Abstract
The majority of patients with traumatic brain injury (TBI) are classified as having a mild TBI. Despite being categorized as mild, these individuals report ongoing and complex symptoms, which negatively affect their ability to complete activities of daily living and overall quality of life. Some of the major symptoms include anxiety, depression, sleep problems, headaches, light sensitivity, and difficulty reading. The root cause for these symptoms is under investigation by many in the field. Of interest, several of these symptoms such as headaches, ocular pain, light sensitivity, and sleep disturbances may overlap and share underlying circuitry influenced by the intrinsically photosensitive retinal ganglion cells (ipRGCs). These cells are light sensing, but non–image forming, and they influence corneal function, pupillary constriction, and circadian rhythm. In this review, we discuss these symptoms and propose a role of the ipRGCs as at least one underlying and unifying cause for such symptoms.
Traumatic brain injury (TBI) is a significant health issue; it was reported that in 2013, 2.8 million people had a TBI that lead to an emergency department visit, hospitalization, and/or death.1 TBIs are classified as mild, moderate, or severe based on factors assessed at the time of injury using the Glasgow Coma Scale (GCS) and/or the Full Scale of Unresponsiveness (FOUR) score, in combination with findings or the lack thereof on neuroimaging, within the cases of mild TBI (mTBI).2–4 TBI symptoms are widespread and can last only a few days (acute) or cause disabilities that affect survivors for months to years (chronic). Reported TBI symptoms include headache, nausea/vomiting, convulsions, inability to awaken, dilation of one or both pupils, slurred speech, aphasia, dysarthria, weakness/numbness of limbs, loss of coordination, restlessness, and agitation.5 Patients with mTBI exhibit many of the same symptoms but may differ in severity. Regardless of the magnitude of injury, those who have chronic TBI can develop endocrine dysfunction and behavioral/emotional disorders, along with other cognitive and sensory conditions, which negatively affect the everyday lives of such individuals.6
Mild TBI accounts for 80% of all TBI cases in the United States, and as of 2018, it is reported that it represents 86% of all US military TBIs.7,8 We, therefore, focus on adult mTBI in this review. Despite the lack of detection of neuronal and physical damage on clinical MRI in patients with mTBI, research studies have demonstrated changes in glucose metabolism, cognition, and cerebral blood flow in this population.4,9–13 In mTBI, axonal damage is often diffuse, likely explaining the wide range of symptoms.14 According to a CDC report from 2019, effects of mTBI include headache, sensitivity to noise or light, sleeping more or less than usual, difficulty falling asleep, and other emotional and physical symptoms.15
Visual dysfunctions, which refer to functional deficits in vision, and in the absence of detectable degeneration, also occur at a particularly high rate in patients with mTBI. An estimated 80% of patients with mTBI have normal best-corrected visual acuity, and yet 75% of those individuals self-report visual complaints.16 Furthermore, 85% of patients with mTBI experience the same degree of photosensitivity chronically after TBI.17 Insufficiencies in convergence or accommodation are detected in 48.4% of patients with mTBI, indicating that at least some vision deficits originate in the oculomotor system.18 Patients with TBI with visual dysfunction are often impeded from pursuing educational goals and work opportunities.19
In this review, we examine 3 commonly reported mTBI symptoms: sleep disturbance, posttraumatic headache (PTH)/migraine, and dry eye syndrome (DES). On the surface, these conditions may appear to be unrelated to the visual system and its functions, but we hypothesize that at least a portion of these symptoms are derived from a disruption in retinal-brain circuitry involving a subset of retinal ganglion cells that are light responsive and non–image forming, that is, the intrinsically photosensitive retinal ganglion cells (ipRGCs). Developing a better understanding of the physiologic basis for TBI symptoms and possible commonalities between them, such as the ipRGCs, will lead to effective diagnosis and treatment of these conditions in patients with TBI.
PTH and photosensitivity in subjects with mTBI
PTH is reported in 60% of patients with TBI regardless of the severity level or phase (acute vs chronic), making it the most commonly reported TBI symptom.20 Although the majority of PTH resolve on their own within 6 months, symptoms persist on 400,000 people in the United States annually.20 Most PTH resemble tension or migraine headaches or a mixture of the 2; the exact distribution varies, but it is estimated that approximately 49% of PTH have migraine features and about 40% have characteristics of tension-type headaches.21
One known cause of headaches in general is photosensitivity, which is broadly defined as a visual symptom of mild-to-extreme discomfort that is experienced by an individual in the presence of normal light levels (e.g., light levels that would not cause visual discomfort in normal population).17 Photosensitivity is a common symptom for patients with mTBI, and based on self-report, the incidence of photosensitivity in the TBI population is 50% compared with an incidence of 10% in healthy controls.17,22 Even with these data, there is still a shortage of literature on the quantification of photosensitivity in the mTBI diagnostic group due to the lack of objective diagnostic assessments.
