Abstract
Objective
To determine whether orthostatic hypotension (OHYPO) and visit-to-visit blood pressure (BP) postural changes variability are associated with incident dementia.
Methods
We studied 2,131 older adults from the Health, Aging, and Body Composition cohort study. Orthostatic BP was repeatedly assessed over a 5-year baseline period. OHYPO was defined as a fall ≥15 mm Hg in systolic or ≥7 mm Hg in diastolic BP after standing from a sitting position for one-third or more of the visits. Systolic OHYPO and diastolic OHYPO were also examined separately. BP postural changes variability over time was evaluated with several indicators, including SD and coefficient of variation (CV). Incident dementia was determined over 12 years after the baseline period by dementia medication use, ≥1.5 SD decline in Modified Mini-Mental State Examination score, or hospitalization records.
Results
Of 2,131 participants (mean age 73 years, 53% female, 39% Black), 309 (14.5%) had OHYPO, 192 (9.0%) had systolic OHYPO, 132 (6.2%) had diastolic OHYPO, and 462 (21.7%) developed dementia. After adjustment for demographics, seated systolic BP (SBP), antihypertensive drugs, cerebrovascular disease, diabetes mellitus, depressive symptoms, smoking, alcohol, body mass index, and presence of 1 or 2 APOE ε4 alleles, systolic OHYPO was associated with greater dementia risk (adjusted hazard ratio [HR] 1.37, 95% confidence interval [CI] 1.01–1.88), unlike diastolic OHYPO and OHYPO. SBP postural changes variability was also associated with higher dementia risk (highest tertile of variability [CV]: adjusted HR 1.35, 95% CI 1.06–1.71).
Conclusion
Systolic OHYPO and visit-to-visit SBP postural changes variability were associated with greater dementia risk. Our findings raise the question of potential preventive interventions to control orthostatic SBP and its fluctuations.
Because postural changes in blood pressure (BP) affect 20% to 30% of the elderly population1–3 and multiple pharmacologic and nonpharmacologic interventions may improve orthostatic symptoms,4 the question of whether postural changes in BP over time are risk factors for dementia has major public health implications.
Orthostatic hypotension (OHYPO) has been associated with an increased risk of cardiovascular events and all-cause mortality,5 but the relationship with cognitive outcomes is less clear. The few studies examining the impact of OHYPO on incident dementia6–11 reported conflicting results, potentially because of limited follow-up time, age at which OHYPO was ascertained, and measurement of OHYPO at a single time point, which may not be as sensitive as several time points.
Over the past decade, higher BP variability has been increasingly recognized, beyond hypertension, as a predictor of clinical events such as stroke, myocardial infarction, and cardiovascular and all-cause mortality12–15 and has recently been suggested to be potentially associated with an increased risk of dementia.16 However, the specific impact of visit-to-visit variability of postural changes in BP on incident dementia has never been considered.
Thus, the aim of our study was to investigate the association between repeatedly assessed postural changes in BP, OHYPO, and visit-to-visit variability of postural changes in BP and risk of dementia in the prospective Health, Aging, and Body Composition (Health ABC) study, a biracial cohort of community-dwelling older adults who were cognitively normal at baseline and followed up for more than a decade.
Methods
Standard protocol approvals, registrations, and patient consents
This study was approved by the institutional review boards of the 2 clinic sites and that of the coordinating center, the University of California, San Francisco. All participants signed an informed written consent, approved by the institutional review boards at the clinical sites. This study was conducted in compliance with the principles of the Declaration of Helsinki and followed the international standards pertaining to protection of human research participants.
Study population
Starting in 1997, participants were enrolled in the Health ABC study, a prospective cohort of 3,075 community-dwelling White (58%) and Black (42%) older adults 70 to 79 years of age who were randomly sampled from Medicare enrollees living in Memphis, TN, or Pittsburgh, PA. Subjects were all dementia-free at baseline and were followed up for up to 17 years. Eligibility criteria included no self-reported mobility difficulty (walking a quarter mile and climbing 10 steps without resting) or functional disability, no known life-threatening cancers, and no plans to leave the area within 3 years.
Assessment of orthostatic BP changes
Orthostatic BP changes were measured at baseline and follow-up years 1, 3, and 5, making up a 5-year baseline period. Given that older adults often have comorbid conditions that may affect mobility and have a higher fall risk, a sit-to-stand measure, as used the in Health ABC study, often offers a safer method to assess OHYPO.17 BP was measured twice in the seated position at the brachial artery with a mercury sphygmomanometer after 5 minutes of quiet rest and once in the standing position after 1 minute. All BP measurements were obtained by centrally trained and certified clinic staff using standardized protocols. Recently, diagnostic OHYPO thresholds with a sit-to-stand test have been proposed18 and used in studies such as the Hypertension in the Very Elderly Trial (HYVET).19 We therefore defined OHYPO as a decrease in systolic BP (SBP) of at least 15 mm Hg or a decrease in diastolic BP (DBP) of at least 7 mm Hg after postural change at any of the 2 measurements. In addition, systolic OHYPO and diastolic OHYPO were examined separately. OHYPO over the 5-year baseline period was defined as having OHYPO for at least one-third of visits.
