Abstract
Objective
To investigate whether sex and race differences exist in dementia diagnosis risk associated with traumatic brain injury (TBI) among older veterans.
Methods
Using Fine-Gray regression models, we investigated incident dementia diagnosis risk with TBI exposure by sex and race.
Results
After the exclusion of baseline prevalent dementia, the final sample (all veterans ≥55 years of age diagnosed with TBI during the 2001–2015 study period and a random sample of all veterans receiving Veterans Health Administration care) included nearly 1 million veterans (4.3% female; 81.8% White, 11.5% Black, and 1.25% Hispanic), 96,178 with TBI and 903,462 without TBI. Compared to those without TBI, Hispanic veterans with TBI were almost 2 times more likely (17.0% vs 10.3%; hazard ratio [HR] 1.74, 95% confidence interval [CI] 1.51–2.01), Black veterans with TBI were >2 times more likely (11.2% vs 6.4%; HR 2.15, 95% CI 2.02–2.30), and White veterans with TBI were nearly 3 times more likely to receive a dementia diagnosis (12.0% vs 5.9%; HR 2.71, 95% CI 2.64–2.77). A significant interaction between TBI and race for dementia diagnosis was observed (p < 0.001). Both male and female veterans with TBI were more than twice as likely (men 11.8% vs 5.9%, HR 2.60, 95% CI 2.54–2.66; women 6.3% vs 3.1%, HR 2.36, 95% CI 2.08–2.69) to receive a diagnosis of dementia compared to those without. There was a significant interaction effect between sex and TBI (p = 0.02), but the magnitude of differences was small.
Conclusions
In this large, nationwide cohort of older veterans, all race groups with TBI had increased risk of dementia diagnosis, but there was an interaction effect such that White veterans were at greatest risk for dementia after TBI. Further research is needed to understand the mechanisms for this discrepancy. Differences in dementia diagnosis risk for men and women after TBI were significant but small, and male and female veterans had similarly high risks of dementia diagnosis after TBI.
Traumatic brain injury (TBI), including mild TBI,1 is a well-known risk factor for dementia.2–8 Veterans are particularly at risk for TBI and therefore may be more vulnerable to developing dementia.9 Most existing research on TBI and risk of dementia diagnosis in veterans has been conducted with predominately male and White participants. As the US military becomes more diverse, understanding the outcomes that female and non-White service members may face after TBI is essential. Women are increasingly involved in combat and at risk for TBI,10 and the number of female veterans, particularly those >55 years of age, is expected to rise sharply in the coming years.11 The proportion of Black, Hispanic, and other (including American Indian/Alaska Native, Asian, and Pacific Islander) minority veterans is also expected to climb.12
The few studies that directly examine the effect of sex on risk of dementia diagnosis after TBI2,13,14 have shown small increases in risk for men but not women. Most existing studies of risk for dementia diagnosis after TBI do not directly examine race differences, and many do not report the racial makeup of their samples.15 Understanding the possible impact of sex- and race-based health differences on dementia diagnosis risk is key to improving care for the growing population of diverse older veterans with TBI.
The goal of our study was to examine whether differences in TBI-associated risk of dementia diagnosis by sex and race exist among a large cohort of older veterans and to evaluate the impact of other demographic factors, medical comorbid conditions, and psychiatric conditions on this relationship.
Methods
Standard protocol approvals, registrations, and patient consents
All study procedures were approved by the institutional review boards of the University of California, San Francisco and San Francisco Veterans Affairs (VA) Medical Center and by the US Army Medical Research and Material Command, Office of Research Protections, Human Research Protection Office. Informed consent was waived because of the use of deidentified archival data. In addition, many participants were deceased or no longer receiving medical care through the VA at the time of study completion.
Study population
We identified all Veterans Health Administration (VHA) patients ≥55 years of age who received a TBI diagnosis between October 1, 2001, and September 30, 2015, and a 2% random sample of patients who received VHA care within the same time frame (n = 1,024,601). Data were sourced from 2 nationwide VHA system databases: the inpatient and outpatient visits database (National Patient Care Database) and the Vital Status File. We excluded veterans with prevalent dementia during the 2-year baseline period (defined as within 2 years before the index date, i.e., the date of TBI diagnosis or random selection date) (n = 24,959). The final sample size was 999,642.
