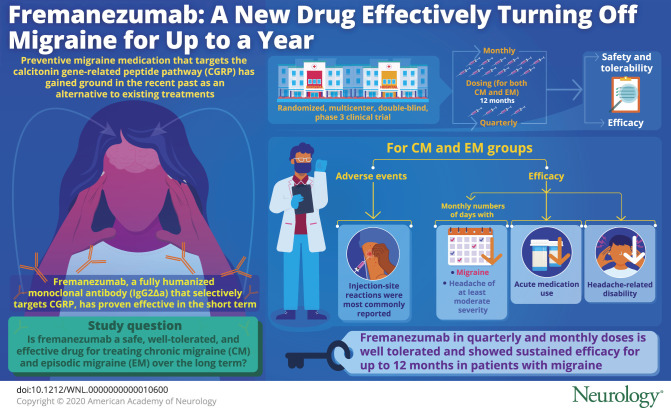
Abstract
Objective
To assess the long-term safety, tolerability, and efficacy of fremanezumab, a fully humanized monoclonal antibody approved for the preventive treatment of migraine.
Methods
A 52-week, multicenter, randomized, double-blind, parallel-group study evaluated fremanezumab monthly or quarterly in adults with chronic migraine (CM) or episodic migraine (EM). Safety and tolerability were assessed by adverse event (AE) monitoring (performed by the investigators), systematic local injection-site assessments (immediately and 1 hour after injection), laboratory/vitals assessments, and immunogenicity testing. Prespecified exploratory evaluations included change from baseline in the monthly number of migraine days, headache days of at least moderate severity, and days with any acute headache medication use. Change from baseline in headache-related disability (6-item Headache Impact Test scores) was also measured.
Results
Of 1,890 patients enrolled, 551 and 559 patients with CM received quarterly and monthly dosing; 394 and 386 patients with EM received quarterly or monthly, respectively. The most commonly reported AEs were injection-site reactions (induration 33%, pain 31%, and erythema 26%). Fremanezumab reduced monthly migraine days (CM quarterly −7.2 days, CM monthly −8.0 days, EM quarterly −5.2 days, EM monthly −5.1 days) and headache days of at least moderate severity (CM quarterly −6.4 days, CM monthly −6.8 days, EM quarterly −4.4, EM monthly −4.2 days) from baseline to 12 months. Reductions in any acute headache medication use and headache-related disability were also maintained over 12 months.
Conclusions
Fremanezumab quarterly and fremanezumab monthly were well tolerated and demonstrated sustained improvements in monthly migraine days, headache days, and headache-related disability for up to 12 months in patients with migraine.
ClinicalTrials.gov
Classification of evidence
This study provides Class IV evidence that long-term fremanezumab treatment is safe, well tolerated, and effective at sustaining reductions in monthly migraine and headache days.
Oral medications currently used for migraine preventive treatment were not originally developed to target migraine pathophysiology.1,2 They are often associated with significant side effects, leading to discontinuation and poor treatment outcomes.3,4 Observational studies have shown that persistence varies widely, ranging from as low as 19% to 79% at 6 months and from 7% to 55% at 12 months.4 Switching or reinitiating treatment after initial discontinuation of an oral migraine preventive medication leads to even lower persistence rates.5
Monoclonal antibodies targeting the calcitonin gene-related peptide (CGRP) pathway are a new class of preventive therapy that specifically target the pathophysiology of migraine.6–8 They have notable advantages over conventional oral migraine preventive medications such as not requiring dose titration, having a long half-life that enables monthly or quarterly administration, and having a favorable safety profile.1,6,9 Fremanezumab, a fully humanized monoclonal antibody (IgG2Δa)10 that selectively targets CGRP, is approved in the United States, the European Union, and Australia for the preventive treatment of migraine in adults. The efficacy and safety of fremanezumab were demonstrated in 2 pivotal 12-week phase 3 randomized, double-blind, placebo-controlled efficacy studies (HALO) in patients with chronic migraine (CM) (HALO CM) and episodic migraine (EM) (HALO EM).11,12 The long-term safety, tolerability, and efficacy of fremanezumab in adults with CM or EM from the 12-month, randomized, double-blind, parallel-group, phase 3 study are reported here.
Methods
Standard protocol approvals, registrations, and patient consents
This clinical study (NCT02638103) was conducted in accordance with the International Conference on Harmonisation guidelines for Good Clinical Practice, principles of the Declaration of Helsinki, and local and national regulations. The protocol was approved by the relevant national/local health authorities and each Independent Ethics Committee/Institutional Review Board. All participants provided written informed consent.
Study design
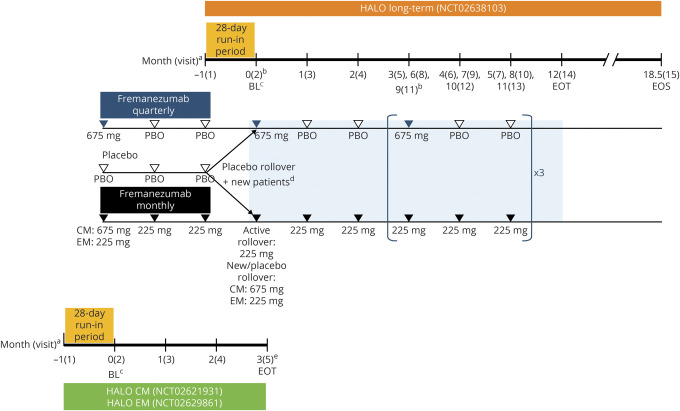
This multicenter, randomized, double-blind, parallel-group, phase 3 study consisted of a screening visit, 28-day run-in period (new patients only), 12-month double-blind treatment period, and 6.5-month follow-up period for antidrug antibody (ADA) assessment (figure 1). The study was conducted from March 2016 to December 2018 at 135 sites, including clinical research centers, academic medical centers, and neurology/headache practices in the United States, Japan, Czech Republic, Russia, Canada, Finland, Poland, Israel, and Spain.
Figure 1. Study design for the long-term safety and efficacy study of fremanezumab.
