Abstract
Objective
To determine the small vessel disease spectrum associated with cysteine-altering NOTCH3 variants in community-dwelling individuals by analyzing the clinical and neuroimaging features of UK Biobank participants harboring such variants.
Methods
The exome and genome sequencing datasets of the UK Biobank (n = 50,000) and cohorts of cognitively healthy elderly (n = 751) were queried for cysteine-altering NOTCH3 variants. Brain MRIs of individuals harboring such variants were scored according to Standards for Reporting Vascular Changes on Neuroimaging criteria, and clinical information was extracted with ICD-10 codes. Clinical and neuroimaging data were compared to age- and sex-matched UK Biobank controls and clinically diagnosed patients from the Dutch cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) registry.
Results
We identified 108 individuals harboring a cysteine-altering NOTCH3 variant (2.2 of 1,000), of whom 75% have a variant that has previously been reported in CADASIL pedigrees. Almost all variants were located in 1 of the NOTCH3 protein epidermal growth factor–like repeat domains 7 to 34. White matter hyperintensity lesion load was higher in individuals with NOTCH3 variants than in controls (p = 0.006) but lower than in patients with CADASIL with the same variants (p < 0.001). Almost half of the 24 individuals with brain MRI had a Fazekas score of 0 or 1 up to age 70 years. There was no increased risk of stroke.
Conclusions
Although community-dwelling individuals harboring a cysteine-altering NOTCH3 variant have a higher small vessel disease MRI burden than controls, almost half have no MRI abnormalities up to age 70 years. This shows that NOTCH3 cysteine altering variants are associated with an extremely broad phenotypic spectrum, ranging from CADASIL to nonpenetrance.
Cysteine-altering NOTCH3 variants cause cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), the most common hereditary cerebral small vessel disease, characterized by stroke and dementia.1 CADASIL disease severity is variable, ranging from first stroke in the third decade to a stroke-free survival up to the eighth decade. On brain MRI, however, all individuals with such NOTCH3 variants have been described to have at least a high white matter hyperintensity (WMH) burden, even those who are not, or not yet, clinically manifest.2–4
Recently, cysteine-altering NOTCH3 variants identical to those associated with CADASIL were shown to be surprisingly frequent in the Genome Aggregation Database.2,5 This suggests that patients diagnosed with CADASIL represent only the small minority of individuals with such variants, namely those at the very severe end of the small vessel disease spectrum. The neuroimaging and clinical features of community-dwelling individuals with cysteine-altering NOTCH3 variants could not be studied until now because large population exome and genome databases did not include health records or neuroimaging data. In 2019, the UK Biobank (UKB) released whole-exome sequences of 50,000 UKB participants, providing the unique opportunity to link genetic information of a large community-dwelling cohort to individual health records and neuroimaging data.
The aim of this study was to determine the small vessel disease phenotype associated with cysteine-altering NOTCH3 variants in community-dwelling individuals. We analyzed the clinical and neuroimaging features of individuals with such a variant in UKB and compared this to UKB controls and to patients from the Dutch CADASIL registry.
Methods
Standard protocol approvals, registrations, and patient consents
For all UKB and Alzheimer's Disease Neuroimaging Initiative (ADNI) participants, signed consent was obtained according to the Declaration of Helsinki. All CADASIL studies performed at the Leiden University Medical Center have been approved by the local ethics committee. The 100-Plus Study and Leiden Longevity Study (LLS) have been approved by their respective local ethics committees.