To assess the visit-to-visit variability of postural changes in SBP over the 5-year baseline period, we first computed at each time point (baseline, years 1, 3, and 5) an orthostatic SBP ratio = (seated SBP/standing SBP) × 100.20 Standing SBP may be higher or lower than seated SBP. Thus, participants with an orthostatic SBP ratio >100% have a seated SBP that exceeds standing SBP. We then investigated the visit-to-visit variability of postural changes in SBP (variability of the orthostatic SBP ratio over the 5-year baseline period of orthostatic assessment) according to indicators used in previous studies12,21: mainly SD and coefficient of variation (CV) but also variation independent of mean (VIM), residual SD (RSD), average real variability, and successive variation (data available from Dryad, appendix e-1 and table e-1, doi.org/10.5061/dryad.dncjsxkw6). Correlations between indicators are shown in table e-2 (data available from Dryad). A similar method was used to assess the visit-to-visit variability of postural changes in DBP.
Assessment of dementia
Global cognition was assessed repeatedly with the Modified Mini-Mental State Examination (3 MS), a cognitive measure that assesses concentration, orientation, language, praxis, and immediate and delayed memory.22 The 3 MS has been shown to be more sensitive in detecting dementia than other cognitive screening instruments.23 After the 5-year baseline period of orthostatic assessment, incident dementia was evaluated over a 12-year period (total follow-up 17 years). Dementia was determined if any of the following qualifications were met: record of a hospitalization with dementia listed as a primary or secondary diagnosis, a documented prescription for dementia medication (i.e., galantamine, rivastigmine, donepezil, memantine), or ≥1.5 SD race-stratified decline in 3 MS score on repeated measures from participants' baseline through the last visit.24 After exclusion of individuals who did not attend year 5 or developed dementia during the 5-year baseline orthostatic assessment period (n = 839) and those who did not have at least 2 measurements of postural BP changes (n = 105), our final analytic cohort consisted of 2,131 Health ABC participants.
Assessment of covariates
Several covariates such as demographics (age, race, sex, and education) were considered potential confounders. Average seated SBP or DBP was calculated as the mean of seated SBP or DBP measurements over the 5-year period of orthostatic assessment. Hypertension was also defined as prevalent or incident over the 5-year baseline with the use of a ≥140/90 mm Hg cutoff. Antihypertensive drug use was collected over the same period. Diabetes mellitus, coronary heart disease, and cerebrovascular disease were determined from a combination of self-report, physician diagnosis, medications, and laboratory values and were coded according to baseline prevalence and incidence during follow-up. Depressive symptoms were defined as a score ≥10 on the Center for Epidemiologic Studies Depression scale.25 Participants self-reported current smoking and alcohol use (≤1 vs >1 drink a day). Body mass index (BMI; kilograms per meters squared) was categorized as normal (BMI <25 kg/m2), overweight (25 ≤ BMI < 30 kg/m2), and obesity (≥30 kg/m2) according to NIH guidelines.26 APOE genotype was considered the presence of 1 or 2 APOE ε4 alleles.
Statistical analyses
Baseline characteristics were compared in patients with and without baseline OYPO with Student tests for continuous variables and χ2 tests for categorical variables. Comparisons between tertile of variability of postural changes in BP over time (CV) were done with an analysis of variance for continuous variables and χ2 tests for categorical variables. Cox proportional hazards models were performed to assess the association of OHYPO and visit-to-visit variability of postural changes in BP with incident dementia over 12 years. Time to dementia was coded as first record of diagnosis or censored from observation at the end of the period for which data were available. The proportional hazard assumption was checked graphically for all covariates and using Schoenfeld residuals. Log-linearity hypothesis was also tested for all covariates. A significance threshold of 0.2 (based on either systolic OHYPO or variability comparisons according to baseline characteristics; table 1 and dementia univariate Cox models, data available from Dryad, table e-3, doi.org/10.5061/dryad.dncjsxkw6) was considered to select potential confounding factors. Adjusted dementia-free survival curves by the presence of systolic OHYPO and tertile of visit-to-visit variability (CV) of postural changes in SBP were also drawn. Interactions between primary predictors and potential confounding factors were checked. Sensitivity analyses with adjustment for baseline cognition (3 MS score) were conducted. All statistical analyses were performed with STATA software version 15 (StataCorp, College Station, TX).
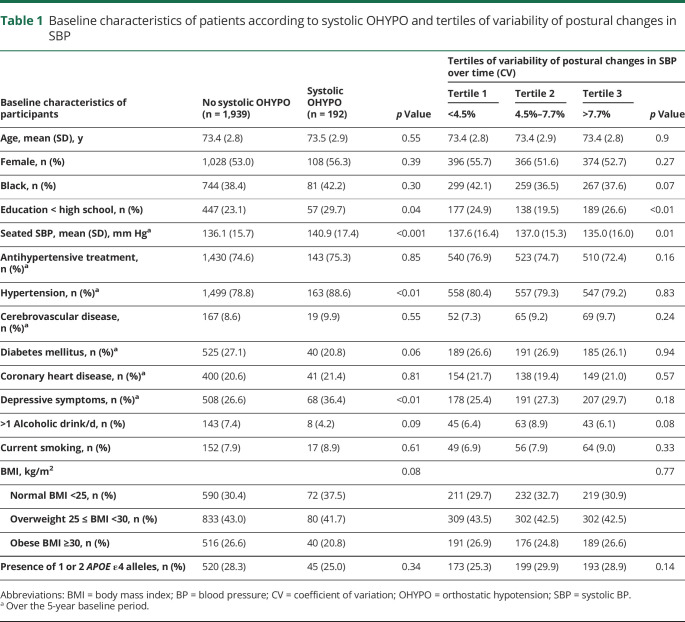
Table 1.
