Abstract
Background
We sought to determine whether laparoscopic hyperthermic intraperitoneal chemoperfusion (LS-HIPEC) improves overall survival (OS) in patients with gastric and gastroesophageal adenocarcinoma and low-volume peritoneal metastasis compared with standard of care treatment.
Methods
We reviewed data from a prospectively maintained database of patients with gastric and gastroesophageal adenocarcinoma to identify patients with radiologically occult carcinomatosis or positive peritoneal cytology, no evidence of distant metastasis, and without disease progression during initial chemotherapy or observation. Univariate and multivariable analyses were performed to evaluate the impact of LS-HIPEC on OS.
Results
We identified 25 patients who underwent LS-HIPEC and 27 treated with a standard of care approach due to patient (33.3%) or provider (51.9%) preference or financial limitations/lack of insurance coverage (14.8%). Resection was ultimately performed in 28% of LS-HIPEC patients and no standard care patients. At a median follow-up of 18.9 months, median OS was 24.7 (IQR 20.8-34.2) months in LS-HIPEC patients and 21.3 (IQR 12.3-23.1) months in standard care patients (p=0.08). Three-year OS in the LS-HIPEC group was 19.1%, compared with 9.6% (p=0.08). Patients who underwent resection had a median OS of 25.3 (IQR 22.6-47.1) months compared with 21.3 months in standard care patients (p=0.05).
Conclusion
Neoadjuvant LS-HIPEC for the treatment of low-volume peritoneal disease in gastric and gastroesophageal cancer patients did not significantly improve OS compared with standard care. Multi-institutional studies are necessary to further elucidate the benefit of LS-HIPEC for this patient population.
INTRODUCTION
Peritoneal metastasis from gastric or gastroesophageal cancer represents advanced disease, with a poor prognosis.1-3 As patients with peritoneal metastasis at diagnosis are unlikely to gain significant benefit from gastrectomy, the National Comprehensive Cancer Network (NCCN) currently recommends palliative systemic therapy or best supportive care for this group of patients.4
Hyperthermic intraperitoneal chemoperfusion (HIPEC), an investigational regional therapy, has been studied for the treatment of peritoneal metastasis and has shown some survival benefit in other malignancies, namely appendiceal,5 ovarian,6,7 and colorectal cancers.8-12 HIPEC for the treatment of peritoneal metastasis in gastric cancer is less widely accepted. Multiple meta-analyses have provided evidence for survival benefit in gastric cancer patients with a high risk of peritoneal recurrence who are treated with various intraperitoneal chemotherapy strategies at the time of potentially curative gastrectomy.13-17 These results are supported by findings that HIPEC has the potential to convert positive cytology to negative.18 Further, there is evidence to support treatment with HIPEC and cytoreductive surgery (CRS) in patients with known peritoneal carcinomatosis (PC) at the time of gastrectomy,16,17,19 although such treatment is associated with significant morbidity.16,20 Laparoscopic HIPEC (LS-HIPEC) has been investigated as a less invasive treatment option in patients with PC or positive peritoneal cytology (PPC) at the time of diagnosis. LS-HIPEC has a generally acceptable safety profile and has potential to convert positive cytology to negative and to decrease or eradicate PC burden to increase eligibility for CRS.18,21-23
Recently, we reported the outcomes of a single-institution, single-arm phase II clinical trial (NCT02092298) to evaluate the safety and efficacy of LS-HIPEC without CRS for gastric and gastroesophageal adenocarcinoma patients with low-volume PC or PPC.22 The trial allowed for patients to undergo up to five LS-HIPEC procedures and potentially become eligible for curative resection, depending on disease regression. The results of the study were promising, with five of 19 patients undergoing resection and a median overall survival (OS) of 30 months for the entire study group. Interpretation of the potential survival benefit of LS-HIPEC was limited by the lack of a control group treated with the standard of care.
The purpose of the present study was to perform a retrospective case-control study to test the hypothesis that LS-HIPEC without CRS will yield better OS outcomes in gastric and gastroesophageal adenocarcinoma patients with low-volume PC or PPC only compared with a standard care (SC) approach.
METHODS
Patient Selection
A prospectively maintained institutional database was queried to identify patients diagnosed with biopsy-proven gastric and gastroesophageal adenocarcinoma between January 2013 and October 2018. Patients were eligible for inclusion in the study if they had radiologically occult PC or PPC on staging diagnostic laparoscopy, no evidence of distant metastasis on imaging, and no imaging evidence of disease progression during initial chemotherapy or the first 3 months of observation. Patients were excluded if the peritoneal carcinomatosis index (PCI) was greater than 7 at the time of either diagnostic laparoscopy or LS-HIPEC. We also excluded patients with gastric outlet obstruction at presentation and any who had received cancer-specific therapy prior to diagnosis of peritoneal disease.
Clinicopathologic characteristics and outcomes were obtained from the electronic medical record and compared between patients who underwent LS-HIPEC and those who did not. SC included palliative systemic chemotherapy or, in some patients with minimal symptoms, an initial period of observation followed by chemotherapy when progression occurred as documented by imaging findings or progressive symptoms.
