Abstract
Objectives:
In humans, depending on dose, blocking the N-methyl-d-aspartate receptor (NMDAR) with ketamine can cause psychomimetic or antidepressant effects. The overall outcome for drugs such as ketamine depends on dose and the number of its available binding sites in the central nervous system, and to understand something of the latter variable we measure NMDAR in the frontal pole, dorsolateral prefrontal, anterior cingulate and parietal cortices from people with schizophrenia, bipolar disorder, major depressive disorders and age/sex matched controls.
Method:
We measured levels of NMDARs (using [3H]MK-801 binding) and NMDAR sub-unit mRNAs (GRINs: using in situ hybridisation) as well as post-synaptic density protein 95 (anterior cingulate cortex only; not major depressive disorders: an NMDAR post-synaptic associated protein) in bipolar disorder, schizophrenia and controls.
Results:
Compared to controls, levels of NMDAR were lower in the outer laminae of the dorsolateral prefrontal cortex (–17%, p = 0.01) in people with schizophrenia. In bipolar disorder, levels of NMDAR binding (laminae IV–VI; –19%, p < 0.01) and GRIN2C mRNA (laminae I–VI; –27%, p < 0.05) were lower in the anterior cingulate cortex and NMDAR binding was lower in the outer lamina IV of the dorsolateral prefrontal cortex (–19%, p < 0.01). In major depressive disorders, levels of GRIN2D mRNA were higher in frontal pole (+22%, p < 0.05). In suicide completers, levels of GRIN2B mRNA were higher in parietal cortex (+20%, p < 0.01) but lower (–35%, p = 0.02) in dorsolateral prefrontal cortex while post-synaptic density protein 95 was higher (+26%, p < 0.05) in anterior cingulate cortex.
Conclusion:
These data suggest that differences in cortical NMDAR expression and post-synaptic density protein 95 are present in psychiatric disorders and suicide completion and may contribute to different responses to ketamine.
Keywords: Schizophrenia, major depressive disorders, bipolar disorders, cortex, NMDA
Introduction
In humans, ketamine has been shown to have rapid acting antidepressant effects and to be psychomimetic (Abdallah et al., 2015; Malhotra et al., 1997). The major action of ketamine is to block the N-methyl-d-aspartate receptor (NMDAR), an ionotropic glutamate receptor (Sucher et al., 1996).
The NMDAR is a ligand-gated ion channel made up of heteromeric complexes formed from different combinations of three families of sub-units; the families of sub-units have been given the nomenclatures of GRIN1, GRIN2 and GRIN3 (Paoletti and Neyton, 2007). It is now generally accepted that a functional NMDAR is a tetramer of subunits that must include at least one GRIN1 and one GRIN2 with differing combinations of sub-units conferring different functionality (Paoletti and Neyton, 2007). This growing understanding of the structure of the NMDAR has allowed increasingly sophisticated studies using postmortem central nervous system (CNS) tissue designed to understand how the NMDAR may be affected by the aetiology of psychiatric disorders. The neuropsychopharmacological outcome from administering any drug is dependent on dose and the number of available target sites for the drug in CNS (Ruffolo, 1982). Thus, while the clinical useful antidepressant outcome of ketamine is known to be dose-dependent (Abdallah et al., 2015), less is known as to whether any of its effects could be due to diagnostic-dependent variation in levels of NMDAR.
Studies in schizophrenia have reported higher (Deakin et al., 1989; Grimwood et al., 1999; Ishimaru et al., 1994; Newell et al., 2005; Nudmamud and Reynolds, 2001; Zavitsanou et al., 2002), unchanged (Beneyto and Meador-Woodruff, 2008; Dean et al., 1999a; Kornhuber et al., 1989; Scarr et al., 2005) or lower (Nudmamud et al., 2003) levels of NMDAR in different cortical regions. There are equally varied outcomes from studies measuring levels of NMDAR sub-unit mRNA in the cortex of subjects with the disorder (Supplementary Table 1). Changes in the level of NMDAR sub-unit expression are only one mechanism by which the levels or activity of the NMDAR can occur (Gladding and Raymond, 2011). It is therefore significant that more recent studies have suggested that changes in the post-translational processing of NMDAR sub-units may be occurring in the CNS of people with schizophrenia. For example, in the cortex of people with schizophrenia it has been reported that there is a decreased GRIN2B and post-synaptic density protein 95 (PSD 95) in the endoplasmic reticulum (Kristiansen et al., 2010), changes in N-linked glycosylation of NMDAR sub-units (Tucholski et al., 2013) and changes in the types of cells expressing NMDAR sub-units (Bitanihirwe et al., 2010; Woo et al., 2008). These post-translational modifications and/or differential cellular expression of NMDAR would clearly alter glutamate homeostasis in the CNS of people with the disorder.
There have been fewer studies of NMDAR in mood disorders. However, one study has reported lower levels of radioligand binding to the glycine binding site on the NMDAR in people with bipolar disorders (BPD) (Nudmamud-Thanoi and Reynolds, 2004). Outcomes from studies on NMDAR sub-units in BPD are varied, but the majority report that levels of NMDAR sub-unit mRNA are not changed in the disorder (Supplementary Table 1). There is also a report that levels of NMDAR are not changed in major depressive disorders (MDD) (Holemans et al., 1993), and most studies report unchanged levels of NMDAR subunit mRNA (Supplementary Table 1).
