Abstract
BACKGROUND:
Recent reports suggest that component plasma products contain significant quantities of cellular contamination. We hypothesized that leukoreduction of whole blood before preparation of derived plasma is an effective method to prevent cellular contamination of stored plasma.
STUDY DESIGN:
Samples of never-frozen liquid plasma prepared by standard methods (n = 25) were obtained from 3 regional blood centers that supply 3 major trauma centers. Samples were analyzed for leukocyte and platelet contamination by flow cytometry. To determine if leukoreduction of whole blood before centrifugation and expression of plasma prevents cellular contamination of liquid plasma, 1 site generated 6 additional units of liquid plasma from leukoreduced whole blood, which were then compared with units of liquid plasma derived by standard processing.
RESULTS:
Across all centers, each unit of never-frozen liquid plasma contained a mean of 12.8 ± 3.0 million leukocytes and a mean of 4.6 ± 2 billion platelets. Introduction of whole blood leukoreduction (LR) before centrifugation and plasma extraction essentially eliminated all contaminating leukocytes (Non-LR: 12.3 ± 2.9 million vs LR: 0.05 ± 0.05 million leukocytes) and platelets (Non-LR: 4.2 ± 0.3 billion platelets vs LR: 0.00 ± 0.00 billion platelets).
CONCLUSIONS:
Despite widespread belief that stored plasma is functionally acellular, testing of liquid plasma from 3 regional blood banks revealed a significant amount of previously unrecognized cellular contamination. Introduction of a leukoreduction step before whole blood centrifugation essentially eliminated detectable leukocyte and platelet contaminants from plasma. Therefore, our study highlights a straightforward and cost-effective method to eliminate cellular contamination of stored plasma.
Blood transfusions are lifesaving in many circumstances, but are also known to be associated with the potential for serious adverse consequences. Significant advancement has been made over the past 3 decades to reduce the risk of transfusion-related complication, particularly by the leukoreduction (LR) of packed red blood cells (PRBC) and platelets.1 Transfusing allogeneic leukocytes causes a wide variety of complications, including transmission of infectious diseases, alloimmunization, graft vs host disease, and transfusion-related immunomodulation (TRIM).2 These and other potentially preventable transfusion-related complications prompted the majority of developed countries to mandate universal leukoreduction (LR) of all transfusion products.3
Blood banking practices in the US have traditionally accepted the notion that plasma products are acellular, containing needed coagulation factors without contamination of cells from the buffy coat (eg leukocytes and/or platelets). The majority of plasma units created for transfusion in the US are derived from whole blood centrifugation, by which components are separated by specific weight into red blood cells, buffy coat (containing leukocytes and platelets), and plasma. The plasma layer is then removed by a technician applying uniform pressure at the bottom of the bag using a “plasma expressor” until all of the plasma is removed without allowing cells from the buffy coat to contaminate the newly formed unit of plasma. As visual buffy coat exclusion during plasma extraction derived from centrifuged whole blood is believed to adequately avoid cellular contamination, filter leukoreduction is not routinely performed on plasma. However, recent reports suggest that stored plasma units reveal previously unrecognized significant leukocyte and platelet contamination.4–6 The combination of a recent paradigm shift in transfusion strategy for patients in hemorrhagic shock resulting large increases of plasma transfusions,7 and the current knowledge that stored plasma products contain significant cellular contamination, provide even more urgency to find a solution to this previously unrecognized problem. Herein, we tested the hypothesis that LR of whole blood before centrifugation is an effective way to prevent cellular contamination of stored plasma.
METHODS
Plasma product preparation
Never-frozen liquid plasma samples from male donors (n = 25) were obtained from regional blood centers (Site A: Mobile, AL; Site B: Baton Rouge, LA; Site C: New Orleans, LA) supplying 3 major trauma centers located in the southeastern US. All samples were transported by the research team to ensure proper temperature conditions and to avoid introducing freeze-thaw events before testing. The methods for plasma generation at these regional blood centers represent standard operating procedure in the blood banking industry. Plasma units were created by single-step whole blood centrifugation and plasma expression without leukocyte reduction, representing standard, never-frozen liquid plasma available for transfusion during resuscitation of trauma patients. Plasma was sampled from the tubular segments of each unit.
To determine if LR of whole blood before centrifugation is effective in eliminating cellular contamination of liquid plasma, Site B created 6 additional units of liquid plasma from leukoreduced whole blood. The leukoreduction step was performed using a commercially available Sepacell RS-2000/RZ-2000 leukocyte reduction filter (Fenwal) before centrifugation. Units created by leukoreduction were then compared to units of liquid plasma derived by standard processing methods.
Flow cytometry measurement of cellular contamination
Samples were analyzed for leukocyte and platelet contamination by flow cytometry (Sysmex XN) in the clinical laboratory at the University of South Alabama University Hospital. Leukocyte count was obtained by analyzing the samples via body fluid mode, and platelet number was obtained via platelet fluorescence. The manufacturer stated linearity, and the institution quality control data were reviewed to ensure result reliability. Acceptable parameter ranges for leukocytes and platelets fall between 0 and 440 cells/μL and 0 and 5,000 cells/μL, respectively.8 All experimental results fell within acceptable ranges for instrument linearity. The number of cells per microliter of sample were extrapolated to determine total leukocytes (expressed as millions of cells per unit of blood product) and platelets (expressed as billions of cells per unit of blood product). Cell numbers were determined by assuming a volume of 300 mL per unit, which reflects the mean volume of plasma product per unit produced by the Site A regional blood center (Mobile, AL).