The treatment algorithm for patients with low-volume peritoneal disease at our institution has been described previously and is depicted in Figure 1.21,22 Briefly, after diagnosis of low-volume PC or PPC, patients are treated first with systemic chemotherapy, at the discretion of the treating medical oncologist. LS-HIPEC is performed at least 3 weeks after completion of chemotherapy. Under the phase II trial protocol, patients were able to undergo up to five LS-HIPEC procedures, a minimum of 3 weeks apart, and chemoradiotherapy was allowed.22 Diagnostic laparoscopy was performed with cytology washings and biopsy of suspicious lesions, at the time of each LS-HIPEC. Patients with negative cytology, no laparoscopic evidence of carcinomatosis and no imaging evidence of solid organ metastases were considered for gastrectomy. All decisions regarding pursuit of LS-HIPEC, chemoradiotherapy, or gastrectomy were discussed in a multidisciplinary gastric cancer tumor board. This treatment algorithm has generally been applied to patients with low-volume peritoneal disease at our institution since the phase II trial.21
Figure 1.
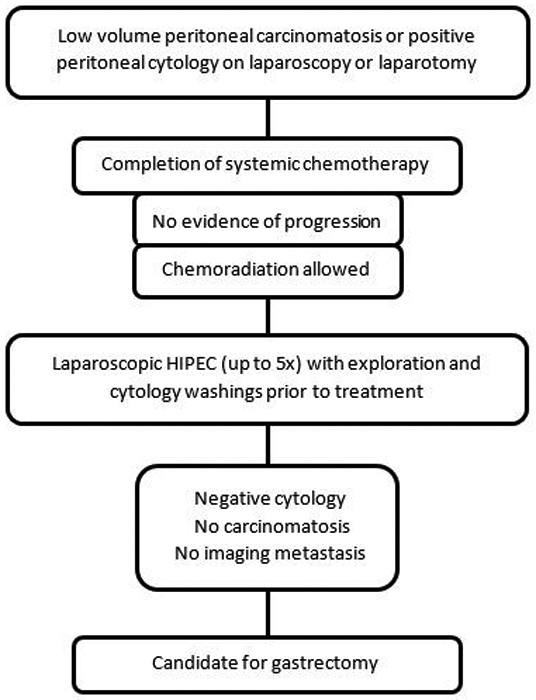
Flowchart depicting the treatment algorithm for patients with low-volume peritoneal disease considered eligible for laparoscopic hyperthermic intraperitoneal chemoperfusion (LS-HIPEC).
The number of chemotherapy cycles reported in our study reflects the number of cycles administered prior to LS-HIPEC, or in the SC group, the number of cycles prior to (1) consideration for HIPEC, (2) initiation of consolidative chemoradiotherapy, (3) a treatment break, or (4) progression after prolonged stability or improvement on systemic treatment.
Operative Techniques
The standard techniques used in our institution for diagnostic laparoscopy and LS-HIPEC have been described elsewhere.21,22,24,25 Diagnostic laparoscopy and peritoneal cytology is performed prior to each LS-HIPEC procedure. After the appropriate samples are obtained, the inflow and outflow catheters are placed into the abdomen through the lateral port sites under laparoscopic visualization. After a preliminary flow infusion of Plasma-Lyte is initiated, 30 mg of mitomycin C and 200 mg of cisplatin are added to the perfusion circuit and the total circuit volume increased to 3 to 7 L in order to establish a constant flow cycle.21,22,25-28 HIPEC is performed for 60 minutes with inflow and outflow temperatures of 41 to 42 °C and 39 to 40 °C, respectively.21,25,28
Peritoneal Carcinomatosis Index
As PCI was not recorded at the time of diagnostic laparoscopy in the large majority of patients in this study, PCI was calculated retrospectively based on staging laparoscopy operative reports at the time of diagnosis and corresponding pathology reports, when necessary details were available. PCI was calculated based the generally accepted scoring system, as has been previously described.29 A score of 0 was given to patients with no macroscopic PC and PPC only.
Statistical Analysis
Continuous variables are reported as mean (95% confidence interval) for parametric variables or median (interquartile range [IQR]) for nonparametric variables. Categorical variables are reported as frequencies and percentages. Differences in continuous variables were assessed with an independent t test for parametric variables or Mann-Whitney U test for nonparametric variables. Chi-square analysis and Fisher’s exact test were used to compare categorical variables between the groups. OS was calculated from the date of diagnosis of peritoneal disease on staging laparoscopy to most recent follow-up or date of death. Kaplan-Meier survival analyses were used to compare median OS between the two groups, and differences were compared using a log rank test. Univariate and multivariable Cox regression analyses were performed to identify factors independently associated with OS. All significance tests were two tailed, and a p value of 0.05 or less was considered statistically significant. Statistical analysis was performed using IBM SPSS Statistics for Windows, Version 26.0 (IBM Corp. Released 2019. Armonk, NY, USA).
The Institutional Review Board at The University of Texas MD Anderson Cancer Center approved this study.
RESULTS
We identified 52 patients meeting eligibility criteria for the study. Twenty-five patients underwent LS-HIPEC, and 27 patients did not and were included in the SC group. Twelve of the patients who underwent LS-HIPEC were included in the previously reported phase II clinical trial for LS-HIPEC for low-volume peritoneal disease.22
Table 1 shows the clinicopathologic characteristics of the LS-HIPEC and SC groups. The LS-HIPEC group was younger, with a mean (SD) age of 57 (10) years compared with 64 (10) years (p = 0.02). Rates of PC were similar between the groups. Operative details were available for calculation of PCI in 37 patients: 20 in the SC group and 17 in the LS-HIPEC group, and there was no significant difference in PCI between the two groups. Limiting the analysis to patients with gross carcinomatosis, PCI remained similar between the groups. Sufficient information was available to calculate the number of peritoneal regions involved in all but three patients, and this was also similar between the two groups. Clinical T stage varied between the two groups, as a large majority (90%) of the SC group had cT3 tumors, while the LS-HIPEC group had a wider variety of clinical T stages, including cT2 (29.4%), cT3 (52.9%), and cT4 (17.6%) tumors (p=0.03).