Baseline characteristics of patients according to systolic OHYPO and tertiles of variability of postural changes in SBP
Data availability
Anonymized data will be shared by request from any qualified investigator.
Results
In our cohort, mean baseline age was 73.4 years, 1,136 (53.3%) participants were female, and 825 (38.7%) were Black. Four hundred sixty-two participants (21.7%) developed incident dementia over 12 years. Of 2,131 participants, 1,978 (93%) had at least 3 orthostatic BP assessments over time. One hundred ninety-two (9.0%) had systolic OHYPO; 132 (6.2%) had diastolic OHYPO; and 309 (14.5%) had OHYPO over the 5-year baseline. Participants with systolic OHYPO were more likely to have higher seated SBP (p < 0.001), hypertension (p < 0.01), and depressive symptoms (p < 0.01) (table 1).
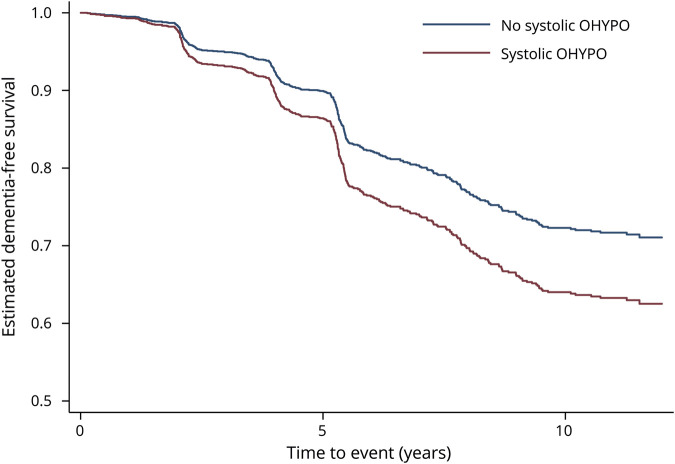
Systolic OHYPO was significantly associated with an increased risk of dementia in unadjusted model (unadjusted hazard ratio [HR] 1.35, 95% confidence interval [CI] 1.01–1.81) (table 2). After adjustment for demographics, seated SBP, antihypertensive drugs, cerebrovascular disease, diabetes mellitus, depressive symptoms, smoking, alcohol, BMI, and APOE, the results remained statistically significant, with systolic OHYPO increasing the risk of dementia by almost 40% (adjusted HR 1.37, 95% CI 1.01–1.88) (table 2). Figure 1 shows the incident dementia risk by systolic OHYPO group over 12 years of follow-up. No interactions were found between systolic OHYPO and age, race, or seated SBP on risk of developing dementia (p > 0.2 for all). Diastolic OHYPO was not significantly associated with increased risk of dementia (adjusted HR 0.92, 95% CI 0.60–1.40) (table 2), nor was OHYPO defined on both systolic and diastolic components (adjusted HR 1.13, 95% CI 0.86–1.48) (table 2). Sensitivity analyses after adjustment for baseline cognition (3 MS score) reported similar findings: systolic OHYPO, adjusted HR 1.35, 95% CI 0.99–1.85; diastolic OHYPO, adjusted HR 0.92, 95% CI 0.60–1.41; and systolic and/or diastolic OHYPO, adjusted HR 1.11, 95% CI 0.85–1.47.
Table 2.
Unadjusted and adjusted Cox proportional hazard models for dementia risk over 12 years according to OHYPO
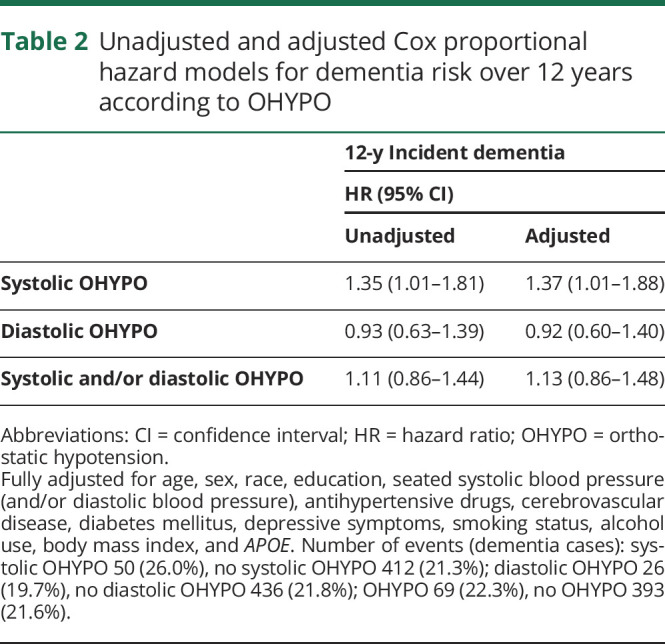
Figure 1. Incident dementia risk over 12 years among those older adults with and without systolic OHYPO: adjusted survival curves.