RESULTS
Across all centers, each unit of never-frozen liquid plasma contained a mean of 12.8 ± 3 million leukocytes (Fig 1.Site A: n = 6, 7.0 ± 1.8 million; Site B: n = 6, 12.3 ± 2.9 million; Site C: n = 13, 15.7 ± 5.5 million), and 4.6 ± 2 billion platelets (Fig 2. Site A: n = 6, 0.35 ± 0.05 billion; Site B: n = 6, 4.2 ± 0.3 billion; Site C: n = 13, 6.7 ± 3.8 billion). Introduction of a whole blood filter leukoreduction (LR) step before plasma extraction essentially eliminated all contaminating leukocytes (Fig 3. Non-LR: 12.3 ± 2.9 million vs LR: 0.05 ± 0.05 million leukocytes) and platelets (Non-LR: 4.2 ± 0.3 billion platelets vs LR: 0.00 ± 0.00 billion platelets).
Figure 1.
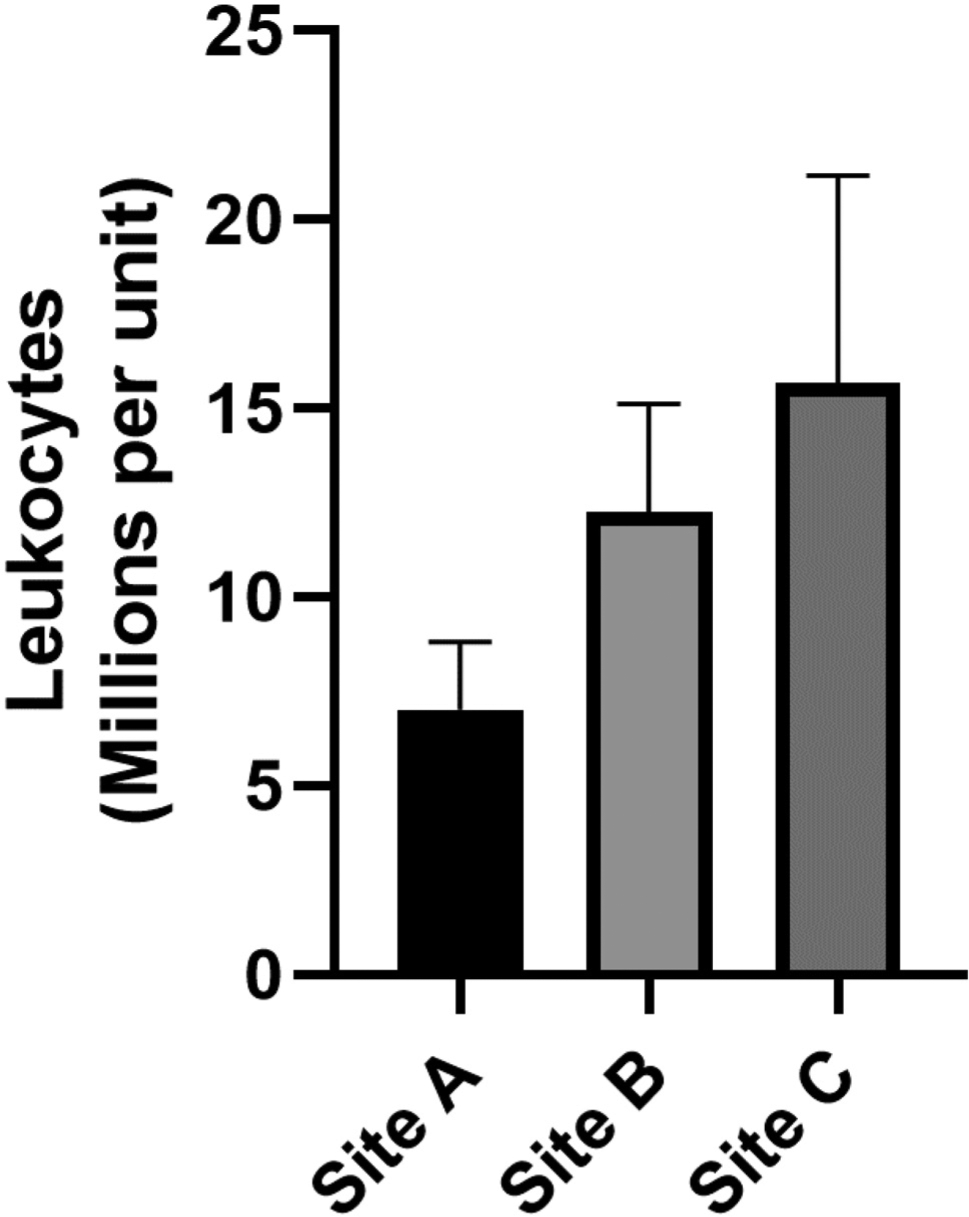
Leukocyte contamination of never-frozen liquid plasma.