We identified all VHA patients who received an inpatient or outpatient TBI diagnosis using the Defense and Veterans Brain Injury Center list of ICD-9 codes for TBI surveillance (data available from Dryad, appendix 1, doi.org/10.7272/Q6V69GSD). We next identified prevalent dementia at baseline using the VA Dementia Steering Committee's recommended list of ICD-9 codes (2016 version)16 (data available from Dryad, appendix 2). For incident dementia diagnoses during the follow-up period, we used a modified version of the same list that excluded prion disease and alcohol- or drug-induced dementia.
Biological sex data were also taken from VHA inpatient or outpatient files in which each veteran was coded as male or female. Two participants had missing sex data, and the final sample size was 999,640 for sex analyses. Race and ethnicity were retrieved from VHA inpatient and outpatient files (supplemented with Medicare data after 2004). Veterans were coded as non-Hispanic Black, non-Hispanic White, Hispanic, or other/unknown. The other/unknown race category was removed from the final unadjusted and adjusted race models because of the likelihood of missing data in the unknown group confounding interpretation of information about respondents in the other category. These codes are based on self-report of patient sex and race. The final sample size was 937,380 for race analyses, reflecting 62,262 participants with missing data (other/unknown) for race.
We obtained data on demographics, medical comorbid conditions, health care visits, and psychiatric conditions using VHA inpatient and outpatient files. Zip codes and 2016 American Community Survey data were used to categorize veterans' residences into educational and income categories (for education, ≤25% of the adult population has a bachelor's degree or higher vs >25%; income was categorized into median income tertiles). Medical and psychiatric comorbid conditions as identified by ICD-9 codes were assessed during the 2-year baseline. Comorbid conditions included hypertension, diabetes mellitus, myocardial infarction, TIA/stroke, chronic pain, posttraumatic stress disorder, depression, drug/alcohol abuse, and tobacco use or smoking. Health care visits were defined as any inpatient or outpatient visit from VA medical records and included information on date of visit and diagnosis.
Baseline characteristics of veterans in each race and sex group were compared by TBI status with t tests for continuous variables and χ2 tests for categorical variables. Although TBI prevalence by race and sex is reported, these data points represent estimates only; because of the oversampling of patients with TBI in our sample, these figures lack precision. We used Fine-Gray proportional hazards regression models, accounting for the competing risk of death, to examine time to dementia diagnosis according to TBI status for each sex and race group with age as the time scale. Models were unadjusted and adjusted for demographics and medical/psychiatric comorbid conditions that significantly differed between sex/race groups at p < 0.01 (age, race or sex, education, income, hypertension, diabetes mellitus, myocardial infarction, TIA/stroke, chronic pain, posttraumatic stress disorder, depression, drug/alcohol abuse, and tobacco use/smoking). For cumulative incidence graphs (figures 1 and 2), we used 95 years of age as a cutoff point, and 1% of data (n = 9,751) were truncated. Assumptions of the Fine-Gray models were examined and found to be satisfied. We used the cumulative residuals with respect to time (SAS ASSESS statement) to test the proportional hazards assumption.
Figure 1. Adjusted* cumulative incidence of dementia.
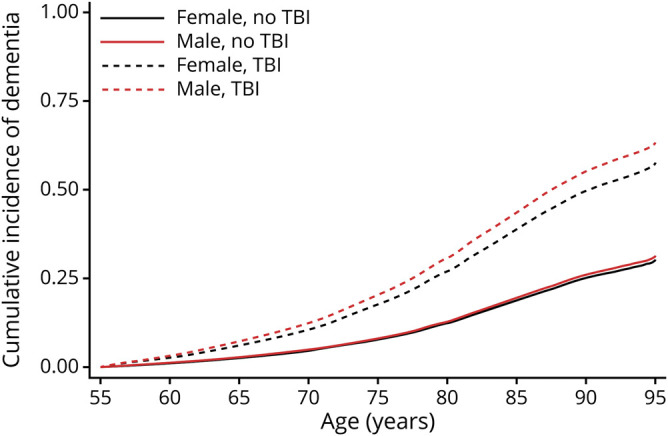
Age at dementia diagnosis with and without traumatic brain injury (TBI), accounting for mortality in male and female veterans. *Adjusted for demographic and health characteristics.
