, Meconi T, Frigieri FC. Evaluation of coagulation function by rotation thromboelastometry in critically ill patients with severe COVID-19 pneumonia. J Thromb Thrombolysis. 2020;50:281–286. doi: 10.1007/s11239-020-02130-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang T, Chen R, Liu C, Liang W, Guan W, Tang R, Tang C, Zhang N, Zhong N, Li S. Attention should be paid to venous thromboembolism prophylaxis in the management of COVID-19. Lancet Haematol. 2020;7:e362–e363. doi: 10.1016/S2352-3026(20)30109-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hottz ED, Azevedo-Quintanilha IG, Palhinha L, Teixeira L, Barreto EA, Pão CRR, Righy C, Franco S, Souza TML, Kurtz P, et al. Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe COVID-19. Blood. 2020;136:1330–1341. doi: 10.1182/blood.2020007252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, Wang T, Zhang X, Chen H, Yu H, et al. Clinical and immunologic features in severe and moderate Coronavirus Disease 2019. J Clin Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaertner F, Massberg S. Patrolling the vascular borders: platelets in immunity to infection and cancer. Nat Rev Immunol. 2019;19:747–760. doi: 10.1038/s41577-019-0202-z [DOI] [PubMed] [Google Scholar]
- 17.Nicolai L, Leunig A, Brambs S, Kaiser R, Weinberger T, Weigand M, Muenchhoff M, Hellmuth JC, Ledderose S, Schulz H, et al. Immunothrombotic dysregulation in COVID-19 pneumonia is associated with respiratory failure and coagulopathy. Circulation. 2020;142:1176–1189. doi: 10.1161/CIRCULATIONAHA.120.048488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackson SP, Darbousset R, Schoenwaelder SM. Thromboinflammation: challenges of therapeutically targeting coagulation and other host defense mechanisms. Blood. 2019;133:906–918. doi: 10.1182/blood-2018-11-882993 [DOI] [PubMed] [Google Scholar]
- 19.Agbani EO, Poole AW. Procoagulant platelets: generation, function, and therapeutic targeting in thrombosis. Blood. 2017;130:2171–2179. doi: 10.1182/blood-2017-05-787259 [DOI] [PubMed] [Google Scholar]
- 20.van der Meijden PEJ, Heemskerk JWM. Platelet biology and functions: new concepts and clinical perspectives. Nat Rev Cardiol. 2019;16:166–179. doi: 10.1038/s41569-018-0110-0 [DOI] [PubMed] [Google Scholar]
- 21.Minuz P, Mansueto G, Mazzaferri F, Fava C, Dalbeni A, Ambrosetti MC, Sibani M, Tacconelli E. High rate of pulmonary thromboembolism in patients with SARS-CoV-2 pneumonia [published online June 18, 2020]. Clin Microbiol Infect. doi: 10.1016/j.cmi.2020.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borghesi A, Maroldi R. COVID-19 outbreak in Italy: experimental chest X-ray scoring system for quantifying and monitoring disease progression. Radiol Med. 2020;125:509–513. doi: 10.1007/s11547-020-01200-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buoro S, Moioli V, Seghezzi M, Previtali G, Alessio MG, Simon Lopez R, Ortolani C, Ottomano C, Lippi G. Evaluation and comparison of automated hematology analyzer, flow cytometry, and digital morphology analyzer for monocyte counting. Int J Lab Hematol. 2018;40:577–585. doi: 10.1111/ijlh.12868 [DOI] [PubMed] [Google Scholar]
- 24.Furman MI, Barnard MR, Krueger LA, Fox ML, Shilale EA, Lessard DM, Marchese P, Frelinger AL, Goldberg RJ, Michelson AD. Circulating monocyte-platelet aggregates are an early marker of acute myocardial infarction. J Am Coll Cardiol. 2001;105:2130–2132. doi: 10.1016/S0735-1097(01)01485-1 [DOI] [PubMed] [Google Scholar]