This long-term study was an extension of the Efficacy and Safety of Subcutaneous Administration of Fremanezumab (TEV-48125) for the Preventive Treatment of Migraine (HALO) chronic migraine (CM) and episodic migraine (EM) studies that allowed an additional subset of new patients who had not previously participated in the HALO studies to directly enroll. BL = baseline; EOS = end of study; EOT = end of treatment; PBO = placebo. a Monthly dosing refers to dosing approximately every 4 weeks (28 days). b To maintain the study blind, all patients received 3 injections at visits 2,5, 8, and 11. A single injection was administered all other months. c Baseline refers to the 28-day run-in period (for headache variables only) and day 0 of the HALO CM or EM studies for rollover patients or to the 28-day run-in period (for headache variables only) and day 0 of this long-term study for new patients. d Rollover patients who received placebo during the HALO CM or EM studies and new patients not rolling over from one of the HALO CM or EM studies who met eligibility criteria after completing a 28-day run-in period were randomized in a 1:1 ratio on day 0 of this long-term study to receive fremanezumab quarterly at 675 mg or monthly at 225 mg (CM monthly group received a single 675 mg dose of fremanezumab at baseline). eFor patients who began this long-term study (visit 2) on the same day as the EOT visit of the HALO CM or EM study, the EOT visit procedures/assessments for the HALO CM or EM study were completed before procedures/assessments for this study began.
Participants
Patients who completed HALO CM (NCT02621931) or HALO EM (NCT02629861) had the option to enroll (rollover patients), and new patients could directly enroll (new patients). Eligible patients were adults 18 to 70 years of age, inclusive, with a history of migraine (according to International Classification of Headache Disorders 3rd Edition, beta version [ICHD-3 beta] criteria13) for ≥12 months before screening. Patients were classified as having CM or EM on the basis of headache data recorded daily in an electronic headache diary (e-diary) device during a 28-day run-in period and the following protocol-specified criteria: patients with CM fulfilled ICHD-3 beta criteria for CM and had headache of any severity and duration occurring on ≥15 days, with ≥8 days fulfilling specific ICHD-3 beta criteria for migraine, while patients with EM had headache of any severity and duration occurring on 6 to 14 days (rollover patients) or on 4 to 14 days (new patients), with ≥4 days fulfilling specific ICHD-3 beta criteria for migraine. A maximum of 1 (rollover patients) or 2 (new patients) concomitant migraine preventive medications were allowed, provided that the medication was recognized as having at least moderate efficacy and dosage had been stable for ≥2 consecutive months at screening. Patients could use acute migraine medication as needed.
In the pivotal HALO CM and EM studies, patients were excluded on the basis of use of onabotulinumtoxinA during the 4 months before screening, use of opioids or barbiturates on >4 d/mo for migraine or any other reason, previous failure of ≥2 preventive medications after an adequate therapeutic trial, or use of an intervention or device (e.g., nerve block or transcranial magnetic stimulation) for migraine during the 2 months before screening.11,12 These exclusion criteria did not apply to new patients.
Study treatment
Rollover patients randomized to treatment with fremanezumab in the HALO CM and EM studies continued the same dosing regimen; rollover patients originally randomized to placebo and new patients were randomized 1:1 to treatment with fremanezumab quarterly or monthly administered via subcutaneous injection for 12 months. The quarterly dosing regimen was fremanezumab 675 mg once every 3 months with placebo at intervening monthly visits; the monthly regimen was fremanezumab 675 mg for CM and 225 mg for EM at baseline followed by 225 mg monthly thereafter.
Randomization and blinding
Randomization was performed with interactive response technology, with patients stratified by sex, country, and preventive medication use at baseline (yes/no). The investigators, study staff (excluding those involved in bioanalytical analyses), and patients were blinded to treatment assignment. The sponsor was initially blinded to treatment assignment and then unblinded when the pivotal studies were unblinded. To maintain study blind during this long-term trial, patients received the same number of injections on each visit during which study drug was administered: three 1.5-mL injections (three 225-mg/1.5-mL injections or one 225-mg/1.5-mL injection and 2 placebo 1.5-mL injections) at visits 2, 5, 8, and 11 and 1 placebo 1.5-mL injection or one 225-mg/1.5-mL injection at all other visits.
Outcomes
The primary objective was to evaluate the long-term safety and tolerability of fremanezumab in the preventive treatment of migraine. All additional efficacy analyses described here were prespecified.
Safety and tolerability were assessed via adverse events (AEs), including injection-site monitoring (immediately and 1 hour after study drug administration); clinical laboratory tests, vital signs, and ECGs (12 lead); physical examinations; and electronic Columbia-Suicide Severity Rating Scale (C-SSRS). Injection-site assessments were performed immediately and 1 hour after administration of each dose of study drug. Injection sites were evaluated for erythema, induration, ecchymosis, and pain, with erythema, induration, and ecchymosis graded according to measurements (absent, mild [5–≤50 mm], moderate [>50–≤100 mm], severe [>100 mm]) and pain graded on a 5-point scale (0 = no pain to 4 = worst possible pain). If a patient had severe injection-site induration, erythema, or ecchymosis or severe or worst possible injection-site pain 1 hour after study drug administration, the patient was reassessed by the investigator 3 hours after administration and hourly thereafter until the reaction/pain was of moderate or less severity. For all other AEs, the investigator asked about AEs at each patient contact using an open-ended question concerning any symptoms or medical problems since the last visit. The investigator assessed the relationship of each AE to the study drug and procedures, as well as the severity and seriousness of each AE, according to protocol-specified criteria.
Prespecified exploratory efficacy evaluations included mean change from baseline in the monthly number of migraine days, headache days of at least moderate severity, headache days of any severity, and days with any acute headache medication use at months 3, 6, and 12. The proportions of patients with an ≥50% reduction from baseline in the monthly average number of migraine days at months 6 and 12 were also assessed. e-Diary entries capturing headache data from the previous day were completed daily during the first 3 months beginning on day 1 (rollover patients) or during the first 4 months beginning on day −27 (new patients). All patients completed daily entries during the 4-week periods after visits 7 and 13.
For patients with CM, mean change from baseline in headache-related disability score at months 6 and 12 was measured by the 6-item Headache Impact Test (HIT-6), as specified in the protocol. The HIT-6 is a reliable and validated tool for measuring headache impact, with total scores ranging from 36 to 78 and higher scores reflecting greater impact.14 For patients with EM, the mean change from baseline in headache-related disability score at months 6 and 12 was measured by the Migraine Disability Assessment (MIDAS) questionnaire, as specified in the protocol. The MIDAS is a brief, self-administered questionnaire validated to quantify headache-related disability over a 3-month period. Total scores are used for grading disability, with scores of 0 to 5 indicating little or no disability, 6 to 10 indicating mild disability, 11 to 20 indicating moderate disability, and ≥21 indicating severe disability.15
As described in the protocol, immunogenicity was assessed by evaluating the incidence of ADAs and their potential impact on clinical outcomes, measured from blood samples with an appropriately validated method. Samples were collected at visits 2 (new patients only), 5, 8, 14, and 15.
