Abstract
Purpose:
Metastatic castration resistant prostate cancer (mCRPC) is a lethal, heterogeneous disease with few therapeutic strategies that significantly prolong survival. Innovative therapies for mCRPC are needed, however, the development of new therapies relies on accurate imaging to assess metastasis and monitor response. Standard imaging modalities for prostate cancer require improvement and there remains a need for selective and sensitive imaging probes that can be widely used in mCRPC patients.
Experimental Technique:
We evaluated the transmembrane protease fibroblast activation protein alpha (FAP) as a targetable cell surface antigen for mCRPC. Genomic and IHC analyses were performed to investigate FAP expression in prostate cancer. Our FAP-targeted antibody imaging probe, [89Zr]Zr-B12 IgG, was evaluated by PET/CT imaging in preclinical prostate cancer models.
Results:
Analysis of patient data documented FAP overexpression in metastatic disease across tumor subtypes. PET imaging with [89Zr]Zr-B12 IgG demonstrated high tumor uptake and long-term retention of the probe in the preclinical models examined. FAP-positive stroma tumor uptake of [89Zr]Zr-B12 IgG was five-fold higher than the isotype control with mean %ID/cc of 34.13 ± 1.99 versus 6.12 ± 2.03 (n = 3/group; P = 0.0006) at 72 hours. Ex vivo biodistribution corroborated these results documenting rapid blood clearance by 24 hours and high tumor uptake of [89Zr]Zr-B12 IgG by 72 hours.
Data Interpretation:
Our study reveals FAP as a target for imaging the tumor microenvironment of prostate cancer. Validation of [89Zr]Zr-B12 IgG as a selective imaging probe for FAP-expressing tumors presents a new approach for noninvasive PET/CT imaging of mCRPC.
Introduction
Malignant cells are surrounded by a complex and supportive tumor microenvironment that consists of immune cells, extracellular matrix, vasculature, and fibroblasts [1]. Cancer-associated fibroblasts (CAFs) are the major cell type in the reactive stroma and are known to promote tumorigenesis and metastasis [2]. Fibroblast activation protein alpha (FAP) is a transmembrane serine protease expressed by CAFs in the microenvironment of epithelial tumors [3]. Meta-analysis of FAP expression and clinical outcomes in solid cancers indicate that patients with FAP overexpression are at higher risk of tumor invasion, lymph node metastasis, and decreased overall survival [4]. Thus, FAP has been gaining momentum as the next pan-cancer target for diagnostic and therapeutic development given its absence in normal adult tissue, upregulated expression in activated fibroblasts, and localization to the tumor microenvironment. Although FAP expression has been annotated in multiple solid cancers, its presence in metastatic castration resistant prostate cancer (mCRPC) is still largely uncharacterized. Few studies have been performed to accurately define and examine the reactive stroma in prostate cancer. CAFs in the localized prostate tumor microenvironment are known to play an essential role in tumorigenesis [5–8]. Furthermore, FAP-positive CAFs induce epithelial-mesenchymal transition (EMT)-driven gain of cancer stemness, invasiveness, and development of distant metastases [9, 10]. Recruitment of reactive stroma is essential for metastatic tumor survival and growth [11–13], however, not much has been published on the role of FAP or CAFs in prostate cancer metastasis.
Prostate cancer that has metastasized to the bone and visceral tissues is highly lethal with 5-year survival rates remaining at 30%. The development of new therapeutics for mCRPC is dependent on accurate imaging modalities for patient staging and evaluating treatment response. The current standard for imaging disease burden is bone scintigraphy with 99mTc methyldiphosphonate ([99mTc]Tc-MDP.) This imaging modality only detects bone lesions and is plagued by poor sensitivity [14, 15]. Positron emission tomography (PET) imaging agents used in conjunction with either computed tomography (CT) or magnetic resonance imaging (MRI) have had various degrees of success imaging prostate cancer [16]. The commonly used PET radiotracer [18F]fluorodeoxyglucose ([18F]FDG) is of little utility [17, 18] while [18F]sodium fluoride (Na[18F]F) has documented improved sensitivity over bone scans, but it cannot detect visceral lesions [19, 20]. [18F]fluciclovine was recently approved by the FDA for detecting biochemical recurrence, however, its utility in mCRPC has not been investigated [21, 22]. Prostate-specific membrane antigen (PSMA) targeted probes such as, [89Zr]Zr-J591 and [68Ga]Ga-PSMA-11, have met with success in the clinic, but loss of PSMA expression is known to occur in neuroendocrine, AR negative prostate cancer rendering the probes ineffective [23]. Therefore, standard imaging modalities for prostate cancer leave room for improvement and warrant the discovery and validation of new antigens in prostate cancer.