While there have been a number of studies on NMDAR in postmortem CNS, none seem to have measured levels of NMDAR and mRNA for its more abundant sub-units in tissue from subjects with schizophrenia, BPD and MDD to begin to understand the changes in these markers in different cortical regions in people with different psychiatric disorders. We posited that such data could also help understand whether any of the effects of ketamine, or the dose of ketamine required to cause advantageous effects, could be influenced by NMDAR levels. Hence, we decided to measure levels of NMDAR, its sub-units and a protein associated with NMDAR function (PSD 95) in areas of the cortex, obtained postmortem, from people who had had schizophrenia, BPD or MDD (see below).
Materials and methods
Materials
Tissue collection
For these studies, tissue was collected from frontal pole (Brodmann’s Area [BA] 10), the dorsolateral prefrontal cortex (DLPFC: BA 46), the anterior cingulate cortex (ACx: BA 24) and the parietal cortex (BA 40) from 10 people with BPD, 10 people with MDD, 20 people with schizophrenia and 20 subjects with no history of psychiatric illness (controls) matched for age and sex to the psychiatric cases (regional delineation is defined in Supplementary Methods). Suicide completion was recorded when suicide was formally recognised in the Coroner’s report on cause of death. Tissue was obtained from the Victorian Brain Bank Network following approval from the Ethics Committee of the Victorian Institute of Forensic Medicine and the Mental Health Research and Ethics Committee of Melbourne Health.
Diagnoses were made according to Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) criteria, following case history reviews using the Diagnostic Instrument for Brain Studies (DIBS) (Hill et al., 1996; Roberts et al., 1998), by consensus between a psychologist and a senior psychiatrist. While completing the DIBS, duration of illness (DI) was calculated as time from first clinical presentation to a psychiatric service to death. In addition, the final recorded dose of antipsychotic drug (FRADD) was recorded and converted to chlorpromazine equivalents using a well-established methodology (Foster, 1989), as was total lifetime exposure to antipsychotic drugs (LEAP). Notably, all cadavers were refrigerated within 5 hours of being found. When death was witnessed, postmortem interval (PMI) was calculated as the time from death to autopsy. Where death was not witnessed, tissue was only collected from subjects who had been seen alive up to 5 hours prior to being found dead; here, the PMI was taken as the midpoint between the person being found and being last seen alive. As well as maintaining cases at low temperatures for most of their PMI, tissue was rapidly processed and frozen to –70°C within 30 minutes of autopsy (Dean et al., 1999b); processing tissue in this way significantly slows autolytic changes (Ferrer et al., 2007). CNS pH was measured as described previously (Kingsbury et al., 1995) as this provides a good measure of overall tissue quality (Stan et al., 2006).
[3H]MK-801 in situ radioligand binding with autoradiography
The conditions used for these studies were the same as our previous study (Scarr et al., 2003), meaning that [3H] MK-801 would bind with high specificity to the ion channel of the NMDAR (Reynolds, 2001) (for full details, see Supplementary Methods).
In situ hybridisation
Riboprobes for the NMDAR sub-units GRIN1, GRIN2A–2D and GRIN3A were measured as described previously (Beneyto et al., 2007) (for full details, see Supplementary Methods).
Western blotting
As levels of PSD 95 can be associated with NMDAR density, we followed our data showing region-specific changes in [3H]MK-801 binding and measured PSD 95 only in the ACx from people with schizophrenia, BPD and controls. Levels of PSD 95 were essentially measured using methodologies we have developed (Dean et al., 2006, 2010; Scarr et al., 2006) to address the difficulties in finding reference proteins to normalise data (Eaton et al., 2013) in human CNS (for full details see Supplementary Methods).
Statistics
Given well-recognised difficulties in assessing the distribution of data when sample sizes are small (D’Agostino et al., 1990), and that biological variables tend to be normally distributed (McKillup, 2006), statistical analyses were completed using parametric analyses. Thus, variations in experimental, donor demographic, drug and CNS collection data were assessed using Graphpad Prism and either one-way analysis of variance (ANOVA) followed by a post hoc Dunnett’s multiple comparison test comparing data for each diagnoses to control or student t-tests (if two cohorts). The sex ratios and rates of suicide in the different cohorts were compared using a Fisher’s exact test. Relationships between experimental variables and demographic, tissue collection and pharmacological data were assessed using linear regression. Small sample sizes mean that strong relationships, as assessed using linear regression analyses, need to have an r2 > 0.49 (Cook and Weisberg, 1999). If strong relationships were revealed, and there was no variation in the non-experimental data with diagnoses (Miller and Chapman, 2001), the variation in the experimental data was re-assessed using multivariate analyses of covariance with the appropriate non-experimental data as a covariant(s).
Results
Demographic data
Mean age (F = 0.68; df = 3,55; p = 0.57), PMI (F = 0.97; df = 3,55; p = 0.41), CNS pH (F = 0.48; df = 3,55; p = 0.69) and DI (F = 0.27; df = 3,55; p = 0.77) did not vary between diagnostic cohorts (Table 1: full details in Supplementary Table 2).
Table 1.
Summary of demographic and tissue collection data for cases in this study.
| Gender (M/F) | Sui | Age (years) |
DI (years) |
PMI (hours) |
pH |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | |||
| Controls | 11/9 | 0 | 50 | 4.1 | 42 | 3.9 | 6.29 | 0.05 | ||
| Schizophrenia | 11/9 | 5 | 51 | 4.1 | 21 | 2.6 | 40 | 2.6 | 6.30 | 0.05 |
| Bipolar disorders | 5/4 | 1 | 60 | 3.3 | 20 | 4.6 | 38 | 4.7 | 6.33 | 0.06 |
| Major depressive disorders | 5/5 | 8 | 52 | 5.2 | 17 | 3.3 | 49 | 5.1 | 6.4 | 0.01 |
| Suicide completers | 8/6 | 40 | 4.2 | 10 | 2.6 | 48 | 3.8 | 6.39 | 0.08 | |
| Non-suicide including controls | 25/20 | 56 | 2.3 | 40 | 2.2 | 6.30 | 0.03 | |||
| Non-suicide without controls | 14/11 | 60 | 2.4 | 26 | 2.8 | 38 | 2.5 | 6.29 | 0.05 | |
Sui: suicide completion; PMI: postmortem interval; DI: duration of illness; SEM: standard error of the mean.