Although occasionally reported in isolation, photosensitivity is commonly seen in patients with PTH and is frequently associated with migraine-type headaches.23 The primary difference between PTH and migraine is that the PTH is initiated from an mTBI, whereas the migraine is possibly initiated by chemical imbalances, nerve communication errors, and blood vessel damage and can be triggered by hormonal, emotional, or physical causes.24 Similarities, however, between the classic migraine and PTH include headache, nausea, dizziness, insomnia, and many more diverse symptoms.25 Along the lines of classic migraines having several commonalities to PTH, they are also similar in terms of their reported sensitivity to light. Independent of TBI, 80% of patients with migraine are photosensitive, both during and between migraine occurrences.26–28 This high prevalence of photosensitivity can lead one to presume that such mechanisms of migraines and other headache conditions use the same retinal pathways by way of the ipRGCs. This is supported by a study from Noseda et al.29 in 2018. These researchers were able to show that photosensitivity in subjects with migraine does in fact align with the pathway of ipRGCs as a primary source for the complex and recurrent issues of light sensitivity. In addition, in these patients, light can act as the sole trigger for migraine attacks.30 Individuals who have tension-type headaches also report more sensitivity to light compared with controls.17 We postulate an underlying common pathophysiologic explanation for the prevalence and association of PTH and photosensitivity.
ipRGC systems are also likely involved in other headache conditions with significant and ongoing photosensitivity complaints from military and sports-related concussions. These issues are especially prevalent in military members with up to 22% of surviving soldiers treated in hospitals being diagnosed with a TBI.31 Likewise, photosensitivity is recorded as a symptom for 59% of this military TBI population, whereas sleep problems account for 36%.32,33 In terms of sports-related concussions, such injury accounts for 5%–9% of all sports-related injuries, with the highest reports coming from football, wrestling, boys' and girls' soccer, and girls' basketball.34–36 With this, 90% of sports-related concussions will incorporate symptoms that last 7–10 days, whereas the other 10% can incorporate several chronic ailments as previously discussed.37 In an effort to better understand this 10% and establish more solidified answers to the chronic effects, such issues have been well documented in recent years regarding retired professional athletes who encounter mTBI onset throughout their careers. In terms of extreme cases of mTBI in professional athletes who were diagnosed with chronic traumatic encephalopathy on death, 90% demonstrated risk factors that include insomnia, headaches, and general pain, as well as other symptoms such as depression, violence, and drug abuse.38,39 Overall, such information suggests that similar characteristics are present in various causes of mTBI and analogous ipRGC impact that is likely associated with the complex symptoms.
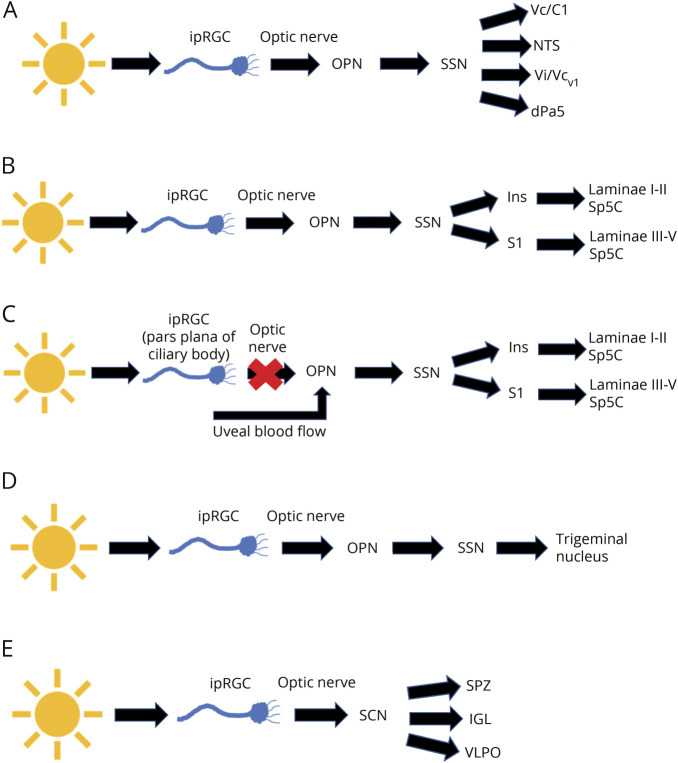
Animal studies have provided insight into 3 pathways that affect PTH and photosensitivity and involve the ipRGCs (figure, A–C). The first pathway begins with a subset of ipRGCs that project via the optic nerve to the olivary pretectal nucleus (OPN) and the superior salivatory nucleus (SSN) (figure, A).26,40,41 This pathway neuronal activity was demonstrated in a rat model using exposure to different light intensities and detection of Fos-like immunoreactivity (Fos-LI) in the caudal brainstem. Fos-LI production was detected in the nucleus tractus solitarius, the trigeminal interpolaris/caudalis transition (Vi/Vcv1), trigeminal caudalis/cervical cord junction region (Vc/C1), and the dorsal paratrigeminal (dPa5) regions, which are all related to nociception.40 In addition, numbing of ocular surface neurons by lidocaine had no effect of the Fos-LI levels, suggesting that ocular surface neurons did not contribute to the light-dependent activation of this central pain response.40 A recent clinical study has specifically investigated changes in brain areas involved in photosensitivity—namely, the trigeminal ganglion and trigeminal nucleus caudalis in the TBI population, which is represented by the first photosensitivity pathway (figure, A). Tensor-based morphometry of the brainstem was measured in patients with TBI who had mild or severe photosensitivity, and the results were compared with healthy controls.42 The authors detected differences in the morphology of the brainstem that varied depending on if the subjects' report of photosensitivity.42
Figure. Schematics of potentially relevant ipRGC pathways and TBI symptoms.