Figure 2. Adjusted* cumulative incidence of dementia.
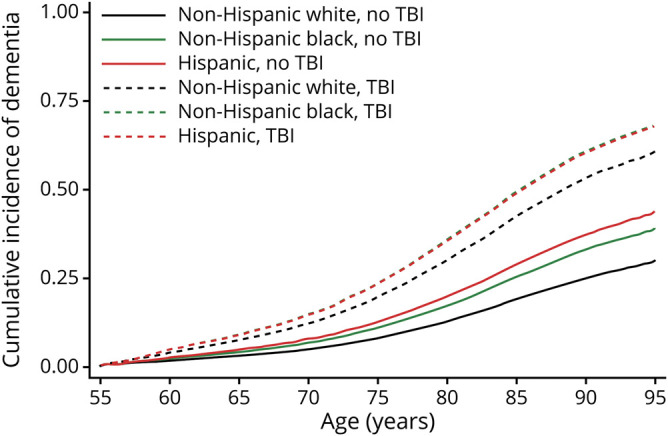
Age at dementia diagnosis with and without traumatic brain injury (TBI), accounting for mortality in White, Black, and Hispanic veterans. *Adjusted for demographic and health characteristics.
We also separately examined the interaction effect of TBI with sex and with race on risk of dementia diagnosis in adjusted models and subsequently conducted stratified analyses to examine the interactions. Finally, in sensitivity analyses, we (1) conducted models in which we excluded veterans receiving a dementia diagnosis within 1 year of TBI diagnosis for a washout period to address concerns about reverse causation and etiology (TBI vs neurodegenerative) and (2) examined the impact of number of health care visits during the follow-up period to determine whether increased involvement in/access to health care accounted for some of the race-based differences in dementia diagnoses we identified. Statistical significance was set at p < 0.05 (2 sided). SAS version 9.4 (SAS Institute Inc, Cary, NC) was used for all analyses.
Data availability
The data are derived from VHA electronic health records and contain protected health information; therefore, the data cannot be placed in a public repository. Please contact the authors for additional details on the process of accessing these data.
Results
The final analytic cohort (all veterans ≥55 years of age with TBI during the study period and a 2% random sample of all veterans in the VHA) included 96,178 veterans with TBI and 903,464 veterans without TBI (4.3% female; 81.8% White, 11.5% Black, and 1.25% Hispanic). The median follow-up was 4.3 years (interquartile range 1.9–7.6 years).
TBI risk for dementia diagnosis by sex
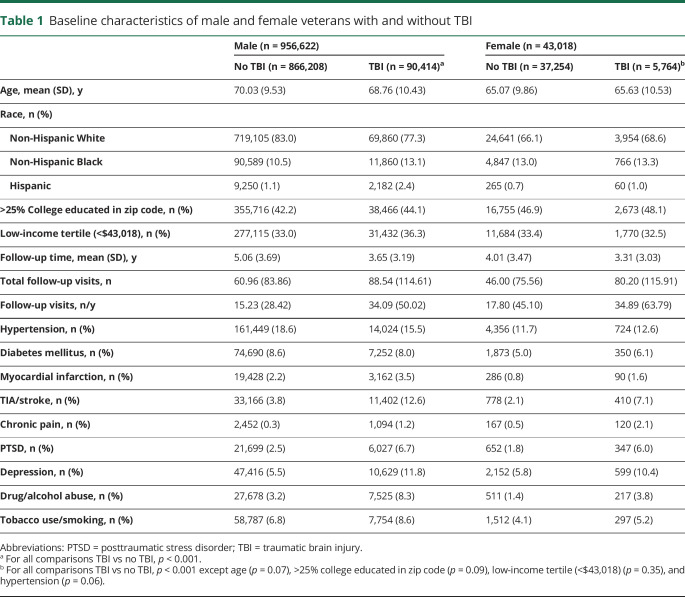
Table 1 shows characteristics of male and female veterans with and without TBI. Male veterans were older (p < 0.001). All veterans with TBI regardless of sex had a higher prevalence of medical and psychiatric comorbid conditions compared to those without TBI history. Among male veterans, those with TBI were more than twice as likely to receive a dementia diagnosis (hazard ratio [HR] 2.87, 95% confidence interval [CI] 2.81–2.94) compared to those without a TBI diagnosis. Among women, those with TBI vs no TBI were more than twice as likely to receive a dementia diagnosis (HR 2.51, 95% CI 2.22–2.84). The difference lessened somewhat after adjustment for demographics and comorbid conditions (men: HR 2.60, 95% CI 2.54–2.66; women: HR 2.36, 95% CI 2.08–2.69). There was a significant interaction effect of sex and TBI on dementia diagnosis risk (p = 0.02) such that men with TBI demonstrated slightly increased risk of receiving a dementia diagnosis compared to women. The interaction between sex and TBI on dementia diagnosis risk remained significant after adjustment (p = 0.03). Adjusted cumulative incidence curves for age at dementia diagnosis accounting for competing risk of mortality are shown in figure 1 for male and female veterans.
