Supplemental Digital Content is available in the text.
Keywords: blood pressure, body weight, cardiology, hypertension, incidence
Abstract
Data regarding health behavior-related factors and systolic or diastolic blood pressure to evaluate the association between blood pressure and kidney cancer are lacking. Using nationally representative data from the Korean National Health Insurance System, 9 746 445 participants without kidney cancer between January 1, 2006 and December 31, 2009 were followed up until December 31, 2017 to obtain data regarding cancer incidence. Participants were categorized, according to blood pressure, as normal (<120/80 mm Hg), elevated (120–129/<80 mm Hg), and hypertensive (≥130/80 mm Hg) with or without antihypertensive medication, according to the 2017 American College of Cardiology and American Heart Association blood pressure guidelines. Kidney cancer was noted in 11 083 participants during the 8-year follow-up. Participants with hypertension were at higher risk for kidney cancer than those without hypertension. Participants with hypertension using medication had a higher cancer risk than those not using medication and those with elevated blood pressure. The risk of kidney cancer significantly increased with higher systolic or diastolic blood pressure, in a dose-dependent manner, even after adjusting for antihypertensive medication use. Therefore, hypertension and high systolic or diastolic blood pressure, compared with normal blood pressure, were associated with an increased risk of kidney cancer.
According to the National Cancer Institute’s Surveillance, Epidemiology, and End-Results program, in the United States, the incidence of kidney cancer has increased for all age groups between 1973 and 2008.1 Although the incidence of renal cancer is lower in Asia than in Europe and North America, the age-standardized incidence rate has also increased nearly 2-fold in Korea in recent decades.2 This increased incidence rate in renal cancer has resulted from an increased public awareness of the importance of periodic health screening and the widespread use of high-resolution imaging methods, such as abdominal ultrasound, computed tomography, magnetic resonance imaging, and contrast-enhanced ultrasound.3 Elevated blood pressure, excess body weight, and cigarette smoking are established factors that increase the risk of kidney cancer, in a dose-dependent manner.4–8 Diabetes mellitus, renal disease, acquired renal cystic disease, viral hepatitis, parity, vitamin D level, and decreased physical activity are suspected to be associated with an increased kidney cancer risk.4,8,9
According to the World Health Organization, hypertension is estimated to affect up to 40% of adults worldwide with the prevalence continuously increasing. Hypertension is often associated with other risk factors that have a high disease burden, such as smoking and obesity.10,11 The association between hypertension and the risk of kidney cancer in adolescents and adults has previously been investigated, with inconclusive results.12–15 A recent population-based Taiwanese cohort study, including 57 961 patients with hypertension, showed that hypertension was associated with a higher risk of genitourinary cancer. However, the study lacked data regarding health behavior-related factors (such as physical activity, alcohol consumption, and smoking), and did not include systolic or diastolic blood pressure to evaluate the specific association between blood pressure and kidney cancer.16
In our study, we evaluated the association of hypertension and systolic or diastolic blood pressure with the risk of kidney cancer, after adjusting for health behavior-related factors and the effects of antihypertensive medications, using the data of ≈9.7 million adults from the Korean National Health Insurance Service (NHIS) database. During our analysis, we used the recent 2017 American College of Cardiology and American Heart Association blood pressure guidelines, which have adopted a lower blood pressure criterion for defining hypertension. Confirming the association between hypertension and the risk of kidney cancer can provide important epidemiological insights and increase awareness of the risk of kidney cancer among patients with hypertension.
Methods
Data Source and Study Population
Anonymized data are publicly available from the National Health Insurance Sharing Service and accessible to all researchers whose research protocols have been approved by their institutional review board. In this study, we used the national health insurance claims database established by the Korea NHIS, which includes all claims data provided by the NHIS program and the Medical Aid program. The Korean NHIS program is a compulsory social insurance program that covers ≈97% of the South Korean population; the remaining 3% are covered under the Medical Aid program.17 Therefore, data extracted from the NHIS database are considered to represent the entire South Korean population (≈50 million).
Among the 10 505 818 participants who underwent at least one health examination in 2009, those younger than 20 years (n=15 327) were excluded because kidney cancer is rare in this population. Information about smoking status, body mass index (BMI), and waist circumference was obtained during health examinations. After excluding participants with missing health examination data (n=510 925) or history of kidney cancer during a washout period (2006–2009; n=233 121), 9 746 445 participants without kidney cancer were followed up, with the registration date of kidney cancer diagnostic codes recorded until December 2017. A flowchart of the selection of study participants is shown in Figure 1.
Figure 1.
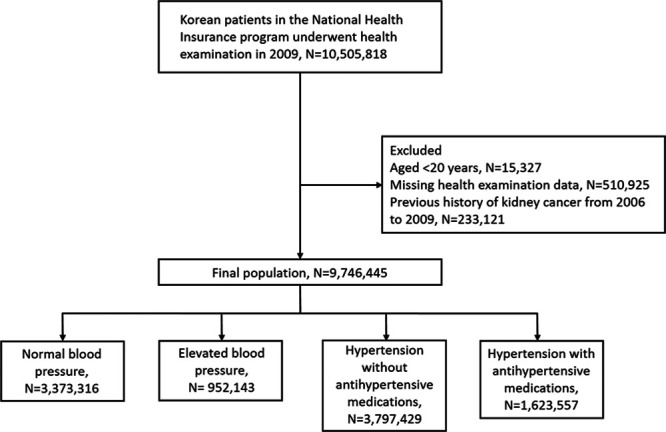
Flowchart of the study population.