The localization of FAP to the tumor microenvironment and its association with aggressive phenotypes make it a compelling imaging and therapeutic target for solid tumors including prostate cancer. Previously, we discovered and characterized a monoclonal antibody (mAb) targeting the extracellular domain of FAP, termed B12 IgG. Here, we describe the development of [89Zr]Zr-B12 IgG, a FAP-targeted probe for the detection of prostate cancer by PET/CT imaging. Genomic and IHC analysis revealed overexpression of FAP in metastatic disease compared to primary prostate tumors. Additionally, FAP expression was found to be uniform across metastatic disease regardless of genomic subtype, patient treatment history, and location of the metastatic lesion. We demonstrate that [89Zr]Zr-B12 IgG can be used for the noninvasive imaging of FAP-expressing cells in preclinical models of prostate cancer. High tumor uptake and retention of [89Zr]Zr-B12 IgG was observed compared to tumors administered the [89Zr]Zr-Isotype Control (IC IgG) suggesting potential for further development of B12 IgG as a platform technology for radioimmunotherapy or antibody drug conjugates. In addition, [89Zr]Zr-B12 IgG detected FAP-expressing stromal cells in a relevant prostate tumor microenvironment model. Overall, our findings suggest that targeting FAP could result in a next-generation targeted imaging paradigm and, thus, positively affect the development of much needed therapeutics for mCRPC.
Methods
Cell Culture
DU145 (#HTB-81) and HEK293T (#CRL-11268) cell lines were purchased from American Type Culture Collection (ATCC) and were maintained according to ATCC guidelines. hPrCSC-44 cells were obtained from Dr. W. Nathaniel Brennen (Johns Hopkins Medicine, Baltimore, MD) and maintained in RoosterNourish-MSC media (RoosterBio). CWR-R1-luciferase cells were obtained from Dr. Scott Dehm (University of Minnesota, Minneapolis, MN) and maintained with 10 μM enzalutamide. CWR-R1 cells were lentivirally transduced to express FAP as previously described [24]. FAP-expressing cells were continuously supplemented with 3 μg/mL puromycin to ensure stable levels of FAP expression. All cell experiments were performed within 4 months of thawing cell lines from frozen cell stocks. Cell lines were authenticated using short-tandem repeat analysis and routinely monitored for mycoplasma contamination prior to our studies.
Antibody Production
For antibody expression, the heavy chain (342 bp) and light chain (324 bp) variable domains of the B12 sequence were cloned separately into pFUSEss human IgG1 expression vectors (pFUSEss-CHIg-hG1 and pFUSE2ss-CLIg-hk, Invitrogen) and co-transfected into HEK293T cells. The serum was collected after 72 hours, filtered through a 0.45 μm filter, and purified using a 5 mL HiTrap Protein A HP column (GE Healthcare). The column was equilibrated with 20 mM sodium phosphate at pH 6.8. The serum was loaded onto the column, washed with equilibration buffer for 10 column volumes, and bound protein was eluted with 0.1M citric acid at pH 3.5. Eluted IgG was collected, concentrated using a 50 kDa centrifugal filter (Millipore), and buffer exchanged into PBS using a Sephadex G-25 PD-10 desalting column (GE Healthcare). The purity of the final B12 IgG was analyzed by reduced and nonreduced SDS-PAGE, and protein concentration was measured based on absorbance at 280 nm using a NanoDrop One UV – Vis Spectrophotometer (Thermo-Fisher).
Genomic Analysis
RNA-seq data was extracted from previously published studies of individuals with primary prostate cancer [25] or mCRPC bone and soft tissue tumor biopsies [26]. Capture of clinical samples was part of standard-of-care approaches for mCRPC that included treatment with abiraterone acetate or enzalutamide followed by taxane. RNA-seq fragments per kilobase million (FPKM) values were obtained using the previously described methods [27]. Microarray data was extracted from previously published studies of a set of metastatic tumors from men with CRPC [28]. The dataset is available in the Gene Expression Omnibus under accession GSE77930.