Table 1.
Baseline characteristics of male and female veterans with and without TBI
TBI risk for dementia diagnosis by race
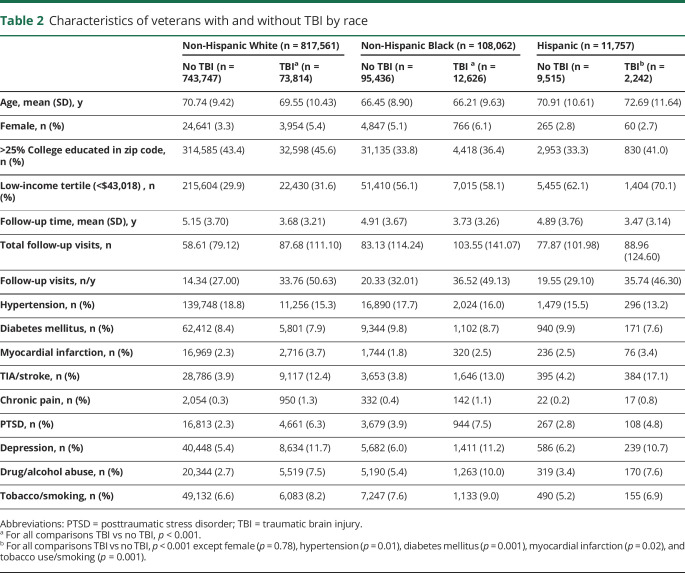
Table 2 shows characteristics of White, Black, and Hispanic veterans with and without TBI. Across all race groups, those with TBI were generally younger, more likely to be female, better educated, more likely to fall in the low-income group, and less likely to be diagnosed with hypertension and diabetes mellitus but otherwise had more health and psychological comorbid conditions compared to those without TBI history. The Hispanic group was unique, however, in that Hispanic veterans with TBI were older (p < 0.001) and did not differ from Hispanic veterans without TBI on sex (p = 0.78). All veterans with TBI were much more likely to fall in the low-income group, but low-income group membership was most likely in the Black and Hispanic groups (31.6% of White veterans with TBI compared to 58.1% of Black veterans and 70.1% of Hispanic veterans with TBI).
Table 2.
Characteristics of veterans with and without TBI by race
White veterans with TBI had an almost 3-fold increased risk of dementia diagnosis (HR 2.93, 95% CI 2.86–3.00) compared to those without TBI, while Black and Hispanic veterans with TBI had an ≈2-fold increased risk (Blacks: HR 2.27, 95% CI 2.13–2.41; Hispanics: HR 1.98, 95% CI 1.74–2.24). There was a significant interaction between TBI and race on risk of receiving a dementia diagnosis (p < 0.001) such that White veterans with TBI were at the highest risk for dementia diagnosis with similar risks for Blacks and Hispanics. After adjustment for demographics and medical and psychiatric conditions, White veterans with TBI remained at higher risk (HR 2.71, 95% CI 2.64–2.77) compared to Black and Hispanic veterans with TBI (Blacks: HR 2.15, 95% CI 2.02–2.30; Hispanics: HR 1.74, 95% CI 1.51–2.01). The interaction between TBI and race on risk of dementia diagnosis remained after full adjustment (p < 0.001). Adjusted cumulative incidence curves for age at dementia diagnosis accounting for competing risk of mortality are shown in figure 2 for White, Black, and Hispanic veterans. Results of 1-year lag washout sensitivity analyses showed slightly attenuated HRs, but the pattern of sex and race results was identical. Additional adjustment for number of clinic visits slightly attenuated risk estimates but did not change the pattern of results (Whites: HR 2.33, 95% CI 2.26–2.39; Blacks: HR 1.94, 95% CI 1.82–2.08; and Hispanics: HR 1.63, 95% CI 1.41–1.89).