This study was approved by the institutional review board of Chonnam National University Hospital, Korea (CNUH-EXP-2018-233). Patient identification numbers were anonymized to protect individual privacy. Anonymized and de-identified data were used for analysis. Therefore, the institutional review board waived the need for informed consent. All procedures were performed in accordance with the ethical standards of the committee responsible for human experimentation and the Helsinki Declaration of 1964 and later versions.
Definitions
Data on age, sex, and diagnostic codes, based on the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM), were retrieved. The diagnostic codes for kidney cancer were C64.1, C64.2, and C64.9.
Hypertension was defined as systolic blood pressure ≥130 mm Hg or diastolic blood pressure ≥80 mm Hg in the health examination database or a history of using at least one antihypertensive drug (diuretic, calcium channel blocker, β-blocker, α-blocker, ACE [angiotensin-converting enzyme] inhibitor, and/or ARB [angiotensin receptor blocker]). Moreover, diagnoses of normal blood pressure (<120/80 mm Hg), elevated blood pressure (120–129/<80 mm Hg), and hypertension (≥130/80 mm Hg) were made according to the 2017 American College of Cardiology/American Heart Association guidelines.18 During the health examination, blood pressure was measured twice or more, using a mercury or automatic sphygmomanometer, after a minimum rest period of 5 minutes, and with the individual in a sitting position. Participants who were prescribed antihypertensive medications within the first 5 years of follow-up were categorized as using antihypertensive medications.
The following health behavior factors (physical activity, alcohol consumption, and smoking) and comorbidities (diabetes mellitus and obesity) were included based on their known association with kidney cancer.4–8 Health behavior factors, namely smoking, alcohol consumption, and exercise, were defined as follows. Regarding smoking status, participants were categorized into 3 groups: nonsmokers, former smokers, or current smokers. Heavy alcohol consumption was defined as intake of ≥30 g of alcohol per day. Exercise habits of individuals were categorized based on their answer to the following question: During the last week, did you exercise ≥5 days for over 30 minutes until you were substantially short of breath?
The following comorbidities were included in our analysis. Diabetes mellitus was identified using the diagnostic codes E10–14 or a fasting serum glucose level ≥126 mg/dL in the health examination database. Dyslipidemia was identified by the diagnostic code E78, the use of lipid-lowering drugs, or a total cholesterol level ≥240 mg/dL.19 Both fasting blood glucose (mg/dL) and total cholesterol (mg/dL) level were obtained after a minimum of 8 hours of overnight fasting. Chronic kidney disease was defined as an estimated glomerular filtration rate <60 mL/minute per 1.73 m2, calculated using the Modification of Diet in Renal Disease formula.20 Stroke was defined by diagnostic codes I63–64.
The quality of all laboratory tests was confirmed by the Korean Association for Laboratory Medicine, and the NHIS certified that the hospitals participate in the NHI health checkup programs.
The following general demographic data were also included in our analysis. BMI was calculated by dividing body weight (kilogram) by the square of body height (square meters). Waist circumference was measured, by trained examiners, at the mid-point between the lower rib cage and the iliac crest.
Regarding socioeconomic factors, we considered residence in urban or rural areas, and income divided into 4 quartiles (Q), with Q1 being the lowest and Q4 the highest. Low income was defined as being in the Q1 group or a medical aid benefit recipient.
Statistical Analyses
Data are presented as the mean±SD for continuous variables and proportions for categorical variables. To compare characteristics between individuals with and without hypertension, Student t-test was used for continuous variables and χ2 test for binary and categorical variables. The incidence rate of kidney cancer was calculated per 100 000 person-years. To calculate the attributable risk, the risk for the nonhypertension group was subtracted from the risk for the group.21 A multivariable Cox proportional hazard regression analysis was conducted to determine the hazard ratios (HRs) and the associated 95% CIs of the association of hypertension (including systolic or diastolic blood pressure categories) with kidney cancer. Calculations were adjusted for age, sex, smoking, alcohol drinking, exercise, BMI, and history of diabetes mellitus, and use of antihypertensive drug therapy. The cumulative incidence probability of kidney cancer was plotted using Kaplan-Meier curves with log-rank test used for between-group comparisons. Age was stratified into the following age groups (20–39, 40–59, 60–74, and ≥75 years) and the association between hypertension and kidney cancer was examined within each group. Potential interactions were formally tested by including interaction terms. All statistical tests were 2-tailed, with a P value <0.05 considered significant. All data analyses were conducted using SAS software (version 9.4; SAS Institute, Cary, NC).
