Abstract
Objective
To describe short-term and 5-year rates of mortality and poor outcome in patients with spontaneous aneurysmal subarachnoid hemorrhage (aSAH) who received repair treatment.
Methods
In this prospective observational study, mortality and poor outcome (modified Rankin Scale score 3–6) were analyzed in 311 patients with aSAH at 3 months, 1 year, and 5 years follow-up. Sensitivity analysis was performed according to treatment modality. In-hospital and 5-year complications were analyzed.
Results
Of 476 consecutive patients with spontaneous subarachnoid hemorrhage, 347 patients (72.9%) had aSAH. Of these, 311 (89.6%) were treated (242 endovascular, 69 neurosurgical), with a mean follow-up of 43.4 months (range, 1 to 145). Three-month, 1-year, and 5-year mortality was 18.4%, 22.9%, and 29.0%, and poor outcome was observed in 42.3%, 36.0%, and 36.0%, respectively. Adjusted poor outcome was lower in endovascular than in neurosurgical treatment at 3 months (odds ratio [OR] 0.36 [95% confidence interval [CI] 0.18-0.74]), with an absolute difference of 15.8% (number needed to treat = 6.3), and at 1 year (OR = 0.40 [95% CI 0.20-0.81]), with an absolute difference of 15.9% (number needed to treat = 6.3). Complications did not differ between the 2 procedures. However, mechanical ventilation was less frequent with the endovascular technique (OR 0.67 [95% CI 0.54–0.84]).
Conclusions
Patients with aSAH treated according to current guidelines had a short-term mortality of 18.4% and 5-year mortality of 29%. The majority (64.0%) of patients remained alive without disabilities at 5-year follow-up. Patients prioritized to endovascular treatment had better outcomes than those referred to neurosurgery because endovascular coiling was not feasible.
Spontaneous aneurysmal subarachnoid hemorrhage (aSAH) carries a high risk of early mortality and of long-term disability in survivors. Available outcome data, mainly for long-time follow-up, are considered scarce1 or very poor.2 Short-term mortality has declined3–6 to approximately 25%–30%.7,8 Long-term mortality, as reported in 2014, is 17.9% at 10 years, 29.5% at 15 years, and 43.6% at 20 years after subarachnoid hemorrhage (SAH).9 Other available information is based on the International Subarachnoid Aneurysm Trial (ISAT)10,11 and Barrow Ruptured Aneurysm Trial (BRAT) results.12–14 Little information is available regarding functional outcome. At 1-year follow-up, poor overall quality of life has been described in 35% of patients after SAH,15 and poor prognosis (modified Rankin Scale [mRS] score 3 to 6) was observed in 42.4% of patients with SAH.16 In studies using home discharge data as an indicator, “good outcome” rates have ranged from 43.3%17 to 54.4%.7
The aim of our study was to describe the short- and long-term clinical evolution during comprehensive follow-up of a prospective series of patients with aSAH treated by a multidisciplinary expert team, following the current guidelines.2,18 A secondary aim was to analyze mortality and poor outcome according to endovascular or neurosurgical repair technique.
Methods
Standard protocol approvals, registrations, and patient consent
The information used in this study was collected from the prospective BASICMAR Register with the approval of our local ethics committee (CEIC PSMAR). We obtained written informed consent from all patients or a designated representative, as appropriate. There were no losses due to lack of informed consent or to study withdrawal.
Study population
Hospital del Mar is a tertiary stroke center included in the Catalan SAH care system. One weekend each month, it serves as the only referral center for all patients with a diagnosis of SAH in Catalonia (7,400,000 inhabitants). This was a prospective observational study of consecutive patients admitted to our hospital from January 2007 to December 2018 with a diagnosis of aSAH (n = 476). Data were obtained from the BASICMAR Register maintained by our center.
Patients with confirmed aSAH by cerebral angiography admitted and treated in our center were considered for inclusion in the study. Patients with trauma-related SAH are not included in the SAH-BASICMAR Register. Exclusion criteria were nonaneurysmal SAH, SAH due to another cerebral vascular malformation, or lack of angiography data.
A structured questionnaire (SAH-BASICMAR) was used to record the following variables: age, sex, hypertension, diabetes mellitus, current smoking habit, previous antithrombotic treatment, initial clinical severity (Hunt and Hess grade), and CT findings (Fisher scale) in the first study performed at admission. Following the Fisher scale definition, any intraventricular hemorrhage was considered grade 4, irrespective of SAH characteristics; thick SAH was also classified as grade 4, and grade 2 was assigned to thin or no SAH. Other variables recorded were the presence, number, size, and location of cerebral aneurysms (anterior or posterior circulation), treatment performed (endovascular coiling or neurosurgical clipping), and in-hospital and long-term complications.