Discussion
In this diverse sample of older veterans, we show an increased risk of dementia diagnosis in those with a diagnosis of TBI compared to those without for veterans of both sexes and all major race groups, consistent with previous work on this topic in the veteran population.9 We also identified differences in the risk of dementia diagnosis after TBI according to race. Specifically, older White veterans appear to have an elevated risk of receiving a dementia diagnosis after TBI compared to Blacks and Hispanics. Sex differences in dementia diagnosis risk after TBI observed in this large sample, although statistically significant, were small and of unclear clinical significance.
The limited available data on the effect of sex on dementia risk after TBI show increased risk for men but not women. For example, an older meta-analysis of 11 case-control studies conducted before 1991 suggested that there is an increased risk of dementia (specifically Alzheimer disease) after TBI for men but not women2; another meta-analysis published in 2003 examining 7 additional studies replicated that finding.13 A recent population-based study in Denmark conducted in 2018 similarly found a slightly increased risk of dementia after TBI in men compared to women (30% vs 19% increased risk).14 Our results showing a similarly high risk for both men and women are inconsistent with this prior work. Therefore, further exploration of sex-based differences in dementia risk after TBI for veterans is indicated. For example, it is possible that although the TBIs of female civilians may be less severe on average than those of male civilian, male and female veterans may have TBIs of similar severity. In addition, women in the military may experience a unique profile of injuries in which repeated injuries caused by intimate partner violence are superimposed on single or multiple concussive or subconcussive head injuries, conferring elevated dementia risk compared to female civilians.
Most existing studies of the risk for dementia after TBI do not directly examine race differences, and many do not report the racial makeup of their samples.15 For example, in a recent review of the evidence for the association between TBI and dementia, race was not listed as a known demographic factor affecting that relationship.17 Therefore, our finding that White veterans may be at increased risk for dementia after TBI is intriguing. Our findings stand in contrast to previous research that has shown that Black and Hispanic adults have worse functional outcomes (as defined by standardized measures such as the Disability Rating Scale, Functional Independence Measure, and Community Integration Questionnaire) compared to White adults 1 year after moderate to severe TBI.18 However, the different methodologic approach in our work, which uses medical record data including diagnostic codes rather than standardized measures of functional outcome, may account for some of these discrepancies. Our results may also be explained by race-based differences in the documentation of dementia diagnoses by health care providers; if providers are, for example, more likely to consider dementia as a diagnosis for White patients, that could account for our findings of increased dementia diagnosis risk for White veterans.
It is clear that more research is needed to understand the impact of race on dementia diagnosis risk after TBI. Differential risk for dementia by race among veterans is unknown and a topic of current ongoing research, and it may be the case that non-White veterans have higher baseline risk such that having a TBI may not lead to increased risk for these race groups as it does for Whites. Health disparities research suggests that White individuals may be more likely to interact with health care and to receive a diagnosis,19,20 which may result in inflated rates of TBI and dementia diagnoses for White veterans compared to other groups. However, in our sample, White veterans had fewer follow-up visits compared to Black and Hispanic veterans, and after adjustment for number of visits, the increased risk of dementia after TBI for White veterans persisted. It is also possible that Black and Hispanic individuals, who are significantly more likely to live in multigenerational households with high levels of family support compared to White individuals,21 may function well independently in the community for longer because of this increased support and therefore delay receiving a dementia diagnosis. However, all veterans were followed up at VA; therefore, cognitive problems were likely to have been detected, even in the absence of concern from patients or families. In addition, there may be unmeasured and unrecognized social factors affecting differences in medical care and driving differences in outcomes between race groups that deserve further study in the future.