There was no difference in the ratio of males to females between the diagnostic groups (p = 0.99: Table 1). There were significant differences in rates of suicide between diagnostic cohorts (p < 0.0001) due to a significantly higher incidence of suicide in subjects with schizophrenia and MDD compared to controls. As suicide was an unresolvable confound, all experimental data were also analysed with suicide completion, independent of diagnoses, as the primary variable.
General findings
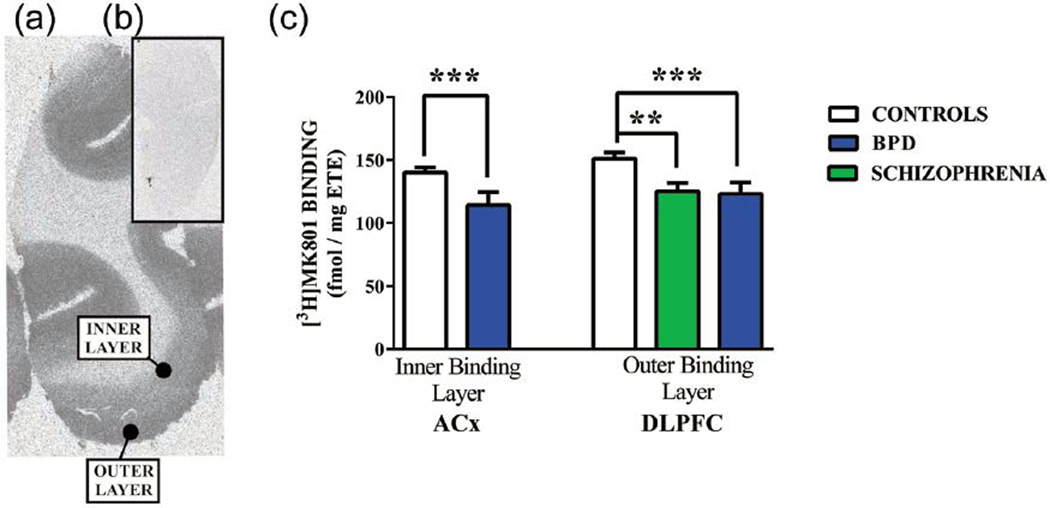
In the human cortex, two layers of [3H]MK-801 binding were detectable (Figure 1(a) and (b)), and thus a section from each case was stained with Nissl stain and viewed under a microscope to determine which laminae of the cortex were encompassed by the outer and inner layers of [3H] MK-801 binding. This showed that the outer layer of [3H] MK-801 binding overlaid cortical laminae I to III plus a portion of lamina IV. The inner layer of [3H]MK-801 binding overlaid the remainder of lamina IV plus laminae V and VI.
Figure 1.
A typical autoradiograph showing the binding of [3H]MK-801 to the NMDAR in human cortex in the absence (a) (total binding [TB]) or presence (b) (non-specific binding [NSB]) of 100-μM MK-801 and (c) the levels of specific binding of [3H]MK-801 (mean ± standard error of the mean [SEM]) in the inner radioligand binding layer of the anterior cingulate (ACx) and the outer radioligand binding layer of the dorsolateral prefrontal cortex (DLPFC) from the controls and people with schizophrenia (Sz) and bipolar disorder (BPD). Specific binding was calculated as TB – NSB.
**p = 0.01; ***p < 0.01.
As described previously, levels of GRIN1 mRNA were highest in all cortical regions studied (Scherzer et al., 1998). Consistent with this previous study, levels of the other NMDAR sub-unit mRNA varied between cortical regions where they were present at low to medium levels.
Schizophrenia
There were lower levels of [3H]MK-801 binding (–17%, p = 0.01) in the outer, but not inner, layer of the DLPFC from people with schizophrenia (Figure 1(c); Supplementary Table 3). There were no other significant variations in levels of [3H]MK-801 binding in people with the disorder (Supplementary Table 3). There were no changes in the levels of hybridisation of radioactive probes bound to NMDAR sub-unit mRNA in cortex from people with schizophrenia (Supplementary Table 4). Levels of PSD 95 were not significantly different in the ACx from people with the disorder (Mean ± SEM: Control = 0.62 ± 0.05 vs schizophrenia = 0.70 ± 0.06 ratio internal control [IC]; p = 0.31).
Bipolar disorders
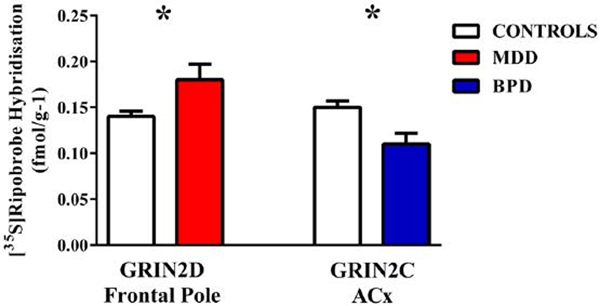
There were lower levels of [3H]MK-801 binding in the outer, but not inner, layers of the DLPFC (–19%, p < 0.01) and the inner layer of the ACx (–19%, p < 0.01) from people with BPD (Figure 1(c); Supplementary Table 3). There was no other significant variation in [3H]MK-801 binding in BPD (Supplementary Table 3). The level of hybridisation of radioactive probes to GRIN2C mRNA was lower (–27%, p < 0.05) in ACx from people with BPD (Figure 2; Supplementary Table 4); there were no other significant changes in the levels of hybridisation to NMDAR sub-unit mRNA. Levels of PSD 95 were not significantly different in the ACx from people with the disorder (Control = 0.62 ± 0.05 vs BPD = 0.57 ± 0.08 ratio IC; p = 0.61).