Figure 2.
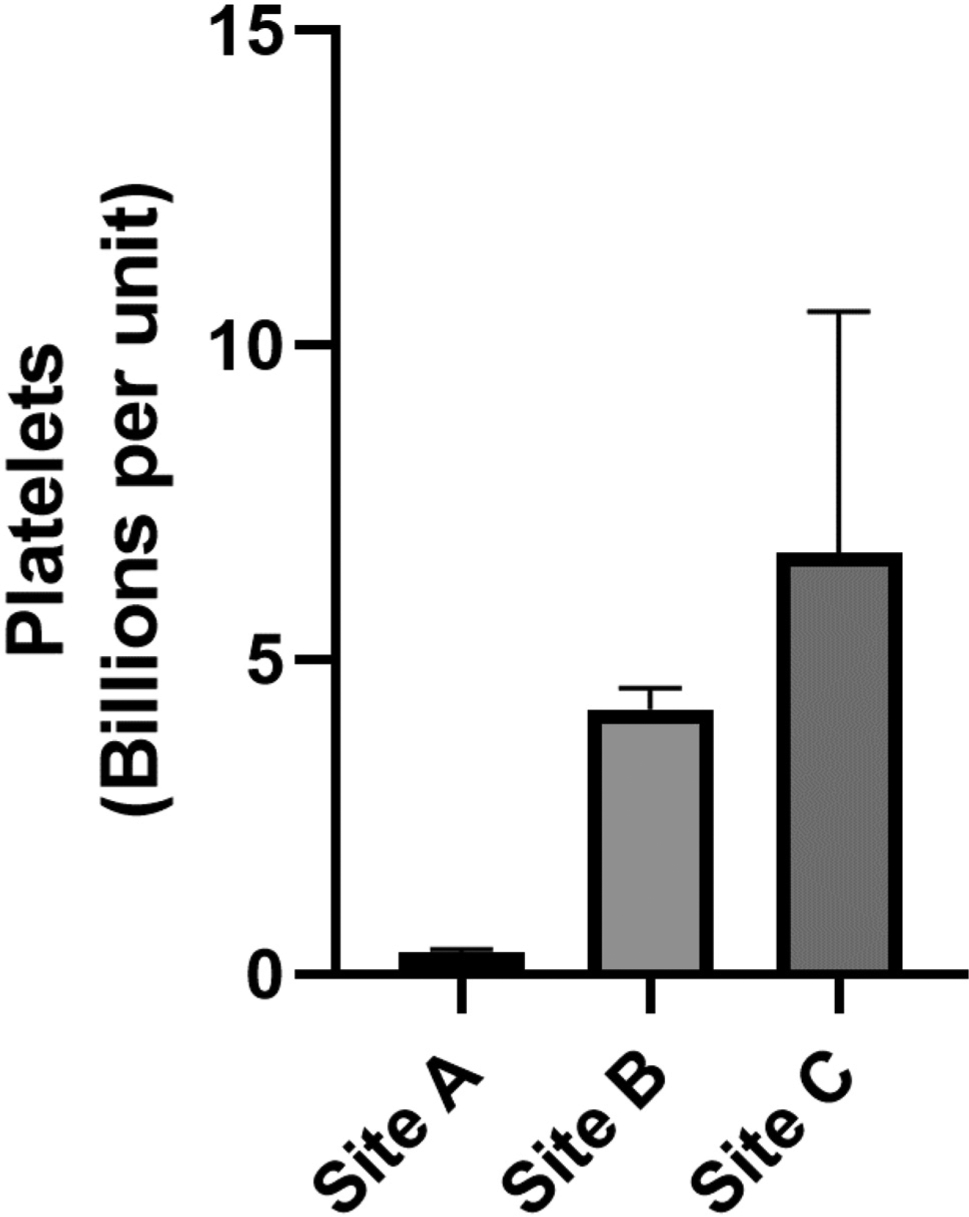
Platelet contamination of never-frozen liquid plasma.
Figure 3.