Furthermore, we did not measure APOE ε4 allele status, which differs by race and increases risk for dementia.22 Although the allele is more common among individuals of African descent,23 White individuals have a greater increased risk for dementia with APOE ε4 compared to other racial groups.24 These findings support our results showing increased risk for White veterans. There is also some evidence that APOE ε4 increases risk for negative outcome, including dementia, after TBI,25–27 which may be related to its decreased ability to effectively protect and repair neural tissue after trauma compared to APOE ε3.28 Other unknown and unmeasured genetic factors may play a role in the race differences and the increased risk of dementia diagnosis for White veterans after TBI seen here, and further research is required to identify such mechanisms.
Although our study was not designed to precisely measure the prevalence of TBI among older veterans, the TBI prevalence estimates we report suggest differential patterns in the prevalence of TBI by both sex and race in our sample that are clinically interesting and bear further study. Our results suggest a greater prevalence of TBI in female veterans compared to male veterans. These results may reflect a departure from civilian findings, which generally show higher rates of TBI in men.29 Our results also suggest the possibility of increased prevalence of TBI among Hispanic veterans compared to Black and White veterans. This pattern may represent previously unacknowledged health disparity, and in fact there is a dearth of research available on the prevalence or risk of TBI by race among veterans and civilians, an area clearly requiring further study. Existing VA research shows that Hispanic Operation Enduring Freedom/Operation Iraqi Freedom veterans are less likely to receive care for a TBI and that Hispanic veterans of all eras are at higher risk for mortality after a TBI,30,31 but these studies do not report prevalence of TBI among Hispanic veterans. In contrast, our results suggest that Hispanic veterans receive more follow-up care compared to White veterans but less than Black veterans. These patterns demonstrate that further research focused specifically on investigating race and sex differences in TBI prevalence among veterans is clearly indicated.
There are some important limitations to our study that affect the interpretation and generalizability of our results. For example, sex and race were based on self-report, and sex was coded as a binary variable only (i.e., transgender individuals were not captured), likely excluding some of the true complexity of this variable. Furthermore, our sample had some limitations: we were unable to examine Asian veterans and veterans identifying their race/ethnicity as other because of small sample size. Further research focused on Asian Veteran samples and those who identify their race as other would be helpful and would provide insights for treatment planning and prevention as this growing cohort of veterans ages. Moreover, because of oversampling of patients with TBI, we are unable to precisely measure TBI prevalence in veterans. Although TBI prevalence by race and sex is reported, these data points represent estimates only. In addition, because of our use of medical record data, there are likely to be differences between the sex and race groups studied that we were not able to measure but that are driving differences in risk of dementia after TBI. In addition, we used ICD-9 codes in existing medical records for dementia diagnoses, which may result in less accurate categorization of participants compared to studies in which participants were given a comprehensive dementia examination. Because we included veterans in our sample who may have received a dementia diagnosis shortly after their TBI diagnosis, we are not able to draw conclusions about causality of dementia diagnoses or to make inferences about neurodegenerative vs traumatic etiology. Finally, results may not generalize to veterans who do not receive VA health care.
This is one of the first studies to examine differential risk for dementia diagnosis after TBI according to sex and race. Key contributions of this study include the large sample size and the direct, explicit consideration of race and sex and their impact on dementia risk after TBI among veterans. Our results show a doubling of dementia diagnosis risk after TBI for both men and women and an interesting difference by sex that is small and of unclear clinical significance. Risk of dementia diagnosis was also approximately doubled for all veterans across race categories after TBI, with White veterans showing an even greater increased risk. These findings suggest that understating the possible differential impact of TBI on dementia diagnosis risk according to race is worth exploring. This is of particular importance given the increasing diversity and rapid aging of our military and veteran populations and may provide the VA with an important opportunity to identify and correct possible health disparities in TBI and dementia identification and care.