Table 1.
Clinicopathologic characteristics of the patient cohort
| Characteristic | Overall, No. (%) (n = 52) |
Standard Care, No. (%) (n = 27) |
LS-HIPEC, No. (%) (n = 25) |
p |
|---|---|---|---|---|
| Age, mean (SD) | 61 (11) | 64 (10) | 57±10 | 0.02 |
| Gender, male | 35 (67.3) | 17 (63) | 18 (72) | 0.48 |
| Race/ethnicity | ||||
| White | 28 (53.8) | 13 (48.1) | 15 (60) | 0.24 |
| Black | 5 (9.6) | 2 (7.4) | 3 (12) | |
| Hispanic | 9 (17.3) | 7 (25.9) | 2 (8) | |
| Asian | 8 (15.4) | 3 (11.1) | 5 (20) | |
| Unknown | 2 (3.8) | 2 (7.4) | 0 (0) | |
| Tumor location | ||||
| GEJ | 19 (36.5) | 10 (37) | 9 (36) | 0.73 |
| Fundus/cardia | 4 (7.7) | 1 (3.7) | 3 (12) | |
| Body/antrum | 20 (38.5) | 11 (40.7) | 9 (36) | |
| Total stomach | 9 (17.3) | 5 (18.5) | 4 (16) | |
| Tumor grade | ||||
| Moderate | 9 (17.3) | 4 (14.8) | 5 (20) | 0.62 |
| Poor/undifferentiated | 43 (82.7) | 23 (85.2) | 20 (80) | |
| Signet ring cells | 33 (64.7) | 18 (66.7) | 15 (62.5) | 0.76 |
| Linitis plastica | 14 (32.6) | 7 (31.8) | 7 (33.3) | 0.96 |
| Clinical T stage | ||||
| cT2 | 7 (18.9) | 2 (10) | 5 (29.4) | 0.03 |
| cT3 | 27 (73.0) | 18 (90) | 9 (52.9) | |
| cT4 | 3 (8.1) | 0 (0) | 3 (17.6) | |
| Clinical N stage | ||||
| cN0 | 19 (55.9) | 13 (68.4) | 6 (40) | 0.22 |
| cN1 | 9 (26.5) | 3 (15.8) | 6 (40) | |
| cN2 | 5 (14.7) | 3 (15.8) | 2 (13.3) | |
| cN3 | 1 (2.9) | 0 (0) | 1 (6.7) | |
| LS gross positive | 34 (65.4) | 17 (63) | 17 (68) | 0.70 |
| LS-positive cytology only | 18 (34.6) | 10 (37) | 8 (32) | 0.70 |
| PCI, median (range) | 1.0 (0-6) | 0.5 (0-6) | 1.0 (0-4) | 0.99 |
| Affected regions, median (range) | 1.0 (0-6) | 1.0 (0-6) | 1.0 (0-4) | 0.74 |
| Additional therapy | ||||
| Chemo only | 26 (50.0) | 17 (63.0) | 9 (36.0) | 0.10 |
| Chemoradiation/XRT | 26 (50.0) | 10 (37.0) | 16 (64.0) | |
| Response to therapy | ||||
| Stable | 12 (23.5) | 8 (30.7) | 4 (16.0) | 0.48 |
| Partial Response | 33 (64.7) | 15 (57.7) | 18 (72.0) | |
| Complete Radiologic Response | 6 (11.8) | 3 (11.5) | 3 (12.0) | |
Abbreviations: GEJ, gastroesophageal junction; PCI, peritoneal carcinomatosis index; LS, laparoscopy; XRT, radiation
All patients in the LS-HIPEC group underwent induction chemotherapy. Sixteen patients (64%) were treated with a doublet 5-fluourouracil (5-FU)/platinum regimen, 8 patients (32%) with a triplet 5-FU/taxol/platinum regimen, and 1 patient (4%) with epirubicin/cisplatin/5-FU. The median number of chemotherapy cycles prior to LS-HIPEC was 8.0 (IQR 6.5-11.5). In the SC group, three patients were followed initially with observation due to low-volume disease and minimal symptoms. After an initial period of stability, progression of disease prompted initiation of systemic chemotherapy, which, for all three patients consisted of 5-FU/oxaliplatin. First-line systemic chemotherapy in the rest of the SC group consisted of a doublet 5-FU/platinum regimen in 14 patients (58.3%), and a triplet 5-FU/taxol/platinum regimen in 10 (41.7%) patients. The median number of chemotherapy cycles prior to evaluation for HIPEC, initiation of chemoradiation, a treatment break, or late progression, was 8.0 (IQR 4-12). Two patients had progression of disease after prolonged treatment with systemic chemotherapy, of twelve and sixteen cycles each. There was no difference in number of chemotherapy cycles between the two groups (p=0.66).
Overall, 22 patients (42.3%) were treated with chemoradiotherapy, 16 (64.0%) in the LS-HIPEC group, and 7 (25.9%) in the SC group. The majority of patients who received chemoradiation were treated with a dose of 45Gy. There were 2 patients in the LS-HIPEC group who received a dose of 50.4Gy due to gastroesophageal junction tumors, and treatment under an esophageal chemoradiotherapy regimen. There was 1 patient in the SC group who received a chemoradiation dose of 30Gy. Three others in the SC group received 30Gy of palliative radiation, without concurrent chemotherapy, during their course.