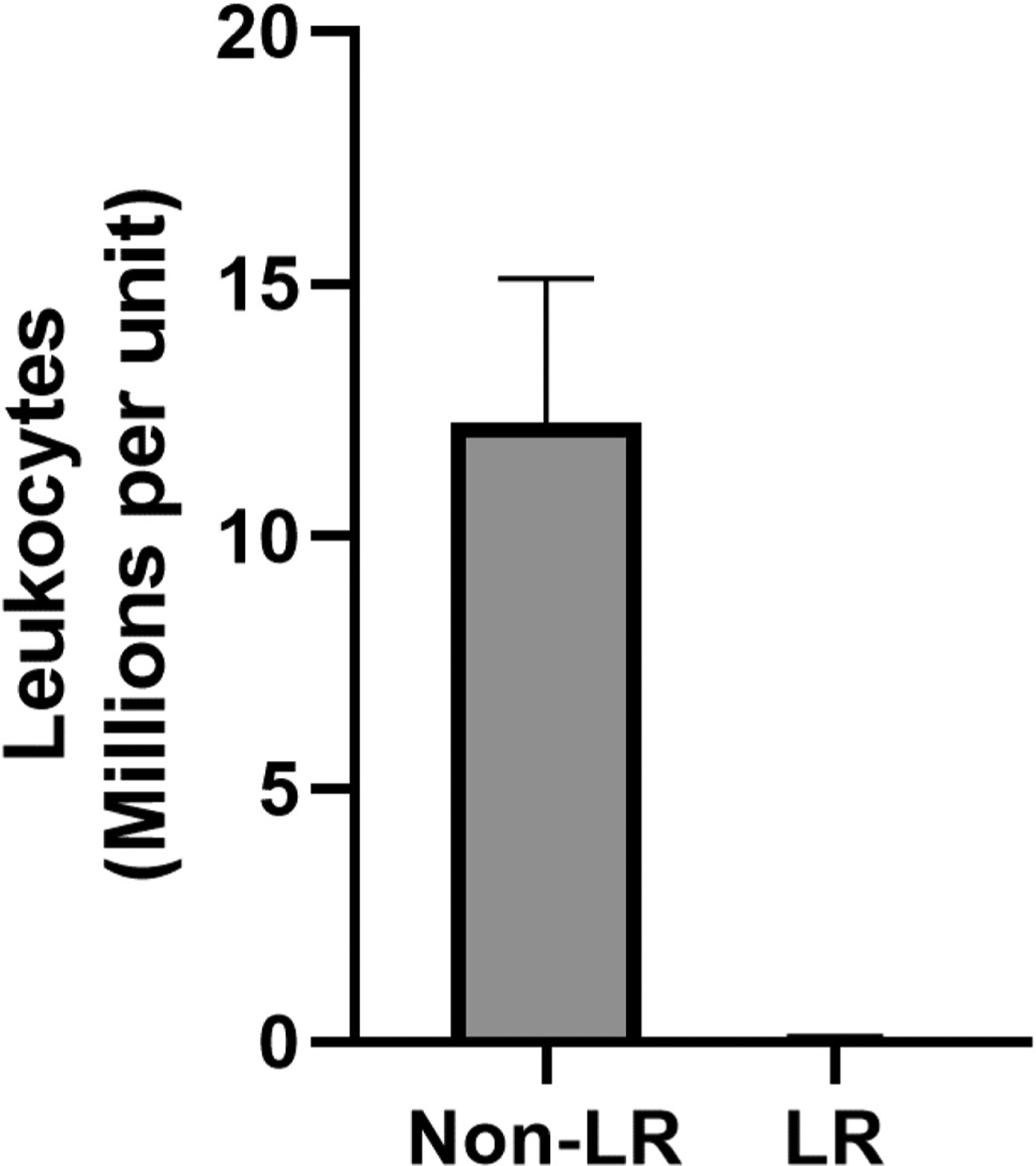
Leukoreduction (LR) of whole blood before centrifugation prevents cellular contamination of plasma units.
DISCUSSION
In this study, we challenged the paradigm that current blood banking practices in the US are sufficient to produce cell-free units of plasma suitable for transfusion. The idea that current practices to produce transfusion-ready plasma products might require reconsideration came from our previous observation that stored plasma products from blood centers servicing 2 regional level 1 trauma centers (Mobile, AL and New Orleans, LA) contained a significant quantity of previously unrecognized cellular contamination.4 The work presented here tested new samples of never-frozen plasma components from the Mobile and New Orleans centers, and also expanded to a third center in Baton Rouge, LA. Plasma units from all 3 centers demonstrated significant levels of contaminating leukocytes and platelets (Figs 1 and 2).
Our studies demonstrate that contaminating leukocytes in plasma are in quantities significantly above the level previously reported to induce alloimmunization responses.9–12 The clinical significance of transfusion-related immunosuppression (TRIM) induced by allogeneic blood transfusion was first reported in 1973, when allograft survival in renal transplant patients was noted to be significantly improved if the patient received a perioperative packed red blood cell (PRBC) transfusion.13 This was later supported with a large prospective analysis revealing that preoperative transfusion significantly improved renal allograft survival, but the survival benefit did not persist in patients receiving a transfusion during the operation.14 Pretransfusion with allogeneic nonleukoreduced PRBC to renal transplant recipients was used as standard immunosuppression technique until the late 1980s, but was subsequently abandoned in favor of more modern immunosuppression strategies.15,16 TRIM was also implicated in significant increases in recurrence rates after resected malignancy17 and postoperative bacterial infection.18
The mechanisms of immunosuppression after transfusion are not known; however, multiple animal studies implicate transfusion-derived donor leukocytes in enhanced cancer progression.19–23 Other authors have specifically postulated that class II major histocompatibility complex (MHC) antigens on allogeneic leukocytes being presented to recipient T lymphocytes24 cause expression of the interleukin (IL)-2 receptor without the required costimulatory signal to induce proliferation and differentiation of alloantigen-specific T-lymphocytes, therefore causing T-cell anergy.25 Regardless of the specific mechanism, the immunosuppression caused by transfusing allogeneic leukocytes has been well documented over the past 4 decades, which suggests the degree of leukocyte contamination reported in the current and previous studies should be alarming, and methods for their removal should be urgently evaluated and implemented.