Ascertainment of NOTCH3 cysteine-altering variants and clinical and neuroimaging analysis in UKB
Details on the UKB study have been described previously.6 In short, UKB is a prospective biobank study in the United Kingdom including ≈500,000 individuals 40 to 69 years of age at initial enrollment in 2006. Approximately 9.2 million individuals who lived within 25 miles of one of the assessment centers were invited to enter the cohort, of whom 5.5% participated in the baseline assessment.7 The ethnic background of UKB participants is White in 95.9%, Asian in 1.9%, Black in 1.6%, and mixed or other in 0.6%. Extensive phenotypic information was collected through touchscreen questionnaires and physical examinations. A subset of ≈20,000 individuals also underwent 3T brain MRI as a part of the UKB Imaging Study. Inclusion in the Imaging Study was based on traveling distance to the imaging center; there was no selection based on clinical information. At the start of 2019, the first 50,000 exomes were released. Methods used for exome sequencing are detailed elsewhere.8 We queried these exomes for CADASIL-associated NOTCH3 variants, i.e., missense variants that lead to a cysteine amino acid alteration in 1 of the 34 epidermal growth factor–like repeat (EGFr) domains of the NOTCH3 protein (amino acid position 40–1373) (uniprot.org/uniprot/Q9UM47). Variants were classified according to their position along the EGFr domains (EGFr 1–6 or 7–34) according to a previous study showing that variants in EGFr 7 to 34 are frequent in the population cohorts and are associated with a broader and milder phenotype than EGFr 1 to 6 variants, which are most frequent in CADASIL cohorts.5 Given the small number of individuals with an EGFr 1 to 6 variant in UKB (n = 3), further analyses included only the individuals with an EGFr 7 to 34 variant. The following clinical information was extracted: main ICD-10 codes, reaction time measured by the mean time to correctly identify matches in the card game Snap, history of smoking, smoking pack-years, blood pressure at intake, diagnosis of diabetes mellitus, cholesterol levels (total, low-density lipoprotein, high-density lipoprotein), and medication. Hypertension was defined as a systolic blood pressure ≥140 mm Hg or a diastolic blood pressure ≥90 mm Hg. Hypercholesterolemia was defined as total cholesterol >6.5 mmol/L or low-density lipoprotein >3.5 mmol/L. History of ischemic stroke was determined from self-report, hospital records, and death records. If a brain MRI was available, it was scored according to the Standards for Reporting Vascular Changes on Neuroimaging guidelines.9 The WMH lesion load in the deep white matter and periventricular white matter was assessed semiquantitatively with the Fazekas scale.10 The following CADASIL/small vessel disease markers were scored as present or absent: lacunes, microbleeds, brain atrophy, and WMH in the external capsules, anterior temporal lobes, frontal subcortical white matter, and basal ganglia. Brain MRI markers were compared to a group of 24 randomly selected age- and sex-matched UKB controls who did not have nonsynonymous NOTCH3 missense variants and to a group of 24 randomly selected age-matched patients with CADASIL with a NOTCH3 EGFr 7 to 34 variant who participated in an ongoing prospective CADASIL study at the Leiden University Medical Center (Disease Variability in NOTCH3-Associated Small Vessel Disease [DiViNAS] study; P18.164), including 3T MRI. Stroke frequency in individuals with a cysteine-altering NOTCH3 variant in UKB was compared to stroke frequency in a previously published sample of 251 Dutch patients with CADASIL.5
Identification of cysteine-altering NOTCH3 variants in cognitively healthy elderly individuals
To study whether cysteine-altering NOTCH3 variants can occur in cognitively healthy elderly individuals, we used the whole-genome sequencing datasets of cognitively healthy controls of the ADNI cohort, the LLS, and the 100-Plus Study. The ADNI cohort (adni.loni.usc.edu) consists of individuals with cognitive impairment and cognitively healthy controls 55 to 90 years of age, excluding a history of serious neurologic disease (e.g., brain infarction, multiple sclerosis, and psychoactive medication use). For full inclusion/exclusion criteria, see adni-info.org. In 2012, 818 individuals from ADNI 1 and ADNI Grand Opportunities (ADNI GO) (128 with Alzheimer disease, 415 with mild cognitive impairment, 267 controls, and 8 with uncertain diagnosis) were selected for whole-genome sequencing. The study design and inclusion criteria of the LLS are detailed elsewhere.11 Whole-genome sequencing was performed for 218 unrelated individuals ≥89 years of age (mean age at inclusion 94.0 years, range 88.9–103.4 years).12 In the 100-Plus Study, whole-exome sequencing (Illumina, San Diego, CA) was performed for 266 unrelated individuals ≥100 years of age with exceptionally good cognitive health.13
Statistical analysis
Differences in WMH lesion load between UKB participants with a cysteine-altering NOTCH3 variant, UKB controls, and patients with CADASIL were analyzed with ordinal logistic regression after correction for hypertension. Differences in age were assessed with unpaired 2-sided samples t tests. Presence or absence of lacunes, microbleeds, brain atrophy, cardiovascular risk factors, and Asian ethnicity were analyzed with Fisher exact tests. Differences in ischemic stroke frequency were analyzed with a log-rank test. All statistical analyses were performed with SPSS 24.0 (Chicago, IL).