Estimated survival curves after Cox proportional hazards models. Adjusted for age, sex, race, education, seated systolic blood pressure, antihypertensive drugs, cerebrovascular disease, diabetes mellitus, depressive symptoms, smoking status, alcohol use, body mass index, and APOE. OHYPO = orthostatic hypotension.
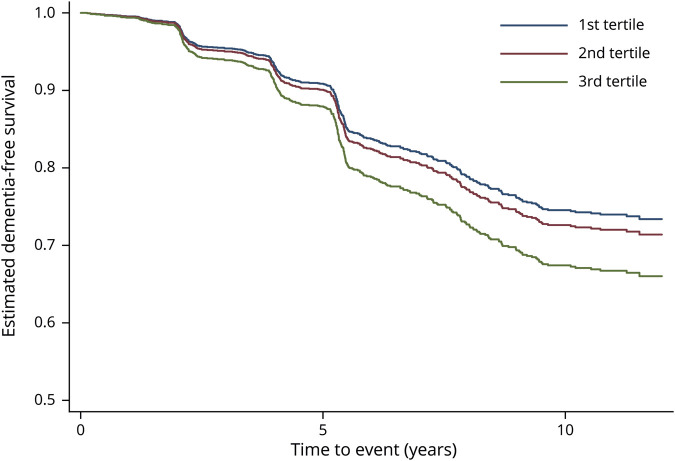
We also investigated the impact of the visit-to-visit variability of postural changes in SBP on incident dementia. Baseline characteristics of patients according to tertile of variability (CV) are presented in table 1, and Cox proportional hazard models for dementia risk over 12 years according to different indicators of variability are shown in table 3. Compared to participants with the lowest variability (CV) of postural changes in SBP (first tertile), participants with the highest variability (third tertile) had a greater risk of developing dementia (unadjusted HR 1.33, 95% CI 1.06–1.66). After adjustment for demographics, seated SBP, antihypertensive drugs, cerebrovascular disease, diabetes mellitus, depressive symptoms, smoking, alcohol, BMI, and APOE, the results remained statistically significant, with greater SBP postural changes variability increasing the risk of dementia by 35% (adjusted HR 1.35, 95% CI 1.06–1.71). Similar patterns were observed for SD, average real variability, successive variation, and VIM. Visit-to-visit variability of postural changes in SBP assessed with RSD did not have significant results (table 3). No interactions were found between visit-to-visit variability of postural changes in SBP and age, race, or seated SBP on risk of developing dementia (p > 0.2 for all). Figure 2 shows the incident dementia risk according to tertile of visit-to-visit variability (CV) of postural changes in SBP. Similar graphs were obtained with other variability indicators. Visit-to-visit variability of postural changes in DBP was not associated with increased risk of dementia (data not shown). Sensitivity analyses after adjustment for baseline cognition (3 MS score) reported similar findings: highest tertile of SBP postural changes variability: CV, adjusted HR = 1.33, 95% CI 1.05–1.69. The results were consistent for all indicators of variability except RSD.
Table 3.
Unadjusted and adjusted Cox proportional hazard models for dementia risk over 12 years according to visit-to-visit variability of postural changes in SBP
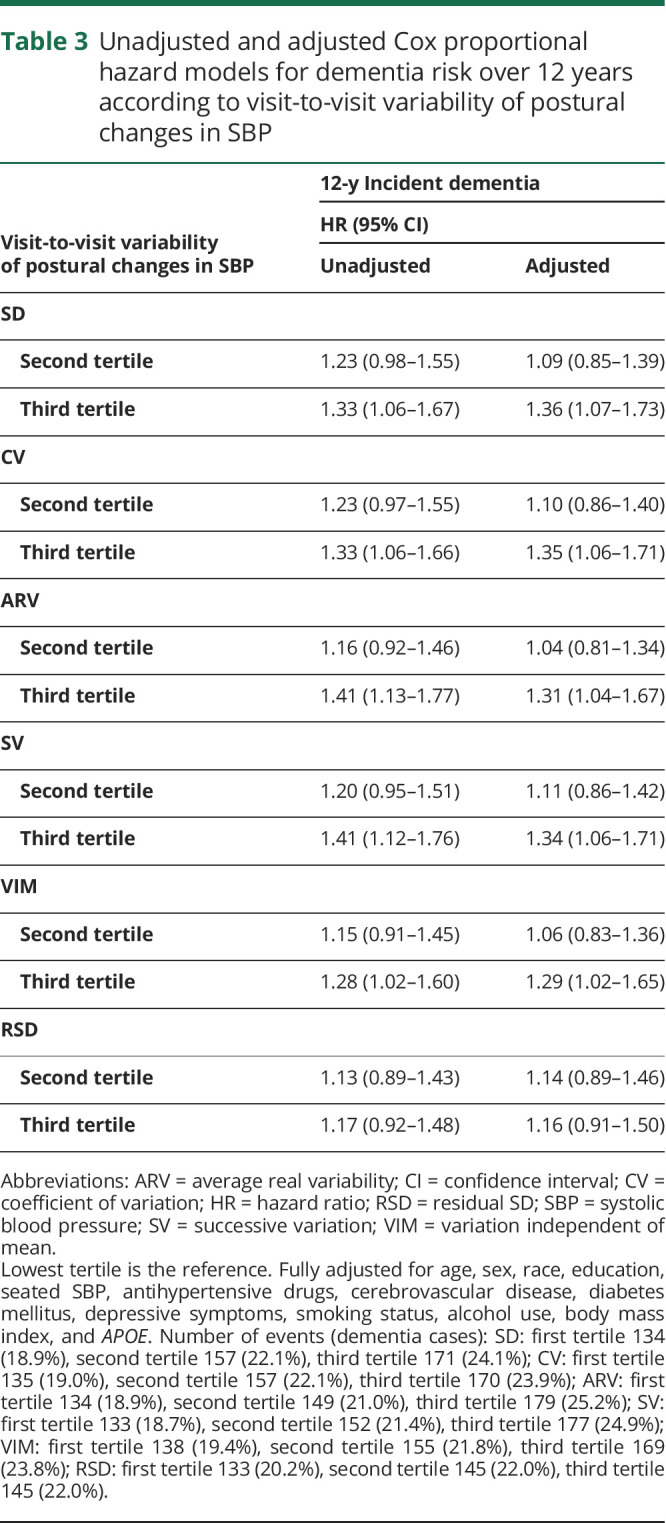
Figure 2. Incident dementia risk over 12 years according to tertile of visit-to-visit variability (coefficient of variation) of postural changes in SBP: adjusted survival curves.