Reasons for which patients in the SC group did not undergo LS-HIPEC included patient (33.3%) and provider (51.9%) preference and financial limitations/lack of insurance coverage (14.8%). Among the 25 patients in the LS-HIPEC group, a total of 39 LS-HIPEC procedures were performed. Eighteen patients underwent a single LS-HIPEC procedure, four patients had two procedures, one patient had three, and two patients had five. Seven LS-HIPEC patients (28%) ultimately underwent resection after clearance of peritoneal disease as demonstrated by diagnostic laparoscopy and negative cytology, compared with zero patients in the SC group.
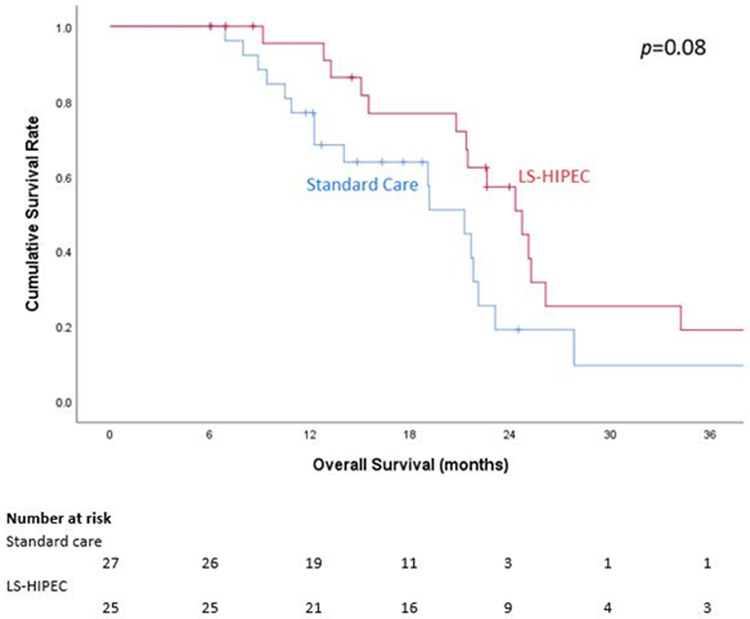
Median follow-up was 18.9 (IQR 14.8-22.5) months for all patients, 14.8 (IQR 10.9-21.6) months for the SC group, and 22.5 (IQR 13.9-25.2) months for the LS-HIPEC group. Median OS by Kaplan-Meier analysis was 21.3 (IQR 12.3-23.1) months in the SC group and 24.7 (IQR 20.8-34.2) months in the LS-HIPEC group, though this difference was not statistically significant (p = 0.08; Figure 2). The respective 1-, 2-, and 3-year OS rates were 76.9%, 19.1%, and 9.6% in the SC group and 95.5%, 57.2%, and 19.1% in the LS-HIPEC group (p = 0.08).
Figure 2.
Kaplan-Meier 3-year overall survival (OS) curve based on treatment group.
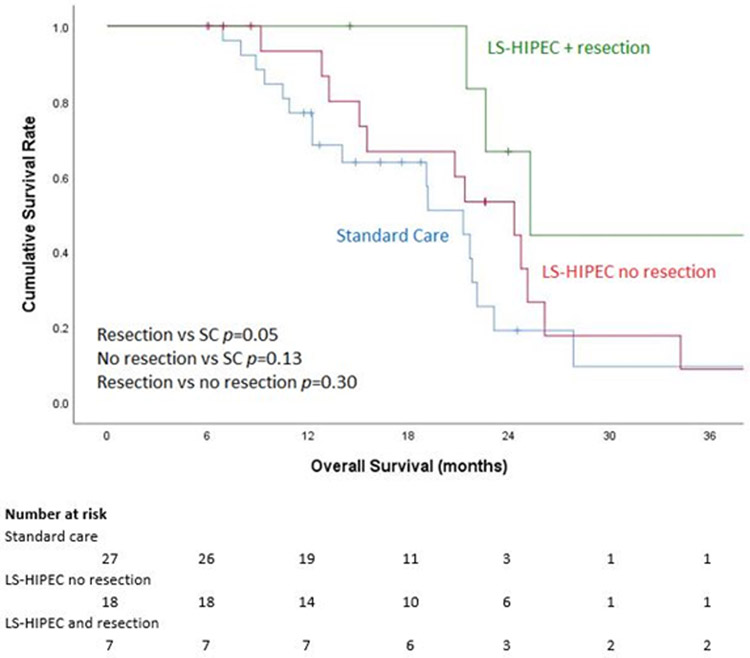
Interestingly, the subset of patients who underwent resection after LS-HIPEC had a median OS of 25.3 (IQR 22.6-47.1) months, which was significantly higher than the SC group’s median OS of 21.3 months (p = 0.05) but no different than that of LS-HIPEC patients who did not undergo resection (p = 0.129; Figure 3). The respective 1-, 2-, and 3-year survival rates were 100%, 66.7%, and 44.4% for patients who underwent resection after LS-HIPEC and 93.3%, 53.3%, and 8.9% for those who underwent only LS-HIPEC.
Figure 3:
Kaplan-Meier 3-year overall survival (OS) curve based on laparoscopic hyperthermic intraperitoneal chemoperfusion (LS-HIPEC) and resection status.
On univariate Cox regression analysis, only clinical T stage of cT4 was found to have a significant influence on OS, compared with cT2 tumors. Variables with p ≤ 0.1 were included in the multivariable analysis; these included histologic grade, clinical T stage, receipt of LS-HIPEC, and resection status. On multivariable analysis, none of the variables were significant independent predictive factors for OS (Table 2).