Data availability
The data that support the findings of this study are available from the corresponding author on reasonable request.
Results
Cysteine-altering NOTCH3 variants in UKB
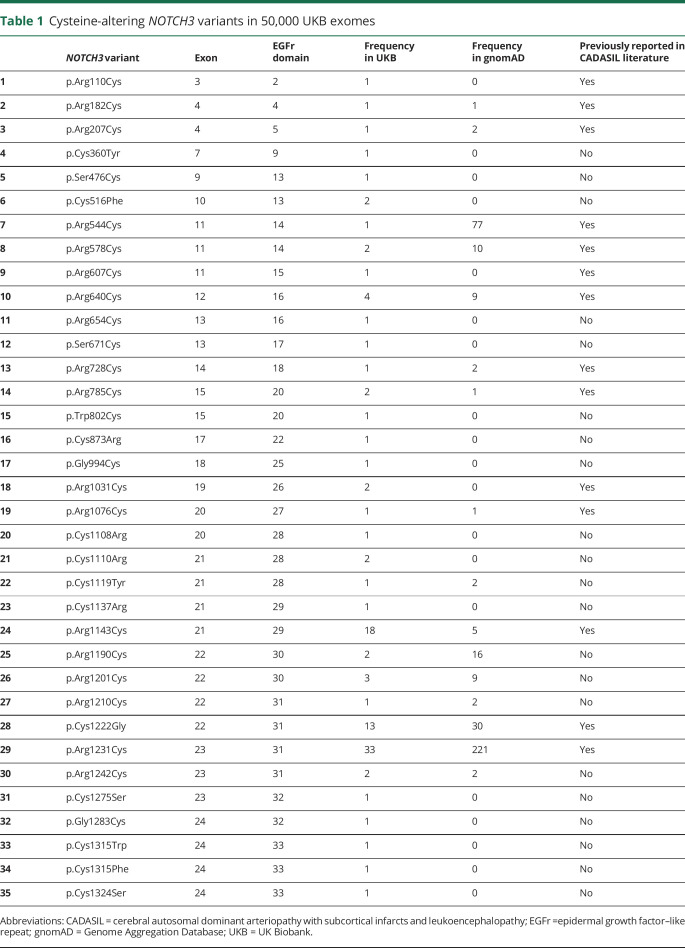
In the 50,000 UKB exomes, we identified 108 individuals (59 male, 49 female) with a cysteine-altering NOTCH3 variant, corresponding to a frequency of 2.2 in 1,000. There were 35 distinct cysteine-altering NOTCH3 variants, and 75% of individuals had a variant that has been previously reported in CADASIL pedigrees (table 1). The mean age of the individuals with a cysteine-altering NOTCH3 variant was 64.9 years (range 49–81 years, SD 8.2 years). In the vast majority of individuals (97.2%), the NOTCH3 variant was located in one of the EGFr domains 7 to 34 (UKBNOTCH3 7-34); only 3 individuals had a NOTCH3 variant located in one of the EGFr domains 1 to 6 (figure 1A). Most individuals (86.1%) with a NOTCH3 variant were White; 9.3% were Asian; and 4.6% had other ethnicities. NOTCH3 variants were relatively enriched in Asians, which represent only 1.9% of the UKB (p < 0.001, Fisher exact test).
Table 1.
Cysteine-altering NOTCH3 variants in 50,000 UKB exomes
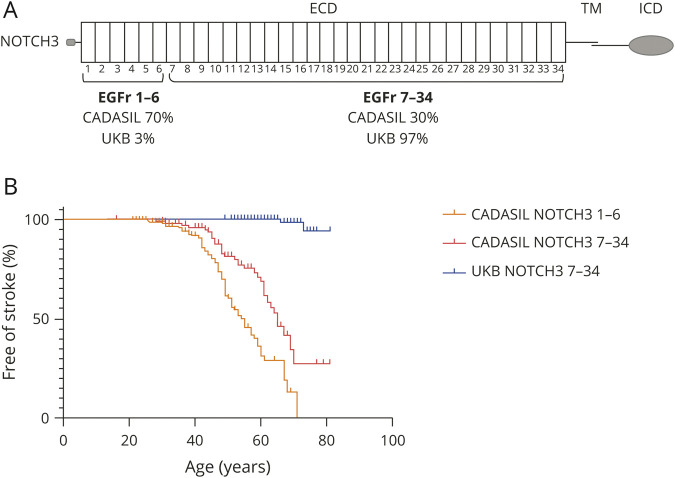
Figure 1. NOTCH3 variant position and stroke frequency in UKB compared to patients with CADASIL.