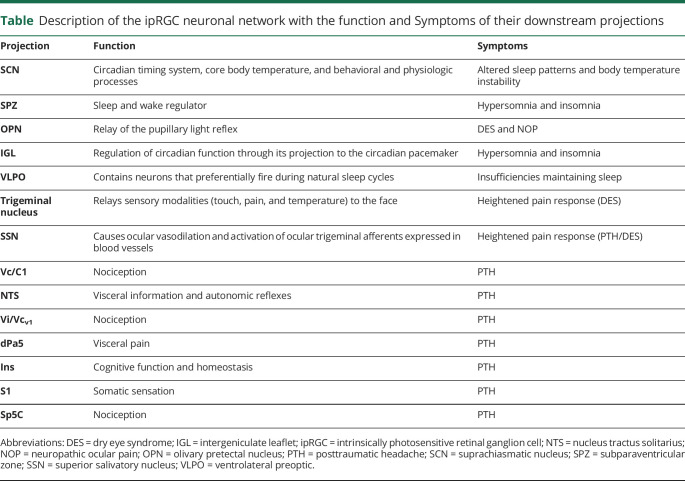
(A–C) Pathways that affect PTH symptoms: (A and B) pathways 1 and 2 showing ipRGC projections to the OPN, SSN, and different downstream pathways; (C) pathway 3 showing ipRGC connections that bypass the optic nerve and alter signaling in the OPN and downstream pathways involved in PTH symptoms. (D) Pathway 4 showing ipRGC projections to the OPN, SSN, and trigeminal nucleus that may contribute to DES/NOP. (E) Pathway 5 showing ipRGC projections to the SCN and downstream pathways including the SPZ, IGL, and VLPO, which are involved in the control of circadian rhythm. DES = dry eye syndrome; ipRGC = intrinsically photosensitive retinal ganglion cell; NOP = neuropathic ocular pain; OPN = olivary pretectal nucleus; PTH = posttraumatic headache; SCN = suprachiasmatic nucleus; SSN = superior salivatory nucleus; VLPO = ventrolateral preoptic.
The second pathway involves the ophthalmic branch of the trigeminal nerve, which is responsible for pain sensation in the eye (figure, B). Noseda et al.43 used a rat model and found that the activity of dura-sensitive neurons in the posterior thalamus was modulated by the presence of light and that their axons projected to the I-V layers of the somatosensory, visual, and associative cortices. Specifically, their axons made synaptic connections in the insular (Ins) and primary somatosensory (S1) cortices, which project to laminae I-II and III-V of the brainstem trigeminocervical complex (Sp5C), another area known to function in nociception.43
Overall, the 2 main pathways of photosensitivity have been well documented and elicit clarity in terms of the mechanisms and subsequent functionality that the ipRGCs obtain. The third pathway, however, has continued to be debated throughout the field in recent years. This third pathway of photosensitivity is still active even if the optic nerve is lesioned (figure, C).44 The results of this mouse study demonstrated that the magnitude of trigeminal reflex blinks continued to increase in response to amplified light levels even when the optic nerve was lesioned.44 There is logical reason to conclude that on an optic nerve lesion, a system of photosensitivity is still intact due to the sustainment of light aversion when a stimulus is present. For this reason, the debate begins as to whether such systems are still incorporating ipRGCs and bypassing the optic nerve altogether or if an alternative pathway is aiding in the PTH and photosensitivity symptoms.
One study by Matynia et al. showed that on optic nerve lesion, light-induced nociception still occurs. This pathway incorporates the trigeminal sensory ganglia and the interaction with the peripheral sensing neurons to produce a direct mechanism of photosensitivity independent of the optic nerve and most notably ipRGC connectivity.45 This is in agreement with other studies showing that the peripheral trigeminal neurons elicit nociception in response to light.40,42,44,46,47 This mode of association incorporates projections from the retinal periphery of the pars plana in the ciliary marginal zones.48 Indirect activation of trigeminal nociceptors can be triggered by the pupillary light reflex, which leads to modulation of uveal blood flow, igniting photosensitivity.49–51 Additional research must be conducted to understand which mechanism is the most efficient and if the 2 interact.