Glossary
- CI
confidence interval
- HR
hazard ratio
- ICD-9
International Classification of Disease, 9th Revision
- TBI
traumatic brain injury
- VA
Veterans Affairs
- VHA
Veterans Health Administration
Appendix. Authors
Footnotes
Editorial, page 561
Study funding
This work was supported by Veterans Affairs Rehabilitation Research and Development Career Development Award 1 IK2 RX003073-01A2 (E.K.). It was also funded by the National Institute on Aging (K24 AG031155 to K.Y.) and by the US Army Medical Research and Material Command and the US Department of Veterans Affairs (Long-Term Impact of Military-Relevant Brain Injury Consortium) under awards W81XWH-18-1-0692, W81XWH-19-2-0067, and 1I01CX002069. The US Army Medical Research Acquisition Activity, Fort Detrick, MD, is the awarding and administering acquisition office. Any opinions, findings, conclusions or recommendations expressed in this publication are those of the authors and do not necessarily reflect the views of the US government or the US Department of Veterans Affairs, and no official endorsement should be inferred.
Disclosure
E. Kornblith, C.B. Peltz, F. Xia, B. Plassman, and T. Novakovic-Apopain report no disclosures relevant to the manuscript. K. Yaffe serves on Data Safety Monitoring boards for Eli Lilly and several National Institute on Aging–sponsored studies, serves on the board of directors for Alector, Inc, and is member of the Beeson Scientific Advisory Board. Go to Neurology.org/N for full disclosures.
References
- 1.Barnes DE, Byers AL, Gardner RC, Seal KH, Boscardin WJ, Yaffe K. Association of mild traumatic brain injury with and without loss of consciousness with dementia in US military veterans. JAMA Neurol 2018;75:1055–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mortimer J, van Duijn CM, Chandra V, et al. Head trauma as a risk factor for Alzheimer's disease: a collaborative re-analysis of case-control studies. Int J Epidemiol 1991;20(suppl 2):S28–S35. [DOI] [PubMed] [Google Scholar]
- 3.Plassman BL, Havlik RJ, Steffens DC, et al. Documented head injury in early adulthood and risk of Alzheimer's disease and other dementias. Neurology 2000;55:1158–1166. [DOI] [PubMed] [Google Scholar]
- 4.Guo Z, Cupples LA, Kurz A, et al. Head injury and the risk of AD in the MIRAGE study. Neurology 2000;54:1316–1323. [DOI] [PubMed] [Google Scholar]
- 5.Wang HK, Lin SH, Sung PS, et al. Population based study on patients with traumatic brain injury suggests increased risk of dementia. J Neurol Neurosurg Psychiatry 2012;83:1080–1085. [DOI] [PubMed] [Google Scholar]
- 6.Gardner RC, Burke JF, Nettiksimmons J, Kaup A, Barnes DE, Yaffe K. Dementia risk after traumatic brain injury vs nonbrain trauma: the role of age and severity. JAMA Neurol 2014;71:1490–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee YK, Hou SW, Lee CC, Hsu CY, Huang YS, Su YC. Increased risk of dementia in patients with mild traumatic brain injury: a nationwide cohort study. PLoS One 2013;8:e62422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nordström P, Michaëlsson K, Gustafson Y, Nordström A. Traumatic brain injury and young onset dementia: a nationwide cohort study. Ann Neurol 2014;75:374–381. [DOI] [PubMed] [Google Scholar]
- 9.Barnes DE, Kaup A, Kirby KA, Byers AL, Diaz-Arrastia R, Yaffe K. Traumatic brain injury and risk of dementia in older veterans. Neurology 2014;83:312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amoroso T, Iverson KM. Acknowledging the risk for traumatic brain injury in women veterans. J Nerv Ment Dis 2017;205:318–323. [DOI] [PubMed] [Google Scholar]
- 11.National Center for Veterans Analysis and Statistics. Living veterans by age, group, gender, 2015–2045. Available at: va.gov/vetdata/veteran_population.asp. Accessed July 2019. [Google Scholar]
- 12.National Center for Veterans Analysis and Statistics. 2014 Minority veterans report. Available at: va.gov/vetdata/docs/SpecialReports/Minority_Veterans_2014.pdf. Accessed July 2019. [Google Scholar]