Estimated survival curves after Cox proportional hazards models. Adjusted for age, sex, race, education, seated systolic blood pressure (SBP), antihypertensive drugs, cerebrovascular disease, diabetes mellitus, depressive symptoms, smoking status, alcohol use, body mass index, and APOE.
Discussion
In this biracial cohort of community-dwelling older adults, systolic but not diastolic OHYPO assessed at several time points was associated with a higher risk of incident dementia over the 12 years of follow-up. This relationship was independent of demographics and medical comorbid conditions. Systolic OHYPO has also been suggested to be a better predictor of cardiovascular events and all-cause mortality than diastolic OHYPO or the combined criteria.27,28 Our results are supported by several prior studies. An inverse association was found between the average systolic orthostatic BP response and dementia diagnosis in a subsample of 240 participants recruited from the Health ABC Study.20 However, this work did not assess incident dementia risk over time and did not consider a clinical definition of systolic OHYPO. Similarly, systolic but not diastolic OHYPO was associated with greater risk of dementia in the Swedish Good Aging in Skåne study9 and with lower cognition in the Maine-Syracuse Longitudinal Study.29 Other findings suggested that systolic but not diastolic OHYPO was associated with cognitive decline.30
Furthermore, we found a significantly greater risk of dementia with more visit-to-visit variability over time of postural changes in SBP. Although higher visit-to-visit SBP variability has recently been suggested to be, beyond hypertension, a clinical predictor of cognitive impairment and dementia,16 the specific impact of visit-to-visit variability of postural changes in SBP on incident dementia had never been considered. Within-day orthostatic BP variability has only been suggested to be associated with stroke in elderly nursing home residents.31 Other investigators reported an increased risk of dementia associated with greater SBP variability between the SBP measurement in the supine position and the 3 SBP measurements in the standing position after several minutes.6 However, they did not focus on the visit-to-visit variability of repeatedly assessed postural changes in SBP over several years. Consistent with diastolic OHYPO, visit-to-visit variability of postural changes in DBP was not associated with incident dementia. Our findings suggest that the detrimental effects of orthostatic impairment and its variability over time on brain functioning may be practically estimated by systolic reaction only.
Several mechanisms may explain the association between systolic OHYPO, visit-to-visit variability of postural changes in SBP, and dementia. The most frequently mentioned explanation is that OHYPO leads to recurrent transient cerebral hypoperfusion. Cerebral autoregulation may be attenuated with aging, with subsequent failure to adapt to repeated oscillations in BP resulting in impaired cerebral perfusion.32 Previous studies reported that cerebral blood flow is decreased in OHYPO33 predominantly in the hippocampus.34 Excessive fluctuations in BP postural changes also could have greater detrimental consequences on cerebral blood flow and hemodynamics35,36 than isolated OHYPO. Repeated episodes of hypoperfusion may lead to hypoxia with detrimental effects on brain tissue through neuroinflammation and oxidative stress.37 Several studies showed that BP variability and repeated episodes of tissue hypoxia/ischemia could be responsible for cerebral small vessel diseases, including white matter lesions, microbleeds, silent infarcts, and brain atrophy, contributing to explain the association we found between OHYPO, greater BP variability in postural changes, and incident dementia.38,39 BP fluctuations, including those associated with postural changes, may also induce shear stress on the vascular wall, leading to microvascular damage and arterial remodeling.40 Consistent with this notion, higher BP variability has been found to be associated with endothelial injury.41 Lesions in cerebral microvessels may compromise the function of the blood-brain barrier, increasing vascular permeability and protein extravasation in cerebral parenchyma. Accumulation of different vasculotoxic and neurotoxic byproducts such as β-amyloid peptide would result in neuronal dysfunction and neurodegenerative changes.42 Recent studies also have hypothesized a role of arterial stiffness in the pathogenesis of OHYPO and BP fluctuations in elderly individuals.43–46 The stiffness of the large elastic arteries could determine a low adaptive capacity to postural changes probably through a baroreflex dysfunction. Finally, the causality of the relationship between OHYPO or SBP postural changes variability and dementia remains poorly understood but might be bidirectional. The same cerebral areas involved in the neurodegenerative process leading to dementia are implicated in the autonomic control of the cardiovascular system.47 Changes in brain structure related to dementia have been suggested to contribute to neurovascular instability and BP dysregulation.48 Alternatively, OHYPO may result in neurodegeneration mediated by cerebral hypoperfusion.