Statistical analysis
The sample size was not based on any statistical considerations. The safety analysis set included all patients who received ≥1 dose of fremanezumab in this study. The full analysis set included all randomized/rolled over patients (intention-to-treat population) who received ≥1 dose of study drug and had ≥10 postbaseline e-diary efficacy assessments. All safety data and efficacy variables were summarized with the use of descriptive statistics. For withdrawals and patients with missing e-diary days, efficacy variables were prorated to 28 days if the patient had ≥10 days of e-diary data for that month; otherwise, data were treated as missing.
Classification of evidence
This interventional study provides Class IV evidence that subcutaneous administration of either fremanezumab quarterly (675 mg every 3 months) or fremanezumab monthly (225 mg monthly; CM: starting dose of 675 mg) is safe, well tolerated, and effective at sustaining reductions in monthly migraine days, monthly headache days of at least moderate severity, and headache-related disability for up to 12 months compared with baseline in adults with CM or EM.
Data availability
The data described in this report are available by request from the author investigators or Teva Pharmaceuticals Ltd, the company sponsoring the clinical development of fremanezumab for the treatment of migraine.
Results
Patient characteristics and disposition
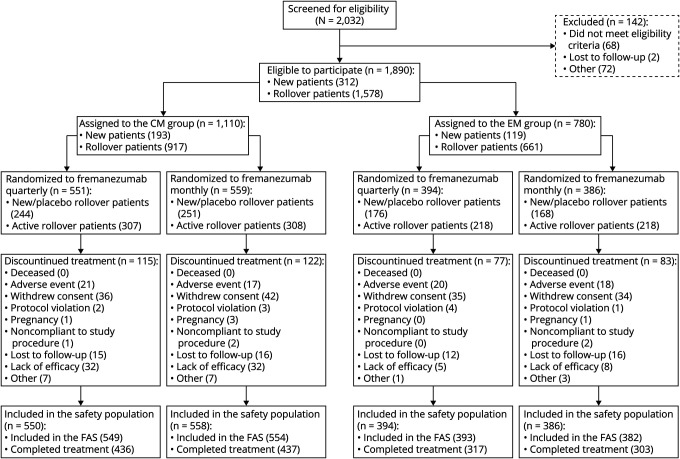
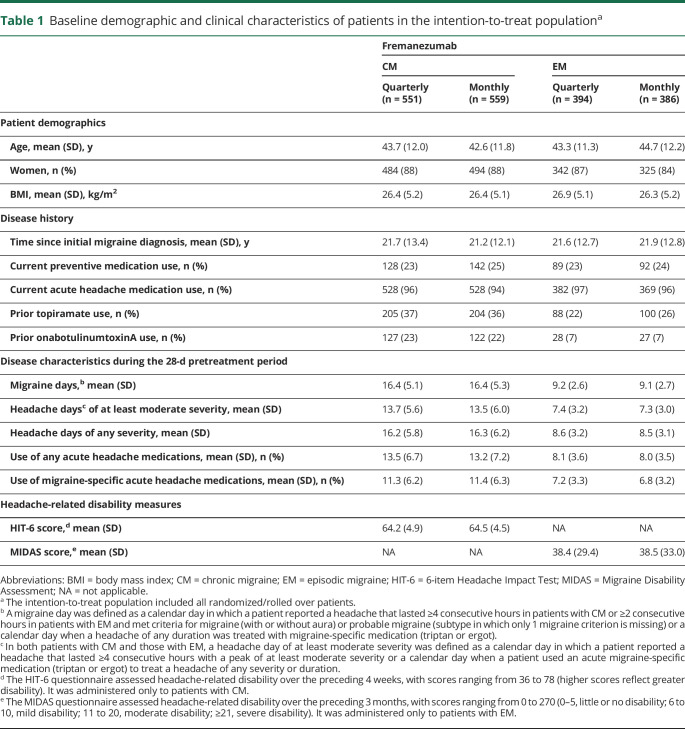
Of 1,890 eligible patients (CM n = 1,110, EM n = 780), 551 and 559 patients with CM received quarterly and monthly dosing; 394 and 386 patients with EM received quarterly and monthly, respectively. A total of 1,493 of 1,890 (79%) completed treatment. Reasons for discontinuing treatment included withdrawal by the patient (147 of 1,890 [8%]), lack of efficacy (77 of 1,890 [4%]; according to patient report, along with the treating physician's assessment), AE (76 of 1,890 [4%]), and loss to follow-up (59 of 1,890 [3%]). Five (<1%) discontinued treatment due to pregnancy (figure 2). Within each migraine diagnosis group, baseline demographics and clinical characteristics were similar across treatment groups (table 1).
Figure 2. Flow of participants in the long-term safety and efficacy study of fremanezumab.
CM = chronic migraine; EM = episodic migraine; FAS = full analysis set.
Table 1.