- 13.Fleminger S, Oliver DL, Lovestone S, Rabe-Hesketh S, Giora A. Head injury as a risk factor for Alzheimer's disease: the evidence 10 years on; a partial replication. J Neurol Neurosurg Psychiatry 2003;74:857–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fann JR, Ribe AR, Pedersen HS, et al. Long-term risk of dementia among people with traumatic brain injury in Denmark: a population-based observational cohort study. Lancet Psychiatry 2018;5:424–431. [DOI] [PubMed] [Google Scholar]
- 15.Bazarian JJ, Zhong J, Blyth B, Zhu T, Kavcic V, Peterson D. Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: a pilot study. J Neurotrauma 2007;24:1447–1459. [DOI] [PubMed] [Google Scholar]
- 16.US Department of Veterans Affairs (VA) Veterans Health Administration (VHA) Dementia Steering Committee (DSC). VHA Dementia Steering Committee recommendations for dementia care in the VHA healthcare system 2016 . 2016. Available at: va.gov/GERIATRICS/docs/VHA_DSC_RECOMMENDATIONS_SEPT_2016_9-12-16.pdf. Accessed September 3, 2019. [Google Scholar]
- 17.Peterson K, Veazie S, Bourne D, Anderson J. Evidence Brief: Traumatic Brain Injury and Dementia. Washington, DC: US Department of Veterans Affairs; 2019. [PubMed] [Google Scholar]
- 18.Arango-Lasprilla JC, Rosenthal M, Deluca J, et al. Traumatic brain injury and functional outcomes: does minority status matter? Brain Inj 2007;21:701–708. [DOI] [PubMed] [Google Scholar]
- 19.Hall WJ, Chapman MV, Lee KM, et al. Implicit racial/ethnic bias among health care professionals and its influence on health care outcomes: a systematic review. Am J Public Health 2015;105:e60–e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boulware LE, Cooper LA, Ratner LE, LaVeist TA, Powe NR. Race and trust in the health care system. Public Health Rep 2016;118:358–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor P, Passel J, Fry R, et al. The Return of the Multi-Generational Family Household. Pew Research Center; 2010. Available at: pewsocialtrends.org/2010/03/18/the-return-of-the-multi-generational-family-household/. Accessed July 2019. [Google Scholar]
- 22.Bookheimer S, Burggren A. APOE-4 genotype and neurophysiological vulnerability to Alzheimer's and cognitive aging. Annu Rev Clin Psychol 2009;5:343–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuller LH, Shemanski L, Manolio T, et al. Relationship between ApoE, MRI findings, and cognitive function in the Cardiovascular Health Study. Stroke 1998;29:388–398. [DOI] [PubMed] [Google Scholar]
- 24.Bretsky P, Guralnik JM, Launer L, Albert M, Seeman TE; MacArthur Studies of Successful Aging. The role of APOE-ε4 in longitudinal cognitive decline: MacArthur Studies of Successful Aging. Neurology 2003;60:1077–1081. [DOI] [PubMed] [Google Scholar]
- 25.Verghese PB, Castellano JM, Holtzman DM. Apolipoprotein E in Alzheimer's disease and other neurological disorders. Lancet Neurol 2011;10:241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou W, Xu D, Peng X, Zhang Q, Jia J, Crutcher K. Meta-analysis of APOE 4 allele and outcome after traumatic brain injury. J neurotrauma 2008;25:279–290. [DOI] [PubMed] [Google Scholar]
- 27.Magnoni S, Brody DL. New perspectives on amyloid-β dynamics after acute brain injury: moving between experimental approaches and studies in the human brain. Arch Neurol 2010;67:1068–1073. [DOI] [PubMed] [Google Scholar]
- 28.Bu G. Apolipoprotein E and its receptors in Alzheimer's disease: pathways, pathogenesis and therapy. Nat Rev Neurosci 2009;10:333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor CA, Bell JM, Breiding MJ, Xu L. Traumatic brain injury–related emergency department visits, hospitalizations, and deaths: United States, 2007 and 2013. MMWR Surveill Summ 2017;66:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arriola V, Rozelle J. Traumatic brain injury in United States Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) Hispanic veterans: a review using the PRISMA method. Behav Sci 2016;6:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Egede LE, Dismuke C, Echols C. Racial/ethnic disparities in mortality risk among US veterans with traumatic brain injury. Am J Public Health 2012;102:S266–S271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are derived from VHA electronic health records and contain protected health information; therefore, the data cannot be placed in a public repository. Please contact the authors for additional details on the process of accessing these data.