Our study has some limitations that are worth noting. First, our findings are based on an observational study, and residual confounding may still exist despite extensive efforts to account for possible confounders. Second, although we believe that our method for identifying cases of dementia was sensitive to capturing people with dementia, our diagnosis was not based on a formal and structured clinical assessment. However, our comprehensive algorithm represents a relatively conservative approach to classifying incident dementia and has been used in previous publications.49,50 We were also not able to distinguish Alzheimer disease from vascular dementia. Third, measuring OHYPO from a seated to standing position after 1 minute may have blunted the full gravitational effect of postural change, resulting in a relatively low prevalence of OHYPO; therefore, the strength of the association between OHYPO and dementia might have been slightly underestimated. Fourth, BP postural changes variability was assessed by a relatively limited number of orthostatic BP measures. However, we were able to find a significant association, and it is likely that a larger number of orthostatic BP assessments would have reinforced the association with dementia risk. Fifth, as in most previous studies, we had no data on heart rate response to orthostatic challenge. We were not able to differentiate neurogenic OHYPO from nonneurogenic OHYPO and to assess the influence of overall autonomic failure in the relationship of OHYPO and postural changes in BP with dementia risk.
Nevertheless, our study has several strengths. It was conducted on a large sample size that has been followed up longitudinally over 17 years. In addition, the repeated measurements of postural BP, rarely available in other datasets, allowed us to define a 5-year baseline period of orthostatic assessment and consecutive evaluation of incident dementia over 12 years, making potential reverse causality less likely. Moreover, we considered a biracial cohort of Black and White individuals. The cohort was well characterized, allowing us to control for important potential confounders. Seated and standing BP measurements were done by rigorously trained staff using standardized protocols, minimizing imprecision and bias. Systolic and diastolic orthostatic responses were also examined separately. To strengthen our findings as sensitivity analyses, we extensively investigated the impact of visit-to-visit variability of postural changes in BP by using several indicators, taking into account the order of orthostatic BP assessments, the influence of orthostatic BP change over time, or uncorrelated to the mean orthostatic BP response. The results were consistent, supporting the robustness of our findings, and it seems unlikely that they have been influenced by the very few numbers of participants who missed baseline visits.
In the present study, systolic OHYPO and visit-to-visit variability over time of postural changes in SBP were associated with an increased risk of dementia of almost 40%. From a preventive medical point of view, orthostatic BP monitoring should be done as accurately as possible to analyze short- and long-term fluctuations. Controlling orthostatic SBP and its variability, including with an optimization of antihypertensive treatment, could be a promising interventional target in preserving cognitive function among older adults.
Glossary
- BMI
body mass index
- BP
blood pressure
- CI
confidence interval
- CV
coefficient of variation
- DBP
diastolic BP
- HEALTH ABC
Health, Aging, and Body Composition
- HR
hazard ratio
- HYVET
Hypertension in the Very Elderly Trial
- OHYPO
orthostatic hypotension
- RSD
residual SD
- SBP
systolic BP
- 3 MS
Modified Mini-Mental State Examination
- VIM
variation independent of mean
Appendix. Authors
Study funding
Health ABC was supported by National Institute on Aging (NIA) contracts N01-AG-6-2101, N01-AG-6-2103, and N01-AG-6-2106; NIA grant R01-AG028050; and National Institute of Nursing Research grant R01-NR012459. This research was funded in part by the Intramural Research Program of the NIH, NIA. This research was also supported by NIA grant K24-AG031155.
Disclosure
L. Rouch, J-S. Vidal, T. Hoang, P. Cestac, and O. Hanon report no disclosures relevant to the manuscript. K. Yaffe serves on Data Safety Monitoring Board for Eli Lilly and several NIA-sponsored studies and is a member of the Beeson Scientific Advisory Board and the Global Council on Brain Health. Go to Neurology.org/N for full disclosures.