Baseline demographic and clinical characteristics of patients in the intention-to-treat populationa
Safety and tolerability
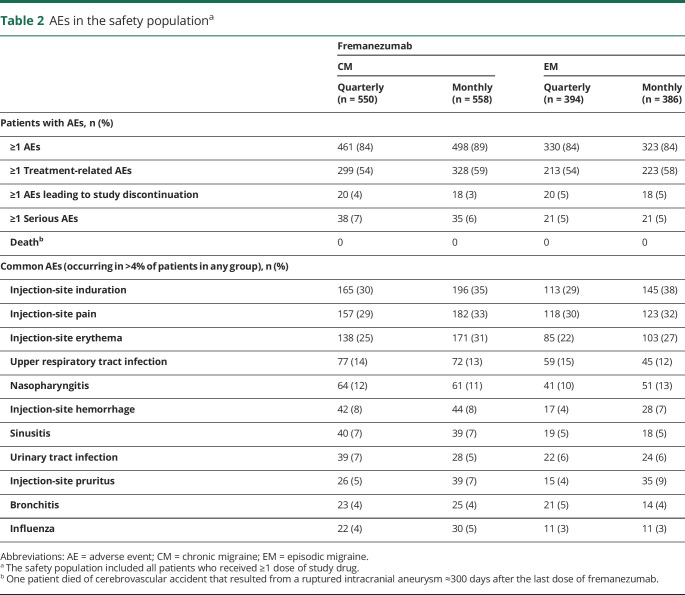
AEs were reported in 84% to 89% of patients in all treatment groups, with injection-site reactions being the most common (table 2). AEs leading to discontinuation occurred in 3% to 5% of patients, and serious AEs occurred in 5% to 7% of patients and were reported for similar proportions of patients across treatment groups (table 2). The most common AEs leading to study discontinuation (occurring in >2 patients) included injection-site erythema (n = 5), injection-site rash (n = 4), injection-site swelling (n = 4), injection-site pruritus (n = 3), and increased weight (n = 3). Serious AEs occurring in >2 patients included status migrainosus (n = 4), basal cell carcinoma (n = 4), cerebrovascular accident (n = 3), migraine (n = 3), malignant melanoma (n = 3), osteoarthritis (n = 3), and retinal tear (n = 3). No serious AEs were assessed by the sponsor as related to study drug, while 9 patients had a serious AE assessed as related to the study drug by the investigator. Serious AEs considered related to the study drug by the investigator included pneumonia, dehydration, suicidal ideation, fibromyalgia, status migrainosus (3 patients), papillary thyroid cancer, transient global amnesia, and pulmonary mass. A single death was reported resulting from a ruptured intracranial aneurysm in a 44-year-old patient ≈300 days after the last of dose of fremanezumab. The patient had a history of atrioventricular block and hypertension. The event was assessed as unrelated to study drug.
Table 2.
AEs in the safety populationa
Cardiovascular safety
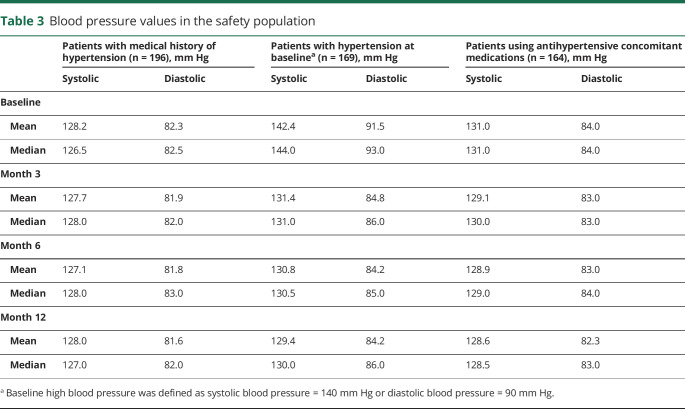
Cardiovascular AEs (CVAEs) were infrequent, were mostly mild to moderate in severity, and occurred in similar percentages of patients across groups, with the most common CVAE being hypertension (42 of 1,888 patients [2%]). The vast majority of these events were single findings of increased blood pressure in 1 patient each, were considered not related to the study drug by the investigator, and occurred in patients with a medical history of hypertension. Of the 196 patients taking part in this study with a history of hypertension, 169 had high blood pressure at baseline (i.e., systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg), and 164 were using concomitant antihypertensive medications. Baseline mean and median blood pressure values did not noticeably change over the 12-month treatment period regardless of the presence of a medical history of hypertension or the use of concomitant antihypertensive medications (table 3). For patients with hypertension at baseline, mean and median blood pressure values at the end of the 12-month treatment period were numerically lower than those at baseline (table 3).
Table 3.
Blood pressure values in the safety population
Fifteen serious CVAEs were reported, including 3 cerebrovascular accidents. One of the cerebrovascular accidents was an acute right frontal stroke that occurred in a 62-year-old patient with a medical history of hypertension and hyperlipidemia. CT results indicated acute and old ischemic changes in the brain. The event was severe and resolved without sequelae. The second cerebrovascular accident was an ischemic left middle cerebral artery stroke that occurred in a 58-year-old postmenopausal patient on hormone replacement therapy. CT showed plaques in the right and left carotid bifurcation extending into proximal left and right internal carotid artery, with <50% diameter narrowing. The patient was overweight and had a medical history of patent foramen ovale, hypertension, and hyperlipidemia. The event was mild and resolved on the same day; the patient discontinued the study because of the event. Per the investigator's assessment, alternative etiologies included suspected atrial fibrillation. The third cerebrovascular accident was the single reported death resulting from a ruptured intracranial aneurysm described above. The remaining serious CVAEs were intracranial aneurysm (n = 2), deep vein thrombosis (n = 2), hypertension (n = 1), hypovolemic shock (n = 1), venous thrombosis limb (n = 1), arteriovenous malformation (n = 1), cerebrovascular arteriovenous malformation (n = 1), atrial fibrillation (n = 1), pericarditis (n = 1), and sinus tachycardia (n = 1). All serious CVAEs were assessed as unrelated to study drug by the sponsor and investigators.
AEs of special interest
Liver AEs of special interest (events of possible drug-induced liver injury defined as aspartate aminotransferase [AST] or alanine aminotransferase [ALT] ≥3 times the upper limit of normal range [ULN], total bilirubin ≥2 times the ULN, or international normalized ratio >1.5) occurred infrequently, with similar frequency across treatment groups. Overall, 39 (2%) of 1,888 patients experienced liver AEs of special interest. Of these, 20 patients had a single mild transient elevation of AST or ALT, and 4 patients had recurrent AST/ALT elevations of 3 to 5 times the ULN; all resolved despite continued treatment with fremanezumab. Five patients had significant (>8 times the ULN) but transient AST/ALT elevations. However, factors in the patients' medical histories such as gall bladder disease, infection with concomitant use of antibiotics known to have liver side effects (e.g., azithromycin), and Epstein-Barr virus infection could better account for these events. No liver enzyme elevations were serious or met the criteria for the Hy law, and none needed treatment.
No severe hypersensitivity events, including anaphylaxis, were observed during the study. No safety signal was observed for ophthalmic AEs, which were monitored due to unconfirmed preclinical findings in monkeys. Individual ophthalmic AEs of at least moderate severity were reported by <1% of patients each and included cataracts and retinal tears (reported by 3 patients each); dry eye, retinal detachment, and vision blurred (reported by 2 patients each); and blepharitis, transient blindness, diplopia, episcleritis, eye irritation, iritis, macular degeneration, meibomian gland dysfunction, ocular hyperemia, punctate keratitis, and retinal cyst (reported by 1 patient each). Six women discontinued the study due to a positive pregnancy test. Among these patients, 1 had a spontaneous abortion, 1 had a premature separation of the placenta (placental abruption) with no fetal loss, 1 had a premature baby, and 1 had a fetal death. These events were assessed to be unrelated to fremanezumab by the investigator.