(A) Schematic representation of the NOTCH3 protein and variant distribution in UK Biobank (UKB) vs cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL). In CADASIL pedigrees, ≈70% of individuals have a cysteine-altering NOTCH3 variant in 1 of the epidermal growth factor–like repeat (EGFr) domains 1 to 6 and ≈30% in 1 of the EGFr domains 7 to 34. In the UKB, the vast majority of cysteine-altering NOTCH3 variants (97%) are located in EGFr 7 to 34. (B) Kaplan-Meier plot showing the difference in age at first stroke between individuals with a NOTCH3 EGFr 7 to 34 variant from UKB, patients with CADASIL with an EGFr 7 to 34 variant, and patients with CADASIL with an EGFr 1 to 6 variant, as determined in a previously published study in 251 individuals from the Dutch CADASIL registry.5 ECD = extracellular domain; ICD = intracellular domain; TM = transmembrane domain.
Stroke and dementia in individuals with a cysteine-altering NOTCH3 variant in UKB
UKBNOTCH3 7-34 cases had a lower stroke frequency and a later onset of stroke than patients with CADASIL with an EGFr 7 to 34 variant (CADASILNOTCH3 7-34 cases) (1.9% vs 30.6% up to age 75 years, hazard ratio 0.03, 95% confidence interval 0.01–0.07, log-rank test) (figure 1B). Stroke frequency in UKBNOTCH3 7-34 cases was not significantly higher than stroke frequency in the whole UKB population (1.9% vs 1.2%, p = 0.375, Fisher exact test). None of the UKBNOTCH3 7-34 cases had an ICD-10 code consistent with dementia, mild cognitive impairment, or migraine with aura.
Neuroimaging phenotype in individuals with a cysteine-altering NOTCH3 variant in UKB
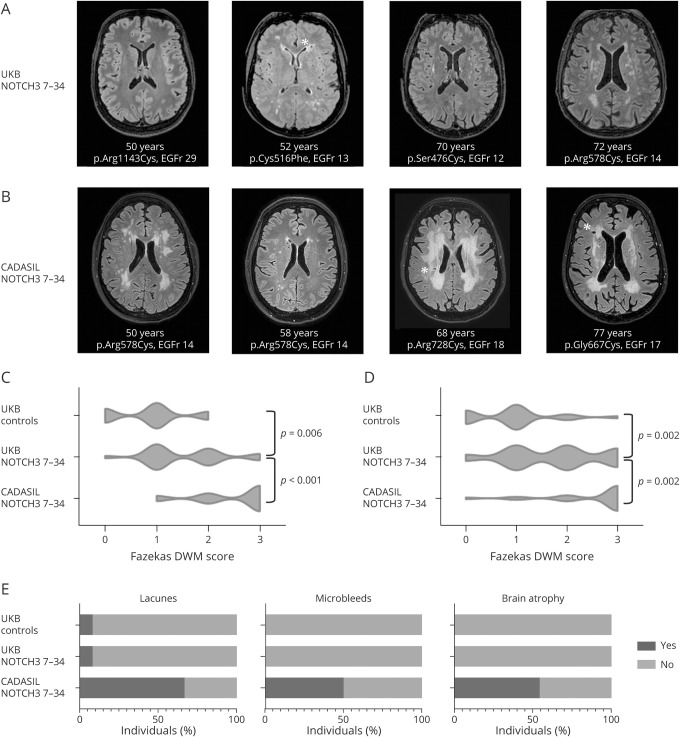
The presence of small vessel disease MRI markers in the 24 UKBNOTCH3 7-34 cases for whom brain MRI was available ranged from an MRI consistent with mid-adult–onset CADASIL to practically nonpenetrance up to age 70 years (figure 2A). The neuroimaging phenotype in UKBNOTCH3 7-34 cases was much milder than in CADASILNOTCH3 7-34 cases (figure 2B), with a lower WMH lesion load and a much lower frequency of lacunes, microbleeds, and brain atrophy (figure 2, C–E and table 2). However, UKBNOTCH3 7-34 cases did have a significantly higher WMH lesion load compared to UKB controls (table 2). There was no difference in WMH lesion load between cases with a previously unreported cysteine-altering variant and those with a variant that has previously been described in CADASIL pedigrees.14 UKBNOTCH3 7-34 cases with a Fazekas score of 2 or 3 for both deep white matter and periventricular white matter had a significantly longer reaction time than UKBNOTCH3 7-34 cases with a Fazekas score of 0 or 1 (553 ± 107 vs 477 ± 55 milliseconds, p = 0.03, independent-samples t test).