Although never-frozen liquid plasma is used in many trauma centers as the initial plasma product of choice for urgent transfusion, most plasma units produced nationally are stored as fresh frozen plasma (FFP, frozen within 8 hours of procurement) or PF-24 (frozen within 24 hours of procurement). The freeze-thaw process required for FFP and PF-24 would rupture any contaminating cells (ie platelets and leukocytes), thereby releasing their contents, potentially leading to activation of the innate immune system distinct from the adaptive immunosuppression seen after transfusion of intact allogenic leukocytes.4 Collectively, the inflammatory elements of cellular debris are termed damage associated molecular patterns (DAMPs).26 Evidence that DAMPs contribute to human disease is compelling.6,27–30 Indeed, the transfusion of inflammatory DAMPs to rodents,30,31 pigs,32 and humans4,6 recapitulates many elements of acute respiratory distress syndrome (ARDS) and multiorgan failure. Because patients in hemorrhagic shock are already immunologically primed and receive multiple units of plasma transfusions, the effect from transfusion-associated DAMPs is likely exaggerated when compared with patients who are not in shock.4
Solutions to the potential problems associated with cellular contamination of plasma units may require re-evaluation of current blood banking practices. The majority of plasma units created for transfusion in the US are derived from whole blood centrifugation. The source of the observed cellular contamination seemed most likely to arise during the expression step of plasma preparation. After standard centrifugation of whole blood into 3 distinct layers, the plasma layer is decanted off the top using a hand-operated “plasma expressor” (Fig 4). The remaining red cell mass and buffy coat are put through a cellular reduction filter to eliminate buffy coat contamination (Fig 5). This process is subject to both sample heterogeneity and human error, as the buffy coat has varying levels of distinctness, and blood bank technicians are often responsible for processing multiple units simultaneously. We