Table 2:
Multivariable Cox Regression Analysis for overall survival.
| Variable | HR | 95% CI | p |
|---|---|---|---|
| Clinical T stage | |||
| T2 | 0.203 | 0.037-1.104 | 0.065 |
| T3 | 0.303 | 0.059-1.555 | 0.152 |
| T4 | Reference | 0.178 | |
| Histologic grade | |||
| Moderate | Reference | ||
| Poor/undifferentiated | 2.595 | 0.602-11.189 | 0.201 |
| LS-HIPEC | 0.863 | 0.278-2.672 | 0.798 |
| Resection | 0.601 | 0.132-2.742 | 0.511 |
Abbreviations: CI, confidence interval; HR, hazard ratio; LS-HIPEC, laparoscopic hyperthermic intraperitoneal chemoperfusion.
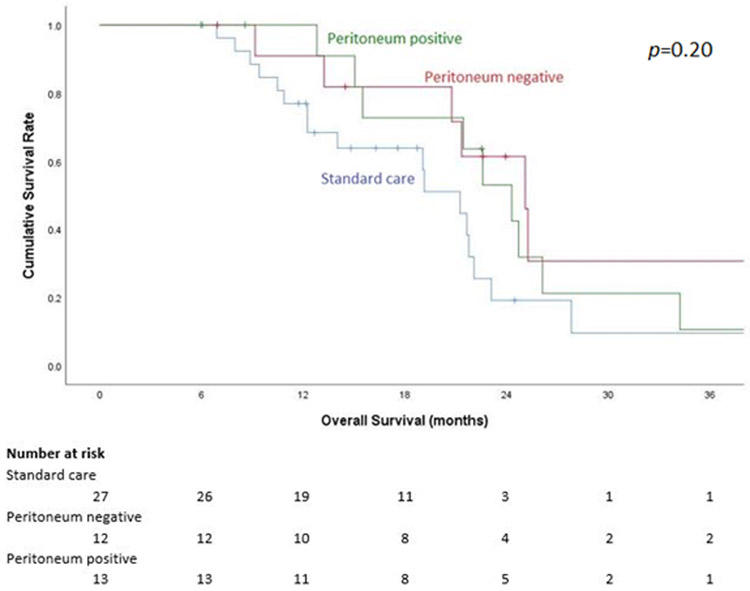
Notably, of the 25 patients who underwent LS-HIPEC after systemic chemotherapy, 12 (48%) had a negative exploration and negative cytology at the time of their first LS-HIPEC. Seven patients (28%) had evidence of gross carcinomatosis and 6 patients (24%) had PPC only at the time of their first LS-HIPEC. In those with gross carcinomatosis, PCI score was less than 7 and in most cases, there was minimal residual disease after excisional biopsy of the lesions, and treatment with HIPEC was appropriate. In rare cases of gross residual disease after excisional biopsy, these patients were treated with LS-HIPEC in the context of a clinical trial, and thus were treated despite the gross residual disease. Of the seven patients who underwent resection, five had no peritoneal disease at the time of their first LS-HIPEC. However, the status of the peritoneum at the time of first LS-HIPEC did not have an effect on OS (Figure 4).
Figure 4:
Kaplan-Meier 3-year overall survival curve based on status of peritoneal disease at the time of laparoscopic hyperthermic intraperitoneal chemoperfusion (LS-HIPEC).
DISCUSSION
Our results indicate that LS-HIPEC shows promise for improving OS in patients with low-volume PC or PPC in gastric and gastroesophageal cancer. However, in the present study, we were unable to demonstrate a significant survival advantage in those treated with LS-HIPEC compared to the SC group. It is possible that this represents a type II error, as the sample size was small. Patients who underwent resection after LS-HIPEC did demonstrate significantly longer OS compared with the SC group.
While current NCCN guidelines recommend palliative chemotherapy or a supportive care approach for patients with peritoneal metastasis, HIPEC has emerged as a promising regional treatment modality in both the prophylactic setting at the time of gastrectomy in high-risk patients, or as adjuvant therapy in patients with synchronous peritoneal disease or peritoneal recurrence. HIPEC provides multiple theoretical benefits over systemic chemotherapy for peritoneal metastasis. HIPEC delivers greater concentrations of chemotherapeutic agents to the tumor cells on the peritoneal surface while minimizing the concentration in systemic circulation, thus minimizing potential side effects.30,31 Further, the addition of hyperthermia has direct cytotoxic effects while also augmenting the effects of some chemotherapeutic agents.30
Level I evidence for HIPEC for peritoneal metastasis from gastric cancer is limited to a single randomized controlled trial in China which assigned patients to undergo CRS with or without HIPEC and found a significant OS benefit in the CRS-HIPEC group.26 However, data from studies in Asian populations are difficult to apply directly to Western gastric cancer patient populations because of known differences in epidemiology, tumor biology, genetic predisposition, screening and treatment practice patterns, which influence the difference in outcomes between Eastern and Western populations.32 There have been no randomized trials for HIPEC in gastric and gastroesophageal adenocarcinoma patients in Western countries. A recently published French study performed propensity-score matching in 277 patients who underwent CRS with or without HIPEC for gastric cancer with peritoneal metastasis and found a survival benefit for CRS-HIPEC, despite a higher PCI in the CRS-HIPEC group.33 This study was limited to patients in whom a completeness of cytoreduction score of 0 or 1 was achieved.