Results
General Characteristics of the Hypertensive Group
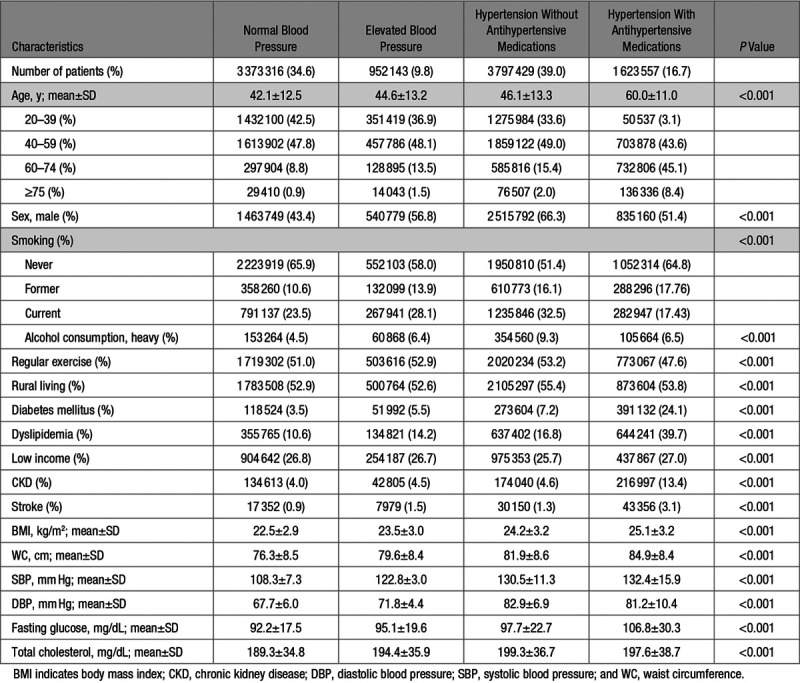
General baseline characteristics of the study population are shown in Table 1. Among our study cohort, 3 373 316 (34.6%) individuals had a normal blood pressure; 952 143 (9.8%) an elevated blood pressure; 3 797 429 (39.0%) had hypertension but did not use antihypertensive drugs; and 1 623 557 (16.7%) had hypertension and used antihypertensive drugs. Compared with individuals with normal blood pressure, those with hypertension tended to be older, male, smokers, and have a heavy alcohol consumption, and be more likely to have diabetes mellitus, dyslipidemia, chronic kidney disease, and stroke, and have a higher BMI, waist circumference, fasting serum glucose level, and total cholesterol level.
Table 1.
Descriptive Baseline Characteristics of the Study Population
Risk of Kidney Cancer According to Hypertension Groups
Kidney cancer was noted in 11 083 of the participants over the 8-year medical follow-up. The attributable risk associated with hypertension was 11.7 (95% CI, 11.1–12.3) cases of kidney cancer per 100 000 person-years of follow-up, with 56.0% (95% CI, 53.1–58.8) of these kidney cancer cases among individuals with hypertension. Results of the multivariable Cox proportional hazard regression analysis evaluating the risk of kidney cancer associated with hypertension, adjusted for other potential predictors of kidney cancer, are shown in Table 2. Compared with individuals without hypertension, those with hypertension were at increased risk of kidney cancer (incidence rate, 20.9 versus 9.2 cases per 100 000 person-years, respectively; adjusted HR, 1.12 [95% CI, 1.06–1.17]). The Kaplan-Meier curve for the incidence probability of kidney cancer according to hypertension is shown in Figure 2, with similar results were obtained. Moreover, elevated blood pressure was associated with a higher risk of kidney cancer than normal blood pressure (incidence rate, 11.8 versus 8.4 cases per 100 000 person-years, respectively; adjusted HR, 1.10 [95% CI, 1.01–1.19]).
Table 2.
Hazard Ratios of Kidney Cancer Risk According to Hypertension Categories and Blood Pressure
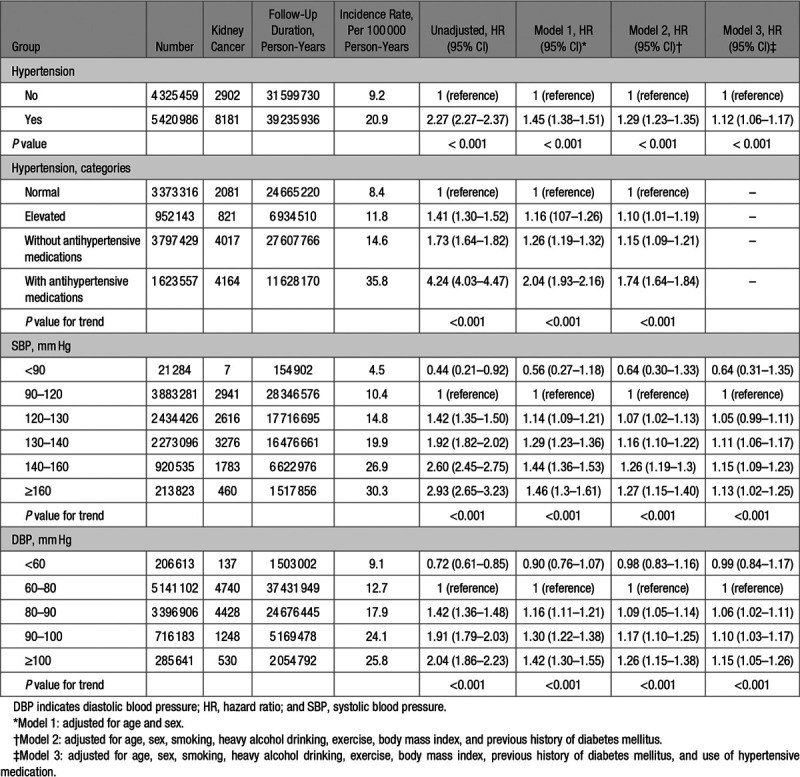
Figure 2.