References
- 1.Nardo CJ, Chambless LE, Light KC, et al. Descriptive epidemiology of blood pressure response to change in body position: the ARIC study. Hypertension 1999;33:1123–1129. [DOI] [PubMed] [Google Scholar]
- 2.Kario K. Orthostatic hypertension: a new haemodynamic cardiovascular risk factor. Nat Rev Nephrol 2013;9:726–738. [DOI] [PubMed] [Google Scholar]
- 3.Alagiakrishnan K, Patel K, Desai RV, et al. Orthostatic hypotension and incident heart failure in community-dwelling older adults. J Gerontol A Biol Sci Med Sci 2014;69:223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayakawa T, McGarrigle CA, Coen RF, et al. Orthostatic blood pressure behavior in people with mild cognitive impairment predicts conversion to dementia. J Am Geriatr Soc 2015;63:1868–1873. [DOI] [PubMed] [Google Scholar]
- 5.Angelousi A, Girerd N, Benetos A, et al. Association between orthostatic hypotension and cardiovascular risk, cerebrovascular risk, cognitive decline and falls as well as overall mortality: a systematic review and meta-analysis. J Hypertens 2014;32:1562–1571. [DOI] [PubMed] [Google Scholar]
- 6.Wolters FJ, Mattace-Raso FUS, Koudstaal PJ, Hofman A, Ikram MA; Heart Brain Connection Collaborative Research Group. Orthostatic hypotension and the long-term risk of dementia: a population-based study. PLoS Med 2016;13:e1002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rawlings AM, Juraschek SP, Heiss G, et al. Association of orthostatic hypotension with incident dementia, stroke, and cognitive decline. Neurology 2018;91:e759–e768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holm H, Nägga K, Nilsson ED, et al. Longitudinal and postural changes of blood pressure predict dementia: the Malmö Preventive Project. Eur J Epidemiol 2017;32:327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elmståhl S, Widerström E. Orthostatic intolerance predicts mild cognitive impairment: incidence of mild cognitive impairment and dementia from the Swedish general population cohort Good Aging in Skåne. Clin Interv Aging 2014;9:1993–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cremer A, Soumaré A, Berr C, et al. Orthostatic hypotension and risk of incident dementia: results from a 12-year follow-up of the Three-City Study cohort. Hypertension 2017;70:44–49. [DOI] [PubMed] [Google Scholar]
- 11.Min M, Shi T, Sun C, et al. The association between orthostatic hypotension and dementia: a meta-analysis of prospective cohort studies. Int J Geriatr Psychiatry 2018;33:1541–1547. [DOI] [PubMed] [Google Scholar]
- 12.Rothwell PM, Howard SC, Dolan E, et al. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet 2010;375:895–905. [DOI] [PubMed] [Google Scholar]
- 13.Tai C, Sun Y, Dai N, et al. Prognostic significance of visit-to-visit systolic blood pressure variability: a meta-analysis of 77,299 patients. J Clin Hypertens 2015;17:107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehlum MH, Liestøl K, Kjeldsen SE, et al. Blood pressure variability and risk of cardiovascular events and death in patients with hypertension and different baseline risks. Eur Heart J 2018;39:2243–2251. [DOI] [PubMed] [Google Scholar]
- 15.Laurent S, Boutouyrie P. Visit-to-visit blood pressure variability: added “VALUE” as a risk marker in low- and high-risk patients. Eur Heart J 2018;39:2252–2254. [DOI] [PubMed] [Google Scholar]
- 16.Alpérovitch A, Blachier M, Soumaré A, et al. Blood pressure variability and risk of dementia in an elderly cohort, the Three-City Study. Alzheimers Dement J Alzheimers Assoc 2014;10:S330–S337. [DOI] [PubMed] [Google Scholar]
- 17.2011 ENA Emergency Nursing Resources Development Committee; Naccarato M, Leviner S, Proehl J, et al. Emergency Nursing Resource: orthostatic vital signs. J Emerg Nurs 2012;38:447–453. [DOI] [PubMed] [Google Scholar]
- 18.Shaw BH, Garland EM, Black BK, et al. Optimal diagnostic thresholds for diagnosis of orthostatic hypotension with a “sit-to-stand test.” J Hypertens 2017;35:1019–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peters R, Anstey KJ, Booth A, et al. Orthostatic hypotension and symptomatic subclinical orthostatic hypotension increase risk of cognitive impairment: an integrated evidence review and analysis of a large older adult hypertensive cohort. Eur Heart J 2018;39:3135–3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Hare C, Kenny R-A, Aizenstein H, et al. Cognitive status, gray matter atrophy, and lower orthostatic blood pressure in older adults. J Alzheimers Dis 2017;57:1239–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fischer U, Webb AJS, Howard SC, Rothwell PM. Reporting of consistency of blood pressure control in randomized controlled trials of antihypertensive drugs: a systematic review of 1372 trial reports. J Hypertens 2012;30:1271–1276. [DOI] [PubMed] [Google Scholar]
- 22.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) Examination. J Clin Psychiatry 1987;48:314–318. [PubMed] [Google Scholar]
- 23.Holsinger T, Plassman BL, Stechuchak KM, Burke JR, Coffman CJ, Williams JW. Screening for cognitive impairment: comparing the performance of four instruments in primary care. J Am Geriatr Soc 2012;60:1027–1036. [DOI] [PubMed] [Google Scholar]
- 24.Hong CH, Falvey C, Harris TB, et al. Anemia and risk of dementia in older adults: findings from the Health ABC study. Neurology 2013;81:528–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale). Am J Prev Med 1994;10:77–84. [PubMed] [Google Scholar]