believe that leukoreduction to remove white blood cells and platelets at the level of whole blood before centrifugation (Fig 6) provides a more distinct interface between the plasma and RBC layers, allowing the technician to more easily visually recognize when to stop the plasma expression step, or facilitating automated termination with existing optical sensor expressor systems. Herein, we have provided an easy and cost-effective solution to this previously unrecognized problem. Indeed, placing a standard, low-cost leukoreduction filter before the centrifugation step completely prevented leukocyte and platelet contamination of stored plasma components when compared to the traditional methods from the same blood bank.
Figure 4.

Plasma expressor.
Figure 5.
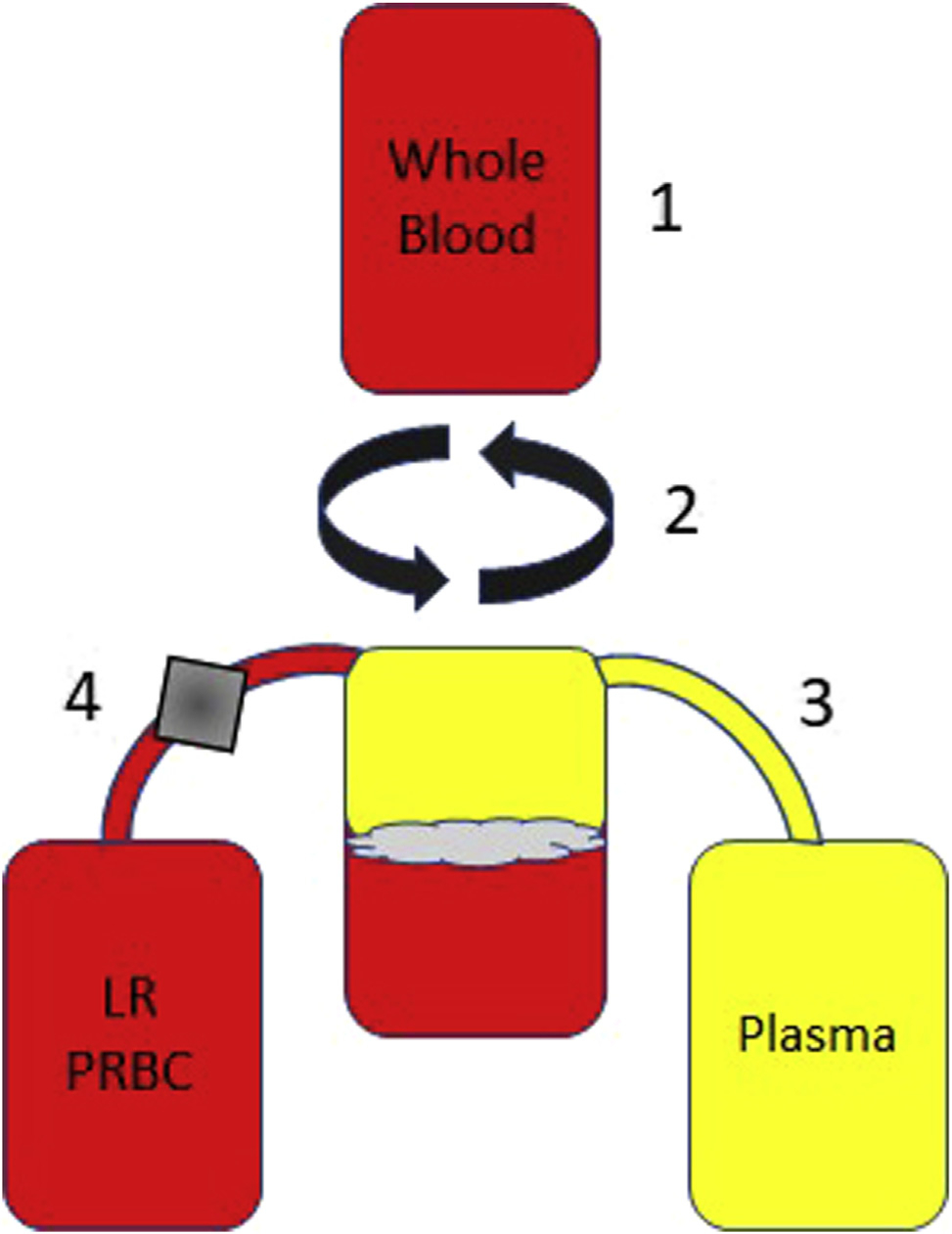
Standard method for creation of non-leukoreduction (non-LR) plasma from whole blood centrifugation. (1) Whole blood is donated, (2) the whole blood is centrifuged, causing the unit to separate into 3 layers (plasma, buffy coat, and red blood cells), (3) the plasma layer is squeezed out of the top of the bag using a plasma expresser (Fig 4), and the technician will stop the expression when cells from the buffy coat enter the line, (4) the remaining buffy coat and red blood cells are put through a cellular reduction filter (grey box represents the position of the LR filter), which removes all leukocytes and platelets. The final products of this method create a unit of LR-packed red blood cells (PRBC) and unit of non-LR plasma.
Figure 6.
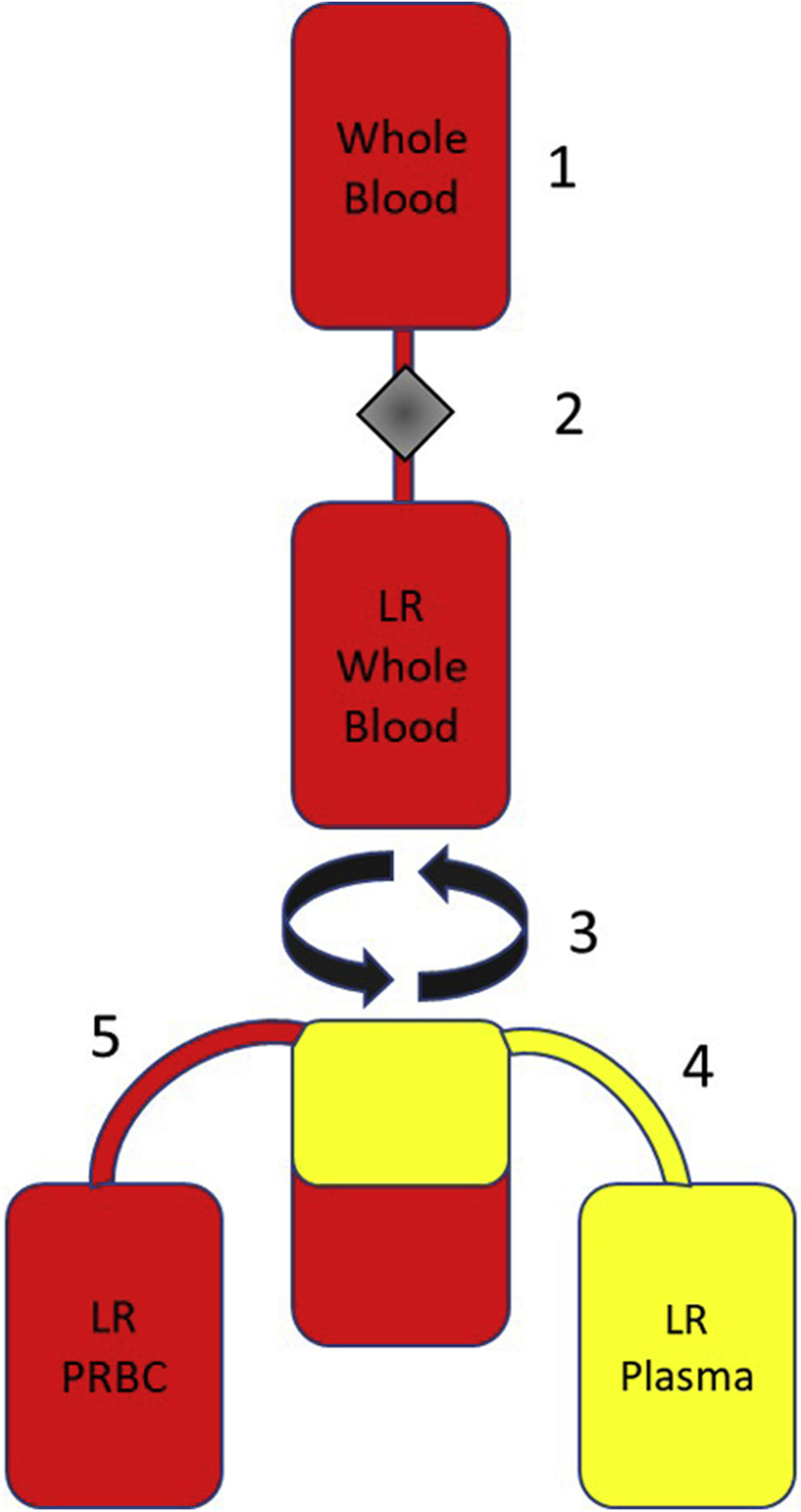
Method for creation of LR plasma from whole blood centrifugation. (1) Whole blood is donated, (2) the whole blood is then put through a cellular reduction filter before (3) centrifugation, which separates the LR whole blood unit into 2 distinct layers (plasma and red blood cells), (4) the plasma layer is squeezed out of the top of the bag using a plasma expresser until the red blood cells are in the line, (5) the remaining red blood cells are already LR. The final products of this method create a unit of LR-PRBC and unit of LR plasma.