Immunohistochemistry
Human studies were conducted in accordance with the Belmont Report. Samples for IHC analysis were obtained from the Prostate Cancer Biorepository Network (PCBN) and the University of Minnesota Masonic Cancer Center (Minneapolis, MN) using a University of Minnesota Human Subjects Division–approved IRB protocol for tissue acquisition (IRB#1604M86269) and with written patient consent. IHC was performed on formalin-fixed paraffin-embedded tissue sections using (1:100) mouse anti- FAP (Abcam, 227703 clone SP325). Unstained sections (4 μm) were deparaffinized and rehydrated using standard methods. For antigen retrieval, slides were incubated in 6.0 pH buffer (Reveal Decloaking reagent, Biocare Medical) in a steamer for 30 min at 95–98 °C, followed by a 20-minute cool down period. A serum-free blocking solution (Sniper, Biocare Medical) was placed on sections for 30 minutes. Blocking solution was removed and slides were incubated in primary antibody diluted in 10% blocking solution/90% TBST. The antibody was used according to the manufacturer’s protocol.
Quantitative Real-Time PCR
RNA was prepared from tissue using a RNeasy kit (Qiagen) and synthesized to cDNA using the High Capacity RNA-to-cDNA kit (Applied Biosystems). Taqman qRT-PCR was performed using the Taqman Universal PCR Master Mix (Applied Biosystems) and Taqman Gene Expression Assay Probes: human FAP Hs00990807_m1 and 18s ribosomal 1 Hs03928985_g1. 18s ribosomal 1 was used as a normalization control. All qPCR was performed on a StepOnePlus Real-Time PCR system instrument (Applied Biosystems). Each sample had three technical replicates, ans each reaction was performed in three biological replicates. The data was analyzed using the comparative Ct method (fold change = 2−ΔΔCt) as previously described. All data are presented as mean ± standard error of the mean (SEM).
Western Blot
Cell lysates were prepared from tissue using 1x laemmli buffer. 20 μg of total protein per sample was denatured with 5% β-mercaptoethanol and separated on a 4% to 12% Bis-Tris Plus precast gel (NW04122, Invitrogen) with sodium dodecyl sulfate polyacrylamide gel electrophoresis and then transferred at 20 V onto a nitrocellulose transfer membrane (ThermoFisher) with an iBlot 2 Dry Blotting System (ThermoFisher) for 7 min. Membranes were blocked in 5% milk in TBS, 1% Tween20 and probed with murine anti- human FAP mAb (1:500, sc-100528, Santa Cruz Biotechnology) overnight at 4°C, washed, and then incubated in rabbit anti- murine mAb conjugated to peroxidase (1:1000, sc-516102, Santa Cruz Biotechnology) for 1 h at room temperature. Equal protein loading was confirmed using murine anti- human α-tubulin (1:10000, sc-23948, Santa Cruz Biotechnology,) and anti-murine conjugated to peroxidase (1:10000). Binding was detected using the SuperSignal West Pico PLUS Chemiluminescent Substrate (ThermoFisher) and blots were imaged with a MyECL imager (ThermoFisher) and Image Studio 2.0 software (Li-Cor).
Animal Models
All animal studies were approved by the University of Minnesota Institutional Animal Care and Use Committee (IACUC). All animal studies were performed in three-to-four-week old Athymic Nude-Foxn1nu mice (Envigo). For subcutaneous tumor implantation, cells suspended in a 1:1 mixture of PBS and Matrigel (Corning) were injected into the shoulders of the mice using a 26 gauge needle. For the engineered cell line model, animals (n = 3 for experimental and control groups) received subcutaneous injections of CWR-R1FAP cells (1 × 106 cells in 100 μL). For the CAF model, animals (n = 3 for experimental and control groups) received subcutaneous injections of a 2:1 mixture of hPrCSC-44 and DU145 cells (2 × 106 hPrCSC-44 cells and 1 × 106 DU145 cells in 100 μL). Tumors volumes were measured twice weekly with calipers and tumors were allowed to grow to a size of 100 – 300 mm3 before nuclear imaging and biodistribution experiments. For intratibial tumor implantation, luciferase-expressing CWR-R1FAP cells suspended in PBS (2.5 × 105 cells in 10 μL) were injected into the tibia of one leg of each mouse (n = 3 for experimental and control groups). Tumors were allowed to grow for five weeks before bioluminescent imaging (BLI) and nuclear imaging experiment. For BLI, the mice were injected intraperitoneally with D-luciferin (150 mg/kg). Images were acquired 10 min after the injection of D-luciferin using an IVIS Spectrum (PerkinElmer).