Figure 2.
Levels of [35S]riboprobe hybridisation (mean ± standard error of the mean [SEM]) to the GRIN2D sub-unit in the frontal pole from people with major depressive disorders (MDD) and GRIN2D sub-unit in the ACx from people with bipolar disorder BPD) compared to that in the same regions from age/sex matched controls.
*p < 0.05.
Major depressive disorders
There was no significant variation in levels of [3H]MK-801 binding in any cortical region from people with MDD (Supplementary Table 3). By contrast, levels of radioactive probe hybridisation to GRIN2D mRNA was higher (+22%, p < 0.05) in frontal pole from people with MDD (Figure 2; Supplementary Table 4); there were no changes in the levels of hybridisation in MDD.
Suicide
Dividing subjects into suicide completers and those who died by other causes meant the suicide completers were significantly younger than those who died by other causes, whether (p = 0.002) or not (p < 0.001) the control subjects were included (Table 1). PMI between suicide completers and death by other causes was significantly different when controls were excluded (p = 0.02 vs p = 0.06). Brain pH did not differ with cause of death whether or not controls were included. DI was significantly shorter in subjects with psychiatric disorders who were suicide completers (p = 0.0008). The ratio between male and female did not vary whether (p = 1.00) or not (p = 0.01) the control subjects were included. Given no experimental variable correlated with donor age, PMI or DI, none of these differences were regarded as confounds in the analyses of data from suicide completers and those who died by other causes.
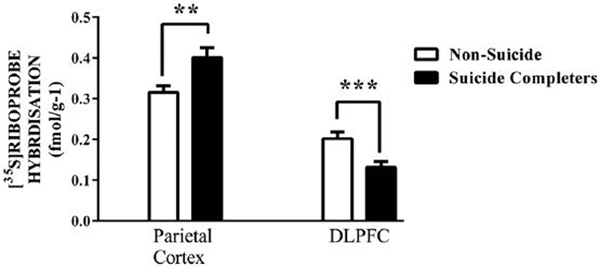
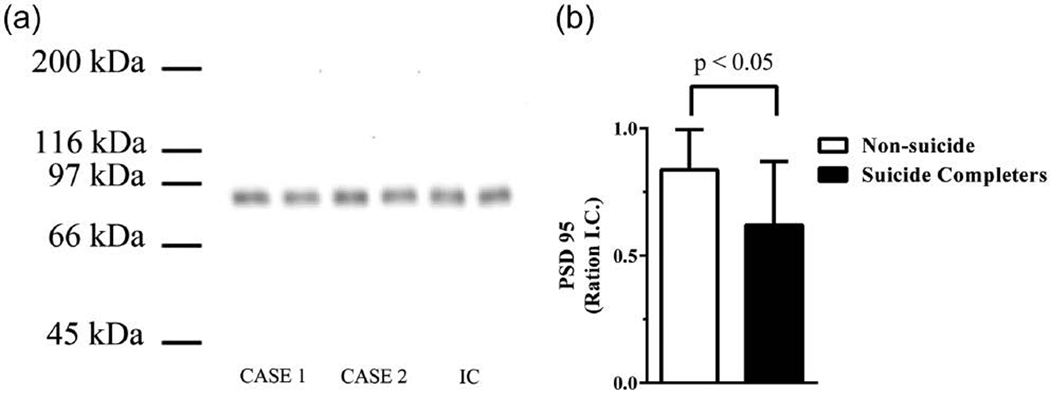
Levels of [3H]MK-801 binding did not differ between suicide completers and people who died by other causes (Supplementary Table 5). By contrast, levels of radioactive probe hybridised to the GRIN2B sub-unit of the NMDAR were higher (20%, p < 0.01) in parietal cortex and lower (–35%, p = 0.02) in DLPFC from suicide completers (Figure 3; Supplementary Table 6); there were no other significant changes in levels of oligonucleotide riboprobes with suicide. The differences in levels of GRIN2B mRNA remained significant when analysed after removal of data from the controls, none of whom had died by suicide (parietal cortex: 0.13 ± 0.01 vs 0.18 ± 0.02, p < 0.01; DLPFC: 0.40 ± 0.02 vs 0.31 ± 0.02, p = 0.05). In the ACx, levels of PSD 95 were higher (26%, p < 0.05) in suicide completers compared to those who had died by other causes (Figure 4). The differences in levels of PSD 95 remained significant after removal of data from the controls, none of whom had died by suicide (ACx: 0.84 ± 0.06 vs 0.60 ± 0.06, p < 0.05). While it would have been interesting to analyse the suicide effect within each diagnosis, sample sizes were not large enough to give such an analysis enough power to be meaningful.
Figure 3.
Levels of [35S]riboprobe hybridisation (mean ± standard error of the mean [SEM]) to the GRIN2B sub-unit in the frontal pole and dorsolateral prefrontal cortex (DLPFC) from suicide completers and people who had died from other causes.