In-hospital complications included seizures, hydrocephalus, CNS infections, acute rebleeding, neurogenic cardiomyopathy, pneumonia, sodium electrolyte disorders, and symptomatic vasospasm (2007–2009), which was revised to delayed cerebral ischemia (DCI) for cases from 2010 to 2018. Vasospasm was considered symptomatic when associated with a pathologic increase of cerebral flow velocity that was congruent with the appearance of focal neurologic signs. Cerebral flow velocity of the middle cerebral artery was evaluated by transcranial Doppler sonography and vasospasm was classified as mild (90–119 cm/s), moderate (120–200 cm/s), or severe (>200 cm/s).19 Beginning in 2010, the consensus definition of DCI was applied: a focal neurologic impairment or a decrease of at least 2 points on the Glasgow Coma Scale, lasting for at least 1 hour, not apparent immediately after aneurysm occlusion, and not attributable to other causes by means of clinical assessment, CT or MRI scanning of the brain, or appropriate laboratory studies.20
Sodium electrolyte disorders were defined as severe hypernatremia (>149 mmol/L sodium) and severe hyponatremia (<130 mmol/L sodium).21
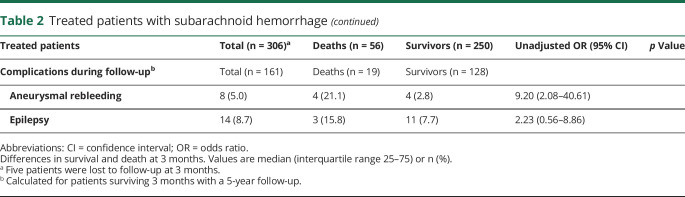
Long-term complications observed in patients who survived at 3-month follow-up and had 5-year follow-up data (n = 161) included rebleeding and epilepsy, defined as seizures recurrence after hospital discharge.
Mortality was recorded in-hospital and at 7 days, 3 months, 1 year, and 5 years after SAH. Poor outcome, defined as mRS score of 3–622 as registered by a neurologist, was recorded at discharge and by clinical visit or telephone interview at 3-month, 1-year, and 5-year follow-up. We also compared outcomes in surviving patients with mRS 0–2 vs those with mRS 3–5.
Patients were treated by a team including a vascular neurologist, neurointensivist, neurosurgeon, and neurointerventionist. After hospital admission, all patients were admitted to the intensive care unit (ICU) and a 4-vessel 3D angiographic study was performed as early as feasible. First intervention procedure was determined by consensus with the neurosurgical team, but the first option was endovascular coiling as soon as possible. A new CT scan was performed within 24 hours after aneurysm treatment. Throughout the hospitalization, the multidisciplinary team monitored each patient. Mechanical ventilation, duration of ventilator uses during ICU admission, and neurosurgical procedures (hematoma evacuation and external ventricular drainage) were recorded.
During at least the first 10 days of hospitalization, vasospasm was monitored daily with transcranial Doppler sonography (DWL Multidop-T) by a trained vascular neurologist. If vasospasm was detected, a new neuroimaging study (CT scan or MRI and/or angiogram and/or perfusion) was performed to confirm the diagnosis. Vasospasm prevention was managed by oral or IV nimodipine treatment and, in case of symptomatic vasospasm, by intra-arterial nimodipine or endoluminal angioplasty.
Statistical analysis
Data are presented as median and interquartile range (25–75) for continuous variables and as a number and percentage for categorical variables. Univariate analysis was performed using the Pearson χ2 (categorical variables) or Mann-Whitney U test for continuous variables. Unadjusted mortality rates were calculated for the hospitalization period and at 7 days, 3 months, 1 year, and 5 years after aSAH. Unadjusted poor outcome rates for each repair treatment were calculated at 3-month, 1-year, and 5-year follow-up.
Sensitivity analysis was performed in patients with aSAH with repair treatment. Patient characteristics were compared by treatment modality, 3-month mortality, and rate of poor outcome at 3-month, 1-year, and 5-year follow-up. Odds ratios (ORs) were estimated with their corresponding 95% confidence interval (CI). Number needed to treat (NNT) and absolute risk reduction were also calculated. Kaplan-Meier survival analysis for mortality and for disability was performed according to treatment modality.
To compare mortality and poor outcome rates between endovascular and neurosurgical techniques, a logistic regression model was adjusted for age, sex, smoking habit, aneurysm location, Hunt and Hess scale, Fisher scale, and associated intracerebral hematoma. The model did not include complications, which were considered intermediate factors related to the repair technique selected.23 Mechanical ventilation, which was less frequent in the coil-technique group, was also considered an intermediate factor (related with the repair technique, not with the therapeutic decision). All analyzed complications showed similar frequency in both treatment groups throughout the follow-up period.
The report of the analysis follows the Strengthening Reporting of Observational Studies in Epidemiology (STROBE) guidelines. Statistical analysis was performed with the Statistical Package for Social Sciences (SPSS, version 20.0 for Windows, Chicago, IL).
Data availability
Anonymized data will be shared upon request from any qualified investigator.
Results
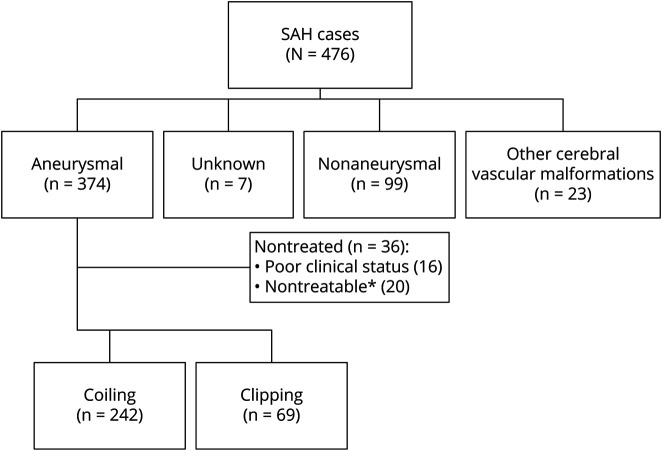
A total of 476 patients were included in the SAH-BASICMAR Register. Figure 1 shows the flowchart of the cohort. In 7 patients (1.5%), no angiographic study was done, due to poor clinical condition; all of these patients died during the first hours post-SAH. In 347 cases (72.9%), the SAH was due to an aneurysmal rupture; in 23 (4.8%), to another cerebral vascular malformation; and in 99 (20.8%), the angiography was normal. The present study analyzed the 347 patients with SAH related to an aneurysm rupture, and focused on the 311 (89.6%) that were treated: 242 by coiling (77.8%) and 69 by clipping (22.2%). No aneurysm repair was done in 36 patients: 16 because of poor clinical condition and 20 because the aneurysm was considered technically not treatable. Treatment by flow diversion was later possible in 4 of the 20 aneurysms initially considered technically nontreatable; these patients were not included in the analysis because the treatment was not done in the acute phase.
