Abstract
Objective
To test the hypothesis that end-stage renal disease (ESRD) risk exposure during young adulthood is related to worse cognitive performance in midlife.
Methods
We included 2,604 participants from the population-based Coronary Artery Risk Development in Young Adults (CARDIA) Study (mean age 35 years, 54% women, 45% Black). Estimated glomerular filtration rate and albumin-to-creatinine ratio were measured every 5 years at year (Y) 10 through Y30. At each visit, moderate/high risk of ESRD according to the Kidney Disease: Improving Global Outcomes guidelines (estimated glomerular filtration rate <60 mL/min/1.73 m2 or albumin-to-creatinine ratio >30 mg/g) was defined, totaled over examinations, and categorized into 0 episodes, 1 episode, and >1 episodes of ESRD risk. At Y30, participants underwent global and multidomain cognitive assessment. We used analysis of covariance to assess the association of ESRD risk categories with cognitive function, controlling for cardiovascular risk factors.
Results
Over the course of 20 years, 427 participants (16% of the study population) had ≥1 episodes of ESRD risk exposure. Individuals with more risk episodes had lower composite cognitive function (p < 0.001), psychomotor speed (p < 0.001), and executive function (p = 0.007). All these associations were independent of sociodemographic status and cardiovascular risk factors.
Conclusions
In this population-based longitudinal study, we show that episodes of decline in kidney function over the young-adulthood course are associated with worse cognitive performance at midlife. Preserving kidney function in young age needs to be investigated as a potential strategy to preserve cognitive function in midlife.
Cognitive impairment is a common and debilitating comorbidity among patients with end-stage renal disease (ESRD).1–5 The prevalence of cognitive impairment is ≈3-fold higher in patients with ESRD than the age-matched general population.1,6 More recent evidence indicates that cognitive impairment is not limited to patients with ESRD and that even mild to moderate degrees of kidney impairment can be toxic for the brain and increase the risk of cognitive decline.5,7 A limitation of most studies is that kidney function is measured once in later life, which does not account for the adverse influence of long-term and repeated exposures to kidney dysfunction.5,8 Kidney function declines with age, but there is a wide variability in the rate and pattern of decline and the risk of adverse clinical outcomes.9 Episodes of decline in kidney function are commonly encountered over the life course and tend to go underrecognized, particularly in community-dwelling individuals. It is unclear whether exposure to such episodes is linked to worse cognitive outcomes.
In this long-term prospective cohort study, we determined the 20-year course of kidney function and associated ESRD risk over young adulthood and studied the link of ESRD with midlife cognitive function in the setting of Coronary Artery Risk Development in Young Adults (CARDIA) Study. We hypothesized that having more episodes of moderate/high risk of ESRD over the young adulthood is related to worse cognitive function in middle age.
Methods
Population
The CARDIA Study is a population-based prospective study started in 1985 to 1986 to investigate the development of cardiovascular risk and disease. In short, 5,115 Black and White women and men 18 to 30 years of age were recruited from 4 urban areas across United States: Birmingham, AL; Chicago, IL; Minneapolis, MN; and Oakland, CA. Participants were recruited by telephone and invited for serial in-person follow-up examinations at year (Y) 2, Y5, Y7, Y10, Y15, Y20, Y25, and Y30 after baseline. A more detailed description of the study design has been published previously.10 Serum creatinine and a random urine albumin-to-creatinine ratio (ACR) were assessed at each visit beginning in Y10; therefore, for this study, we considered Y10 the baseline.
Standard protocol approvals, registrations, and patient consents
All participants provided written informed consent, and institutional review boards at each field center and the coordinating center approved the study annually.
Assessment of kidney function
Serum creatinine concentrations were measured by nephelometry and calibrated to National Institute of Standards and Technology standards as recommended by the National Kidney Disease Education Program Laboratory Working Group. The calibration process has been detailed previously.11 We computed estimated glomerular filtration rate (eGFR) according to the Chronic Kidney Disease Epidemiology Collaboration equation.12 Urine albumin and creatinine were measured with a single, untimed spot urine sample. Albumin was assessed using a nephelometric procedure with a specific anti-albumin monoclonal antibody, and the Jaffe method was used for measuring urine creatinine. ACR (milligrams per gram) was estimated by dividing albumin by creatinine.