Figure 2. Neuroimaging in cases with a cysteine-altering NOTCH3 variant in UKB.
(A) Brain MRI T2–fluid-attenuated inversion recovery (FLAIR) images of 4 representative cases with a cysteine-altering NOTCH3 variant in the UK Biobank (UKB). From left to right: a 50-year old woman with a normal brain MRI; a 52-year old woman with periventricular and subcortical white matter hyperintensities (WMH) (Fazekas deep white matter [DWM] score 2 and periventricular white matter [PVWM] score 3) and a lacune; a 70-year old man with only minimal WMH in the external capsules (Fazekas DWM score 1 and PVWM score 1); and a 72-year old man with subcortical and basal ganglia WMH (Fazekas DWM score 3 and PVWM score 3). (B) Brain MRI T2-FLAIR images of 4 representative cases with a cysteine altering NOTCH3 variant in epidermal growth factor–like repeat (EGFr) 7 to 34 from cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) pedigrees. From left to right: a 50-year old woman with extensive WMH (Fazekas DWM score 3 and PVWM score 3); a 58-year old man with only minimal WMH (Fazekas DWM score 1 and PVWM score 1); a 68-year old woman with extensive WMH (Fazekas DWM score 3 and PVWM score 3) and lacunes; and a 77-year old woman with extensive WMH (Fazekas DWM score 3 and PVWM score 3) and lacunes. (C and D) Violin plots showing Fazekas DWM and PVWM scores of UKB controls, UKBNOTCH3 7-34 cases, and CADASILNOTCH3 7-34 cases. WMH lesion load in UKBNOTCH3 7-34 cases was significantly lower than in CADASILNOTCH3 7-34 cases but significantly higher than in UKB controls (for statistical analyses, see table 2). Almost half (10 of 24) of UKBNOTCH3 7-34 cases had a Fazekas score of 0 or 1 in both DWM and PVWM. In contrast, almost all (21 of 24) CADASILNOTCH3 7-34 cases had a Fazekas score of ≥2 in both DWM and PVWM. (E) Bar charts showing the frequency of lacunes, microbleeds, and brain atrophy in UKB controls, UKBNOTCH3 7-34 cases, and CADASILNOTCH3 7-34 cases. *A lacune.
Table 2.
Characteristics of UKBNOTCH3 7-34 cases, UKB controls, and CADASILNOTCH3 7–34 cases with brain MRI
Cysteine-altering NOTCH3 variants and phenotype in cognitively healthy elderly
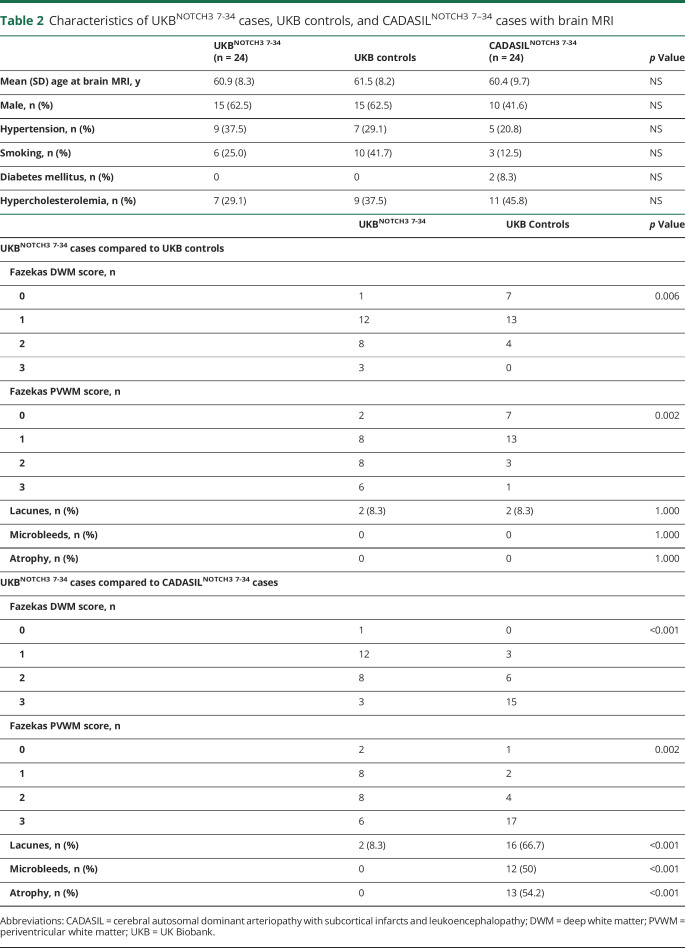
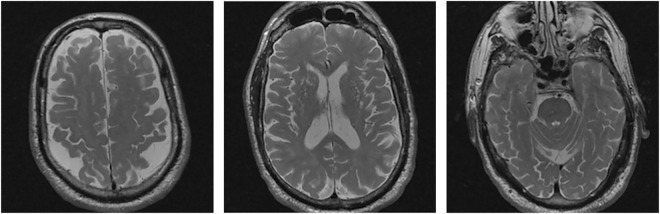
In the total group of 751 cognitively healthy individuals from ADNI, LLS, and the 100-Plus study, 1 individual had a cysteine-altering NOTCH3 variant, which was located in EGFr domain 31. He had been enrolled in ADNI as a cognitively healthy control. At the time of the last study site visit, he was 84 years old and did not have a history of stroke or other neurologic symptoms. His score on the Mini-Mental State Examination was 30 of 30. Brain MRI showed subtle WMH in the external capsules and frontal subcortical areas, dilated perivascular spaces, brain atrophy, and 2 small cerebellar infarcts (figure 3). No cysteine-altering NOTCH3 variants were present in individuals with Alzheimer dementia or cases with mild cognitive impairment from ADNI.