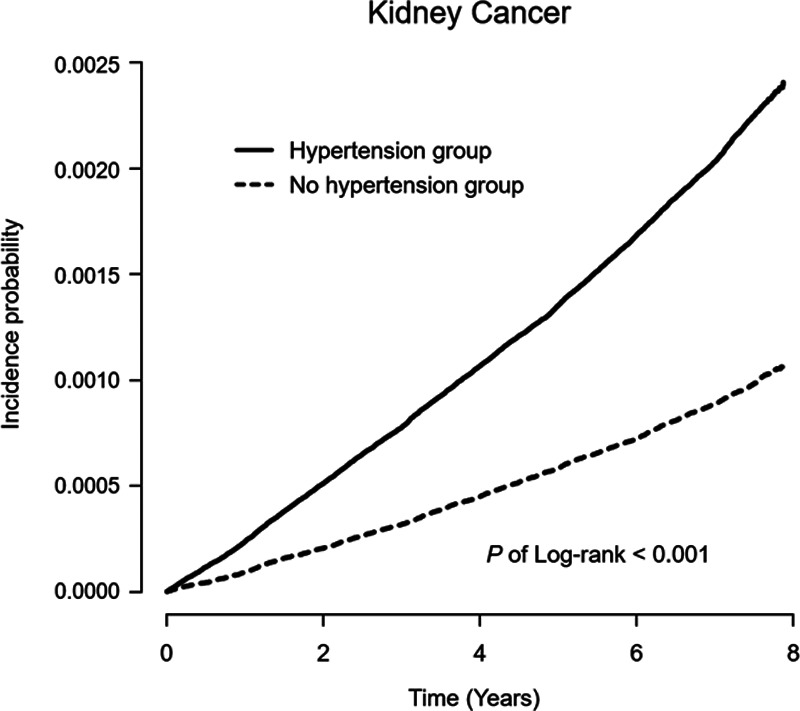
Kaplan–Meier curve for the crude cumulative 8-year incidence probability of kidney cancer with respect to hypertension (P of Log-rank < 0.001).
Risk of Kidney Cancer According to Antihypertensive Medications and Blood Pressure Categories
Although patients with hypertension who were not prescribed antihypertensive medications also were at increased risk for kidney cancer, those using antihypertensive medications were at the highest risk for kidney cancer (incidence rate, 14.6 versus 35.8 cases per 100 000 person-years, respectively; adjusted HR, 1.15 [95% CI, 1.09–1.21] versus adjusted HR, 1.74 [95% CI, 1.64–1.84]; Table 2). Among participants who had used α-blockers, β-blockers, CCBs, diuretics, and ACE inhibitors, and/or ARBs, we identified those who had used one drug class exclusively. The adjusted HRs for kidney cancer were higher for participants using β-blockers or diuretics than for those with α-blockers, CCBs, or ACE inhibitors/ARBs. In addition, users of ≥2 classes of antihypertensive drugs were at higher risk for kidney cancer than those using one drug class (Table S1 in the Data Supplement). To control for the effects of antihypertensive medications on the risk of kidney cancer, participants were categorized according to blood pressure levels, regardless of drug use, with the risk of kidney cancer evaluated within each group, after adjusting for antihypertensive medications. A systolic blood pressure ≥120 mm Hg was associated with an increased risk of kidney cancer, in a dose-dependent manner, even after adjusting for antihypertensive medications (P value for trend <0.001). Similarly, a diastolic blood pressure ≥80 mm Hg showed an increasing trend in the risk of kidney cancer as the diastolic pressure increased. Moreover, the increased risk of kidney cancer noted in the hypertensive and high blood pressure groups were observed regardless of sex (Tables S2 and S3). However, a higher systolic blood pressure, but not diastolic blood pressure, seemed to confer a greater risk of kidney cancer among men.
Subgroup Analyses
The relative risks of kidney cancer stratified by age, sex, smoking, alcohol consumption, exercise, BMI category, and history of chronic kidney disease and stroke are shown in Figure 3. The risk of kidney cancer was higher among patients with hypertension 60–74 years of age than among those in other age groups. In addition, patients who were male, former smokers, and had BMI ≥23 kg/m2 were at a specifically higher risk for kidney cancer. However, the association between hypertension and risk of kidney cancer was not significantly different among the other subgroups.
Figure 3.