Our exposure was defined by creating categories of ESRD risk using both eGFR and ACR according to Kidney Disease: Improving Global Outcomes (KDIGO) guidelines for chronic kidney disease (CKD) prognosis.13,14 According to KDIGO guidelines, individuals are at low ESRD risk if they have an eGFR >60 mL/min/1.73 m2 and ACR <30 mg/g; at moderate risk if they have an eGFR of 45 to 60 mL/min/1.73 m2 and ACR <30 mg/g or ACR of 30 to 300 and eGFR >60 mL/min/1.73 m2; at high risk if they have an eGFR of 45 to 60 mL/min/1.73 m2 and ACR of 30 to 300, eGFR of 30 to 44 mL/min/1.73 m2 and ACR <30 mg/g, or ACR >300 mg/g and eGFR >60 mL/min/1.73 m2; and at very high risk if they have an eGFR <30 mL/min/1.73 m2 and ACR <30 mg/g, ACR of 30 to 300 mg/g and eGFR <45 mL/min/1.73 m2, or ACR >300 mg/g and eGFR <60 mL/min/1.73 m2 (figure 1). We defined 3 categories based on ESRD risk during follow-up as follows: (1) consistent low risk (eGFR >60 mL/min/1.73 m2 and ACR <30 mg/g at all visits (n = 2,177), (2) 1 episode of moderate, high, or very high ESRD risk (5% high/very high episode) (n = 240), and (3) >1 episode of moderate, high, or very high risk during follow-up (55% at least 1 high/very high episode) (n = 187) (figure 1).
Figure 1. Definition of ESRD risk categories based on KDIGO guidelines.
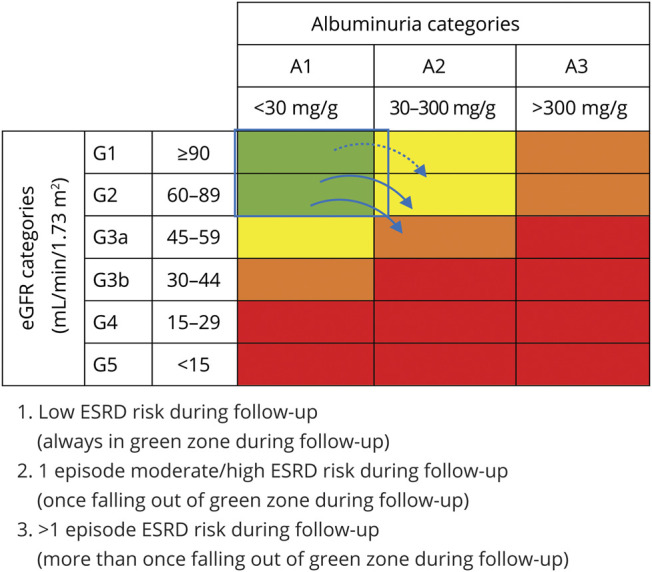
Green indicates low end-stage renal disease (ESRD) risk; yellow indicates moderately increased risk; orange indicates high risk; and red indicates very high risk. eGFR = estimated glomerular filtration rate; KDIGO = Kidney Disease: Improving Global Outcomes.
Given that some participants recovered after 1 or 2 episodes of decline in kidney function, we additionally defined 3 categories as participants (1) with stable kidney function (same ESRD risk in all visits), (2) with recovered kidney function (improvement in ESRD risk after ≥1 episodes of moderate/high ESRD risk), and (3) with constant decline in kidney function.
Cognitive assessment
Cognitive function was assessed at Y30. Multiple standardized tests, including the Stroop test, Rey Auditory Verbal Learning Test (RAVLT), Digit Symbol Substitution Test (DSST), Montreal Cognitive Assessment (MoCA), and category and letter fluency tests, were administered to evaluate multiple domains of cognition. The Stroop test evaluates executive function, including the ability to view complex visual stimuli and respond to 1 stimulus dimension while suppressing the response to another dimension. We used an interference score for this analysis, which is calculated by subtracting the score on subtest II from the score on subtest III. The test is scored by seconds to state spelled-out color words printed in a different color ink, plus number of errors. Stroop scores range from 1 to 160, and higher seconds plus errors score indicates worse performance. To be consistent with other cognitive measure, we inverted the Stroop test so that higher values reflect better cognitive function. The RAVLT assesses verbal memory, including the ability to memorize and retrieve words. The long-delay (10 minutes) free recall (range 0–15 words) was analyzed. RAVLT ranges between 0 and 15; more words recalled indicate better performance. To assess psychomotor speed, the DSST was used. DSST evaluates visual motor speed, sustained attention, and working memory and ranges from 0 to 133; having more correct digits indicates better performance. MoCA is a rapid screening test that evaluates different cognitive domains, including attention, executive function, memory, language, visuospatial skills, calculations, and orientation. The total possible score for MoCA is 30 points. The category and letter fluency tests assess verbal production, semantic memory, phonemic fluency, and language. The category fluency test assessed the total number of unique animals that the participant was able to name in 60 seconds. For the letter fluency test, participants were asked to list as many words as they can that begin with a particular letter in 1 minute. The test was repeated 3 times, and the sum total of words for the 3 letters was used for the score. To compare different cognitive tests, we standardized all test scores using z scores for all cognitive measures. In addition, we combined these z scores to create z scores for composite cognitive function.