Figure 3. Brain MRI in an 84-year-old cognitively healthy control from ADNI with a NOTCH3 p.Cys1222Gly variant.
T2-weighted images showing subtle white matter hyperintensities, dilated perivascular spaces, and brain atrophy. ADNI = Alzheimer's Disease Neuroimaging Initiative.
Discussion
Our investigation of the neuroimaging and clinical features of UKB participants harboring CADASIL-associated cysteine-altering NOTCH3 variants reveals that patients with CADASIL represent only the very severe and rare end of the NOTCH3-associated disease spectrum. The vast majority of community-dwelling individuals harboring such NOTCH3 variants turn out to have a significantly milder small vessel disease phenotype. In fact, in almost half of individuals for whom MRI was available, there were no neuroimaging abnormalities up to age 70 years.
Almost all cysteine-altering NOTCH3 variants found in UKB participants were located in EGFr domains 7 to 34 of the NOTCH3 protein. Roughly one-third of patients diagnosed with CADASIL harbor variants in EGFr domains 7 to 34, which shows that the small vessel disease phenotype associated with NOTCH3 EGFr 7 to 34 variants is very broad, ranging from a severe, mid-adult–onset CADASIL phenotype to nonpenetrance. This suggests an important role for so far unknown genetic modifiers, the identification of which will likely be key for future individualized disease prediction in individuals harboring a cysteine-altering NOTCH3 variant. Until these factors have been elucidated, a positive family history compatible with CADASIL could be used as a proxy for the presence of these exacerbating genetic risk factors. From our findings in the UKB, it seems that individuals with a negative family history and a chance finding of a cysteine-altering NOTCH3 variant, when located in EGFr domains 7 to 34, likely have a very low risk of developing the classic severe CADASIL phenotype. A brain MRI in mid-adulthood could be done to determine whether signs of small vessel disease are present. Awareness of the broad clinical spectrum associated with cysteine-altering NOTCH3 variants is important because whole-exome sequencing is increasingly being implemented in clinical practice, increasing the risk of encountering such a variant as a secondary finding.