- 25.Giacomazzi A, Degan M, Calabria S, Meneguzzi A, Minuz P. Antiplatelet agents inhibit the generation of platelet-derived microparticles. Front Pharmacol. 2016;7:314 doi: 10.3389/fphar.2016.00314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mezger M, Nording H, Sauter R, Graf T, Heim C, von Bubnoff N, Ensminger SM, Langer HF. Platelets and immune responses during thromboinflammation. Front Immunol. 2019;10:1731 doi: 10.3389/fimmu.2019.01731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koupenova M, Corkrey HA, Vitseva O, Manni G, Pang CJ, Clancy L, Yao C, Rade J, Levy D, Wang JP, et al. The role of platelets in mediating a response to human influenza infection. Nat Commun. 2019;10:1780 doi: 10.1038/s41467-019-09607-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bronte V, Ugel S, Tinazzi E, Vella A, De Sanctis F, Canè S, Batani V, Trovato R, Fiore A, Petrova V, et al. Baricitinib restrains the immune dysregulation in severe COVID-19 patients [published online August 18, 2020]. J Clin Invest. doi: 10.1172/jci141772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rondina MT, Brewster BA, Grissom CK, Zimmerman GA, Kastendieck DH, Harris ES, Weyrich AS. In vivo platelet activation in critically ill patients with primary 2009 influenza A(H1N1). Chest. 2012;141:1490–1495. doi: 10.1378/chest.11-2860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pulavendran S, Rudd JM, Maram P, Thomas PG, Akhilesh R, Malayer JR, Chow VTK, Teluguakula N. Combination therapy targeting platelet activation and virus replication protects mice against lethal influenza pneumonia. Am J Respir Cell Mol Biol. 2019;61:689–701. doi: 10.1165/rcmb.2018-0196OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Middleton EA, He X-Y, Denorme F, Campbell RA, Ng D, Salvatore SP, Mostyka M, Baxter-Stoltzfus A, Borczuk AC, Loda M, et al. Neutrophil extracellular traps (NETs) contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136:1169–1179. doi: 10.1182/blood.2020007008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barnes BJ, Adrover JM, Baxter-Stoltzfus A, Borczuk A, Cools-Lartigue J, Crawford JM, Daßler-Plenker J, Guerci P, Huynh C, Knight JS, et al. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J Exp Med. 2020;217:e20200652 doi: 10.1084/jem.20200652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sung PS, Hsieh SL. CLEC2 and CLEC5A: pathogenic host factors in acute viral infections. Front Immunol. 2019;10:2867 doi: 10.3389/fimmu.2019.02867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zellweger RM, Eddy WE, Tang WW, Miller R, Shresta S. CD8 + T cells prevent antigen-induced antibody-dependent enhancement of dengue disease in mice. J Immunol. 2014;193:4117–4124. doi: 10.4049/jimmunol.1401597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cunin P, Nigrovic PA. Megakaryocytes as immune cells. J Leukoc Biol. 2019;105:1111–1121. doi: 10.1002/JLB.MR0718-261RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu K, Fang YY, Deng Y, Liu W, Wang MF, Ma JP, Xiao W, Wang YN, Zhong MH, Li CH, et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl). 2020;133:1025–1031. doi: 10.1097/CM9.0000000000000744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang W, Zhao Y, Zhang F, Wang Q, Li T, Liu Z, Wang J, Qin Y, Zhang X, Yan X, et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the experience of clinical immunologists from China. Clin Immunol. 2020;214:108393 doi: 10.1016/j.clim.2020.108393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Battinelli EM, Thon JN, Okazaki R, Peters CG, Vijey P, Wilkie AR, Noetzli LJ, Flaumenhaft R, Italiano JE. Megakaryocytes package contents into separate a-granules that are differentially distributed in platelets. Blood Adv. 2019;3:3092–3098. doi: 10.1182/bloodadvances.2018020834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Podolnikova NP, Yakovlev S, Yakubenko VP, Wang X, Gorkun OV, Ugarova TP. The interaction of integrin αIIb β3 with fibrin occurs through multiple binding sites in the α IIb β-propeller domain. J Biol Chem. 2014;289:2371–2383. doi: 10.1074/jbc.M113.518126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mattheij NJ, Gilio K, van Kruchten R, Jobe SM, Wieschhaus AJ, Chishti AH, Collins P, Heemskerk JW, Cosemans JM. Dual mechanism of integrin αIIbβ3 closure in procoagulant platelets. J Biol Chem. 2013;288:13325–13336. doi: 10.1074/jbc.M112.428359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raghunathan V, Zilberman-Rudenko J, Olson SR, Lupu F, McCarty OJT, Shatzel JJ. The contact pathway and sepsis. Res Pract Thromb Haemost. 