Laboratory and physical examination assessments, vital signs, and ECG measurements
No clinically meaningful trends were identified in mean changes from baseline for any clinical laboratory variables, vital signs, or ECG. No treatment-emergent clinically significant physical examination findings were reported.
Electronic C-SSRS
Eight patients had treatment-emergent positive electronic C-SSRS responses. An additional 4 patients had suicidal ideation reported as an AE, all of which were assessed by the sponsor as not related to study drug.
Immunogenicity
Overall, 43 (2.3%) of 1,888 patients were identified as having treatment-emergent (n = 42) or treatment-boosted (n = 1) ADA responses. Neutralizing antibody responses developed in 18 of these patients (<1% of all patients tested), 17 of whom were determined to have transient neutralizing antibodies (1 or 2 positive sampling time points followed by negative neutralizing antibody results at later time points). At each visit during which blood samples were collected for ADA assessment, blood samples were also collected for measuring plasma drug concentration. Four patients with treatment-emergent ADAs showed decreased fremanezumab exposure at the same time point that the ADA response occurred. No significant safety consequences of ADA development were identified.
Efficacy
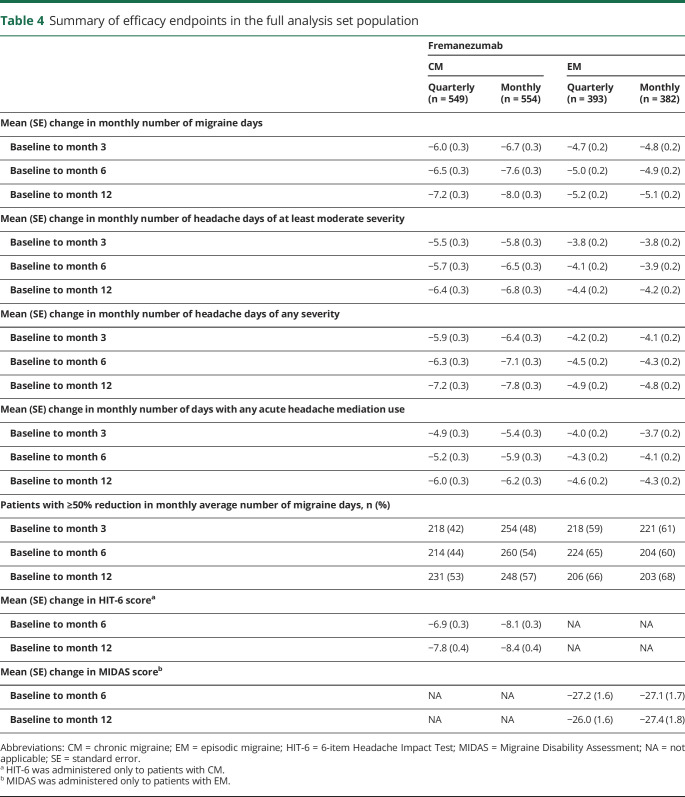
In patients with CM or EM, both fremanezumab quarterly and fremanezumab monthly reduced the monthly number of migraine days from baseline to month 12 (CM quarterly −7.2 days, CM monthly −8.0 days, EM quarterly −5.2 days, EM monthly −5.1 days) (table 4 and figure 3). Reductions from baseline to month 12 were observed in the monthly number of headache days of at least moderate severity (CM quarterly −6.4 days, CM monthly −6.8 days, EM quarterly −4.4, EM monthly −4.2 days) (table 4). Monthly number of headache days of any severity and monthly number of days of any acute headache medication use were also reduced across all treatment groups (table 4). More than half of patients with CM and approximately two-thirds of patients with EM had a ≥50% reduction in monthly average number of migraine days from baseline to month 12 (table 4 and figure 3).
Table 4.
Summary of efficacy endpoints in the full analysis set population
Figure 3. Reduction in monthly migraine days.
Mean change from baseline in monthly number of migraine days for patients in fremanezumab monthly and quarterly groups during the long-term study is shown for (A) patients with chronic migraine (CM) and (B) patients with episodic migraine (EM). Percentage of patients with at least 50% reduction from baseline in monthly average number of migraine days for patients in fremanezumab quarterly and monthly groups during the long-term study is shown for (C) patients with CM and (D) patients with EM. BL = baseline; SE = standard error.
The degree of headache-related disability decreased for patients with CM and patients with EM from baseline to month 12 (table 4). For patients with CM, baseline mean (SD) HIT-6 scores were 64.2 (4.9) and 64.5 (4.6) in the quarterly and monthly groups, respectively. At month 12, mean (SD) HIT-6 scores were 56.2 (7.8) in the quarterly group and 55.8 (8.0) in the monthly group. For patients with EM, baseline mean (SD) MIDAS scores were 38.4 (29.4) and 38.6 (33.1) in the quarterly and monthly groups, respectively. At month 12, mean (SD) MIDAS scores were reduced to 10.3 (15.3) and 9.6 (16.3) for the quarterly and monthly groups.
Discussion
Long-term fremanezumab treatment was well tolerated and resulted in sustained improvements in monthly migraine days, headache days, and headache-related disability for up to 12 months compared with baseline. This is the first long-term study reported with fremanezumab and features double-blind active dose blinding. The data are in line with those for other CGRP pathway monoclonal antibodies.16,17 The AE profile of fremanezumab in this study was consistent with previously reported clinical studies in which no safety signals were identified.11,12,18,19 No clinically significant patterns of AEs were seen in the current study, and no serious CVAEs were considered related to the study drug by the investigators or sponsor. No treatment-emergent, clinically significant laboratory or clinical findings were observed. No significant consequences of ADA development were identified, and 43 (2.3%) patients had treatment-emergent or treatment-boosted ADAs. A pooled analysis of phase 2b and phase 3 studies also showed that fremanezumab quarterly and monthly was generally safe and well tolerated, with very low rates of serious or severe AEs and AEs leading to study discontinuation and no safety signals from aggregate and individual case evaluations.20
Similar to previous studies,11,12,18,19 the most common AEs reported in this study were injection-site reactions. In the HALO CM and EM studies and the current study, injection sites were proactively monitored and graded systematically, as required by the protocols, for pain, erythema, induration, and ecchymosis immediately and at 1 hour after study drug administration.11,12 This proactive monitoring may have resulted in the higher rates of injection-site reactions observed in these studies compared with earlier or subsequent studies. Despite these higher rates, most injection-site reactions were mild or moderate in intensity, and only 3% to 5% of patients discontinued due to an AE.