In contrast to NOTCH3 variants located in EGFr domains 7 to 34, those located in the first 6 EGFr domains are rarely present in population-based cohorts but are found in approximately two-thirds of CADASIL pedigrees. Cysteine-altering NOTCH3 variants in one of the EGFr domains 1 to 6, therefore, seem to be highly penetrant, predisposing to a typical CADASIL disease course in the vast majority of cases.5 Variant location proximal or distal to EGFr domain 6 therefore seems to be a key determinant for disease severity. The molecular mechanisms underlying the difference in disease severity between EGFr 1 to 6 variants and 7 to 34 variants are unknown. Regardless of EGFr location, all these cysteine-altering NOTCH3 variants lead to an unpaired cysteine residue and disrupted disulfide bridge formation. We hypothesize that there may be a difference in the established proaggregatory properties of the mutant NOTCH3 proteins.15,16 Taken together, these findings suggest that NOTCH3-associated small vessel disease severity depends on at least 3 factors: the location of the cysteine-altering NOTCH3 variant in the 34 EGFr domains, unknown genetic modifiers, and the presence or absence of common vascular risk factors such as smoking and hypertension.17
Although we found that the phenotype associated with NOTCH3 EGFr 7 to 34 variants in UKB participants was significantly milder than in patients with CADASIL, there was an increased WMH burden compared to controls. Therefore, individuals with such mutations in the population are likely at increased risk for developing cognitive deficits because WMH are known to be associated with vascular cognitive impairment and dementia.18 Indeed, UKB participants with a NOTCH3 mutation and a high WMH lesion load had increased reaction times, a measure for early cognitive and behavioral alterations, compared to those with no or low WMH burden.19 In UKB and in the Genome Aggregation Database, NOTCH3 EGFr 7 to 34 mutations are especially frequent in Asians (1%),2 suggesting that these variants may contribute to the relatively high prevalence of small vessel disease in Asians.20,21 It is likely that individuals with cysteine-altering NOTCH3 variants are also at increased risk for small vessel stroke; this was shown to be the case for 2 highly frequent NOTCH3 variants (p.Arg544Cys and p.Arg1231Cys).4,22 The fact that we did not find this increased risk of stroke in individuals with a cysteine-altering NOTCH3 variant in UKB may be due to stochastic effects because of relatively low stroke frequencies in community-dwelling volunteer cohorts. Due to the healthy volunteer selection bias in UKB,7 individuals with disability or dementia are likely also underrepresented. Furthermore, mild cognitive impairment, dementia, and migraine with aura were captured by the use of ICD-10 codes, which likely also contributes to an underestimation of these features in the cohort. Finally, most individuals in UKB are White, so other ethnicities are underrepresented.
This study shows that CADASIL constitutes only the very severe and rare end of the NOTCH3-associated small vessel disease spectrum. The majority of individuals harboring cysteine-altering NOTCH3 variants have a significantly milder and later-onset small vessel disease, with a substantial number of individuals having no neuroimaging abnormalities up to age 70 years.
Acknowledgment
This research has been conducted with the UKB Resource.
Glossary
- ADNI
Alzheimer's Disease Neuroimaging Initiative
- ADNI GO
ADNI Grand Opportunities
- CADASIL
cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy
- EGFr
epidermal growth factor–like repeat
- ICD-10
International Classification of Disease, 10th edition
- LLS
Leiden Longevity Study
- UKB
UK Biobank
- WMH
white matter hyperintensity
Appendix. Authors
Footnotes
Editorial, page 565
Study funding
Supported by the Netherlands Organisation for Health Research and Development (ZonMw 91717325) and the Netherlands Brain Foundation (HA2016-02-03).
Disclosure
J.W. Rutten is funded by the Netherlands Brain Foundation (HA2016-02-03) and received institutional support from Leiden University Medical Center. R.J. Hack is funded by the Netherlands Organisation for Health Research and Development (ZonMw 91717325). M. Duering, G. Gravesteijn, J.G. Dauwerse, M. Overzier, E.B. van den Akker, E. Slagboom, H. Holstege, K. Nho, A. Saykin, M. Dichgans, and R. Malik report no disclosures relevant to the manuscript. S.A.J. Lesnik Oberstein is funded by the Netherlands Organisation for Health Research and Development (ZonMw 91717325) and received institutional support from Leiden University Medical Center. Go to Neurology.org/N for full disclosures.