**p < 0.01; ***p = 0.02.
Figure 4.
(a) A typical Western blot showing post-synaptic density protein 95 (PSD 95) immunogenic bands in two cases and the internal control (IC). The IC is run on every gel to control for gel to gel variation and (b) levels of PSD 95 (mean ± standard error of the mean [SEM]) in the ACx from suicide completers and people who had died from other causes.
Potential confounds
Levels of [3H]MK-801 binding, hybridisation of radioactive probes to NMDAR sub-unit mRNA or PSD 95 (male = 0.70 ± 0.05 vs female = 0.58 ± 0.05 ratio IC; p = 0.11) did not differ with gender in any of the cortical regions examined (Supplementary Tables 7 and 8). There were no strong correlations between [3H]MK-801 binding (Supplementary Table 9), hybridisation of radioactive probes to NMDAR subunit mRNA (Supplementary Table 10) or PSD 95 (age [r2 = 0.39, p < 0.001], PMI [r2 < 0.001, p = 0.93], DI [r2 = 0.09, p = 0.93], CNS pH [r2 = 0.003, p = 0.69], FRADD [r2 = 0.03, p = 0.69] or LEAP [r2 = 0.007, p = 0.13]) with any potential confounds. Due to smaller cohort sizes, comparing experimental variables to final recorded or lifetime exposure to antidepressant or mood stabilising drugs standardised to a common drug in the class was not completed as such an analysis would be under-powered.
There were a number of correlations between experimental variables where a regression line defining the relationships deviated significantly from the horizontal (i.e. p < 0.05: Supplementary Table 11). However, the only correlations that would be regarded as strong, given our small cohort sizes, were between the layers of [3H]MK-801 binding in the frontal pole, ACx and DLPFC and levels of GRIN2C and GRIN2D in the DLPFC.
Discussion
A major finding of this study is that there were significantly lower levels of [3H]MK-801 binding to the outer lamina of the DLPFC from people with schizophrenia, and as [3H] MK-801 would have been almost exclusively binding to the NMDAR (Wong et al., 1986), our data suggest there are lower levels of NMDAR in the outer cortical lamina in DLPFC from people with the disorder. As our findings are restricted to specific cortical laminae within certain cortical regions, any comparisons to other studies must be tentative but worthwhile to put our findings into some context. Thus, these data differ from our previous studies that showed no changes in NMDAR in the DLPFC from people with schizophrenia (Dean et al., 1999a; Scarr et al., 2005). This could be because our current study is in BA 46 whereas our previous studies were in BA 9 (Dean et al., 1999a; Scarr et al., 2005) and that loss of NMDAR only occurs in a portion of the DLPFC. Thus, it is also possible that previous reports of higher (Deakin et al., 1989; Grimwood et al., 1999; Ishimaru et al., 1994; Newell et al., 2005; Nudmamud and Reynolds, 2001; Zavitsanou et al., 2002), unchanged (Beneyto and Meador-Woodruff, 2008; Dean et al., 1999a; Kornhuber et al., 1989; Scarr et al., 2005) or lower (Nudmamud et al., 2003) levels of NMDAR in different cortical regions could be reflecting these regional selective changes. Our current data also suggest that the lower levels of NMDAR in the outer lamina of DLPFC from people with schizophrenia are not associated with any significant changes in levels of receptor sub-unit mRNA. This suggests the changes in NMDAR in the outer layers of the DLPFC are not due to decreased NMDAR sub-unit expression and that our data are in agreement with other studies reporting no change in NMDAR sub-unit mRNA in the cortex of people with schizophrenia but do not agree with other studies that report changes in levels of GRIN mRNAs (Supplementary Table 1).
Contrary to a previous study (Funk et al., 2009), we did not find lower levels of PSD 95 in the ACx from people with schizophrenia. Significantly, PSD 95 has been reported to be higher in the occipital cortex (Dracheva et al., 2001) and lower in the DLPFC (Kristiansen et al., 2010) from people with schizophrenia. Thus, it would seem that, as for other components of the glutamatergic system, there are regionally selective changes in PSD 95 in the cortex of people with schizophrenia.
Another major finding of this study is that there were significantly lower levels of NMDAR in the inner lamina of the ACx and outer lamina of the DLPFC from people with BPD. This finding is in line with an earlier study that reported lower levels of radioligand binding to the glycine binding site on the NMDAR in people with BPD (Nudmamud-Thanoi and Reynolds, 2004). We also showed lower levels of GRIN2C in ACx from people with BPD, which raises the possibility that the lower NMDAR in that cortical region, but not the DLPFC, is partly due to decreased gene expression. It has been suggested that the GRIN2 family of sub-units are important in NMDAR modulation and desensitisation (McIlhinney et al., 2003). Thus, our data are consistent with both a lower level of NMDAR and possibly a change in the ability of these receptors to desensitise in the ACx from people with BPD. As our study is the first to report on NMDAR mRNA in the ACx, this is a particularly novel finding. We did not find changes in the levels of PSD 95 in the ACx from people with BPD, which agrees with a previous report of this protein not being changed in the DLPFC from people with that disorder (Beneyto and Meador-Woodruff, 2008).
In MDD, we found higher levels of GRIN2D mRNA in the frontal pole, where there was no difference in levels of NMDAR. These data are consistent with the notion that a change in gene expression will not necessarily be associated with changes for the encoded protein of that gene.