As an initial description of this phenomenon and evaluation of a potential low-cost, implementation-ready solution, several limitations to this work must be acknowledged. Specifically, while we evaluated the effect of pre-centrifugation whole blood leukoreduced plasma, ongoing work will be required to enumerate the effect of this strategy on red cell and platelet units as well. While red cell mass is not generally affected by leukoreduction, the effect of pre-centrifugation whole blood leukocyte reduction on platelet count would likely prohibit platelet extraction from units processed in this way. Furthermore, clotting factor level analysis and functional hemostatic potential remain to be evaluated between these LR-plasma and non-LR plasma units to confirm their equivalence before considering pre-centrifugation whole blood leukoreduction as a safe and effective strategy to address the previously unrecognized cellular contamination of plasma described here.
CONCLUSIONS
Despite widespread belief that stored plasma is functionally acellular, liquid plasma at 3 regional blood banks contained significant amounts of previously unrecognized cellular contamination. Intact leukocytes, or inflammatory DAMPs from thawed, ruptured cells may be drive transfusion-related side effects and end-organ injury. Introduction of a leukoreduction step before whole blood centrifugation essentially eliminated detectable leukocyte and platelet contaminants from plasma, highlighting a straightforward and cost-effective method to mitigate cellular contamination, and improve patient safety.
Support:
Dr Simmons is supported by funding from the NIH (K08 GM109113 and UL1 TR001417) and the American College of Surgeons (Clowes Award 2014-2019).
Abbreviations and Acronyms
- DAMP
damage associated molecular patterns
- LR
leukoreduction
- PRBC
packed red blood cells
- TRIM
transfusion-related immunomodulation
Footnotes
Disclosure information: Nothing to disclose.
Presented at the Southern Surgical Association 131st Annual Meeting, Hot Springs, VA, December 2019.
REFERENCES
- 1.Bassuni WY, Blajchman MA, Al-Moshary MA. Why implement universal leukoreduction? Hematol Oncol Stem Cell Ther 2008;1:106–123. [DOI] [PubMed] [Google Scholar]
- 2.Bordin JO, Heddle NM, Blajchman MA. Biologic effects of leukocytes present in transfused cellular blood products. Blood 1994;84:1703–1721. [PubMed] [Google Scholar]
- 3.Sharma RR, Marwaha N. Leukoreduced blood components: Advantages and strategies for its implementation in developing countries. Asian J Transfus Sci 2010;4:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan YB, Rieske RR, Audia JP, et al. Plasma transfusion products and contamination with cellular and associated proinflammatory debris. J Am Coll Surg 2019;229:252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee YL, King MB, Gonzalez RP, et al. Blood transfusion products contain mitochondrial DNA damage-associated molecular patterns: a potential effector of transfusion-related acute lung injury. J Surg Res 2014;191:286–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simmons JD, Lee YL, Pastukh VM, et al. Potential contribution of mitochondrial DNA damage associated molecular patterns in transfusion products to the development of acute respiratory distress syndrome after multiple transfusions. J Trauma Acute Care Surg 2017;82:1023–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holcomb JB, Tilley BC, Baraniuk S, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA 2015;313:471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sysmex XN-1000 R. Sysmex Available at: https://www.sysmex.com/us/en/Products/Hematology/XNSeries/Pages/XN-1000-Hematology-Analyzer.aspx. Accessed February 18, 2020.