Figure 1. Patient flowchart.
*In 4 cases, delayed treatment occurred (flow diverter) beyond the acute phase. SAH = subarachnoid hemorrhage.
Patients with treated aSAH were followed up for a mean of 43.4 months (range from 1 to 145 months). Mortality was 8.4% at 7 days, 16.7% during hospitalization, 18.4% at 3 months, 22.9% at 1 year, and 29.0% at 5 years. Poor outcome (defined as mRS 3–6) was 42.3% at 3 months, 36.0% at 1 year, and 36.0% at 5 years. Follow-up was very comprehensive, with no losses at 7 days or during hospitalization, and only 5 (1.6%) lost cases at 3 months, all of them because they lived outside of our catchment area and follow-up was not feasible. Of the 311 patients followed up at 1 year post-SAH, only 12 (3.9%) had missing data for mortality and 14 (4.5%) for functional status. After 5 years, mortality and outcome data were missing for 16 of the 216 remaining patients (7.4%).
Given the long follow-up, we compared the outcome data of the 311 treated patients with aSAH for 2 different time periods: 2007–2012 (n = 155) and 2013–2018 (n = 156). There were no significant mortality differences: 19.2% vs 17.4% at 3 months, 23.1% vs 22.4% at 1 year, and 30.0% vs 26.7% at 5 years. No significant differences in poor outcome were found in any follow-up analysis: 39.3% vs 45.2% at 3 months, 33.1% vs 38.8% at 1 year, and 35.0% vs 38.3% at 5 years.
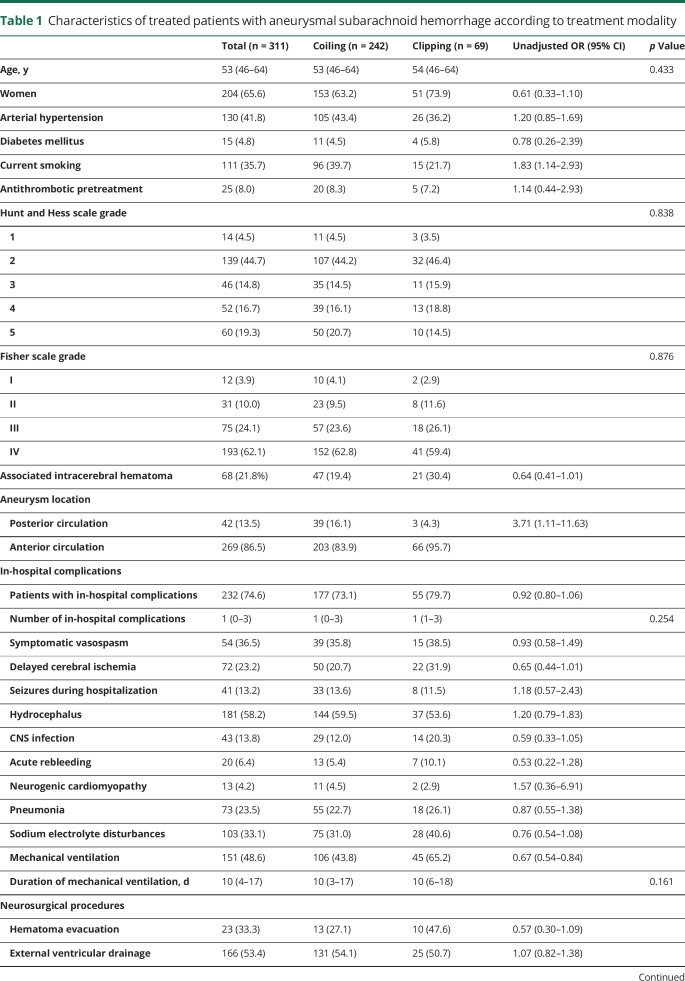
The characteristics of patients with aSAH according to treatment modality are shown in table 1. The endovascular group had fewer women (OR 0.61 [95% CI 0.33–1.10]), more smokers (OR 1.83 [95% CI 1.14–2.93]), more posterior circulation aneurysms (OR 3.71 [95% CI 1.11–11.63]), and a nonsignificant trend toward less associated intracerebral hematoma (OR 0.64 [95% CI 0.41–1.01]), compared to the neurosurgical group. Complications in hospital and during follow-up did not differ by treatment group. Vasospasm was managed by oral or IV nimodipine in 109 patients (73.6%) and by intra-arterial nimodipine or endoluminal angioplasty in 39 (26.4%), without differences between the 2 groups (OR 0.94 [95% CI 0.77–1.16]). Mechanical ventilation was needed more frequently in the neurosurgical (65.2%) than in the endovascular group (43.8%) (OR 0.67 [95% CI 0.54–0.84]), with a mean duration only 2 days longer in surgically treated patients (p = 0.140).