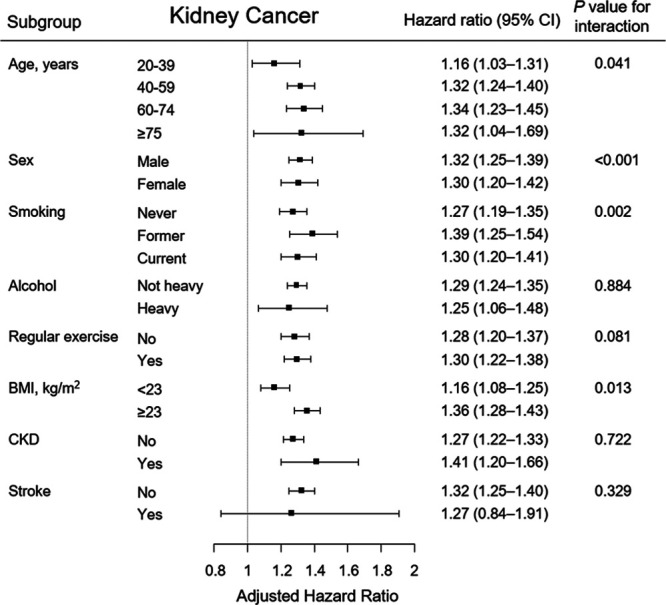
Subgroup analysis of the association between hypertension and kidney cancer in the adjusted Cox-model. Points and bars represent hazard ratio estimates and their associated 95% CIs, respectively. BMI indicates body mass index; CI, confidential interval; and CKD, chronic kidney disease.
Discussion
In this nationwide population-based study, hypertension was found to be associated with a considerable kidney cancer risk. Systolic blood pressure ≥120 mm Hg and diastolic blood pressure ≥80 mm Hg were associated with an increased kidney cancer risk, which was maintained after multivariable adjustment for confounding variables, such as smoking status, alcohol drinking habits, physical activity, BMI, and use of antihypertensive medications.
The results of previous studies are consistent with our findings, which identified the association between hypertension and the risk of kidney cancer. In a previous study involving 77 260 participants aged 50 to 76 years, a 1.70-fold increase in the risk of renal cell carcinoma was observed for patients with hypertension.4 Hidayat et al5 reported that hypertension was associated with a 1.67-fold increased risk of kidney cancer, and that a 10-mm Hg increase in systolic and diastolic blood pressures was significantly associated with 10% and 20% increased risks of kidney cancer, respectively, in both sexes through a meta-analysis of prospective studies. In accordance with previous studies, we found that the risk of kidney cancer increases in a dose-dependent manner with regard to systolic and diastolic blood pressures. A nationwide population-based Taiwanese cohort study involving 11 704 participants showed that younger patients with hypertension (≤49 years) were more likely to develop kidney cancer during a follow-up period of >5 years.16 In the nationwide Swedish Cancer Registry study that used repeated measures of blood pressure over time, a decreased risk was observed with a reduction of blood pressure for 363 992 Swedish men.14 However, previous nationwide studies were limited to men or lacked adjustments for substantial confounders that could affect cancer development, such as smoking and drinking habits and obesity.14,16
Our study targeted a large population, including women, and demonstrated that the high risk of kidney cancer associated with high blood pressure was consistent even after adjusting for health behavior-related factors. Our subgroup analyses showed that the risk of kidney cancer was higher for older (≥40 years) patients with hypertension, indicating that aging is an important risk factor for cancer. However, we do note that younger (20–39 years) patients with hypertension also had a 16% increase in the risk of kidney cancer compared with those without hypertension over our 8-year follow-up period. Therefore, more long-term follow-up studies are needed to confirm whether prolonged exposure to hypertension in younger patients is related to an increased risk of kidney cancer.16 We showed that obese patients with hypertension were at significantly higher risk for kidney cancer than those with a low BMI (<23 kg/m2), in agreement with results from previous studies.14,22,23
The plausible biological mechanisms underlying the association between hypertension and kidney cancer remain unclear. However, it is possible that hypertension might induce a state of chronic inflammation, activating the cellular hypoxia response pathway and promoting hypoxia-induced factor expression in the kidney.24,25 This abnormal accumulation of hypoxia-inducible factors leads to cell growth dysregulation and angiogenesis.26 Moreover, inflammatory conditions accompanying hypertension contribute to renal carcinogenesis and tumor progression, and cancer-related inflammation induces genetic instability due to inflammatory mediators.27,28 It is also possible that hypertension is associated with endothelial dysfunction and altered vascular remodeling, which lead to increased formation of reactive oxygen species in hypertensive individuals.29–31 Reactive oxygen species can promote tumor development and progression through various biological processes.32
A previous case-control study showed that patients using diuretic treatment were at an increased risk for kidney cancer than those not using diuretic treatment.33 Conversion of diuretics to mutagenic nitroso derivatives in the stomach may be underlying mechanisms relating diuretic treatment to kidney cancer risk.34 Similarly, the risk of kidney cancer was increased among users of CCBs in a case-control drug surveillance study.35 It has been suggested that the use of CCBs may influence cancer risk by interfering with apoptosis.36 However, recent large or meta-analysis studies have reported discordant results regarding the association between antihypertensive medications and the risk of cancer.37,38 In a recent population-based case-control study, the long-term use of diuretics or CCBs was associated with papillary renal cell carcinoma among patients with a history of hypertension; however, findings of clear cell renal cell carcinoma were weaker and nonsignificant.37 In addition, results of a network meta-analysis study refuted the association of an increased risk of cancer or cancer-related death with the use of diuretics, CCBs, β-blockers, ACE inhibitors, and ARBs.38 Because antihypertension drugs are frequently used in patients with hypertension, it is difficult to distinguish the effects of high blood pressure from those of antihypertensive medications per se on the increased risk of kidney cancer. Although our results showed that patients with hypertension using antihypertensive medications were at higher risk for kidney cancer than those not using medications, high blood pressure was clearly associated with the risk of kidney cancer in a dose-dependent manner, even after adjusting for antihypertensive drugs. These findings are consistent with those of previous studies that showed that hypertension is more likely to be a risk factor than antihypertensive medications.8,9,13
The limitations of our study need to be acknowledged. First, our study population was from a single country; therefore, the results may not be generalizable to individuals with other racial or ethnic backgrounds. However, the homogeneous population could have removed the effects of ethnic genetic variations on the findings. Second, although we identified the effects of individual antihypertensive drug classes, whether the duration of antihypertensive medications and degree of blood pressure control with drugs affect the risk of kidney cancer in relation to antihypertensive agents could not be determined. Third, there were potential limitations regarding whether the diagnosis of kidney cancer or the timing of the diagnosis of hypertension and antihypertensive drug use could be accurately assessed, because our database is primarily for administrative purposes. However, our database enables us to identify almost all kidney cancer cases detected intentionally or incidentally in the South Korean population as patients diagnosed with kidney cancer are registered in the Korea NHIS database to exempt from copayment.