Finally, we report higher levels of GRIN2B mRNA in parietal cortex and lower levels of that mRNA in the DLPFC from suicide completers. In addition, levels of PSD 95 were increased in ACx from suicide completers. These data open up a possible role for abnormal signalling through the NMDAR in the CNS of suicide completers. It is also intriguing that a previous study had reported lower levels of PSD 95 and GRIN2B mRNA in the hippocampus from suicide completers (Sowa-Kucma et al., 2013). These and our findings suggest that a further investigation into levels of GRIN2B mRNA and PSD 95 in suicide completion would be worthwhile to better understand the extent of changes in these measures across the CNS. Notably, the study in the hippocampus also reported a significant increase in zinc mediated EC50 for [3H]MK-801 binding, but not total [3H] MK-801 binding, in suicide completers (Sowa-Kucma et al., 2013). These latter data suggest a lower bioavailability of the NMDAR in the CNS of suicide completers, possibly due to conformational changes in the assembled receptor.
Our studies have certain limitations. Thus, although our cohort sizes are not unusually small for postmortem CNS studies, they are still a low representation from each diagnosis and our findings need replicating in larger cohorts. Moreover, like many studies in psychiatry, treatment remains a potential confound. We did not observe any strong relationships between types of drug or drug doses and any of our experimental variables; however, at this point, it is not possible to exclude changes in glutamatergic markers in the cortex being part of the neuropsychopharmacological outcome of psychotropic drug treatments. Nonetheless, given the somewhat homogeneous neurochemical nature of the human cortex it is somewhat difficult to explain how psychotropic drugs, acting on the same targets throughout the cortex, could produce the regional and laminae-specific outcomes we observed in different psychiatric disorders. While rates of suicide completion differed between diagnoses, our sample sizes were too small to determine if suicide completion within specific diagnoses was associated with changes in glutamatergic markers in the cortex.
Conclusion
In interpreting the significance of our finding, it is important to note that changed levels of [3H]MK-801 binding in rodents have been linked to changes in functions such as learning and memory (Basham et al., 1996; Stecher et al., 1997). This suggests that the changes in levels of NMDAR in the DLPFC and ACx from people with schizophrenia and BPD could have behavioural/functional consequences. However, the complex changes in glutamatergic markers in schizophrenia, BPD and MDD will require further study to better understand the significance of regional changes in the CNS and how they may be altering glutamatergic function in psychiatric disorders and in suicide. Moreover, given the regional variation in changes in NMDAR in people with different psychiatric disorders, continuing to unravel these complex changes in the glutamatergic system in the CNS should help to better understand the role of NMDAR in the pathophysiologies of schizophrenia, BPD and MDD as well as the dose-dependent outcomes from ketamine administration in humans. The findings are summarized in the following:
Lower NMDAR in laminae I to III plus a portion of lamina IV in dorsolateral prefrontal cortex (DLPFC) from people with schizophrenia (–17%, p = 0.01).
Lower NMDAR in laminae IV–VI in ACx (–19%, p < 0.01) and laminae I to III plus a portion of lamina IV in DLPFC from people with bipolar disorders (–19%, p < 0.01).
Lower GRIN2C mRNA in ACx from people with bipolar disorders (–27%, p < 0.05).
Higher GRIN2D mRNA in frontal pole from major depressive disorders (+22%, p < 0.05).
Higher GRIN2B mRNA in parietal cortex (+20%, p < 0.01) from suicide completers.
Lower GRIN2B mRNA in DLPFC (–35%, p = 0.02) from suicide completers.
Higher levels of PSD 95 (+26%, p < 0.05) in ACx from suicide completers.
Supplementary Material
Acknowledgements
All authors made a substantial contribution to editing the iterations of this manuscript leading to submission for publication. A.G., S.B. and A.U. completed all of the experimental work described in the manuscript. B.D., E.S., J.M.W. and R.M. provided oversight to the experimental activities. B.D. completed all the statistical analyses described in the manuscript and took the lead role in writing the manuscript.
Funding
B.D. is a National Health and Medical Research Council (NHMRC) Senior Research Fellow (APP1002240) and E.S. is an Australian Research Council (ARC) Future Fellow (FT100100689) and was the Royce Abbey Postdoctoral Fellow (Australian Rotary). This work was supported in part by NHMRC project grant (APP628699), the Victorian Government’s Operational Infrastructure Support, the Andrew and Claire Heenan Ride for Ben and the Rebecca Cooper Medical Research Foundation. The Victorian Brain Bank Network is supported by the Florey Institute for Neuroscience and Mental Health, the Alfred Hospital, the Victorian Forensic Institute of Medicine, the University of Melbourne and funded by Australia’s National Health & Medical Research Council, Helen Macpherson Smith Trust, Parkinson’s Victoria and Perpetual Philanthropic Services.
Footnotes
Declaration of interest
None of the authors have any conflict of interest, and the research described in this manuscript was completed in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. E.S. has previously received honorarium from Astra-Zeneca and travel support from GSK that were unrelated to this research.