Table 1.
Characteristics of treated patients with aneurysmal subarachnoid hemorrhage according to treatment modality
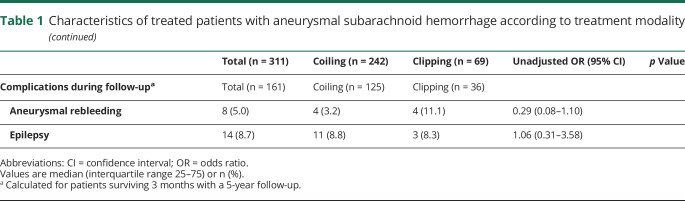
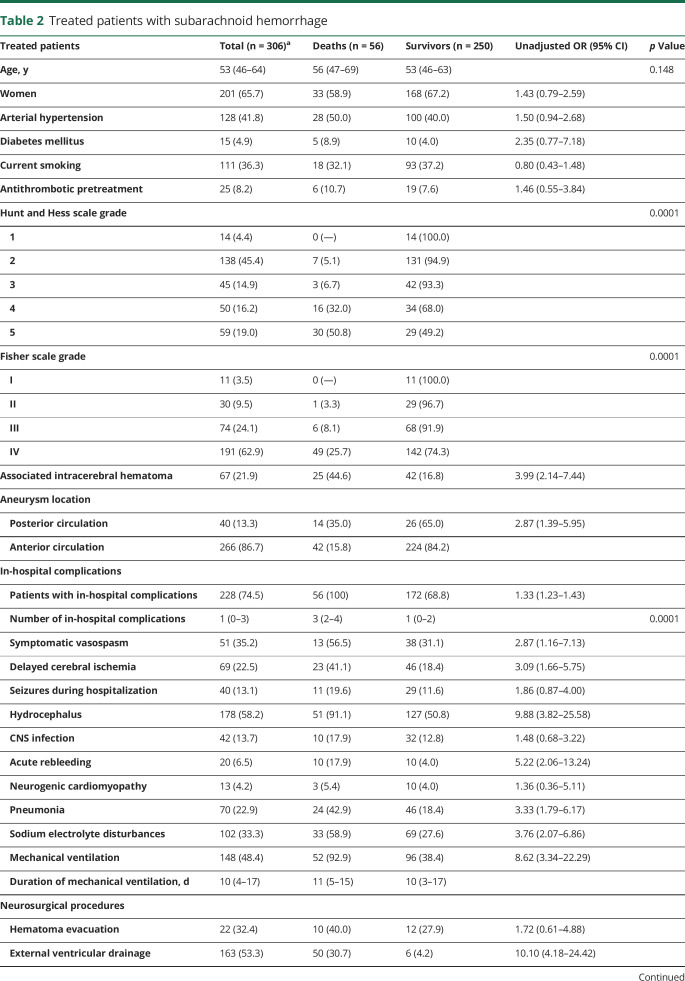
Comparisons by vital status at 3-month follow-up (table 2) show a relationship between mortality and clinical severity by Hunt and Hess scale (p < 0.0001), radiologic severity by Fisher scale (p < 0.0001), associated intracerebral hematoma (OR 3.99 [95% CI 2.14–7.44]), and posterior circulation aneurysm location (OR 2.87 [95% CI 1.39–5.95]). Mortality was very low among patients with low Hunt and Hess (1–3) and Fisher scale (I–III) scores, and the majority of deaths occurred in patients with Hunt and Hess scores of 4 or 5 and a Fisher score of IV (82.1% and 87.5%, respectively). Regarding complications, nonsurviving patients with aSAH more frequently had experienced symptomatic vasospasm (OR 2.87 [95% CI 1.16–7.13]) or DCI (OR 3.09 [95% CI 1.66–5.75]), hydrocephalus (OR: 9.88 [95% CI 3.29–19.20]), acute in-hospital rebleeding (OR 5.22 [95% CI 2.06–13.24]), pneumonia (OR 3.33 [95% CI 1.79–6.17]), sodium electrolyte disturbances (OR 3.84 [95% CI 2.11–7.00]), mechanical ventilation (OR 8.62 [95% CI 3.34–22.29]), or external ventricular drainage (OR 10.10 [95% CI 4.18–24.42]).
Table 2.
Treated patients with subarachnoid hemorrhage
The majority (75%) of patients with DCI had symptomatic vasospasm. The degree of vasospasm detected by Doppler sonography (severe, moderate, mild) was well correlated (p < 0.0001) with DCI prevalence: 67.3% (37/55 patients), 20% (8/40 patients), and 17% (9/44 patients), respectively. Severe hypernatremia was observed in 61 patients (19.6%) and severe hyponatremia in 42 (13.5%). The only complication related with 5-year fatal outcome was rebleeding after hospital discharge (OR 9.20 [95% CI 2.08–40.61]), which occurred in 5.0% of cases.