Despite these limitations, the strength of our study lies in the inclusion of >9.7 million adults and 70 million person-years during the observation period, including 11 083 cases of kidney cancer. The number of participants and the duration of the observation period in our study were higher than those in previous studies that have evaluated the association between hypertension and the risk of kidney cancer. Moreover, we adjusted for as many health behavior-related factors that could affect kidney cancer development as possible. Finally, to our knowledge, this is the first study to use the new definition of hypertension according to the 2017 American College of Cardiology/American Heart Association guidelines.
Perspectives
We provide evidence of an association between hypertension and an increased risk of kidney cancer, compared with normal blood pressure. An association between a higher systolic and a higher diastolic blood pressure and an increased risk of kidney cancer was also observed. Therefore, awareness of the risk of kidney cancer among patients with hypertension is clinically relevant, especially for patients with uncontrolled hypertension, indicating that optimal blood pressure control should be emphasized in these clinical populations. Future prospective studies from a public health perspective are required to further validate the effects of optimal blood pressure control on kidney cancer prevention.
Sources of Funding
This research was supported by the Bio & Medical Development Program through the National Research Foundation (NRF) of Korea funded by the Ministry of Science and ICT (MSIT; NRF-2017M3A9E8023001, NRF-2017M3A9E8023018), by the Basic Science Research Program through the NRF of Korea funded by the Ministry of Education (NRF-2018R1D1A1B07042999), by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI18C0331), and by grant (BCRI19030) from Chonnam National University Hospital Institute for Biomedical Science.
Disclosures
None.
Supplementary Material
Footnotes
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/HYPERTENSIONAHA.120.14820.
Novelty and Significance
What Is New?
Our risk analysis was adjusted for as many health behavior-related factors as possible that could affect kidney cancer development.
This is the first study to use the new hypertension definition, according to the 2017 American College of Cardiology/American Heart Association guidelines, to evaluate the association between hypertension and the risk of kidney cancer.
What Is Relevant?
Confirming the association between hypertension and the risk of kidney cancer can provide important epidemiological insight and increase awareness of the risk of kidney cancer among hypertensive patients. Further studies to determine optimal blood pressure control to prevent kidney cancer are warranted.
Summary
Hypertension and high systolic or diastolic blood pressure, compared with normal blood pressure, were associated with an increased risk of kidney cancer.