In patients with synchronous peritoneal metastasis, past studies have found that performing CRS and HIPEC concurrent with gastrectomy has high rates of morbidity and mortality.16,20,34 Thus, a role for LS-HIPEC with or without CRS has been investigated. LS-HIPEC has been shown to have an acceptable safety profile and is well tolerated.21 Yonemura et al demonstrated the potential for neoadjuvant LS-HIPEC to convert positive peritoneal cytology to negative, as well as an effective reduction of PCI prior to consideration for CRS.18 Additionally, LS-HIPEC may have benefits over the open perfusion procedure, with increased chemotherapy absorption at the peritoneal surface.35
A recently published phase II clinical trial from our institution showed encouraging results with seven of 19 patients being eligible for, and five undergoing gastrectomy after neoadjuvant LS-HIPEC for low-volume peritoneal metastasis or PPC. The median OS in this group of patients was 30 months.22 The current study attempted to further elucidate the benefit of neoadjuvant LS-HIPEC by comparing patients who underwent neoadjuvant LS-HIPEC after induction chemotherapy with a similar group who underwent SC. While we were unable to demonstrate a statistically significant difference in OS between the LS-HIPEC and SC groups, the median OS was significantly improved in those patients who underwent resection of their primary tumor following LS-HIPEC. This suggests that if the peritoneum is found to be negative for metastasis after neoadjuvant systemic and HIPEC therapy and resection is performed, survival may be improved, though resection did not demonstrate statistical significance in our multivariable analysis. Limiting our study to patients with low-volume peritoneal disease may have been a contributing factor to a type II error, as even the median OS in our SC group was longer than previously published survival rates for patients with peritoneal disease.1,2,36
It is interesting to note the rate of conversion from positive cytology to negative with neoadjuvant chemotherapy (NAC) alone. In our study, 12 patients (48%) had negative peritoneal exploration and cytology at the time of their first LS-HIPEC procedure, after NAC. This finding is similar to previously reported rates of peritoneal clearance. Lorenzen et al reported that peritoneal cytology reverted from positive to negative in 37% of patients following NAC.36 Mezhir et al reported a 56% rate of conversion to negative peritoneal cytology in patients who underwent repeat laparoscopy after systemic chemotherapy, with better OS compared with those with persistently positive cytology.2
While peritoneal clearance after NAC alone did not affect OS in our study, the small sample size, as well as the fact that patients in the SC group did not have repeat laparoscopy after systemic chemotherapy, limited this evaluation. Also, while some patients may convert from positive to negative cytology during NAC, Lorenzen et al also reported a rate of conversion from negative to positive cytology of 24% during NAC.36 Knowledge of which tumors or patients are more likely to revert to a negative peritoneum with NAC is lacking and will be an important area of research in the future. Further, the limited sensitivity of peritoneal cytology, reported to be 64%, currently limits clinical evaluation of the peritoneum for true clearance of disease at the time of LS-HIPEC or repeat peritoneal examination.3 Improvement in peritoneal cytology techniques may aid in patient selection for definitive surgical resection, or for peritoneal directed regional therapies, such as HIPEC, in the future.
The potential synergistic benefit with systemic chemotherapy and HIPEC for the treatment of peritoneal disease is another important area of consideration for future research. As we have become more comfortable performing gastrectomy/CRS/HIPEC concurrently through the course of an ongoing phase II trial at our institution,37 the use of HIPEC has continued to expand within our multi-disciplinary treatment paradigm.21 We look forward to reporting the outcomes of this trial soon. Additionally, the PERISCOPE II trial is a multi-center randomized controlled trial currently underway in the Netherlands, which will evaluate NAC followed by gastrectomy/CRS/HIPEC compared to palliative systemic chemotherapy alone in patients with low-volume peritoneal disease or PPC.38 This study will provide valuable information regarding the benefit of an aggressive treatment approach compared with palliative systemic chemotherapy and will further inform our practice in treating gastric cancer patients with peritoneal disease.
Our study has limitations. By nature of a retrospective study, there is an inherent selection bias, which we attempted to mitigate by choosing patients who would have met eligibility criteria for the LS-HIPEC phase II clinical trial. This resulted in narrow inclusion criteria, leading to a smaller sample size than desired and limited power for identifying a potential survival difference between the two groups. This further supports the need for larger, multi-institutional efforts to evaluate the role for LS-HIPEC in the treatment of this patient population through randomized controlled trials. We were able to use the results from our study to inform future prospective, randomized trials regarding the sample size necessary to detect a survival difference between the two groups. Based on our results, the hazard ratio (HR) for OS for LS-HIPEC vs SC is estimated to be between 0.3-0.6, over a range of follow-up times of 12 to 48 months. If we conservatively assume the smallest difference between the groups, with a HR of 0.6 and a power of 0.8, we find that future trials should aim for a total sample size of 128 patients, with 64 patients in each treatment group.
This retrospective analysis of LS-HIPEC for the treatment of low-volume peritoneal disease in gastric and gastroesophageal cancer patients failed to demonstrate a significant survival benefit for patients treated with LS-HIPEC compared to the standard of systemic chemotherapy or best supportive care. Larger, more strongly powered, randomized controlled trials are necessary to assess the role of LS-HIPEC in the treatment of this patient population. Patient selection for treatment with this technique will continue to be of the utmost importance.
SYNOPSIS: This retrospective case-control study evaluates the role and potential benefits of laparoscopic-HIPEC in the treatment of patients with gastric and gastroesophageal adenocarcinoma and low-volume peritoneal metastasis.
Acknowledgments
This work was supported by National Institutes of Health T32 CA 009599 and the MD Anderson Cancer Center support grant (P30 CA016672). This study was also supported by the Holly Clegg Gastric Cancer Research Fund, the No Stomach for Cancer Award for Gastric Cancer Research.