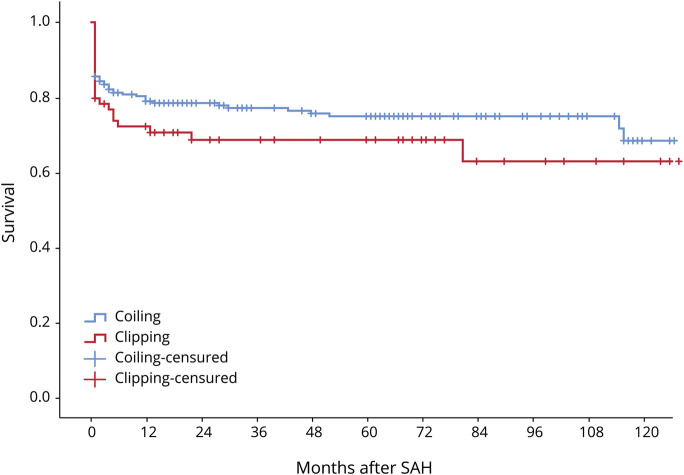
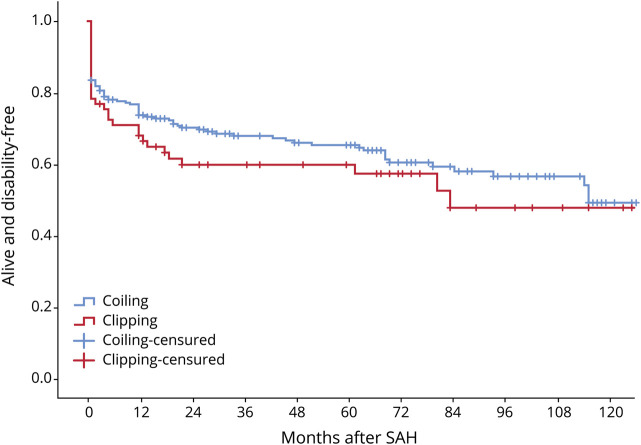
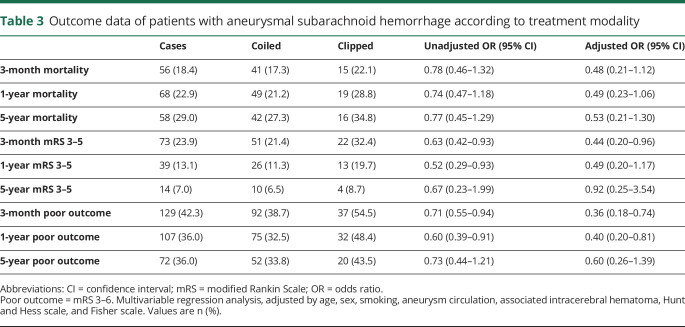
Kaplan-Meier curves for survival and disability according to treatment modality are shown in figures 2 and 3. There were no statistical differences (Mantel-Cox test) between patients in the coiled and clipped groups (p = 0.187 and p = 0.260, respectively). Outcome data, both unadjusted and adjusted, are shown by treatment modality in table 3. In the bivariate analysis, mortality did not differ between the 2 groups, but poor outcome differed at 3 months (absolute difference of 15.8%, OR 0.71 [95% CI 0.55–0.94], NNT of 6.3) and 1-year post-SAH (absolute difference of 15.9%, OR 0.60 [95% CI 0.39–0.91], NNT of 6.3). In multivariate regression analysis, the coiled technique yielded better outcomes than clipping, with significant differences in poor outcome between the 2 treatments at 3-month (OR 0.36 [95% CI 0.18–0.74]) and 1-year follow-up (OR 0.38 [95% CI 0.19–0.78]).
Figure 2. Kaplan-Meier survival curves for all treated cases according to treatment modality (p = 0.187).
SAH = subarachnoid hemorrhage.
Figure 3. Kaplan-Meier curves of disability-free outcome for all treated cases according to treatment modality (p = 0.260).
SAH = subarachnoid hemorrhage.
Table 3.
Outcome data of patients with aneurysmal subarachnoid hemorrhage according to treatment modality
Discussion
Recently published SAH reviews1,24,25 still use outcomes data from decades-old research results and the newest studies5,6,8,16,17,26–28 exhibit many methodologic problems. These range from exclusion of early deaths16 and patients with SAH with poor clinical scores8 to including only patients admitted to an ICU26,27 or only in-hospital data,17,26,27 experiencing excessive losses to follow-up8 and combining retrospective study design with only administrative sources of data.5,6,17,27,28 In addition, the available outcome information from clinical trials does not reflect the aSAH reality because the exclusion criteria lead to underrepresentation of the more severe cases.10,12 In this context, our study results provide reliable and current information on aSAH outcome.
For patients with treated aSAH, 3-month mortality was 18.4% in our cohort. A systematic review29 found 43% mortality in 2002–2008 for patients with aSAH, and a 2009 meta-analysis of 33 studies3 describes a case fatality of 8.3%–66.7%, with higher mortality in Europe (43%–44%) compared to the United States (32%) or Japan (27%). While some studies have suggested a progressive mortality decline,3–6,8,17,18,30 the most recent data show no clear changes, with mortality of 10.9%–27.5% in-hospital,6,17,26,27 22%–28.7% after 30 days,6,8 and 30.7% at 3-month follow-up.8 Compared with these data, our short-term results can be considered good, especially since the clinical severity of our cases (36% had Hunt and Hess score >3) is slightly greater than recent reports,26 and much greater than the reported results from the main 2 clinical trials: BRAT (18.1% with Hunt and Hess >3) and ISAT (5.5% with World Federation of Neurosurgical Societies >3).