CVAEs were mostly mild or moderate in intensity and occurred with similar frequency across treatment groups and patient populations. The most frequent CVAE was hypertension. The vast majority of these events were single occurrences as opposed to sustained blood pressure elevations, were considered unrelated to study drug, and were observed in patients with a history of hypertension. Among patients with hypertension at baseline, mean and median systolic and diastolic blood pressures showed a downward trend across the course of the study.
Two patients had serious CVAEs leading to discontinuation. One patient with CM receiving fremanezumab monthly had venous thrombosis of the left leg that was moderate in severity and resolved 107 days later after treatment with rivaroxaban. The event was not considered related to study drug by the investigator or sponsor because the patient had a recent diagnosis of endometrial cancer (a risk factor for hypercoagulability) and a report of recent air travel, which may be associated with increased venous stasis and risk of lower extremity venous thrombosis. The other patient was in the EM group receiving fremanezumab quarterly and experienced atrial fibrillation that was severe, was not related to study drug, and resolved the same day. This patient was treated with rivaroxaban for atrial fibrillation, acarbose and losartan for elevated blood pressure, and diltiazem and flecainide acetate for the prevention of atrial fibrillation. Due the association of migraine with CVAEs and the theoretical concern of systemic CGRP antagonism to result in vascular effects,21–23 caution is warranted.
Six women were discontinued due to pregnancy during the study. Four of these women had AEs (<1%), with 2 cases resulting in the loss of pregnancy. In an evaluation of the 20 pregnancies that have occurred in the clinical development of fremanezumab (15 in the fremanezumab arms, 5 in the placebo arm), there is no evidence of an increased rate of complications in the fremanezumab vs the placebo arms, nor is there evidence of any dose-response relationship between the occurrence of pregnancy complications and treatment assignment. It is important to note that the rate of fetal defects observed in the general US population is ≈3% to 5%.24 The pregnancy data available for fremanezumab should be considered limited, and as a precautionary measure, pregnant women or women planning to become pregnant should avoid the use of fremanezumab. To provide further safety information, a pregnancy registry and database study are currently planned.
No safety signal was observed for ophthalmic AEs of at least moderate intensity. The study sponsor decided to define any ophthalmic events of at least moderate severity as AEs of special interest on the basis of data from a 3-month repeat-dose toxicity study in monkeys, which indicated slight perivascular inflammation around the ciliary vessels of the eye. The perivascular nature (minimal grade of severity), location, and appearance of the inflammation suggested a species-specific immunogenic response to the humanized monoclonal antibody. Ophthalmic events occurred infrequently, and none were considered related to the study drug by the investigator or the sponsor.
A ≥50% reduction from baseline in the monthly average number of migraine days, a response considered clinically relevant in controlled trials of preventive treatment for migraine,25,26 was observed in more than half of patients with CM and approximately two-thirds of patients with EM at 12 months. Across the different outcomes, including the reduction in monthly number of migraine days and headache-related disability achieved in the placebo-controlled phase, effects were maintained for the additional 12-month treatment period regardless of fremanezumab dosing regimen. Specifically, the proportions of patients who had a ≥50% response rate continued to increase over time. While discontinuations may be responsible for some of the increase in responder rates, the low incidence of discontinuation due to lack of efficacy (4%) suggests that this impact is likely to be small or negligible. Another possibility is that patients may experience a delayed onset of response to therapy or accumulate additional response to therapy over time. In practice, patients who see some improvement but who have not yet achieved desired responses may benefit from a longer trial duration before a clinical decision is made to discontinue the medication. To help guide clinical decisions, future studies should also clarify whether patients who do not benefit from fremanezumab would still be likely to benefit from a different therapy that targets the CGRP pathway (or vice versa) or whether a different class of therapy should be considered.
The safety profile of fremanezumab, combined with its efficacy and flexible dosing regimens, has the potential to improve medication persistence and clinical outcomes, as demonstrated by the low percentage of treatment withdrawal due to tolerability issues (4%) and lack of efficacy (4%). In a pooled analysis of 2 6-month pivotal North American migraine prevention trials of topiramate, which typically has high persistence for an oral migraine preventive medication,5,27 52.9% completed topiramate treatment, with the most common reasons for discontinuing treatment being AEs (22.2%) and lack of efficacy (8.5%).28
Strengths of the current study include blinding of patients to treatment regimen (fremanezumab quarterly or monthly) and allowing patients to remain on concomitant migraine preventive medication. Outcomes for safety, tolerability, and efficacy were similar between quarterly and monthly dosing regimens and between patients taking and not taking concomitant preventive medications.29 Given a lack of outcome differentiation between the quarterly and monthly doses, other patient-related factors may drive decision-making during selection of the optimal dosing option (e.g., patient preference).
There were also several limitations to this study. There was no placebo control. The study was not powered to detect efficacy differences between treatment groups. For patients receiving fremanezumab monthly, the 675-mg starting dose used in the current study for patients with CM is not an approved dosing regimen.30,31 However, the impact of this loading dose over the 12-month treatment period is considered low. Patients with ≥2 failed preventive drug classes and those with continuous headache were excluded, which may limit generalizability. Results from a separate double-blind, parallel-group, placebo-controlled phase 3b study (An Efficacy and Safety Study of Fremanezumab in Adults With Migraine [FOCUS]; NCT03308968) evaluating fremanezumab in patients with CM and EM with documented failure to 2 to 4 classes of preventive therapies showed that, compared with placebo, treatment with fremanezumab quarterly or monthly was associated with significant reductions in the monthly number of migraine days.32 This provides insight into the effectiveness of fremanezumab in a patient population that has been difficult to treat with traditional migraine preventive medications. Furthermore, there were small numbers of patients in the current study with treatment-emergent positive electronic C-SSRS responses (n = 8) or suicidal ideation AEs (n = 4). These AEs were considered not related to study drug. It is worth noting that depression is common in the migraine population.33 The impact of fremanezumab on depression was not systematically evaluated in this study.