- 26.Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—the evidence report: National Institutes of Health. Obes Res 1998;6(suppl 2):51S–209S. [PubMed] [Google Scholar]
- 27.Fedorowski A, Stavenow L, Hedblad B, Berglund G, Nilsson PM, Melander O. Consequences of orthostatic blood pressure variability in middle-aged men (the Malmö Preventive Project). J Hypertens 2010;28:551–559. [DOI] [PubMed] [Google Scholar]
- 28.Fagard RH, De Cort P. Orthostatic hypotension is a more robust predictor of cardiovascular events than nighttime reverse dipping in elderly. Hypertension 2010;56:56–61. [DOI] [PubMed] [Google Scholar]
- 29.Torres RV, Elias MF, Crichton GE, Dore GA, Davey A. Systolic orthostatic hypotension is related to lowered cognitive function: findings from the Maine-Syracuse Longitudinal Study. J Clin Hypertens (Greenwich) 2017;19:1357–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Casiglia E, Tikhonoff V. Orthostatic hypotension, focus on cognitive pattern. J Hypertens 2018;36:1038–1040. [DOI] [PubMed] [Google Scholar]
- 31.Hossain M, Ooi WL, Lipsitz LA. Intra-individual postural blood pressure variability and stroke in elderly nursing home residents. J Clin Epidemiol 2001;54:488–494. [DOI] [PubMed] [Google Scholar]
- 32.Moretti R, Torre P, Antonello RM, Manganaro D, Vilotti C, Pizzolato G. Risk factors for vascular dementia: hypotension as a key point. Vasc Health Risk Manag 2008;4:395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elmstáhl S, Rosén I. Postural hypotension and EEG variables predict cognitive decline: results from a 5-year follow-up of healthy elderly women. Dement Geriatr Cogn Disord 1997;8:180–187. [DOI] [PubMed] [Google Scholar]
- 34.Laosiripisan J, Tarumi T, Gonzales MM, Haley AP, Tanaka H. Association between cardiovagal baroreflex sensitivity and baseline cerebral perfusion of the hippocampus. Clin Auton Res 2015;25:213–218. [DOI] [PubMed] [Google Scholar]
- 35.Buratti L, Cagnetti C, Balucani C, et al. Blood pressure variability and stroke outcome in patients with internal carotid artery occlusion. J Neurol Sci 2014;339:164–168. [DOI] [PubMed] [Google Scholar]
- 36.Lattanzi S, Cagnetti C, Provinciali L, Silvestrini M. Blood pressure variability and clinical outcome in patients with acute intracerebral hemorrhage. J Stroke Cerebrovasc Dis 2015;24:1493–1499. [DOI] [PubMed] [Google Scholar]
- 37.Raz L, Knoefel J, Bhaskar K. The neuropathology and cerebrovascular mechanisms of dementia. J Cereb Blood Flow Metab 2016;36:172–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Havlik RJ, Foley DJ, Sayer B, Masaki K, White L, Launer LJ. Variability in midlife systolic blood pressure is related to late-life brain white matter lesions: the Honolulu-Asia Aging Study. Stroke 2002;33:26–30. [DOI] [PubMed] [Google Scholar]
- 39.Brickman AM, Reitz C, Luchsinger JA, et al. Long-term blood pressure fluctuation and cerebrovascular disease in an elderly cohort. Arch Neurol 2010;67:564–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cecchi E, Giglioli C, Valente S, et al. Role of hemodynamic shear stress in cardiovascular disease. Atherosclerosis 2011;214:249–256. [DOI] [PubMed] [Google Scholar]
- 41.Diaz KM, Veerabhadrappa P, Kashem MA, et al. Visit-to-visit and 24-h blood pressure variability: association with endothelial and smooth muscle function in African Americans. J Hum Hypertens 2013;27:671–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci 2011;12:723–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mattace-Raso FUS, van der Cammen TJM, Knetsch AM, et al. Arterial stiffness as the candidate underlying mechanism for postural blood pressure changes and orthostatic hypotension in older adults: the Rotterdam Study. J Hypertens 2006;24:339–344. [DOI] [PubMed] [Google Scholar]
- 44.Boddaert J, Tamim H, Verny M, Belmin J. Arterial stiffness is associated with orthostatic hypotension in elderly subjects with history of falls. J Am Geriatr Soc 2004;52:568–572. [DOI] [PubMed] [Google Scholar]
- 45.Omboni S, Parati G, Di Rienzo M, Wieling W, Mancia G. Blood pressure and heart rate variability in autonomic disorders: a critical review. Clin Auton Res 1996;6:171–182. [DOI] [PubMed] [Google Scholar]
- 46.Tedla YG, Yano Y, Carnethon M, Greenland P. Association between long-term blood pressure variability and 10-year progression in arterial stiffness: the Multiethnic Study of Atherosclerosis. Hypertension 2017;69:118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Idiaquez J, Roman GC. Autonomic dysfunction in neurodegenerative dementias. J Neurol Sci 2011;305:22–27. [DOI] [PubMed] [Google Scholar]
- 48.Skoog I, Andreasson LA, Landahl S, Lernfelt B. A population-based study on blood pressure and brain atrophy in 85-year-olds. Hypertension 1998;32:404–409. [DOI] [PubMed] [Google Scholar]
- 49.Brenowitz WD, Kaup AR, Lin FR, Yaffe K. Multiple sensory impairment is associated with increased risk of dementia among Black and White older adults. J Gerontol A Biol Sci Med Sci 2019;74:890–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yaffe K, Freimer D, Chen H, et al. Olfaction and risk of dementia in a biracial cohort of older adults. Neurology 2017;88:456–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared by request from any qualified investigator.