References
- Abdallah CG, Sanacora G, Duman RS, et al. (2015) Ketamine and rapid-acting antidepressants: A window into a new neurobiology for mood disorder therapeutics. Annual Review of Medicine 66: 509–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basham ME, Nordeen EJ and Nordeen KW (1996) Blockade of NMDA receptors in the anterior forebrain impairs sensory acquisition in the zebra finch (Poephila guttata). Neurobiology of Learning and Memory 66: 295–304. [DOI] [PubMed] [Google Scholar]
- Beneyto M and Meador-Woodruff JH (2008) Lamina-specific abnormalities of NMDA receptor-associated postsynaptic protein transcripts in the prefrontal cortex in schizophrenia and bipolar disorder. Neuropsychopharmacology 33: 2175–2186. [DOI] [PubMed] [Google Scholar]
- Beneyto M, Kristiansen LV, Oni-Orisan A, et al. (2007) Abnormal glutamate receptor expression in the medial temporal lobe in schizophrenia and mood disorders. Neuropsychopharmacology 32: 1888–1902. [DOI] [PubMed] [Google Scholar]
- Bitanihirwe BK, Lim MP and Woo TU (2010) N-methyl-d-aspartate receptor expression in parvalbumin-containing inhibitory neurons in the prefrontal cortex in bipolar disorder. Bipolar Disorder in Adults 12: 95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook RD and Weisberg S (1999) Applied Regression Including Computing and Graphics. Hoboken, NJ: John Wiley. [Google Scholar]
- D’Agostino RB, Belanger A and D’Agostino RB Jr (1990) A suggestion for using powerful and informative tests of normality. The American Statistician 44: 316–321. [Google Scholar]
- Deakin JF, Slater P, Simpson MD, et al. (1989) Frontal cortical and left temporal glutamatergic dysfunction in schizophrenia. Journal of Neurochemistry 52: 1781–1786. [DOI] [PubMed] [Google Scholar]
- Dean B, Gray L and Scarr E (2006) Regionally specific changes in levels of cortical S100beta in bipolar 1 disorder but not schizophrenia. Australian and New Zealand Journal of Psychiatry 40: 217–224. [DOI] [PubMed] [Google Scholar]
- Dean B, Hussain T, Hayes W, et al. (1999a) Changes in serotonin2A and GABA(A) receptors in schizophrenia: Studies on the human dorsolateral prefrontal cortex. Journal of Neurochemistry 72: 1593–1599. [DOI] [PubMed] [Google Scholar]
- Dean B, Pavey G, Chai SY, et al. (1999b) The localisation and quantification of molecular changes in the human brain using in situ radioligand binding and autoradiography In: Dean B, Kleinman JE and Hyde TM (eds) Using CNS Tissue in Psychiatric Research: A Practical Guide. Amsterdam: Harwood Academic Press, pp. 67–83. [Google Scholar]
- Dean B, Tawadros N, Scarr E, et al. (2010) Regionally-specific changes in levels of tumour necrosis factor in the dorsolateral prefrontal cortex obtained postmortem from subjects with major depressive disorder. Journal of Affective Disorders 120: 245–248. [DOI] [PubMed] [Google Scholar]
- Dracheva S, Marras SA, Elhakem SL, et al. (2001) N-methyl-d-aspartic acid receptor expression in the dorsolateral prefrontal cortex of elderly patients with schizophrenia. The American Journal of Psychiatry 158: 1400–1410. [DOI] [PubMed] [Google Scholar]
- Eaton SL, Roche SL, Llavero HM, et al. (2013) Total protein analysis as a reliable loading control for quantitative fluorescent Western blotting. PLoS ONE 8: e72457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I, Santpere G, Arzberger T, et al. (2007) Brain protein preservation largely depends on the postmortem storage temperature: Implications for study of proteins in human neurologic diseases and management of brain banks: A BrainNet Europe Study. Journal of Neuropathology & Experimental Neurology 66: 35–46. [DOI] [PubMed] [Google Scholar]
- Foster P (1989) Neuroleptic equivalence. The Pharmaceutical Journal 243: 431–432. [Google Scholar]
- Funk AJ, Rumbaugh G, Harotunian V, et al. (2009) Decreased expression of NMDA receptor-associated proteins in frontal cortex of elderly patients with schizophrenia. Neuroreport 20: 1019–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladding CM and Raymond LA (2011) Mechanisms underlying NMDA receptor synaptic/extrasynaptic distribution and function. Molecular and Cellular Neurosciences 48: 308–320. [DOI] [PubMed] [Google Scholar]
- Grimwood S, Slater P, Deakin JF, et al. (1999) NR2B-containing NMDA receptors are up-regulated in temporal cortex in schizophrenia. Neuroreport 10: 461–465. [DOI] [PubMed] [Google Scholar]
- Hill C, Keks N, Roberts S, et al. (1996) Problem of diagnosis in postmortem brain studies of schizophrenia. The American Journal of Psychiatry 153: 533–537. [DOI] [PubMed] [Google Scholar]
- Holemans S, De PF, Horton RW, et al. (1993) NMDA glutamatergic receptors, labelled with [3H]MK-801, in brain samples from drug-free depressed suicides. Brain Research 616: 138–143. [DOI] [PubMed] [Google Scholar]
- Ishimaru M, Kurumaji A and Toru M (1994) Increases in strychnine-insensitive glycine binding sites in cerebral cortex of chronic schizophrenics: Evidence for glutamate hypothesis. Biological Psychiatry 35: 84–95. [DOI] [PubMed] [Google Scholar]
- Kingsbury AE, Foster OJ, Nisbet AP, et al. (1995) Tissue pH as an indicator of mRNA preservation in human post-mortem brain. Brain Research. Molecular Brain Research 28: 311–318. [DOI] [PubMed] [Google Scholar]