This study provides evidence for the long-term safety, tolerability, and efficacy of fremanezumab as either a quarterly or a monthly dosing regimen. Fremanezumab continues to change the landscape of migraine prevention and treatment and provides a treatment option for patients with EM or CM to reduce their pain and disability while improving their quality of life.
Acknowledgment
The authors thank the patients who participated in this study and their families; all site personnel and investigators, including the coordinating investigators. Editorial assistance, including assistance with incorporating the authors' edits, checking references, and comparing figures and tables with the study report, was provided by Lindsay Tannenholz, of Chameleon Communications International. This assistance was in accordance with Good Publication Practice (guidelines and funded by Teva Pharmaceuticals. The authors maintained full editorial control of the manuscript and decision to submit for publication.
Glossary
- ADA
antidrug antibody
- AE
adverse event
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- C-SSRS
Columbia-Suicide Severity Rating Scale
- CGRP
calcitonin gene-related peptide
- CM
chronic migraine
- CVAE
cardiovascular AE
- e-diary
electronic headache diary
- EM
episodic migraine
- FOCUS
An Efficacy and Safety Study of Fremanezumab in Adults With Migraine
- HALO
Efficacy and Safety of Subcutaneous Administration of Fremanezumab (TEV-48125) for the Preventive Treatment of Migraine
- HIT-6
6-item Headache Impact Test
- ICHD-3 beta
International Classification of Headache Disorders 3rd Edition, beta version
- MIDAS
Migraine Disability Assessment
- ULN
upper limit of normal range
Appendix. Authors
Footnotes
Class of Evidence: NPub.org/coe
Study funding
Funding provided by Teva Pharmaceutical Industries Ltd., Petach Tikva, Israel.
Disclosure
P.J. Goadsby reports grants and personal fees from Amgen and Eli Lilly and Co and personal fees from Alder Biopharmaceuticals, Allergan, Autonomic Technologies Inc, Biohaven Pharmaceuticals Inc, Electrocore LLC, eNeura, Impel Neuropharma, Mundipharma, Novartis, Teva Pharmaceuticals, Trigemina Inc, WL Gore, medicolegal work, the Massachusetts Medical Society, UpToDate, Oxford University Press, and Wolters Kluwer; he holds a patent for magnetic stimulation for headache assigned to eNeura without fee. S.D. Silberstein provides consultation to Alder, Allergan, Amgen, Autonomic Technologies, Avanir, Curelater Inc, Depomed, Dr. Reddy's Laboratories, Ensured Inc, ElectroCore Medical LLC, eNeura Therapeutics, INSYS Therapeutics, Lilly USA LLC, Supernus Pharmaceuticals Inc., Teva Pharmaceuticals, Theranica, and Trigemina Inc. P.P. Yeung is a former employee of Teva Branded Pharmaceutical Products R&D, Inc. J.M. Cohen, X. Ning, and R. Yang are employees of Teva Branded Pharmaceutical Products R&D, Inc. D.W. Dodick reports personal fees from Amgen, AEON, the Association of Translational Medicine, the University Health Network, Daniel Edelman Inc, Autonomic Technologies, Axsome, Allergan, Alder BioPharmaceuticals, Biohaven, Charleston Laboratories, Clexio, Dr. Reddy's Laboratories/Promius, Electrocore LLC, Eli Lilly, eNeura, Neurolief, Novartis, Ipsen, Impel, Satsuma, Supernus, Sun Pharma (India), Theranica, Teva, Vedanta, WL Gore, Nocira, PSL Group Services, XoC, Zosano, ZP Opco, Foresite Capital, Oppenheimer, Upjohn (Division of Pfizer), Pieris, Revance, Equinox, Salvia, and Amzak Health; speaking fees from Eli Lilly, Novartis Canada, Amgen, and Lundbeck; continuing medical education fees or royalty payments from HealthLogix, Medicom Worldwide, MedLogix Communications, Mednet, Miller Medical, PeerView, WebMD Health/Medscape, Chameleon, the Academy for Continued Healthcare Learning, Universal Meeting Management, Haymarket, Global Scientific Communications, Global Life Sciences, Global Access Meetings, Catamount, UpToDate (Elsevier), Oxford University Press, Cambridge University Press, and Wolters Kluwer Health; stock options from Precon Health, Aural Analytics, Healint, Theranica, Second Opinion/Mobile Health, Epien, Nocira, Matterhorn, Ontologics, and King-Devick Technologies; consulting without fee for Aural Analytics, Healint, Second Opinion/Mobile Health, Epien; serving on the Board of Directors for Precon Health, Epien, Matterhorn, Ontologics, and King-Devick Technologies; holding patent No. 17189376.1-1466 (Botulinum Toxin Dosage Regimen for Chronic Migraine Prophylaxis) without fee; research funding from the American Migraine Foundation, US Department of Defense, Patient-Centered Outcomes Research Institute, and Henry Jackson Foundation; and professional society fees or reimbursement for travel from the American Academy of Neurology, the American Brain Foundation, the American Headache Society, the American Migraine Foundation, the International Headache Society, and the Canadian Headache Society. Go to Neurology.org/N for full disclosures.