Covariates
We used covariates assessed at the Y10 and Y30 visits. Covariates were selected on the basis of previously published data and previous knowledge. Age, race, sex, education levels, and cigarette smoking (current, former, and never smokers) were obtained from questionnaires. Body mass index was calculated as measured body weight in kilograms divided by height in meters squared. Total cholesterol was measured enzymatically with the Abbott Spectrum diagnostic system (Abbot Laboratories, Abbott Park, IL) using the Trinder-type method for lipoproteins standard laboratory technique from blood stored at −70°C. Hypertension was defined as a systolic blood pressure ≥140 mm Hg, a diastolic blood pressure ≥90 mm Hg (according to the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure guidelines), or the use of antihypertensive medications. We chose these cut points to correspond to diagnosis and treatment guidelines that were contemporaneous with our assessment of participants. Cumulative systolic blood pressure was calculated as millimeters of mercury times year across the follow-up (Y10–Y30) to represent long-term exposure to blood pressure levels.15 Diabetes mellitus was defined as fasting blood glucose ≥126 mg/dL or the use of insulin and/or oral hypoglycemic agents. Cumulative fasting glucose was calculated as milligrams per deciliter times year across the follow-up (Y10–Y30) to represent long-term exposure to high glucose level.
Analytical sample
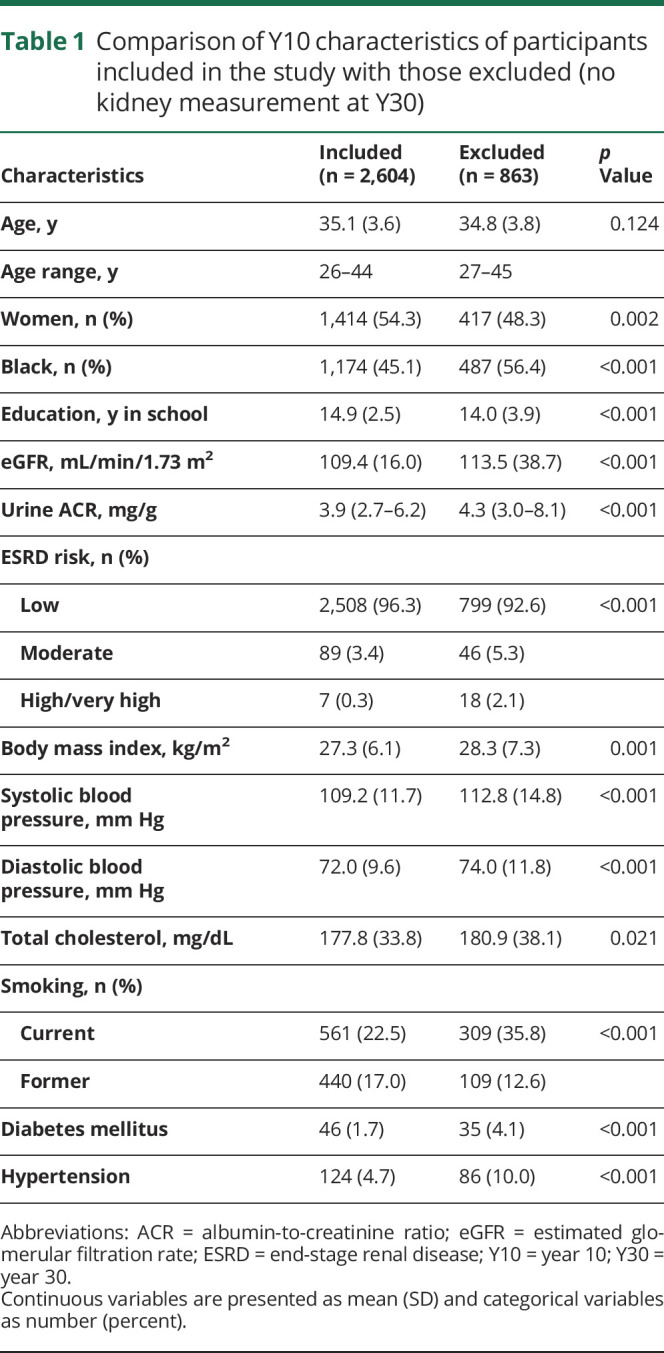
We restricted our analysis sample to those with information on kidney function at both Y10 and Y30 and at least 1 more visit information during the follow-up (75% of total at Y30). The strategy we used to account for missing data in kidney function was to assume no change in kidney measures if participants had no kidney measures on 1 or 2 examinations. We excluded participants with missing cognitive assessment at Y30 (figure 2). Participants included in this study, compared with those excluded, were more likely to be women and White and had higher education, lower eGFR and ACR levels, and better profiles of cardiovascular risk factors and disease (table 1).
Figure 2. Study sample.