Editorial support was provided by Bryan Tutt in Scientific Publications Services, Research Medical Library.
Footnotes
DISCLOSURES: None of the authors have any financial or commercial interests to disclose.
Portions of these data were presented at the Society of Surgical Oncology’s Advanced Cancer Therapies, February 15-17, 2020, in Orlando, Florida.
References
- 1.Badgwell B, Cormier JN, Krishnan S, et al. Does neoadjuvant treatment for gastric cancer patients with positive peritoneal cytology at staging laparoscopy improve survival? Ann Surg Oncol. 2008;15(10):2684–2691. [DOI] [PubMed] [Google Scholar]
- 2.Mezhir JJ, Shah MA, Jacks LM, Brennan MF, Coit DG, Strong VE. Positive peritoneal cytology in patients with gastric cancer: natural history and outcome of 291 patients. Ann Surg Oncol. 2010;17(12):3173–3180. [DOI] [PubMed] [Google Scholar]
- 3.Allen CJ, Newhook TE, Vreeland TJ, et al. Yield of peritoneal cytology in staging patients with gastric and gastroesophageal cancer. J Surg Oncol. 2019;120(8):1350–1357. [DOI] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network Guidelines Version 1.2017, Gastric Cancer. www.nccn.org. Accessed
- 5.Dehal A, Smith JJ, Nash GM. Cytoreductive surgery and intraperitoneal chemotherapy: an evidence-based review-past, present and future. J Gastrointest Oncol. 2016;7(1):143–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang G, Zhu Y, Liu C, Chao G, Cui R, Zhang Z. The prognosis impact of hyperthermic intraperitoneal chemotherapy (HIPEC) plus cytoreductive surgery (CRS) in advanced ovarian cancer: the meta-analysis. Journal of ovarian research. 2019;12(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Q, Wu Q, Xu J, et al. Efficacy of hyperthermic intraperitoneal chemotherapy in patients with epithelial ovarian cancer: a meta-analysis. Int J Hyperthermia. 2019;36(1):562–572. [DOI] [PubMed] [Google Scholar]
- 8.Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21(20):3737–3743. [DOI] [PubMed] [Google Scholar]
- 9.Verwaal VJ, Bruin S, Boot H, van Slooten G, van Tinteren H. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2008;15(9):2426–2432. [DOI] [PubMed] [Google Scholar]
- 10.Elias D, Lefevre JH, Chevalier J, et al. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J Clin Oncol. 2009;27(5):681–685. [DOI] [PubMed] [Google Scholar]
- 11.Hornung M, Werner JM, Schlitt HJ. Applications of hyperthermic intraperitoneal chemotherapy for metastatic colorectal cancer. Expert Rev Anticancer Ther. 2017;17(9):841–850. [DOI] [PubMed] [Google Scholar]
- 12.Behrenbruch C, Hollande F, Thomson B, et al. Treatment of peritoneal carcinomatosis with hyperthermic intraperitoneal chemotherapy in colorectal cancer. ANZ journal of surgery. 2017;87(9):665–670. [DOI] [PubMed] [Google Scholar]
- 13.Xu DZ, Zhan YQ, Sun XW, Cao SM, Geng QR. Meta-analysis of intraperitoneal chemotherapy for gastric cancer. World J Gastroenterol. 2004;10(18):2727–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan TD, Black D, Sugarbaker PH, et al. A systematic review and meta-analysis of the randomized controlled trials on adjuvant intraperitoneal chemotherapy for resectable gastric cancer. Ann Surg Oncol. 2007;14(10):2702–2713. [DOI] [PubMed] [Google Scholar]
- 15.Mi DH, Li Z, Yang KH, et al. Surgery combined with intraoperative hyperthermic intraperitoneal chemotherapy (IHIC) for gastric cancer: a systematic review and meta-analysis of randomised controlled trials. Int J Hyperthermia. 2013;29(2):156–167. [DOI] [PubMed] [Google Scholar]
- 16.Coccolini F, Cotte E, Glehen O, et al. Intraperitoneal chemotherapy in advanced gastric cancer. Meta-analysis of randomized trials. Eur J Surg Oncol. 2014;40(1):12–26. [DOI] [PubMed] [Google Scholar]
- 17.He Z, Zhao TT, Xu HM, et al. Efficacy and safety of intraperitoneal chemotherapy in patients with advanced gastric cancer: a cumulative meta-analysis of randomized controlled trials. Oncotarget. 2017;8(46):81125–81136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yonemura Y, Ishibashi H, Hirano M, et al. Effects of Neoadjuvant Laparoscopic Hyperthermic Intraperitoneal Chemotherapy and Neoadjuvant Intraperitoneal/Systemic Chemotherapy on Peritoneal Metastases from Gastric Cancer. Ann Surg Oncol. 2017;24(2):478–485. [DOI] [PubMed] [Google Scholar]
- 19.Scaringi S, Kianmanesh R, Sabate JM, et al. Advanced gastric cancer with or without peritoneal carcinomatosis treated with hyperthermic intraperitoneal chemotherapy: a single western center experience. Eur J Surg Oncol. 2008;34(11):1246–1252. [DOI] [PubMed] [Google Scholar]