Dry eye syndrome in subjects with mTBI
DES is clinically defined as a multifactorial disease of the tears, lids, and ocular surface, which results in symptoms of discomfort, visual disturbance, and tear film instability with the potential for damage to the ocular surface.52,53 In terms of DES and TBI, a meta-analysis showed that US military veterans with TBI were more likely to have a diagnosis of DES (37.2%) compared with their counterparts without TBI (29.1%).54 DES is a heterogeneous diagnosis, especially in patients with TBI, and as such, this umbrella term covers a host of symptoms with many potential underlying etiologies.55 First, DES may be primarily symptomatic but without obvious signs of disease on the ocular surface.52,53,56 For example, many patients with TBI describe ocular irritation and nonspecific ocular pain without corresponding clinically described signs. Typically, the initial approach to management of DES is lubrication of the ocular surface. However, it is well documented that symptom severity correlates poorly with ocular surface signs and tear film parameters, and current treatments focused on tear replacement do not adequately control symptoms in many patients.55,57
There is a growing body of literature suggesting that DES symptoms in these affected individuals may be better conceptualized as neuropathic ocular pain (NOP). NOP may be the expression of a central pain processing disorder, where eye pain is just one of multiple overlapping peripheral manifestations. To test this, a cross-sectional analysis was performed to assess the symptoms of NOP and to what degree it associates with the sensitivity levels of DES through the use of a questionnaire and ocular surface and corneal sensitivity examinations in the presence or absence of the corneal numbing agent, proparacaine.58 Subjects classified as having DES continued to report ocular pain even when the ocular surface was numbed, suggesting that that the pain sensation was centralized.58 In addition, there was significant overlap in corneal pathway sensitivity and overall sensitivity to light in these patient populations.
Lee et al.54 investigated the relationship between DES and general pain diagnoses in US veterans with and without TBI. The authors concluded that the association was stronger between TBI and NOP with an odds ratio of 3.08 at a 95% CI compared with TBI and tear film dysfunction with an odds ratio of 1.09 at a 95% CI.54 On further analysis of TBI, it was characterized that DES and pain diagnoses of interest revealed that central pain syndrome, cluster headache, sicca syndrome, keratoconjunctivitis sicca, and late effect of injury to the nervous system that can be seen after TBI were all closely clustered together.54 Of interest, certain features are shared between TBI and DES, including predilection to light sensitivity.17,23,54,59 Therefore, when patients with TBI present with eye pain in the absence of ocular surface damage or tear film disruption, clinicians should consider the possibility of NOP.
There are several ipRGC axons that afferently project to various components that are responsible for proper functioning pupillary reflex and pain responses that can been seen in DES/NOP (pathway 4; figure, D). Such ipRGC axons project to the OPN, which then lead downstream to the SSN causing ocular vasodilation and activation of ocular trigeminal afferents, which are heavily expressed in blood vessels (figure, D).60,61 These afferents further project to the trigeminal nucleus and function in the relaying of sensory information to the face that in response to light and pain stimuli.23,60
Sleep disturbance in subjects with mTBI
The incidence of sleep disorders is greater in patients with mTBI than in the general population.4,62 A recent meta-analysis of patients with mTBI revealed that 50% of patients with mTBI reported sleep complaints and approximately 30% were diagnosed with a clinical sleep disorder.4,63 These include insomnia (29%), hypersomnia (28%), and sleep apnea (25%).4,63 Other mTBI symptoms, such as anxiety, headaches, fatigue, and irritability, can result from as well as exacerbate sleep disturbances in a vicious cycle.4 For individuals with mTBI, problems with sleep can compromise the recovery process and impede social reintegration.
We will focus on circadian rhythm, which is altered in patients with mTBI and animal models of TBI, is centrally controlled by input from ipRGCs (pathway 5; figure, E), and disruption of it can lead to sleep disorders detected in patients with TBI.64,65 Posttraumatic hypersomnia is clinically derived from damage to the brainstem reticular formation, posterior hypothalamus, and an area near the third ventricle.64 Posttraumatic insomnia is clinically derived from damage to the interior frontal and anterior temporal regions of the brain, as well as the basal forebrain.64 In an animal model of TBI, increased expression of the circadian clock genes, Cry1 and Bma1 were detected in neurons of the suprachiasmatic nucleus (SCN).64 The SCN has been linked to hypersomnia and insomnia in both mice and humans.64,65 In addition, both clock genes had decreased expression in the hippocampus.65 The changes in expression patterns between the hippocampus and SCN may lead to dysregulation of the sleep cycle and cognitive function.
The ipRGC axons of the retina exit the eye via the optic nerve and project to the SCN, which is located in the hypothalamus and is responsible for the circadian timing system, core body temperature, and other behavioral and physiologic processes (table).6 One such afferent projection of the pathway circadian rhythm pathway leads to the subparaventricular zone (SPZ), which acts as a sleep and wake regulator (table; pathway 5; figure, E).7 Another connection downstream of the SCN in the circadian rhythm pathway is the ventrolateral preoptic, which is in the hypothalamic region and contains neurons that preferentially fire during natural sleep cycles (figure, E).6 An additional ipRGC projection that is afferent to the SCN includes the intergeniculate leaflet (IGL) of the lateral geniculate nucleus (LGN), which is responsible for the regulation of circadian function through its projection to the circadian pacemaker (table and figure, E).6 Several ipRGC projections are involved in circadian regulation, with their primary functionality being depicted in terms of the major symptom of sleep cycle disturbances.23,60
Table.
Description of the ipRGC neuronal network with the function and Symptoms of their downstream projections
Discussion
The purpose of this review was to bring together current knowledge on the potential overlap in a subset of symptoms observed in patients with mTBI and correlate with the functional roles of the ipRGCs and their downstream pathways. Most studies to date report on each symptom independently, but do not provide data on the coincidence of symptoms within subjects. We posit that in particular, the coincidence of symptoms stated in this review should be reported, as this would provide important data to prove or disprove a role for ipRGCs circuits and guide treatment strategies.