References
- 1.Chabriat H, Joutel A, Dichgans M, Tournier-Lasserve E, Bousser MG. CADASIL. Lancet Neurol 2009;8:643–653. [DOI] [PubMed] [Google Scholar]
- 2.Rutten JW, Dauwerse HG, Gravesteijn G, et al. Archetypal NOTCH3 mutations frequent in public exome: implications for CADASIL. Ann Clin Transl Neurol 2016;3:844–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pescini F, Bianchi S, Salvadori E, et al. A pathogenic mutation on exon 21 of the NOTCH3 gene causing CADASIL in an octogenarian paucisymptomatic patient. J Neurol Sci 2008;267:170–173. [DOI] [PubMed] [Google Scholar]
- 4.Lee YC, Chung CP, Chang MH, Wang SJ, Liao YC. NOTCH3 cysteine-altering variant is an important risk factor for stroke in the Taiwanese population. Neurology 2020;94:e87–e96. [DOI] [PubMed] [Google Scholar]
- 5.Rutten JW, Van Eijsden BJ, Duering M, et al. The effect of NOTCH3 pathogenic variant position on CADASIL disease severity: NOTCH3 EGFr 1-6 pathogenic variant are associated with a more severe phenotype and lower survival compared with EGFr 7-34 pathogenic variant. Genet Med 2019;21:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bycroft C, Freeman C, Petkova D, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 2018;562:203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fry A, Littlejohns TJ, Sudlow C, et al. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am J Epidemiol 2017;186:1026–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Hout CV, Tachmazidou I, Backman JD, et al. Whole exome sequencing and characterization of coding variation in 49,960 individuals in the UK Biobank. bioRxiv 2019:572347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013;12:822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol 1987;149:351–356. [DOI] [PubMed] [Google Scholar]
- 11.Schoenmaker M, de Craen AJ, de Meijer PH, et al. Evidence of genetic enrichment for exceptional survival using a family approach: the Leiden Longevity Study. Eur J Hum Genet 2006;14:79–84. [DOI] [PubMed] [Google Scholar]
- 12.van den Akker EB, Pitts SJ, Deelen J, et al. Uncompromised 10-year survival of oldest old carrying somatic mutations in DNMT3A and TET2. Blood 2016;127:1512–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holstege H, Beker N, Dijkstra T, et al. The 100-Plus Study of cognitively healthy centenarians: rationale, design and cohort description. Eur J Epidemiol 2018;33:1229–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rutten JW, Haan J, Terwindt GM, van Duinen SG, Boon EM, Lesnik Oberstein SA. Interpretation of NOTCH3 mutations in the diagnosis of CADASIL. Expert Rev Mol Diagn 2014;14:593–603. [DOI] [PubMed] [Google Scholar]
- 15.Joutel A, Andreux F, Gaulis S, et al. The ectodomain of the Notch3 receptor accumulates within the cerebrovasculature of CADASIL patients. J Clin Invest 2000;105:597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duering M, Karpinska A, Rosner S, et al. Co-aggregate formation of CADASIL-mutant NOTCH3: a single-particle analysis. Hum Mol Genet 2011;20:3256–3265. [DOI] [PubMed] [Google Scholar]
- 17.Adib-Samii P, Brice G, Martin RJ, Markus HS. Clinical spectrum of CADASIL and the effect of cardiovascular risk factors on phenotype: study in 200 consecutively recruited individuals. Stroke 2010;41:630–634. [DOI] [PubMed] [Google Scholar]
- 18.Alber J, Alladi S, Bae HJ, et al. White matter hyperintensities in vascular contributions to cognitive impairment and dementia (VCID): knowledge gaps and opportunities. Alzheimers Dementia 2019;5:107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jouvent E, Reyes S, De Guio F, Chabriat H. Reaction time is a marker of early cognitive and behavioral alterations in pure cerebral small vessel disease. J Alzheimers Dis 2015;47:413–419. [DOI] [PubMed] [Google Scholar]
- 20.Hilal S, Mok V, Youn YC, Wong A, Ikram MK, Chen CL. Prevalence, risk factors and consequences of cerebral small vessel diseases: data from three Asian countries. J Neurol Neurosurg Psychiatry 2017;88:669–674. [DOI] [PubMed] [Google Scholar]
- 21.Wolma J, Nederkoorn PJ, Goossens A, Vergouwen MD, van Schaik IN, Vermeulen M. Ethnicity a risk factor? The relation between ethnicity and large- and small-vessel disease in White people, Black people, and Asians within a hospital-based population. Eur J Neurol 2009;16:522–527. [DOI] [PubMed] [Google Scholar]
- 22.Masoli JAH, Pilling LC, Kuchel GA, Melzer D. Clinical outcomes of CADASIL-associated NOTCH3 mutations in 451,424 European ancestry community volunteers. Transl Stroke Res 2019;10:339–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on reasonable request.