- 20.Gill RS, Al-Adra DP, Nagendran J, et al. Treatment of gastric cancer with peritoneal carcinomatosis by cytoreductive surgery and HIPEC: a systematic review of survival, mortality, and morbidity. J Surg Oncol. 2011;104(6):692–698. [DOI] [PubMed] [Google Scholar]
- 21.Newhook TE, Agnes A, Blum M, et al. Laparoscopic Hyperthermic Intraperitoneal Chemotherapy is Safe for Patients with Peritoneal Metastases from Gastric Cancer and May Lead to Gastrectomy. Ann Surg Oncol. 2019;26(5):1394–1400. [DOI] [PubMed] [Google Scholar]
- 22.Badgwell B, Blum M, Das P, et al. Phase II Trial of Laparoscopic Hyperthermic Intraperitoneal Chemoperfusion for Peritoneal Carcinomatosis or Positive Peritoneal Cytology in Patients with Gastric Adenocarcinoma. Ann Surg Oncol. 2017;24(11):3338–3344. [DOI] [PubMed] [Google Scholar]
- 23.Takeshita K, Liu Y, Ishibashi H, Yonemura Y. Laparoscopic Hyperthermic Intraperitoneal Chemotherapy for Peritoneal Carcinomatosisfrom Gastric Cancer: Its Beneficial Effects on Reduction and Exact Evaluation of the Peritoneal Cancer Index. Am Surg. 2017;83(11):1315–1320. [PubMed] [Google Scholar]
- 24.Ikoma N, Blum M, Chiang YJ, et al. Yield of Staging Laparoscopy and Lavage Cytology for Radiologically Occult Peritoneal Carcinomatosis of Gastric Cancer. Ann Surg Oncol. 2016;23(13):4332–4337. [DOI] [PubMed] [Google Scholar]
- 25.Badgwell B, Blum M, Das P, et al. Lessons learned from a phase II clinical trial of laparoscopic HIPEC for gastric cancer. Surg Endosc. 2018;32(1):512. [DOI] [PubMed] [Google Scholar]
- 26.Yang XJ, Huang CQ, Suo T, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: Final results of a phase III randomized clinical trial. Ann Surg Oncol. 2011;18(6):1575–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blum Murphy M, Ikoma N, Wang X, et al. Phase I Trial of Hyperthermic Intraperitoneal Chemoperfusion (HIPEC) with Cisplatin, Mitomycin, and Paclitaxel in Patients with Gastric Adenocarcinoma and Associated Carcinomatosis or Positive Cytology. Ann Surg Oncol. 2020. [DOI] [PubMed] [Google Scholar]
- 28.Kapoor R, Robinson KA, Cata JP, et al. Assessment of nephrotoxicity associated with combined cisplatin and mitomycin C usage in laparoscopic hyperthermic intraperitoneal chemotherapy. Int J Hyperthermia. 2019;36(1):493–498. [DOI] [PubMed] [Google Scholar]
- 29.Sugarbaker PH. Peritoneal surface oncology: review of a personal experience with colorectal and appendiceal malignancy. Tech Coloproctol. 2005;9(2):95–103. [DOI] [PubMed] [Google Scholar]
- 30.Yonemura Y, Canbay E, Endou Y, et al. Peritoneal cancer treatment. Expert Opin Pharmacother. 2014;15(5):623–636. [DOI] [PubMed] [Google Scholar]
- 31.Yonemura Y, Canbay E, Li Y, et al. A comprehensive treatment for peritoneal metastases from gastric cancer with curative intent. Eur J Surg Oncol. 2016;42(8):1123–1131. [DOI] [PubMed] [Google Scholar]
- 32.Badgwell B Multimodality Therapy of Localized Gastric Adenocarcinoma. JNCCN. 2016;14(10):1321–1326. [DOI] [PubMed] [Google Scholar]
- 33.Bonnot P-E, Piessen G, Kepenekian V, et al. Cytoreductive Surgery With or Without Hyperthermic Intraperitoneal Chemotherapy for Gastric Cancer With Peritoneal Metastases (CYTO-CHIP study): A Propensity Score Analysis. J Clin Oncol. 2019:JCO.18.01688. [DOI] [PubMed] [Google Scholar]
- 34.Glehen O, Gilly FN, Arvieux C, et al. Peritoneal carcinomatosis from gastric cancer: a multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann Surg Oncol. 2010;17(9):2370–2377. [DOI] [PubMed] [Google Scholar]
- 35.Gesson-Paute A, Ferron G, Thomas F, de Lara EC, Chatelut E, Querleu D. Pharmacokinetics of oxaliplatin during open versus laparoscopically assisted heated intraoperative intraperitoneal chemotherapy (HIPEC): an experimental study. Ann Surg Oncol. 2008;15(1):339–344. [DOI] [PubMed] [Google Scholar]
- 36.Lorenzen S, Panzram B, Rosenberg R, et al. Prognostic significance of free peritoneal tumor cells in the peritoneal cavity before and after neoadjuvant chemotherapy in patients with gastric carcinoma undergoing potentially curative resection. Ann Surg Oncol. 2010;17(10):2733–2739. [DOI] [PubMed] [Google Scholar]
- 37.A Phase II Study of Cytoreduction, Gastrectomy, and Hyperthermic Intraperitoneal Chemoperfusion (HIPEC) in Patients With Gastric Adenocarcinoma and Carcinomatosis or Positive Cytology. https://www.clinicaltrials.gov/ct2/show/NCT02891447. Accessed 5/5/2020.
- 38.Koemans WJ, van der Kaaij RT, Boot H, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy versus palliative systemic chemotherapy in stomach cancer patients with peritoneal dissemination, the study protocol of a multicentre randomised controlled trial (PERISCOPE II). BMC Cancer. 2019;19(1):420. [DOI] [PMC free article] [PubMed] [Google Scholar]