Some studies indicate an adjusted 1.5- to 2-fold increase in mortality after surviving SAH, compared with healthy individuals.31–33 According to our data, the 3-month to 5-year mortality increase for treated patients with aSAH was 10.6% (table 3), very similar to the ISAT results,10,11 which show an 11% increase for endovascular-treated patients (10.0% in our study) and 14% for neurosurgical-treated cases (12.7% in our study). These similar data, showing a moderate increase in mortality after the first 3 months, emphasize the importance of a good short-term outcome. Further research is needed to determine the persistence of this effect over time, as long-term mortality of 17.9% at 10 years, 29.5% at 15 years, and 43.6% at 20 years has been reported in patients with aSAH.9
Functional outcome data in population-based SAH studies are particularly scant. A decade ago, it was estimated that 55% of patients with SAH regain independent function, 8%–20% remain dependent, and 26% do not survive.3 Recent data on short- and long-term disability are unavailable, incomplete, or obtained from clinical trials. One study observed a poor quality of life in 35% of patients with SAH at 1-year follow-up.15 Another reported poor prognosis, defined as mRS of 3–6, in 42.4% of patients with aSAH at 1-year follow-up.16 Other recent studies have used the percentage of patients discharged home, which ranges from 43.3%17 to 54.4%,8 with good recovery or moderate disability in 71.4% of these cases.8
A Cochrane review of 3 aSAH clinical trials, which excluded the BRAT trial, found that 24% of participants randomized to coiling and 32% of participants randomized to clipping had poor functional outcome at 1-year follow-up. The authors note that these data cannot be considered representative of long-term prognosis due to methodologic bias.23 According to our 3-month follow-up data (table 3), 57.7% of treated patients with aSAH were functionally independent, 23.9% remained dependent, and 18.4% were deceased. After 5 years, the vast majority of surviving patients did not have major disabilities: nearly two-thirds of all treated aneurysmal patients (64.0%) remained alive and independent, 29.0% had died, and only 7.0% were physically dependent.
The complications observed are shown in table 1. The majority of patients (74.2%) had at least one in-hospital complication. Globally, the figures are in line with previous reports.18,20,21,25,26,34,35 The treatment groups did not differ in number of complications or the specific complications analyzed. As described in the ISAT study,36 we found a nonsignificant increase in DCI in the clipped (31.9%) vs coil technique group (20.7%). We also observed more rebleeding in the clipped group, which did not reach significance but was double that of coiled cases (10.1% vs 5.4%). In the majority of the clipped cases (4/7), rebleeding occurred when coiling was attempted unsuccessfully and before surgery could be done. These patients were included in the clipped group because clipping was the final procedure applied.
During ICU admission, mechanical ventilation was needed significantly less often in coiled than in clipped cases (43.8% vs 65.2%) (OR 0.67 [95% CI 0.54–0.84]). No differences in surgical procedures were observed between the 2 techniques. It is well established that complications after SAH increase mortality and poor outcome.21 Table 2 compares 3-month survival of patients with aSAH according to in-hospital complications in our cohort. DCI, hydrocephalus, acute rebleeding, pneumonia, and sodium disturbances were significantly associated with fatal outcome, as were the number of complications, need for mechanical ventilation, and external ventricular drainage procedures. In the bivariate analysis, the factors most strongly associated with 3-month mortality were mechanical ventilation (OR 8.62 [95% CI 3.34–22.29]), hydrocephalus (OR 9.88 [95% CI 3.82–25.28]), and rebleeding (OR 5.22 [95% CI 2.06–13.24]). Analysis of the determinants of mortality would require a multivariable study, which exceeds the study objectives to describe complications and perform a bivariate analysis of clinical outcomes.
We analyzed 2 late complications, epilepsy and rebleeding, in 161 patients who were discharged alive and followed up for 5 years. Nineteen patients (11.8%) died during follow-up. The rate of epilepsy was low (8.7%) in both groups and had no impact on long-term outcome. Rebleeding rate was 5.0%, similar to that reported in the Cochrane review,23 and clearly increased the odds of poor outcome after hospital discharge (OR 9.20 [95% CI 2.08–40.61]).
It is widely accepted that coiling is preferred when either coiling or clipping appear to be equally effective in treating an aneurysm.2,18 A Cochrane review23 supports this recommendation but points out that, even though all trials found better outcomes for patients randomized to coiling,10,37,38 the evidence comes mainly from one clinical trial.10 According to this review, the risk ratio of poor outcome (death or dependency) for coiling vs clipping was 0.77, with an absolute risk reduction of 7%. We obtained very similar results in our series of patients, in which the clinical characteristics of both groups were balanced, with no differences in complications during hospitalization or follow-up. Also, in line with our results (under a protocol that prioritizes endovascular treatment over surgery), the ISAT investigators reported greater 10-year probability of death or dependency in the neurosurgical than in the endovascular group.39
This study was carried out at a single tertiary stroke center. However, the experienced and diverse multidisciplinary team involved in SAH care remained stable throughout the study period, delivering optimal patient care and follow-up. As 1 of 5 reference centers in Catalonia for SAH treatment, overrepresentation of more severe cases in our patient population is possible. Slight or moderate cognitive deficit after SAH may have been underestimated because neuropsychological evaluation was not done in all cases. Among the 250 who survived at 3-month follow-up, neuropsychological assessment was done in 90 cases (36%). Normal cognitive function was observed in 31 patients (34.4%), mild cognitive deficit in 27 patients (30.0%), and moderate to severe deficit in 32 patients (35.6%). Although the adjusted analysis suggested superiority of the endovascular treatment, our study was not randomized and the majority of patients treated with neurosurgery (95.7%) were clipped because coiling was not considered suitable or feasible. Therefore, it cannot be inferred that one treatment is superior to another. The study aim was descriptive, and we did not perform an exhaustive analysis of the determinants of aSAH mortality. Our results regarding outcome differences between the coiling and clipping techniques must be interpreted with caution and we cannot infer causality between in-hospital or late complications and outcome.