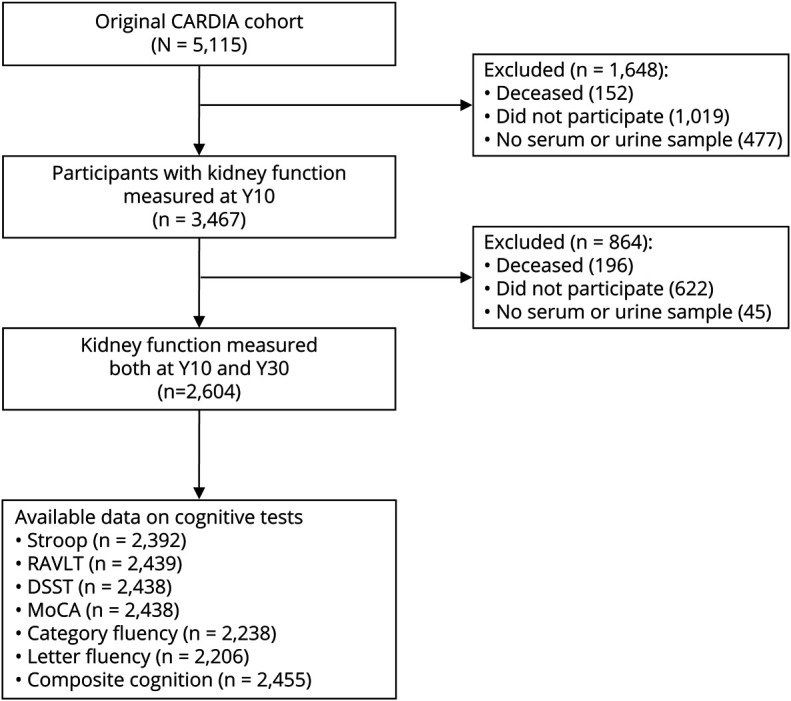
Flowchart of participant selection from the Coronary Artery Risk Development in Young Adults (CARDIA) cohort for the analysis in this study. DSST = Digit Symbol Substitution Test; MoCA = Montreal Cognitive Assessment; RAVLT = Rey Auditory Verbal Learning Test.
Table 1.
Comparison of Y10 characteristics of participants included in the study with those excluded (no kidney measurement at Y30)
Statistical analysis
We used alluvial plots to show change in participants kidney function during the follow-up. Associations between kidney function measures and cognitive function were evaluated with analysis of covariance. Mean and standard error were estimated for difference in z scores of cognitive function. All analyses were adjusted for age, sex, race, education, and recruitment center. We further adjusted the analyses for body mass index, total cholesterol, smoking, hypertension, and diabetes mellitus (model 2). We carried out several sensitivity analyses. We repeated the analyses of model 2 adjusting for covariates assessed at Y30. Because elevated glucose and blood pressure levels are known risk factor for cognitive impairment and major comorbid conditions of CKD, we investigated whether adjusting for exposure to high glucose and systolic blood pressure levels during follow-up time (cumulative exposure of glucose and systolic blood pressure) changed our findings. We also assessed interactions with sex and race by stratification and tested for multiplicative interactions by including multiplicative interaction terms in models. To assess the sensitivity of our results to missing data, we repeated the analyses with the inverse probability weighting method.16 With this method, observations are weighted by the inverse of the probability of an individual who is included in the study. We repeated the analyses excluding kidney function measured at Y30 to eliminate the role of simultaneous kidney and cognitive function impairment. Data analyses were performed with R version R-3.4.2 (R Foundation for Statistical Computing, Vienna, Austria).
Data availability
Anonymized data are available from the CARDIA Coordinating Center (cardia.dopm.uab.edu/contact-cardia). A description of the National Heart, Lung, and Blood Institute policies governing the data and describing access to the data can be found online (cardia.dopm.uab.edu/study-information/nhlbi-data-repository-data).
Results
Figure 3 shows the change in each participant’s kidney function over time. Over the course of 20 years, 427 (16%) participants had ≥1 episodes of ESRD risk exposure. At baseline 2,508 (96.3%) participants had low ESRD risk, 89 (3.4%) had moderately increased risk, 7 (0.2%) had high risk, and none had very high risk. At the end of the follow-up (Y30), 2,347 (90.1%) participants had low ESRD risk, 196 (7.5%) had moderately increased risk, 37 (1.4%) participants had high risk, and 24 (0.9%) participants had very high risk.
Figure 3. Alluvial plot depicting kidney function trajectories during follow-up.