2019;3:331–339. doi: 10.1002/rth2.12217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Renné T, Pozgajová M, Grüner S, Schuh K, Pauer HU, Burfeind P, Gailani D, Nieswandt B. Defective thrombus formation in mice lacking coagulation factor XII. J Exp Med. 2005;202:271–281. doi: 10.1084/jem.20050664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nechipurenko DY, Receveur N, Yakimenko AO, Shepelyuk TO, Yakusheva AA, Kerimov RR, Obydennyy SI, Eckly A, Léon C, Gachet C, et al. Clot contraction drives the translocation of procoagulant platelets to thrombus surface. Arterioscler Thromb Vasc Biol. 2019;39:37–47. doi: 10.1161/ATVBAHA.118.311390 [DOI] [PubMed] [Google Scholar]
- 46.Ruiz FA, Lea CR, Oldfield E, Docampo R. Human platelet dense granules contain polyphosphate and are similar to acidocalcisomes of bacteria and unicellular eukaryotes. J Biol Chem. 2004;279:44250–44257. doi: 10.1074/jbc.M406261200 [DOI] [PubMed] [Google Scholar]
- 47.Verhoef JJ, Barendrecht AD, Nickel KF, Dijkxhoorn K, Kenne E, Labberton L, McCarty OJ, Schiffelers R, Heijnen HF, Hendrickx AP, et al. Polyphosphate nanoparticles on the platelet surface trigger contact system activation. Blood. 2017;129:1707–1717. doi: 10.1182/blood-s2016-08-734988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zilberman-Rudenko J, Reitsma SE, Puy C, Rigg RA, Smith SA, Tucker EI, Silasi R, Merkulova A, McCrae KR, Maas C, et al. Factor XII activation promotes platelet consumption in the presence of bacterial-type long-chain polyphosphate in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2018;38:1748–1760. doi: 10.1161/ATVBAHA.118.311193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lefrançais E, Ortiz-Muñoz G, Caudrillier A, Mallavia B, Liu F, Sayah DM, Thornton EE, Headley MB, David T, Coughlin SR, et al. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature. 2017;544:105–109. doi: 10.1038/nature21706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coenen DM, Mastenbroek TG, Cosemans JMEM. Platelet interaction with activated endothelium: mechanistic insights from microfluidics. Blood. 2017;130:2819–2828. doi: 10.1182/blood-2017-04-780825 [DOI] [PubMed] [Google Scholar]
- 51.Gros A, Syvannarath V, Lamrani L, Ollivier V, Loyau S, Goerge T, Nieswandt B, Jandrot-Perrus M, Ho-Tin-Noé B. Single platelets seal neutrophil-induced vascular breaches via GPVI during immune-complex-mediated inflammation in mice. Blood. 2015;126:1017–1026. doi: 10.1182/blood-2014-12-617159 [DOI] [PubMed] [Google Scholar]
- 52.Antoniak S, Owens AP, III, Baunacke M, Williams JC, Lee RD, Weithäuser A, Sheridan PA, Malz R, Luyendyk JP, Esserman DA, et al. PAR-1 contributes to the innate immune response during viral infection. J Clin Invest. 2013;123:1310–1322. doi: 10.1172/JCI66125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sexton TR, Zhang G, Macaulay TE, Callahan LA, Charnigo R, Vsevolozhskaya OA, Li Z, Smyth S. Ticagrelor reduces thromboinflammatory markers in patients with pneumonia. JACC Basic Transl Sci. 2018;3:435–449. doi: 10.1016/j.jacbts.2018.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nickel KF, Long AT, Fuchs TA, Butler LM, Renné T. Factor XII as a therapeutic target in thromboembolic and inflammatory diseases. Arterioscler Thromb Vasc Biol. 2017;37:13–20. doi: 10.1161/ATVBAHA.116.308595 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