The current mortality and prognosis of patients with aSAH is clearly better than previously reported in the available systematic reviews, which are based on outcome data from old studies with care models that differ from current clinical guidelines. Regardless of whether a coiling or clipping technique was used, in-hospital complications affected around three-quarters of repair-treated patients. At 5-year follow-up, nearly two-thirds of our patients with repair-treated aSAH survived without functional limitations.
Acknowledgment
Elaine M. Lilly, PhD, provided English language assistance.
Glossary
- aSAH
aneurysmal subarachnoid hemorrhage
- BRAT
Barrow Ruptured Aneurysm Trial
- CI
confidence interval
- DCI
delayed cerebral ischemia
- ICU
intensive care unit
- OR
odds ratio
- ISAT
International Subarachnoid Aneurysm Trial
- mRS
modified Rankin Scale
- NNT
number needed to treat
- SAH
subarachnoid hemorrhage
Appendix. Authors
Footnotes
Patient page e1915
Study funding
Supported in part by Spain's Ministry of Health (Ministerio de Sanidad y Consumo, Instituto de Salud Carlos III FEDER, RD16/0019/0002).
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.MacDonald RL, Schweizer TA. Spontaneous subarachnoid hemorrhage. Lancet 2017;389:655–666. [DOI] [PubMed] [Google Scholar]
- 2.Steiner T, Juvela S, Unterberg A, Jung C, Forsting M, Rinkel G. European Stroke Organization guidelines for the management of intracranial aneurysms and subarachnoid haemorrhage. Cerebrovasc Dis 2013;35:93–112. [DOI] [PubMed] [Google Scholar]
- 3.Nieuwkamp DJ, Setz LE, Algra A, Linn FH, de Rooij NK, Rinkel GJ. Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: a meta-analysis. Lancet Neurol 2009;8:635–642. [DOI] [PubMed] [Google Scholar]
- 4.Vergouwen MD, Jong-Tjien-Fa AV, Algra A, Rinkel GJ. Time trends in causes of death after aneurysmal subarachnoid hemorrhage: a hospital-based study. Neurology 2016;86:59–63. [DOI] [PubMed] [Google Scholar]
- 5.Mackey J, Khoury JC, Alwell K, et al. Stable incident but declining case fatality rates of subarachnoid haemorrhage in a population. Neurology 2016;87:2192–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Worthington JM, Goumas C, Jalaludin B, Gattellari M. Decreasing risk of fatal subarachnoid hemorrhage and other epidemiological trends in the era of coiling implementation in Australia. Front Neurol 2017;8:424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol 2009;8:355–369. [DOI] [PubMed] [Google Scholar]
- 8.Galea JP, Dulhanty L, Patel HC; on behalf of the UK and Ireland Subarachnoid Hemorrhage Database Collaborators. Predictors of outcome in aneurysmal subarachnoid hemorrhage patients: observations from a multicenter data set. Stroke 2017;48:2958–2963. [DOI] [PubMed] [Google Scholar]
- 9.Nieuwkamp DJ, de Wilde A, Wermer MJ, Algra A, Rinkel GJ. Long-term outcome after aneurysmal subarachnoid hemorrhage-risks of vascular events, death from cancer and all-cause death. J Neurol 2014;261:309–315. [DOI] [PubMed] [Google Scholar]
- 10.Molyneux A, Kerr R, Stratton I, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomized trial. Lancet 2002;360:1267–1274. [DOI] [PubMed] [Google Scholar]
- 11.Molyneux AJ, Kerr RSC, Birks J, et al. Risk of recurrent subarachnoid haemorrhage, death, or dependence and standardised mortality ratios after clipping or coiling of an intracranial aneurysm in the International Subarachnoid Aneurysm Trial (ISAT): long-term follow-up. Lancet Neurol 2009;8:427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDougall CG, Spetzler RF, Zabramski JM, et al. The Barrow Ruptured Aneurysm Trial. J Neurosurg 2012;116:135–144. [DOI] [PubMed] [Google Scholar]
- 13.Spetzler RF, McDougall CG, Zabramski JM, et al. The Barrow Ruptured Aneurysm Trial: 6-year results. J Neurosurg 2015;123:609–617. [DOI] [PubMed] [Google Scholar]
- 14.Spetzler RF, McDougall CG, Zabramski JM, et al. Ten-year analysis of saccular aneurysms in the Barrow Ruptured Aneurysm Trial. J Neurosurg 2019;8:1–6. [DOI] [PubMed] [Google Scholar]
- 15.Taufique Z, May T, Meyers E, et al. Predictors of poor quality of life 1 year after subarachnoid hemorrhage. Neurosurgery 2016;78:256–264. [DOI] [PubMed] [Google Scholar]
- 16.Hammer A, Steiner A, Ranaie G, et al. Impact of comorbidities and smoking on the outcome in aneurysmal subarachnoid hemorrhage. Sci Rep 2018;8:12335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan V, Lindsay P, McQuiggan J, Zagorski B, Hill MD, O'Kelly C. Declining admission and mortality rates for subarachnoid hemorrhage in Canada between 2004 and 2015. Stroke 2019;50:181–184. [DOI] [PubMed] [Google Scholar]