References
- 1.Tyson MD, Humphreys MR, Parker AS, Thiel DD, Joseph RW, Andrews PE, Castle EP. Age-period-cohort analysis of renal cell carcinoma in United States adults. Urology. 2013;82:43–47. doi: 10.1016/j.urology.2013.02.065. doi: 10.1016/j.urology.2013.02.065. [DOI] [PubMed] [Google Scholar]
- 2.Song W, Jeon HG. Incidence of kidney, bladder, and prostate cancers in Korea: an update. Korean J Urol. 2015;56:422–428. doi: 10.4111/kju.2015.56.6.422. doi: 10.4111/kju.2015.56.6.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ziegelmüller BK, Spek A, Szabados B, Casuscelli J, Clevert DA, Staehler M. [Epidemiology and diagnostic assessment of small renal masses]. Urologe A. 2018;57:274–279. doi: 10.1007/s00120-018-0585-7. doi: 10.1007/s00120-018-0585-7. [DOI] [PubMed] [Google Scholar]
- 4.Macleod LC, Hotaling JM, Wright JL, Davenport MT, Gore JL, Harper J, White E. Risk factors for renal cell carcinoma in the VITAL study. J Urol. 2013;190:1657–1661. doi: 10.1016/j.juro.2013.04.130. doi: 10.1016/j.juro.2013.04.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hidayat K, Du X, Zou SY, Shi BM. Blood pressure and kidney cancer risk: meta-analysis of prospective studies. J Hypertens. 2017;35:1333–1344. doi: 10.1097/HJH.0000000000001286. doi: 10.1097/HJH.0000000000001286. [DOI] [PubMed] [Google Scholar]
- 6.Oh SW, Yoon YS, Shin SA. Effects of excess weight on cancer incidences depending on cancer sites and histologic findings among men: Korea National Health Insurance Corporation Study. J Clin Oncol. 2005;23:4742–4754. doi: 10.1200/JCO.2005.11.726. doi: 10.1200/JCO.2005.11.726. [DOI] [PubMed] [Google Scholar]
- 7.Hunt JD, van der Hel OL, McMillan GP, Boffetta P, Brennan P. Renal cell carcinoma in relation to cigarette smoking: meta-analysis of 24 studies. Int J Cancer. 2005;114:101–108. doi: 10.1002/ijc.20618. doi: 10.1002/ijc.20618. [DOI] [PubMed] [Google Scholar]
- 8.Scelo G, Larose TL. Epidemiology and risk factors for kidney cancer. J Clin Oncol. 2018;36:3574–3581. doi: 10.1200/JCO.2018.79.1905. doi: 10.1200/JCO.2018.79.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chow WH, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nat Rev Urol. 2010;7:245–257. doi: 10.1038/nrurol.2010.46. doi: 10.1038/nrurol.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.GBD 2016 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2016: A systematic analysis for the global burden of disease study 2016. Lancet. 2017;390:1345–1422. doi: 10.1016/S0140-6736(17)32366-8. doi: 10.1016/S0140-6736(17)32366-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee JH, Kim SH, Kang SH, Cho JH, Cho Y, Oh IY, Yoon CH, Lee HY, Youn TJ, Chae IH, et al. Blood pressure control and cardiovascular outcomes: real-world implications of the 2017 ACC/AHA hypertension guideline. Sci Rep. 2018;8:13155. doi: 10.1038/s41598-018-31549-5. doi: 10.1038/s41598-018-31549-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanfilippo KM, McTigue KM, Fidler CJ, Neaton JD, Chang Y, Fried LF, Liu S, Kuller LH. Hypertension and obesity and the risk of kidney cancer in 2 large cohorts of US men and women. Hypertension. 2014;63:934–941. doi: 10.1161/HYPERTENSIONAHA.113.02953. doi: 10.1161/HYPERTENSIONAHA.113.02953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weikert S, Boeing H, Pischon T, Weikert C, Olsen A, Tjonneland A, Overvad K, Becker N, Linseisen J, Trichopoulou A, et al. Blood pressure and risk of renal cell carcinoma in the European prospective investigation into cancer and nutrition. Am J Epidemiol. 2008;167:438–446. doi: 10.1093/aje/kwm321. doi: 10.1093/aje/kwm321. [DOI] [PubMed] [Google Scholar]
- 14.Chow WH, Gridley G, Fraumeni JF, Jr, Järvholm B. Obesity, hypertension, and the risk of kidney cancer in men. N Engl J Med. 2000;343:1305–1311. doi: 10.1056/NEJM200011023431804. doi: 10.1056/NEJM200011023431804. [DOI] [PubMed] [Google Scholar]
- 15.Goon PK, Stonelake PS, Lip GY. Hypertension, anti-hypertensive therapy and neoplasia. Curr Pharm Des. 2007;13:2539–2544. doi: 10.2174/138161207781663073. doi: 10.2174/138161207781663073. [DOI] [PubMed] [Google Scholar]
- 16.Sun LM, Kuo HT, Jeng LB, Lin CL, Liang JA, Kao CH. Hypertension and subsequent genitourinary and gynecologic cancers risk: a population-based cohort study. Medicine (Baltimore) 2015;94:e753. doi: 10.1097/MD.0000000000000753. doi: 10.1097/MD.0000000000000753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi JB, Lee EJ, Han KD, Hong SH, Ha US. Estimating the impact of body mass index on bladder cancer risk: stratification by smoking status. Sci Rep. 2018;8:947. doi: 10.1038/s41598-018-19531-7. doi: 10.1038/s41598-018-19531-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whelton PK, Carey RM, Aronow WS, Casey DE, Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the american college of cardiology/american heart association task force on clinical practice guidelines. Hypertension. 2018;71:e13–e115. doi: 10.1161/HYP.0000000000000065. doi: 10.1161/HYP.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 19.Committee for the Korean Guidelines for the Management of Dyslipidemia. 2015 korean guidelines for the management of dyslipidemia: executive summary (english translation). Korean Circ J. 2016;46:275–306. doi: 10.4070/kcj.2016.46.3.275. doi: 10.4070/kcj.2016.46.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mariat C, Alamartine E, Berthoux F. Measured and estimated glomerular filtration rate. N Engl J Med. 2006;355:1068–1069. author reply 1067–1070. [PubMed] [Google Scholar]