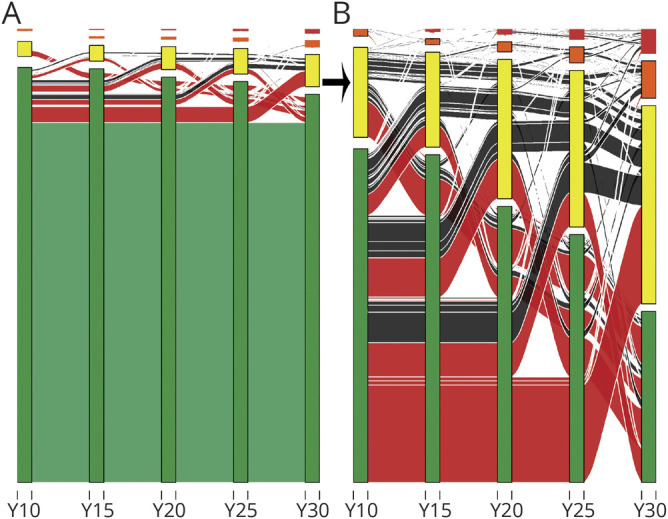
(A) All participants and (B) participants with ≥1 episodes of moderate/high end-stage renal disease (ESRD risk). The y-axis shows ESRD risk categories based on Kidney Disease: Improving Global Outcomes guidelines. Green indicates low risk; yellow indicates moderately increased risk; orange indicates high risk; and red indicates very high risk. The x-axis shows follow-up examinations in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Green lines indicate participants with no episode of ESRD risk; red indicates 1 episode; and black indicates >1 episode of ESRD risk. Thicker lines indicate a higher number of participants with corresponding pattern of change in kidney function. Y = year.
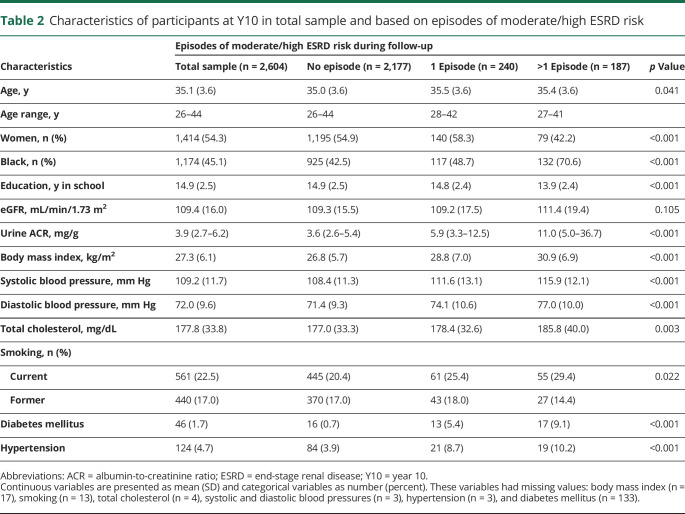
Table 2 presents the characteristics of the total study sample and across categories of number of moderate/high ESRD risk measures based on both eGFR and ACR according to the KDIGO guideline. Average ± SD age of the participants was 35 ± 4 years; 54% were female; and 45% were Black. Mean ± SD eGFR at Y10 was 109.4 ± 16.0 mL/min/1.73 m2, and median (interquartile range) ACR was 3.9 (2.7–6.2) mg/g.
Table 2.
Characteristics of participants at Y10 in total sample and based on episodes of moderate/high ESRD risk
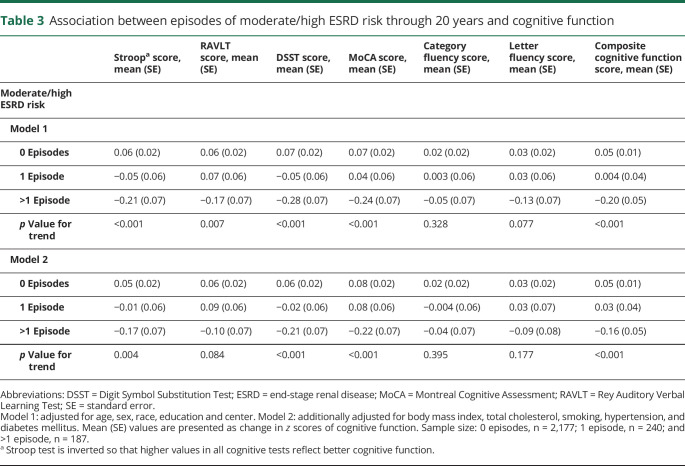
We observed worse cognitive performance in participants with >1 episode of moderate/high ESRD risk than those with 1 or 0 episodes (table 3). Participants with >1 episode of moderate/high ESRD risk had a significantly lower performance in executive function (Stroop), verbal memory (RAVLT), psychomotor speed (DSST), MoCA, and composite cognitive function (table 3, model 1). After adjustment for cardiovascular risk factors, effect estimates were attenuated, and there was no association with RAVLT (table 3, model 2). There was no difference between categories of ESRD risk in relation to category and letter fluency tests.
Table 3.