We present data linking ipRGC circuits to pain, including NOP and PTH, and to sleep disturbances. Identification of these pathways are significant advances in the field, but more investigation is needed into how they are affected in patients with TBI. For example, how are pain regions such as the trigeminal pathway and the corresponding structures in the brainstem nuclei affected as a result of mTBI? We would argue that the TBI literature suggests that DES in these patients has more to do with NOP than ocular surface diseases. More research on ipRGC dysfunction and other mechanisms as potential explanations for TBI-associated eye pain is needed. Furthermore, does TBI cause damage to downstream projections of ipRGCs, such as the SCN, which is the brain region that controls circadian rhythm? Ultimately, it will be of great interest to directly determine whether ipRGCs themselves are damaged in patients with TBI. Although only 5% of all RGCs are ipRGCs, there is a possibility that they could all be degenerated or lost, and the effect would be subclinical (e.g., the optic nerve and retinal nerve fiber layer [RNFL] could potentially appear unchanged). Of interest, a recent case report on a patient with an isolated occipital lesion and a history of headaches with visual acuity of 20/20 reported thinning of the RNFL in both eyes by optical coherence tomography, suggesting retrograde degeneration.68 This could be a potential mechanism in patients with TBI.
Furthermore, there are data showing that damage to the ipRGCs results in symptoms such as those reported in patients with TBI. For example, a recent study showed that patients with advanced glaucoma have higher daytime sleepiness as well as postillumination pupil response.69,70 And a correlation between the mean RNFL thickness and the overall pupillary light response was detected in patients with glaucoma, suggesting that the ipRGCs were affected.71 Finally, patients with advanced age-related macular degeneration have a decreased pupil response and poorer sleep efficiency, demonstrating a role for the ipRGCs.72 Along these lines, with the main pathways of the ipRGCs going through the optic nerve, a few pathologic conditions that have the ability to destroy such systems as mentioned above in glaucoma and other optic neuropathies.73 These ailments are associated with degeneration of the optic tract and therefore limited the projections from the retina to the associated regions of the brain that generate mTBI symptoms. Additional pathologies that do not incorporate the optic nerve, and therefore elicit connection for the 3rd pathway of photosensitivity, are not well known due to the limited research established with these alternative ipRGC collaterals. Thus, future studies should more closely seek to understand ipRGCs and their potential role in the pathophysiology of mTBI.
Assessing coincidence of symptoms would help the field to discern if there is a common link between them, such as ipRGC dysfunction. An increased understanding of the underlying etiology of these TBI-associated symptoms will improve efforts to properly diagnose and treat these patients.
Acknowledgment
DoD W81XWH-15-1-0096, W81XWH-17-2-0055, NEI R01 EY022349, NEI U24 EY29893, NEI P30 EY008126 (VVRC), Research to Prevent Blindness Unrestricted Funds (VEI), Ret. Maj. General Stephen L. Jones, MD Fund, Mark Pigott Fund, Potoscnak Family-CSC Research Fund, Ayers Research Fund in Regenerative Visual Neuroscience.
Glossary
- DES
dry eye syndrome
- Fos-LI
Fos-like immunoreactivity
- ipRGC
intrinsically photosensitive retinal ganglion cell
- NOP
neuropathic ocular pain
- OPN
olivary pretectal nucleus
- PTH
posttraumatic headache
- RNFL
retinal nerve fiber layer
- SCN
suprachiasmatic nucleus
- SSN
superior salivatory nucleus
- TBI
traumatic brain injury
- mTBI
mild TBI
Appendix. Authors
Study funding
Support for this work included funding from DoD grants W81XWH-15-1-0096, W81XWH-17-2-0055, NEI grants R01 EY022349, U24EY029893, P30EY008126 (VVRC), Retired Major General Stephen L. Jones, MD Fund, and Research Prevent Blindness, Inc (VEI).