References
- 1.American Headache Society. The American Headache Society position statement on integrating new migraine treatments into clinical practice. Headache 2019;59:1–18. [DOI] [PubMed] [Google Scholar]
- 2.Sprenger T, Viana M, Tassorelli C. Current prophylactic medications for migraine and their potential mechanisms of action. Neurotherapeutics 2018;15:313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blumenfeld AM, Bloudek LM, Becker WJ, et al. Patterns of use and reasons for discontinuation of prophylactic medications for episodic migraine and chronic migraine: results from the Second International Burden of Migraine Study (IBMS-II). Headache 2013;53:644–655. [DOI] [PubMed] [Google Scholar]
- 4.Hepp Z, Bloudek LM, Varon SF. Systematic review of migraine prophylaxis adherence and persistence. J Manag Care Pharm 2014;20:22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hepp Z, Dodick DW, Varon SF, et al. Persistence and switching patterns of oral migraine prophylactic medications among patients with chronic migraine: a retrospective claims analysis. Cephalalgia 2017;37:470–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dodick DW. CGRP ligand and receptor monoclonal antibodies for migraine prevention: evidence review and clinical implications. Cephalalgia 2019;39:445–458. [DOI] [PubMed] [Google Scholar]
- 7.Jain S, Silberstein SD. Invited commentary on preventive anti-migraine therapy (PAMT). Curr Treat Options Neurol 2019;21:14. [DOI] [PubMed] [Google Scholar]
- 8.Tso AR, Goadsby PJ. Anti-CGRP monoclonal antibodies: the next era of migraine prevention? Curr Treat Options Neurol 2017;19:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tepper SJ. History and review of anti-calcitonin gene-related peptide (CGRP) therapies: from translational research to treatment. Headache 2018;58(suppl 3):238–275. [DOI] [PubMed] [Google Scholar]
- 10.Armour KL, Clark MR, Hadley AG, Williamson LM. Recombinant human IgG molecules lacking Fcγ receptor I binding and monocyte triggering activities. Eur J Immunol 1999;29:2613–2624. [DOI] [PubMed] [Google Scholar]
- 11.Dodick DW, Silberstein SD, Bigal ME, et al. Effect of fremanezumab compared with placebo for prevention of episodic migraine: a randomized clinical trial. JAMA 2018;319:1999–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silberstein SD, Dodick DW, Bigal ME, et al. Fremanezumab for the preventive treatment of chronic migraine. N Engl J Med 2017;377:2113–2122. [DOI] [PubMed] [Google Scholar]
- 13.Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd Edition (beta version). Cephalalgia 2013;33:629–808. [DOI] [PubMed] [Google Scholar]
- 14.Yang M, Rendas-Baum R, Varon SF, Kosinski M. Validation of the Headache Impact Test (HIT-6™) across episodic and chronic migraine. Cephalalgia 2011;31:357–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stewart WF, Lipton RB, Dowson AJ, Sawyer J. Development and testing of the Migraine Disability Assessment (MIDAS) questionnaire to assess headache-related disability. Neurology 2001;56:S20–S28. [DOI] [PubMed] [Google Scholar]
- 16.Camporeale A, Kudrow D, Sides R, et al. A phase 3, long-term, open-label safety study of galcanezumab in patients with migraine. BMC Neurol 2018;18:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashina M, Goadsby PJ, Reuter U, et al. Long-term safety and tolerability of erenumab: three-plus year results from a five-year open-label extension study in episodic migraine. Cephalalgia 2019;39:1455–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bigal ME, Dodick DW, Rapoport AM, et al. Safety, tolerability, and efficacy of TEV-48125 for preventive treatment of high-frequency episodic migraine: a multicentre, randomised, double-blind, placebo-controlled, phase 2b study. Lancet Neurol 2015;14:1081–1190. [DOI] [PubMed] [Google Scholar]
- 19.Bigal ME, Edvinsson L, Rapoport AM, et al. Safety, tolerability, and efficacy of TEV-48125 for preventive treatment of chronic migraine: a multicentre, randomised, double-blind, placebo-controlled, phase 2b study. Lancet Neurol 2015;14:1091–1100. [DOI] [PubMed] [Google Scholar]
- 20.Silberstein SD, McAllister P, Ning X, et al. Safety and tolerability of fremanezumab for the prevention of migraine: a pooled analysis of phases 2b and 3 clinical trials. Headache 2019;59:880–890. [DOI] [PubMed] [Google Scholar]
- 21.Kurth T, Winter AC, Eliassen AH, et al. Migraine and risk of cardiovascular disease in women: prospective cohort study. BMJ 2016;353:i2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MaassenVanDenBrink A, Meijer J, Villalon CM, Ferrari MD. Wiping out CGRP: potential cardiovascular risks. Trends Pharmacol Sci 2016;37:779–788. [DOI] [PubMed] [Google Scholar]
- 23.Ohlsson L, Kronvall E, Stratton J, Edvinsson L. Fremanezumab blocks CGRP induced dilatation in human cerebral, middle meningeal and abdominal arteries. J Headache Pain 2018;19:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mai CT, Isenburg J, Langlois PH, et al. Population-based birth defects data in the United States, 2008 to 2012: presentation of state-specific data and descriptive brief on variability of prevalence. Birth Defects Res A Clin Mol Teratol 2015;103:972–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peres MF, Silberstein S, Moreira F, et al. Patients' preference for migraine preventive therapy. Headache 2007;47:540–545. [DOI] [PubMed] [Google Scholar]
- 26.Tfelt-Hansen P, Pascual J, Ramadan N, et al. Guidelines for controlled trials of drugs in migraine, third edition: a guide for investigators. Cephalalgia 2012;32:6–38. [DOI] [PubMed] [Google Scholar]
- 27.Yaldo AZ, Wertz DA, Rupnow MF, Quimbo RM. Persistence with migraine prophylactic treatment and acute migraine medication utilization in the managed care setting. Clin Ther 2008;30:2452–2460. [DOI] [PubMed] [Google Scholar]
- 28.Rapoport A, Mauskop A, Diener HC, Schwalen S, Pfeil J. Long-term migraine prevention with topiramate: open-label extension of pivotal trials. Headache 2006;46:1151–1160. [DOI] [PubMed] [Google Scholar]
- 29.Yeung PP, Goadsby PJ, Jann AE, Cohen JM, Yang R, Ning X. Long-term efficacy of fremanezumab in patients with chronic migraine with concomitant preventive medication use. Presented at American Academy of Neurology 71st Annual Meeting; May 4–10, 2019; Philadelphia.
- 30.AJOVY® (fremanezumab) [summary of product characteristics]. Jona, Switzerland: Teva Pharmaceuticals GmbH; 2019. [Google Scholar]
- 31.AJOVY® (fremanezumab) [prescribing information]. Overland Park, KS: Teva Pharmaceuticals USA, Inc.; 2019. [Google Scholar]
- 32.Ferrari MD, Diener HC, Ning X, et al. Fremanezumab versus placebo for migraine prevention in patients with documented failure to up to four migraine preventive medication classes (FOCUS): a randomised, double-blind, placebo-controlled, phase 3b trial. Lancet 2019;394:1030–1040. [DOI] [PubMed] [Google Scholar]
- 33.Minen MT, Begasse De Dhaem O, Kroon Van Diest A, et al. Migraine and its psychiatric comorbidities. J Neurol Neurosurg Psychiatry 2016;87:741–749. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data described in this report are available by request from the author investigators or Teva Pharmaceuticals Ltd, the company sponsoring the clinical development of fremanezumab for the treatment of migraine.