Association between episodes of moderate/high ESRD risk through 20 years and cognitive function
Repeating the analyses using covariates assessed at Y30 did not alter the associations. In addition, after adjustment for cumulative glucose and systolic blood pressure, the effect estimates were minimally attenuated and remained statistically significant. We found no race or sex interaction with kidney function in relation to cognitive assessment (all p for interaction >0.05). In a series of extra analyses, we tested the associations using the inverse probability weighting method. Repeating the analyses with this method revealed similar findings. Furthermore, excluding kidney function at Y30 did not change the association between episodes of ESRD risk and cognitive function (data not shown).
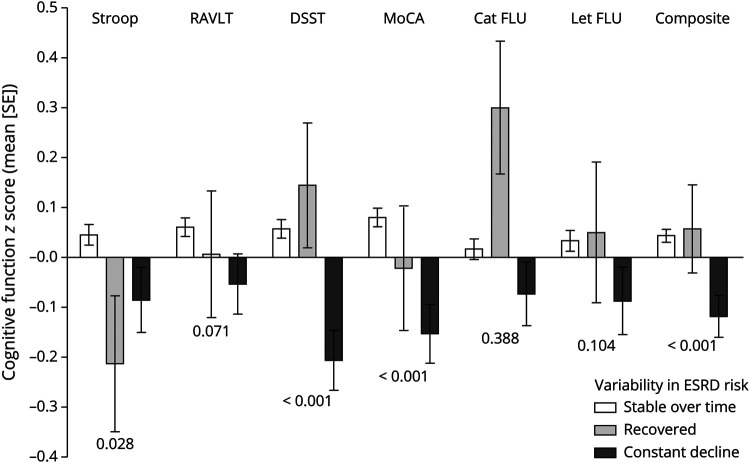
Figure 4 shows cognitive performance in participants with stable ESRD risk over the course of follow-up, participants with constant decline in kidney function, and those with recovered 4 kidney function. Participants who had constant kidney function decline performed worse in DSST, MoCA, and composite cognitive function compared with participants who recovered and those with stable kidney function (figure 4).
Figure 4. Association between variability in ESRD risk during the follow-up and cognitive function at year 30.
Adjusted for age, sex, race, education, center, hypertension, body mass index, total cholesterol, smoking, and diabetes mellitus. Mean (standard error) are presented as difference in z scores of cognitive function. The Stroop test is inverted so that higher values in all cognitive tests reflect better cognitive function. The p values presented are for trend. Cat FLU = category fluency; DSST = Digit Symbol Substitution Test; ESRD = end-stage renal disease; Let FLU = Letter fluency; MoCA = Montreal Cognitive Assessment; RAVLT = Rey Auditory Verbal Learning Test.
Discussion
In this population-based study, we observed that adults between 26 and 44 years of age who experience more episodes of moderate/high ESRD risk across 20 years of follow-up have worse performance in various cognitive domains in middle age, particularly in executive function, psychomotor speed domains, and composite function. In addition, participants with constant decline in their kidney function performed worse in cognitive function than those with stable risk over time or those who recovered from 1 or 2 episodes of moderate/high ESRD risk.
Higher stages of CKD are associated with severity of cognitive impairment.2,3 Several studies showed that even a mild to moderate decrease in eGFR is associated with poor cognitive performance independently of cardiovascular risk factors.17–21 Limited evidence exists on the relation between longitudinal changes in kidney function and cognitive outcomes. In the volunteer cohort of the Baltimore Longitudinal Study of Aging (BLSA), middle-aged adults (mean age 54 years) were followed up over an average of 7.7 years.22 Declines in kidney function were independently associated with greater long-term declines in visual memory and verbal memory and learning.22 In another longitudinal study of middle-aged adults (mean age 62 years), change in renal function over 5 years was related to declines in global cognitive ability.23 The current study with a longer follow-up time and younger adults suggests that changes in kidney function during young adulthood is related to cognitive outcomes in midlife, opening a window for early detection of high-risk individuals and implementing preventive measures to preserve cognitive function.
Cardiovascular risk factors such as hypertension, diabetes mellitus, and dyslipidemia may accelerate the rate of decline in kidney function and increase the risk of cognitive impairment.24,25 In particular, elevated blood pressure and serum glucose may be in the causal pathway for the association of kidney function measures with cognitive impairment. In this study, adjusting for baseline or Y30 cardiovascular risk factors attenuated the effect estimates but did not change the association. To take into account the long-term exposure to high blood pressure and elevated serum glucose, we also adjusted the analyses for cumulative exposure to high systolic blood pressure and elevated glucose. After this adjustment, the effect estimates were slightly attenuated, suggesting that part of this association can be explained by cardiovascular risk factors and long-term exposure to elevated blood pressure and glucose in young adulthood. Rather than shared risk factors, kidney function decline can lead to cognitive impairment through other mechanisms such as promotion of chronic microinflammation, oxidative stress, direct metabolic toxic effect, and hemodynamic dysregulations.26,27 Cognitive impairment is a major consequence of kidney impairment, and future studies are required to determine the mechanisms linking kidney function, cognitive function, and these factors.