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Taylor C, Bell J, Breiding M, Xu L. Traumatic brain injury-related emergency department visits, hospitalizations, and deaths - United States, 2007 and 2013. MMWR Surveill Summ 2017;66:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teasdale G, Jennett B. Assessment of coma and impaired consciousness: a practical scale. Lancet 1974;2:81–84. [DOI] [PubMed] [Google Scholar]
- 3.Wijdicks E, Bamlet W, Maramattom B, Manno E, McClelland R. Validation of a new coma scale: the FOUR score. Ann Neurol 2005;58:585–593. [DOI] [PubMed] [Google Scholar]
- 4.Wickwire E, Williams S, Roth T, et al. Sleep, sleep disorders, and mild traumatic brain injury. What we know and what we need to know: findings from a national working group. Neurotherapeutics 2016;13:403–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstrong R. Visual problems associated with traumatic brain injury. Clin Exp Optom 2018;101:716–726. [DOI] [PubMed] [Google Scholar]
- 6.Driver S, Juengst S, McShan E, et al. A randomized controlled trial protocol for people with traumatic brain injury enrolled in a healthy lifestyle program (GLB-TBI). Contemp Clin trials Commun 2019;14:100328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arciniegas D, Anderson C, Topkoff J, McAllister T. Mild traumatic brain injury: a neuropsychiatric approach to diagnosis, evaluation, and treatment. Neuropsychiatr Dis Treat 2005;1:311–327. [PMC free article] [PubMed] [Google Scholar]
- 8.DVBIC. Defense and Veterans Brain Injury Center. (DoD Numbers for Traumatic Brain Injury Worldwide. 2018. Available at: dvbic.dcoe.mil/system/files/tbi-numbers/worldwide-totals-2018Q1_jun-21-2018_v1.0_2018-07-26_0.pdf. Accessed October 16, 2019. [Google Scholar]
- 9.Giza C, Hovda D. The new neurometabolic cascade of concussion. Neurosurgery 2014;75:S24–S33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peskind E, Petrie E, Cross D, et al. Cerebrocerebellar hypometabolism associated with repetitive blast exposure mild traumatic brain injury in 12 Iraq war Veterans with persistent post-concussive symptoms. Neuroimage 2011;54:S76–S82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Komura A, Kawasaki T, Yamada Y, Uzuyama S, Asano Y, Shinoda J. Cerebral glucose metabolism in patients with chronic mental and cognitive sequelae after a single blunt mild traumatic brain injury without visible brain lesions. J Neurotrauma 2019;36:641–649. [DOI] [PubMed] [Google Scholar]
- 12.Ito K, Asano Y, Ikegame Y, Shinoda J. Differences in brain metabolic impairment between chronic Mild/Moderate TBI patients with and without visible brain lesions based on MRI. BioMed Res Int 2017;2017:1–8. [Google Scholar]
- 13.Nakayama N, Okumura A, Shinoda J, Nakashima T, Iwama T. Relationship between regional cerebral metabolism and consciousness disturbance in traumatic diffuse brain injury without large focal lesions: an FDG-PET study with statistical parametric mapping analysis. J Neurol Neurosurg Psychiatry 2006;77:856–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirov I, Tal A, Babb J, Lui Y, Grossman R, Gonen O. Diffuse axonal injury in mild traumatic brain injury: a 3D multivoxel proton MR spectroscopy study. J Neurol 2013;260:242–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Facts about Concussion and Brain Injury: Where to Get Help. 2010. 2019. Centers for Disease Control and Prevention, U.S. Department of Health and Human Services. Available at: cdc.gov/headsup/pdfs/providers/facts_about_concussion_tbi-a.pdf. Accessed January 18, 2020. [Google Scholar]
- 16.Goodrich G, Kirby J, Cockerham G, Ingalla S, Lew H. Visual function in patients of a polytrauma rehabilitation center: a descriptive study. J Rehabil Res Dev 2007;44:929–936. [DOI] [PubMed] [Google Scholar]
- 17.Truong J, Ciuffreda K, Han M, Suchoff I. Photosensitivity in mild traumatic brain injury (mTBI): a retrospective analysis. Brain Inj 2014;28:1283–1287. [DOI] [PubMed] [Google Scholar]
- 18.Brahm K, Wilgenburg H, Kirby J, Ingalla S, Chang C, Goodrich G. Visual impairment and dysfunction in combat-injured service members with traumatic brain injury. Optom Vis Sci 2009;86:817–825. [DOI] [PubMed] [Google Scholar]
- 19.Lemke S, Cockerham G, Glynn-Milley C, Cockerham K. Visual quality of life in veterans with blast-induced traumatic brain injury. JAMA Ophthalmol 2013;131:1602–1609. [DOI] [PubMed] [Google Scholar]
- 20.Erickson J, Neely E, Theeler B. Posttraumatic headache. Continuum 2010;16:55–78. [DOI] [PubMed] [Google Scholar]
- 21.Lucas S, Hoffman J, Bell K, Dikmen S. A prospective study of prevalence and characterization of headache following mild traumatic brain injury. Cephalalgia 2014;34:93–102. [DOI] [PubMed] [Google Scholar]
- 22.Capo-Aponte J, Urosevich T, Temme L, Tarbett A, Sanghera H. Visual dysfunctions and symptoms during the subacute stage of blast-induced mild traumatic brain injury. Mil Med 2012;177:804–813. [DOI] [PubMed] [Google Scholar]
- 23.Katz B, Digre K. Diagnosis, pathophysiology, and treatment of photophobia. Surv Ophthalmol 2016;61:466–477. [DOI] [PubMed] [Google Scholar]