- Kornhuber J, Mack-Burkhardt F, Riederer P, et al. (1989) [3H]MK-801 binding sites in postmortem brain regions of schizophrenic patients. Journal of Neural Transmission 77: 231–236. [DOI] [PubMed] [Google Scholar]
- Kristiansen LV, Patel SA, Haroutunian V, et al. (2010) Expression of the NR2B-NMDA receptor subunit and its Tbr-1/CINAP regulatory proteins in postmortem brain suggest altered receptor processing in schizophrenia. Synapse 64: 495–502. [DOI] [PubMed] [Google Scholar]
- McIlhinney RA, Philipps E, Le BB, et al. (2003) Assembly of N-methylD-aspartate (NMDA) receptors. Biochemical Society Transactions 31: 865–868. [DOI] [PubMed] [Google Scholar]
- McKillup S (2006) Statistics Explained: An Introductory Guide for Life Scientists. Cambridge: Cambridge University Press. [Google Scholar]
- Malhotra AK, Pinals DA, Adler CM, et al. (1997) Ketamine-induced exacerbation of psychotic symptoms and cognitive impairment in neuroleptic-free schizophrenics. Neuropsychopharmacology 17: 141–150. [DOI] [PubMed] [Google Scholar]
- Miller GA and Chapman JP (2001) Misunderstanding analysis of covariance. Journal of Abnormal and Social Psychology 110: 40–48. [DOI] [PubMed] [Google Scholar]
- Newell KA, Zavitsanou K and Huang XF (2005) Ionotropic glutamate receptor binding in the posterior cingulate cortex in schizophrenia patients. Neuroreport 16: 1363–1367. [DOI] [PubMed] [Google Scholar]
- Nudmamud S and Reynolds GP (2001) Increased density of glutamate/Nmethyl-d-aspartate receptors in superior temporal cortex in schizophrenia. Neuroscience Letters 304: 9–12. [DOI] [PubMed] [Google Scholar]
- Nudmamud S, Reynolds LM and Reynolds GP (2003) N-acetylaspartate and N-Acetylaspartylglutamate deficits in superior temporal cortex in schizophrenia and bipolar disorder: A postmortem study. Biological Psychiatry 53: 1138–1141. [DOI] [PubMed] [Google Scholar]
- Nudmamud-Thanoi S and Reynolds GP (2004) The NR1 subunit of the glutamate/NMDA receptor in the superior temporal cortex in schizophrenia and affective disorders. Neuroscience Letters 372: 173–177. [DOI] [PubMed] [Google Scholar]
- Paoletti P and Neyton J (2007) NMDA receptor subunits: Function and pharmacology. Current Opinion in Pharmacology 7: 39–47. [DOI] [PubMed] [Google Scholar]
- Reynolds IJ (2001) [3H](+)MK801 radioligand binding assay at the N-methyl-d-aspartate receptor. Current Protocols in Pharmacology Chapter 1: Unit 1.20. [DOI] [PubMed] [Google Scholar]
- Roberts SB, Hill CA, Dean B, et al. (1998) Confirmation of the diagnosis of schizophrenia after death using DSM-IV: A Victorian experience. Australian and New Zealand Journal of Psychiatry 32: 73–76. [DOI] [PubMed] [Google Scholar]
- Ruffolo RR Jr (1982) Review important concepts of receptor theory. Journal of Autonomic Pharmacology 2: 277–295. [DOI] [PubMed] [Google Scholar]
- Scarr E, Beneyto M, Meador-Woodruff JH, et al. (2005) Cortical glutamatergic markers in schizophrenia. Neuropsychopharmacology 30: 1521–1531. [DOI] [PubMed] [Google Scholar]
- Scarr E, Gray L, Keriakous D, et al. (2006) Increased levels of SNAP-25 and synaptophysin in the dorsolateral prefrontal cortex in bipolar I disorder. Bipolar Disorders 8: 133–143. [DOI] [PubMed] [Google Scholar]
- Scarr E, Pavey G, Sundram S, et al. (2003) Decreased hippocampal NMDA, but not kainate or AMPA receptors in bipolar disorder. Bipolar Disorders 5: 257–264. [DOI] [PubMed] [Google Scholar]
- Scherzer CR, Landwehrmeyer GB, Kerner JA, et al. (1998) Expression of N-methyl-d-aspartate receptor subunit mRNAs in the human brain: Hippocampus and cortex. Journal of Comparative Neurology 390: 75–90. [DOI] [PubMed] [Google Scholar]
- Sowa-Kucma M, Szewczyk B, Sadlik K, et al. (2013) Zinc, magnesium and NMDA receptor alterations in the hippocampus of suicide victims. Journal of Affective Disorders 151: 924–931. [DOI] [PubMed] [Google Scholar]
- Stan AD, Ghose S, Gao XM, et al. (2006) Human postmortem tissue: What quality markers matter? Brain Research 1123: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecher J, Muller WE and Hoyer S (1997) Learning abilities depend on NMDA-receptor density in hippocampus in adult rats. Journal of Neural Transmission 104: 281–289. [DOI] [PubMed] [Google Scholar]
- Sucher NJ, Awobuluyi M, Choi YB et al. (1996) NMDA receptors: from genes to channels. Trends in Pharmacological Sciences 17: 348–355. [PubMed] [Google Scholar]
- Tucholski J, Simmons MS, Pinner AL, et al. (2013) N-linked glycosylation of cortical N-methyl-d-aspartate and kainate receptor subunits in schizophrenia. Neuroreport 24: 688–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong EH, Kemp JA, Priestley T, et al. (1986) The anticonvulsant MK-801 is a potent N-methyl-d-aspartate antagonist. Proceedings of the National Academy of Sciences of the United States of America 83: 7104–7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo TU, Shrestha K, Lamb D, et al. (2008) N-methyl-d-aspartate receptor and calbindin-containing neurons in the anterior cingulate cortex in schizophrenia and bipolar disorder. Biological Psychiatry 64: 803–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavitsanou K, Ward PB and Huang XF (2002) Selective alterations in ionotropic glutamate receptors in the anterior cingulate cortex in schizophrenia. Neuropsychopharmacology 27: 826–833. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.