We observed more prominent associations of episodes of ESRD risk with cognitive domains known to be vulnerable to cerebrovascular pathology (executive function and psychomotor speed) rather than domains typically linked with Alzheimer disease pathology (verbal memory and language). This is in line with previous literature on the stronger association of measures of kidney function, in particular microalbuminuria, with vascular dementia.28–30 A high ACR is the result of endothelial damage in the kidneys leading to the leakage of serum proteins in the urine. This can reflect a systemic phenomenon; the leakage of serum proteins into the extracellular space of the brain was suggested to causes cognitive problems and cerebrovascular disease.31
As a limitation, in this study we included CARDIA participants who had kidney function measures available on both the Y10 and Y30 examination visits, which was >50% of initial CARDIA cohort sample. As demonstrated, participants in CARDIA who had missing kidney function data had a greater burden of cardiovascular risk factors, which can result in underestimating the strength of the associations. Second, although we adjusted for various cardiovascular risk factors, residual confounding cannot be ruled out. Third, we did not have information on repeated measurements of cognitive function during follow-up; therefore, the temporality of the association needs further research. Fourth, this is the first study to use repeated assessment of ESRD risk; future studies are needed to assess how these categories are related to higher risk of ESRD and mortality. The population-based design of this study, large sample size of young Black and White Americans, follow-up time of >2 decades, and availability of extensive data on various sociodemographic and cardiovascular factors, which enabled us to control for various potential confounders, can be stated as the main strengths of this study.
We observed that having more episodes of impaired kidney function in CARDIA participants during young adulthood was associated with worse performance in various cognitive domains in midlife. The observed association between kidney function and cognitive impairment at such an early stage in life warrants clinical attention and further investigations. Dementia is a devastating medical condition, and extensive efforts to treat this clinical entity have been unsuccessful. This calls for the early prevention of cognitive decline decades before full-blown manifestation of dementia. Our finding highlights the importance of early detection of kidney dysfunction and implementation of appropriate interventions to prevent adverse brain function in later life.
Glossary
- ACR
albumin-to-creatinine ratio
- BLSA
Baltimore Longitudinal Study of Aging
- CARDIA
Coronary Artery Risk Development in Young Adults
- CKD
chronic kidney disease
- DSST
Digit Symbol Substitution Test
- eGFR
estimated glomerular filtration rate
- ESRD
end-stage renal disease
- KDIGO
Kidney Disease: Improving Global Outcomes
- MoCA
Montreal Cognitive Assessment
- RAVLT
Rey Auditory Verbal Learning Test
- Y
year
Appendix. Authors
Footnotes
CME Course: NPub.org/cmelist
Study funding
CARDIA is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with the University of Alabama at Birmingham (HHSN268201800005I and HHSN268201800007I), Northwestern University (HHSN268201800003I), University of Minnesota (HHSN268201800006I), and Kaiser Foundation Research Institute (HHSN268201800004I). Cognitive function assessment was supported by NHLBI grant R01HL122658-04. This manuscript has been reviewed by CARDIA for scientific content. Sanaz Sedaghat is supported by a Rubicon fellowship of the Netherlands Organization for Scientific Research and McKnight Clinical Translational Research Scholarship in Cognitive Aging and Age-Related Memory Loss. Farzaneh Sorond was supported by National Institute of Neurological Disorders and Stroke (R01-NS085002).