- 24.Mayo Clinic. Disease Conditions of Migraine-Headaches- Symptoms and Causes. Mayo Foundation for Medical Education and Research (MFMER). 2020. Available at: mayoclinic.org/diseases-conditions/migraine-headache/symptoms-causes/syc-20360201. Accessed January 18, 2020. [Google Scholar]
- 25.American Migraine Foundation. Timeline of a Migraine Attack. 2018. Available at: americanmigrainefoundation.org/resource-library/timeline-migraine-attack/. Accessed January 22, 2020. [Google Scholar]
- 26.Albilali A, Dilli E. Photophobia: when light hurts, a review. Curr Neurol Neurosci Rep 2018;18:1–6. [DOI] [PubMed] [Google Scholar]
- 27.Choi J, Oh K, Kim B, Chung C, Koh S, Park K. Usefulness of a photophobia questionnaire in patients with migraine. Cephalalgia 2009;29:953–959. [DOI] [PubMed] [Google Scholar]
- 28.Robbins M, Lipton R. The epidemiology of primary headache disorders. Semin Neurol 2010;30:107–119. [DOI] [PubMed] [Google Scholar]
- 29.Noseda R, Copenhagen D, Burstein R. Current understanding of photophobia, visual networks and headaches. Cephalalgia 2019;13:1623–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vincent A, Spierings E, Messinger H. A controlled study of visual symptoms and eye strain factors in chronic headache. Headache 1989;29:523–527. [DOI] [PubMed] [Google Scholar]
- 31.Department of Veterans Affairs. Visual Problems in Traumatic Brain Injury: A Systematic Review of Sequelae and Intervention for the Beater Population. 2009. Available at: va.gov/OPTOMETRY/docs/VISTBI-Vision-tbi-final-report-9-09.pdf. Accessed November 4, 2019. [Google Scholar]
- 32.Lew H, Poole J, Vanderploeg R, et al. Program development and defining characteristics of returning military in a VA Polytrauma Network Site. J Rehabil Res Develop 2007;44:1027- 1034. [PubMed] [Google Scholar]
- 33.Kraus J, Schaffer K, Ayers K, Stenehjem J, Shen H, Afifi AA. Physical complaints, medical service use, and social and employment changes following mild traumatic brain injury: a 6-month longitudinal study. J Head Trauma Rehabil 2005;20:239–256. [DOI] [PubMed] [Google Scholar]
- 34.Powell J, Barber-Foss K. Injury patterns in selected high school sports: a review of the 1995–1997 seasons. J Athletic Trainers 1999;34:277–284. [PMC free article] [PubMed] [Google Scholar]
- 35.Hobbs J, Young J, Bailes J. Sports-related concussions: diagnosis, complication, and current management strategies. Neurosurg Focus 2016;40:E5. [DOI] [PubMed] [Google Scholar]
- 36.Gessel L, Fields S, Collins C, Dick R, Comstock R. Concussions among United States high school and collegiate athletes. J athletic Train 2007;42:495–503. [PMC free article] [PubMed] [Google Scholar]
- 37.McCREA M, Barr W, Guskiewicz K, et al. Standard regression-based methods for measuring recovery after sport-related concussion. J Int Neuropsychol Soc 2005;11:58–69. [DOI] [PubMed] [Google Scholar]
- 38.Omalu B, Bailes J, Hammers J, Fitzsimmons R. Chronic traumatic encephalopathy, suicides and parasuicides in professional American athletes: the role of the forensic pathologist. Am J Forensic Med Pathol 2010;31:130–132. [DOI] [PubMed] [Google Scholar]
- 39.Moscicki E. Identification of suicide risk factors using epidemiologic studies. Psychiatr Clinic of North Am 1997;20:499–517. [DOI] [PubMed] [Google Scholar]
- 40.Okamoto K, Thompson R, Tashiro A, Chang Z, Bereiter DA. Bright light produces Fos-positive neurons in caudal trigeminal brainstem. Neuroscience 2009;160:858–864. [DOI] [PubMed] [Google Scholar]
- 41.Okamoto K, Tashiro A, Chang Z, Bereiter DA. Bright light activates a trigeminal nociceptive pathway. Pain 2010;149:235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Likova L, Tyler C. When light hurts: comparative Morphometry of human brainstem in traumatic photalgia. Sci Rep 2018;8:10–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Noseda R, Constandil L, Bourgeais L, Chalus M, Villanueva L. Changes of meningeal excitability mediated by corticotrigeminal networks: a link for the endogenous modulation of migraine pain. J Neurosci 2010;30:14420–14429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dolgonos S, Ayyala H, Evinger C. Light-induced trigeminal sensitization without central visual pathways: another mechanism for photophobia. Invest Ophthalmol Vis Sci 2011;52:7852–7858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matynia A, Nguyen E, Sun E, et al. Peripheral sensory neurons expressing melanopsin respond to light. Front Neural Circuits 2016;60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Semo M, Gias C, Ahmado A, Vugler A. A role for the ciliary marginal zone in the melanopsin-dependent intrinsic pupillary light reflex. Exp Eye Res 2014;119:8–18. [DOI] [PubMed] [Google Scholar]
- 47.Xue T, Do M, Roccio A, Jiang Z, Hsieh J, Wang H. Melanopsin signaling in mammalian iris and retina. Nature 2011;479:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yazulla S, Studholme K. Vanilloid receptor like 1 (VRL1) immunoreactivity in mammalian retina: colocalization with somatostatin and purinergic P2X1 receptors. J Comp Neurol 2004;474:407–418. [DOI] [PubMed] [Google Scholar]