- 9.Brand A, Claas FH, Voogt PJ, et al. Alloimmunization after leukocyte-depleted multiple random donor platelet transfusions. Vox Sang 1988;54:160166. [DOI] [PubMed] [Google Scholar]
- 10.Eernisse JG, Brand A. Prevention of platelet refractoriness due to HLA antibodies by administration of leukocyte-poor blood components. Exp Hematol 1981;9:77–83. [PubMed] [Google Scholar]
- 11.Oksanen K, Kekomaki R, Ruutu T, et al. Prevention of alloimmunization in patients with acute leukemia by use of white cell-reduced blood components-a randomized trial. Transfusion 1991;31:588–594. [DOI] [PubMed] [Google Scholar]
- 12.van Marwijk Kooy M, van Prooijen HC, Moes M, et al. Use of leukocyte-depleted platelet concentrates for the prevention of refractoriness and primary HLA alloimmunization: a prospective, randomized trial. Blood 1991;77:201205. [PubMed] [Google Scholar]
- 13.Opelz G, Sengar DP, Mickey MR, Terasaki PI. Effect of blood transfusions on subsequent kidney transplants. Transplant Proc 1973;5:253–259. [PubMed] [Google Scholar]
- 14.Opelz G, Terasaki PI. Improvement of kidney-graft survival with increased numbers of blood transfusions. N Engl J Med 1978;299:799–803. [DOI] [PubMed] [Google Scholar]
- 15.Opelz G, The role of HLA matching and blood transfusions in the cyclosporine era. Collaborative Transplant Study. Transplant Proc 1989;21:609–612. [PubMed] [Google Scholar]
- 16.Ross WB, Yap PL. Blood transfusion and organ transplantation. Blood Rev 1990;4:252e258. [DOI] [PubMed] [Google Scholar]
- 17.Heiss MM, Mempel W, Delanoff C, et al. Blood transfusion-modulated tumor recurrence: first results of a randomized study of autologous versus allogeneic blood transfusion in colorectal cancer surgery. J Clin Oncol 1994;12:1859–1867. [DOI] [PubMed] [Google Scholar]
- 18.Tartter PI, Mohandas K, Azar P, et al. Randomized trial comparing packed red cell blood transfusion with and without leukocyte depletion for gastrointestinal surgery. Am J Surg 1998;176:462–466. [DOI] [PubMed] [Google Scholar]
- 19.Shirwadkar S, Blajchman MA, Frame B, et al. Effect of blood transfusions on experimental pulmonary metastases in mice. Transfusion 1990;30:188–190. [DOI] [PubMed] [Google Scholar]
- 20.Shirwadkar S, Blajchman MA, Frame B, Singal DP. Effect of allogeneic blood transfusion on solid tumor growth and pulmonary metastases in mice. J Cancer Res Clin Oncol 1992; 118:176–180. [DOI] [PubMed] [Google Scholar]
- 21.Blajchman MA, Bardossy L, Carmen R, et al. Allogeneic blood transfusion-induced enhancement of tumor growth: two animal models showing amelioration by leukodepletion and passive transfer using spleen cells. Blood 1993;81:1880–1882. [PubMed] [Google Scholar]
- 22.Bordin JO, Bardossy L, Blajchman MA. Growth enhancement of established tumors by allogeneic blood transfusion in experimental animals and its amelioration by leukodepletion: the importance of the timing of the leukodepletion. Blood 1994; 84:344–348. [PubMed] [Google Scholar]
- 23.Francis DM, Clunie GJ. Influence of the timing of blood transfusion on experimental tumor growth. J Surg Res 1993; 54:237–241. [DOI] [PubMed] [Google Scholar]
- 24.Meryman HT. Transfusion-induced alloimmunization and immunosuppression and the effects of leukocyte depletion. Transfus Med Rev 1989;3:180–193. [DOI] [PubMed] [Google Scholar]
- 25.Mincheff MS, Meryman HT. Costimulatory signals necessary for induction of T cell proliferation. Transplantation 1990;49: 768–772. [DOI] [PubMed] [Google Scholar]
- 26.Krysko DV, Agostinis P, Krysko O, et al. Emerging role of damage-associated molecular patterns derived from mitochondria in inflammation. Trends Immunol 2011;32:157–164. [DOI] [PubMed] [Google Scholar]
- 27.Simmons JD, Lee YL, Mulekar S, et al. Elevated levels of plasma mitochondrial DNA DAMPs are linked to clinical outcome in severely injured human subjects. Ann Surg 2013; 258:591e596; discussion 596–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamanouchi S, Kudo D, Yamada M, et al. Plasma mitochondrial DNA levels in patients with trauma and severe sepsis: time course and the association with clinical status. J Crit Care 2013;28:1027–1031. [DOI] [PubMed] [Google Scholar]
- 29.Schumacker PT, Gillespie MN, Nakahira K, et al. Mitochondria in lung biology and pathology: more than just a powerhouse. Am J Physiol Lung Cell Mol Physiol 2014;306:L962–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuck JL, Obiako BO, Gorodnya OM, et al. Mitochondrial DNA damage-associated molecular patterns mediate a feed-forward cycle of bacteria-induced vascular injury in perfused rat lungs. Am J Physiol Lung Cell Mol Physiol 2015;308: L1078–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Q, Raoof M, Chen Y, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 2010; 464:104–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanchez PG, Pasrija C, Mulligan MJ, et al. A novel large animal model of acute respiratory distress syndrome induced by mitochondrial products. Ann Surg 2017;266:1091–1096. [DOI] [PubMed] [Google Scholar]