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Bronas UG, Puzantian H, Hannan M. Cognitive impairment in chronic kidney disease: vascular milieu and the potential therapeutic role of exercise. Biomed Res Int 2017;2017:2726369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bugnicourt JM, Godefroy O, Chillon JM, Choukroun G, Massy ZA. Cognitive disorders and dementia in CKD: the neglected kidney-brain axis. J Am Soc Nephrol 2013;24:353–363. [DOI] [PubMed] [Google Scholar]
- 3.Lu R, Kiernan MC, Murray A, Rosner MH, Ronco C. Kidney-brain crosstalk in the acute and chronic setting. Nat Rev Nephrol 2015;11:707–719. [DOI] [PubMed] [Google Scholar]
- 4.Murray AM. The brain and the kidney connection: a model of accelerated vascular cognitive impairment. Neurology 2009;73:916–917. [DOI] [PubMed] [Google Scholar]
- 5.Berger I, Wu S, Masson P, et al. Cognition in chronic kidney disease: a systematic review and meta-analysis. BMC Med 2016;14:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurella Tamura M, Yaffe K. Dementia and cognitive impairment in ESRD: diagnostic and therapeutic strategies. Kidney Int 2011;79:14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurella M, Chertow GM, Fried LF, et al. Chronic kidney disease and cognitive impairment in the elderly: the health, aging, and body composition study. J Am Soc Nephrol 2005;16:2127–2133. [DOI] [PubMed] [Google Scholar]
- 8.Elias MF, Dore GA, Davey A. Kidney disease and cognitive function. Contrib Nephrol 2013;179:42–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Darmady EM, Offer J, Woodhouse MA. The parameters of the ageing kidney. J Pathol 1973;109:195–207. [DOI] [PubMed] [Google Scholar]
- 10.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol 1988;41:1105–1116. [DOI] [PubMed] [Google Scholar]
- 11.Muntner P, Newsome B, Kramer H, et al. Racial differences in the incidence of chronic kidney disease. Clin J Am Soc Nephrol 2012;7:101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012;367:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inker LA, Astor BC, Fox CH, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis 2014;63:713–735. [DOI] [PubMed] [Google Scholar]
- 14.Mahmoodi BK, Yatsuya H, Matsushita K, et al. Association of kidney disease measures with ischemic versus hemorrhagic strokes: pooled analyses of 4 prospective community-based cohorts. Stroke 2014;45:1925–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kishi S, Teixido-Tura G, Ning H, et al. Cumulative blood pressure in early adulthood and cardiac dysfunction in middle age: the CARDIA study. J Am Coll Cardiol 2015;65:2679–2687. [DOI] [PubMed] [Google Scholar]
- 16.Seaman SR, White IR. Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res 2013;22:278–295. [DOI] [PubMed] [Google Scholar]
- 17.Helmer C, Stengel B, Metzger M, et al. Chronic kidney disease, cognitive decline, and incident dementia: the 3C Study. Neurology 2011;77:2043–2051. [DOI] [PubMed] [Google Scholar]
- 18.Sedaghat S, Ding J, Eiriksdottir G, et al. The AGES-Reykjavik Study suggests that change in kidney measures is associated with subclinical brain pathology in older community-dwelling persons. Kidney Int 2018;94:608–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buchman AS, Tanne D, Boyle PA, Shah RC, Leurgans SE, Bennett DA. Kidney function is associated with the rate of cognitive decline in the elderly. Neurology 2009;73:920–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiner DE, Gaussoin SA, Nord J, et al. Cognitive function and kidney disease: baseline data from the Systolic Blood Pressure Intervention Trial (SPRINT). Am J Kidney Dis 2017;70:357–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurella Tamura M, Wadley V, Yaffe K, et al. Kidney function and cognitive impairment in US adults: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. Am J Kidney Dis 2008;52:227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seliger SL, Wendell CR, Waldstein SR, Ferrucci L, Zonderman AB. Renal function and long-term decline in cognitive function: the Baltimore Longitudinal Study of Aging. Am J Nephrol 2015;41:305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davey A, Elias MF, Robbins MA, Seliger SL, Dore GA. Decline in renal functioning is associated with longitudinal decline in global cognitive functioning, abstract reasoning and verbal memory. Nephrol Dial Transpl 2013;28:1810–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seliger SL, Longstreth WT Jr. Lessons about brain vascular disease from another pulsating organ, the kidney. Stroke 2008;39:5–6. [DOI] [PubMed] [Google Scholar]
- 25.Vanholder R, De Deyn PP, Van Biesen W, Lameire N. Marconi revisited: from kidney to brain: two organ systems communicating at long distance. J Am Soc Nephrol 2008;19:1253–1255. [DOI] [PubMed] [Google Scholar]
- 26.Kurella Tamura M, Chertow GM, Depner TA, et al. Metabolic profiling of impaired cognitive function in patients receiving dialysis. J Am Soc Nephrol 2016;27:3780–3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lau WL, Huisa BN, Fisher M. The cerebrovascular-chronic kidney disease connection: perspectives and mechanisms. Transl Stroke Res 2017;8:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sedaghat S, Cremers LG, de Groot M, et al. Lower microstructural integrity of brain white matter is related to higher mortality. Neurology 2016;87:927–934. [DOI] [PubMed] [Google Scholar]
- 29.Georgakis MK, Dimitriou NG, Karalexi MA, et al. Albuminuria in association with cognitive function and dementia: a systematic review and meta-analysis. J Am Geriatr Soc 2017;65:1190–1198. [DOI] [PubMed] [Google Scholar]
- 30.Weiner DE, Bartolomei K, Scott T, et al. Albuminuria, cognitive functioning, and white matter hyperintensities in homebound elders. Am J Kidney Dis 2009;53:438–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knopman DS. Invited commentary: albuminuria and microvascular disease of the brain: a shared pathophysiology. Am J Epidemiol 2010;171:287–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data are available from the CARDIA Coordinating Center (cardia.dopm.uab.edu/contact-cardia). A description of the National Heart, Lung, and Blood Institute policies governing the data and describing access to the data can be found online (cardia.dopm.uab.edu/study-information/nhlbi-data-repository-data).