- 49.Lehtosalo J, Uusitalo H, Palkama A. Sensory supply of the anterior uvea: a light and electron microscope study. Exp Brain Res 1984;55:562–569. [DOI] [PubMed] [Google Scholar]
- 50.Terenghi G, Polak JM, Ghatei MA, et al. Distribution and origin of calcitonin gene-related peptide (CGRP) immunoreactivity in the sensory innervation of the mammalian eye. J Comp Neurol 1985;233:506–516. [DOI] [PubMed] [Google Scholar]
- 51.Uusitalo H, Krootila K, Palkama A. Calcitonin gene-related peptide (CGRP) immunoreactive sensory nerves in the human and Guinea pig uvea and cornea. Exp Eye Res 1989;48:467–475. [DOI] [PubMed] [Google Scholar]
- 52.Mcmonnies C. The potential role of neuropathic mechanisms in dry eye syndromes. J Optom 2016;10:5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lemp M, Baudouin C, Baum J, et al. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye Work- shop. Ocul Surf 2007;5:75–92. [DOI] [PubMed] [Google Scholar]
- 54.Lee C, Felix E, Levitt R, et al. Traumatic brain injury, dry eye and comorbid pain diagnoses in US veterans. Br J Ophthalmol 2018;102:667–673. [DOI] [PubMed] [Google Scholar]
- 55.Levitt A, Galor A, Levitt R. Evidence that dry eye represents a chronic overlapping pain condition. Mol Pain 2017;13:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosenthal P, Borsook D. The corneal pain system. Part 1: the missing piece of the dry eye puzzle. Ocul Surf 2012;10:2–14. [DOI] [PubMed] [Google Scholar]
- 57.Stonecipher K, Perry H, Gross R, Kerney D. The impact of topical cyclosporine A emulsion 0.05% on the outcomes of patients with keratoconjunctivitis sicca. Curr Med Res Opin 2005;21:1057–1063. [DOI] [PubMed] [Google Scholar]
- 58.Crane A, Feuer W, Felix E, et al. Evidence of central sensitisation in those with dry eye symptoms and neuropathic-like ocular pain complaints: incomplete response to topical anaesthesia and generalised heightened sensitivity to evoked pain British. J Ophthalmol 2017;101:1238–1243. [DOI] [PubMed] [Google Scholar]
- 59.Kalangara J, Galor A, Levitt R, et al. Characteristics of ocular pain complaints in patients with idiopathic dry eye symptoms. Eye Contact Lens 2017;43:192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Digre K, Brennan K. Shedding light on photophobia. J Neuroophthalmol 2012;32:68–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mares C, Dagher J, Harissi-Dagher M. Narrative review of the pathophysiology of headaches and photosensitivity in mild traumatic brain injury and concussion. Can J Neurol Sci 2019;46:14–22. [DOI] [PubMed] [Google Scholar]
- 62.Shekleton J, Parcell D, Redman J, Phipps-Nelson J, Ponsford J, Rajaratnam S. Sleep disturbance and melatonin levels following traumatic brain injury. Neurology 2010;74:1732–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mathias J, Alvaro P. Prevalence of sleep disturbances, disorders, and problems following traumatic brain injury: a meta-analysis. Sleep Med 2012;13:898–905. [DOI] [PubMed] [Google Scholar]
- 64.Viola-Saltzman M, Watson N. Traumatic brain injury and sleep disorders. Neurol Clin 2012;30:1299–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boone D, Sell S, Micci M, et al. Traumatic brain injury-induced dysregulation of the circadian clock. PLoS One 2012;7:e46204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moore R Suprachiasmatic nucleus in sleep–wake regulation. Sleep Med 2007;827–33, ISSN 1389-9457. [DOI] [PubMed] [Google Scholar]
- 67.Gooley J, Saper C. Anatomy of the Mammalian Circadian System, Chapter 33. Principles and Practice of Sleep Medicine Vol 5. W.B. Saunders, 2011:376–389, ISBN 9781416066453. [Google Scholar]
- 68.Meier P, Maeder P, Kardon R, Borruat F. Homonymous ganglion cell layer thinning after isolated occipital lesion: macular OCT demonstrates transsynaptic retrograde retinal degeneration. J Neuroophthalmol 2015;35:112–116. [DOI] [PubMed] [Google Scholar]
- 69.Gracitelli C, Duque-Chica G, Moura A, et al. Relationship between daytime sleepiness and intrinsically photosensitive retinal ganglion cells in glaucomatous disease. J Ophthalmol 2016;2016:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Feigl B, Mattes D, Thomas R, Zele A. Intrinsically photosensitive (melanopsin) retinal ganglion cell function in glaucoma. Invest Opthalmology Vis Sci 2011;52:4362–4367. [DOI] [PubMed] [Google Scholar]
- 71.Gracitelli C, Duque-Chica G, Moura A, et al. A positive association between intrinsically photosensitive retinal ganglion cells and retinal nerve fiber layer thinning in glaucoma. Invest Ophthalmol Vis Sci 2014;55:7997–8005. [DOI] [PubMed] [Google Scholar]
- 72.Maynard M, Zele A, Kwan A, Feigl B. Intrinsically photosensitive retinal ganglion cell function, sleep efficiency and depression in advanced age-related macular degeneration. Invest Ophthalmol Vis Sci 2017;58:990–996. [DOI] [PubMed] [Google Scholar]
- 73.Guo Z, Jiang S, Zeng L, et al. ipRGCs: possible causation accounts for the higher prevalence of sleep disorders in glaucoma patients. Int J Ophthalmol 2017;10:1163–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]