- 21.Walter SD. The estimation and interpretation of attributable risk in health research. Biometrics. 1976;32:829–849. [PubMed] [Google Scholar]
- 22.Arnold M, Pandeya N, Byrnes G, Renehan PAG, Stevens GA, Ezzati PM, Ferlay J, Miranda JJ, Romieu I, Dikshit R, et al. Global burden of cancer attributable to high body-mass index in 2012: a population-based study. Lancet Oncol. 2015;16:36–46. doi: 10.1016/S1470-2045(14)71123-4. doi: 10.1016/S1470-2045(14)71123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, Smeeth L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5·24 million UK adults. Lancet. 2014;384:755–765. doi: 10.1016/S0140-6736(14)60892-8. doi: 10.1016/S0140-6736(14)60892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dinh QN, Drummond GR, Sobey CG, Chrissobolis S. Roles of inflammation, oxidative stress, and vascular dysfunction in hypertension. Biomed Res Int. 2014;2014:406960. doi: 10.1155/2014/406960. doi: 10.1155/2014/406960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haase VH. Inflammation and hypoxia in the kidney: friends or foes? Kidney Int. 2015;88:213–215. doi: 10.1038/ki.2015.89. doi: 10.1038/ki.2015.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Vivar Chevez AR, Finke J, Bukowski R. The role of inflammation in kidney cancer. Adv Exp Med Biol. 2014;816:197–234. doi: 10.1007/978-3-0348-0837-8_9. doi: 10.1007/978-3-0348-0837-8_9. [DOI] [PubMed] [Google Scholar]
- 27.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–1081. doi: 10.1093/carcin/bgp127. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 28.Fox P, Hudson M, Brown C, Lord S, Gebski V, De Souza P, Lee CK. Markers of systemic inflammation predict survival in patients with advanced renal cell cancer. Br J Cancer. 2013;109:147–153. doi: 10.1038/bjc.2013.300. doi: 10.1038/bjc.2013.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Popolo A, Autore G, Pinto A, Marzocco S. Oxidative stress in patients with cardiovascular disease and chronic renal failure. Free Radic Res. 2013;47:346–356. doi: 10.3109/10715762.2013.779373. doi: 10.3109/10715762.2013.779373. [DOI] [PubMed] [Google Scholar]
- 30.Crowley SD. The cooperative roles of inflammation and oxidative stress in the pathogenesis of hypertension. Antioxid Redox Signal. 2014;20:102–120. doi: 10.1089/ars.2013.5258. doi: 10.1089/ars.2013.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gago-Dominguez M, Castelao JE, Yuan JM, Ross RK, Yu MC. Lipid peroxidation: a novel and unifying concept of the etiology of renal cell carcinoma (United States). Cancer Causes Control. 2002;13:287–293. doi: 10.1023/a:1015044518505. doi: 10.1023/a:1015044518505. [DOI] [PubMed] [Google Scholar]
- 32.Sosa V, Moliné T, Somoza R, Paciucci R, Kondoh H, LLeonart ME. Oxidative stress and cancer: an overview. Ageing Res Rev. 2013;12:376–390. doi: 10.1016/j.arr.2012.10.004. doi: 10.1016/j.arr.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Grossman E, Messerli FH, Goldbourt U. Does diuretic therapy increase the risk of renal cell carcinoma? Am J Cardiol. 1999;83:1090–1093. doi: 10.1016/s0002-9149(99)00021-1. doi: 10.1016/s0002-9149(99)00021-1. [DOI] [PubMed] [Google Scholar]
- 34.Gold B, Mirvish SS. N-Nitroso derivatives of hydrochlorothiazide, niridazole, and tolbutamide. Toxicol Appl Pharmacol. 1977;40:131–136. doi: 10.1016/0041-008x(77)90124-7. doi: 10.1016/0041-008x(77)90124-7. [DOI] [PubMed] [Google Scholar]
- 35.Rosenberg L, Rao RS, Palmer JR, Strom BL, Stolley PD, Zauber AG, Warshauer ME, Shapiro S. Calcium channel blockers and the risk of cancer. JAMA. 1998;279:1000–1004. doi: 10.1001/jama.279.13.1000. doi: 10.1001/jama.279.13.1000. [DOI] [PubMed] [Google Scholar]
- 36.Ray SD, Kamendulis LM, Gurule MW, Yorkin RD, Corcoran GB. Ca2+ antagonists inhibit DNA fragmentation and toxic cell death induced by acetaminophen. FASEB J. 1993;7:453–463. doi: 10.1096/fasebj.7.5.8462787. doi: 10.1096/fasebj.7.5.8462787. [DOI] [PubMed] [Google Scholar]
- 37.Colt JS, Hofmann JN, Schwartz K, Chow WH, Graubard BI, Davis F, Ruterbusch J, Berndt S, Purdue MP. Antihypertensive medication use and risk of renal cell carcinoma. Cancer Causes Control. 2017;28:289–297. doi: 10.1007/s10552-017-0857-3. doi: 10.1007/s10552-017-0857-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bangalore S, Kumar S, Kjeldsen SE, Makani H, Grossman E, Wetterslev J, Gupta AK, Sever PS, Gluud C, Messerli FH. Antihypertensive drugs and risk of cancer: network meta-analyses and trial sequential analyses of 324,168 participants from randomised trials. Lancet Oncol. 2011;12:65–82. doi: 10.1016/S1470-2045(10)70260-6. doi: 10.1016/S1470-2045(10)70260-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