- 18.Connolly ES Jr, Rabinstein AA, Carhuapoma JR, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage. Stroke 2012;43:1711–1737. [DOI] [PubMed] [Google Scholar]
- 19.Rigamonti A, Ackery A, Baker AJ. Transcranial doppler monitoring in subarachnoid hemorrhage: a critical tool in critical care. Can J Anesth 2008;55:112–123. [DOI] [PubMed] [Google Scholar]
- 20.Vergouwen MD, Vermeulen M, van Gijn J, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke 2010;41:2391–2395. [DOI] [PubMed] [Google Scholar]
- 21.Wartenberg KE, Schmidt JM, Claassen J, et al. Impact of medical complications on outcome after subarachnoid hemorrhage. Crit Care Med 2006;34:617–623. [DOI] [PubMed] [Google Scholar]
- 22.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604–607. [DOI] [PubMed] [Google Scholar]
- 23.Lindgren A, Vergouwen MDI, van der Schaaf IC, et al. Endovascular coiling versus neurosurgical clipping for people with aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev 2018;8:CD003085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lawton MT, Vates GE. Subarachnoid hemorrhage. N Engl J Med 2017;377:257–266. [DOI] [PubMed] [Google Scholar]
- 25.Muehlschlegel S. Subarachnoid hemorrhage. Continuum 2018;24:1623–1657. [DOI] [PubMed] [Google Scholar]
- 26.Lantigua H, Ortega-Gutierrez S, Schmidt JM, et al. Subarachnoid hemorrhage: who dies, and why? Crit Care 2015;19:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Udy A, Vladic C, Saxby ER, et al. Subarachnoid hemorrhage patients admitted to intensive care in Australia and New Zealand: a multi-centre cohort analysis of in-hospital mortality over 15 years. Crit Care Med 2017;45:e138–e145. [DOI] [PubMed] [Google Scholar]
- 28.Huhtakangas J, Lehto H, Seppä K, et al. Long-term excess mortality after aneurysmal subarachnoid hemorrhage: patients with multiple aneurysms at risk. Stroke 2015;46:1813–1818. [DOI] [PubMed] [Google Scholar]
- 29.Lovelock CE, Rinkel GJ, Rothwell PM. Time trends in outcome of subarachnoid hemorrhage: population-based study and systematic review. Neurology 2010;74:1494–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phillips TJ, Dowling RJ, Yan B, Laidlaw JD, Mitchell PJ. Does treatment of ruptured intracranial aneurysms within 24 hours improve clinical outcome? Stroke 2011;42:1936–1945. [DOI] [PubMed] [Google Scholar]
- 31.Nieuwkamp DJ, Algra A, Blomqvist P, et al. Excess mortality and cardiovascular events in patients surviving subarachnoid hemorrhage: a nationwide study in Sweden. Stroke 2011;42:902–907. [DOI] [PubMed] [Google Scholar]
- 32.Ronkainen A, Niskanen M, Rinne J, Koivisto T, Hernesniemi J, Vapalahti M. Evidence for excess long-term mortality after treated subarachnoid hemorrhage. Stroke 2001;32:2850–2853. [DOI] [PubMed] [Google Scholar]
- 33.Wermer MJH, Greebe P, Algra A, Rinkel GJE. Long-term mortality and vascular event risk after aneurysmal subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry 2009;80:1399–1401. [DOI] [PubMed] [Google Scholar]
- 34.Sherlock M, O'Sullivan E, Agha A, et al. The incidence and pathophysiology of hyponatraemia after subarachnoid haemorrhage. Clin Endocrinol 2006;64:250–254. [DOI] [PubMed] [Google Scholar]
- 35.Rowland MJ, Hadjipavlou G, Kelly M, Westbrook J, Pattinson KTS. Delayed cerebral ischaemia after subarachnoid haemorrhage: looking beyond vasospasm. Br J Anaesth 2012;109:315–329. [DOI] [PubMed] [Google Scholar]
- 36.Dorhout Mees SM, Kerr RS, Rinkel GJ, Algra A, Molyneux AJ. Occurrence and impact of delayed cerebral ischemia after coiling and after clipping in the International Subarachnoid Aneurysm Trial (ISAT). J Neurol 2012;259:679–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koivisto T, Vanninen R, Hurskainen H, Saari T, Hernesniemi J, Vapalahti M. Outcomes of early endovascular versus surgical treatment of ruptured cerebral aneurysms. A prospective randomized study. Stroke 2000;31:2369–2377. [DOI] [PubMed] [Google Scholar]
- 38.Li ZQ, Wang QH, Chen G, Quan Z. Outcomes of endovascular coiling versus surgical clipping in the treatment of ruptured intracranial aneurysms. J Int Med Res 2012;40:2145–2151. [DOI] [PubMed] [Google Scholar]
- 39.Molyneux AJ, Birks J, Clarke A, Sneade M, Kerr RS. The durability of endovascular coiling versus neurosurgical clipping of ruptured cerebral aneurysms: 18-year follow-up of the UK cohort of the International Subarachnoid Aneurysm Trial (ISAT). Lancet 2015;385:691–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared upon request from any qualified investigator.