Bioconjugation and Radiochemistry
For nuclear imaging studies, the B12 IgG and IC IgG were conjugated to p-SCN-Bn-Deferoxamine (DFO, Macrocyclic) as previously described [29]. Zirconium-89 (89Zr) was purchased from the University of Wisconsin Medical Physics Department (Madison, WI). [89Zr]Zr-oxalate (277 MBq) in 1.0 mol/L oxalic acid (600 μL) was adjusted to pH 7.5 with 1.0 mol/L Na2CO3. To radiolabel the B12 IgG and IC IgG, the DFO-B12 IgG conjugate (272 μL, 2.2 mg/mL, 600 μg mAb) or DFO-IC IgG conjugate (600 μg mAb) in 0.5 mol/L HEPES (pH 7.5) was added to the neutralized [89Zr]Zr-oxalate solution and incubated at room temperature with rotation for 1 hour. The labeled product was purified using a size-exclusion PD-10 column preequilibrated with PBS buffer and evaluated via radioactive TLC for purity using standard methods as previously described [30]. Size-exclusion HPLC analysis with a radioactive detector was not performed on any of the radiolabeled products.
Biodistribution
Acute in vivo biodistribution studies were conducted to evaluate the uptake of [89Zr]Zr-B12 IgG in mice bearing subcutaneous CWR-R1FAP tumors. Mice were randomized before the study and administered [89Zr]Zr-B12 IgG (1.15 MBq, 1 – 2 μg mAb, in 100 μL of PBS) via tail vein injection. Mice (n = 3 mice for each tissue per time point) were euthanized at 24, 72, and 144 hours post-injection. Organs (including the tumor) were excised and counted on an automatic gamma-counter (Hidex). The total number of counts (counts per minute, cpm) of each organ was compared with a standard syringe of known activity and mass. Count data were background- and decay-corrected, and the percentage injected dose per gram (%ID/g) for each tissue sample was calculated by normalization to the total amount of activity injected into each mouse.
μPET/CT imaging
Positron emission tomography (PET) imaging experiments were conducted on an Inveon μPET/CT Scanner (Siemens Medical Solutions). Mice (n = 3 for experimental and control groups) were administered [89Zr]Zr-B12 IgG or [89Zr]Zr-Isotype Control formulations (4.3 MBq, 25 – 30 μg of mAb, in 100 μL of PBS) via tail vein injection. Mice were anesthetized by inhalation of 2% isoflurane and PET images were recorded at 24 hour time points out to 144 hours post-injection. PET list-mode data were acquired for 30 minutes using a gamma ray energy window of 350–650 keV and a coincidence timing window of 3.438 ns. CT acquisition was performed for 5 minutes at 80 kVp, 500 μA, 384 ms per step, and 340 steps covering 220 degrees. CT images were reconstructed using a Hu scaled Feldkamp algorithm resulting in 192 × 192 matrix and PET utilized Ordered Subset Expectation Maximization (OSEM-3D) with 18 subsets and 2 iterations resulting in a 128 × 128 matrix. Two-dimensional (2D) images were prepared in Inveon Research Workplace and quantified using AMIDE. An empirically determined system calibration factor was used to convert voxel count rates to activity concentrations and the resulting image data were normalized to the administered activity to parameterize images in terms of %ID/cc. Drawn ellipsoid ROIs (n = 3 for experimental and control groups per time point) were used to determine the mean %ID/cc in various tumors. Three-dimensional (3D) reconstructions were generated using AMIRA.
Statistical Analysis
All statistical analyses were performed using Prism GraphPad software. Patient microarray data were analyzed using a one-way ANOVA and corrected for multiple comparisons using Sidak hypothesis testing. Animal studies were performed with n = 3 for experimental and control groups and the statistical significance of signal intensities were determined using a two-way ANOVA and corrected for multiple comparisons using Sidak hypothesis testing. Quantitative RT-PCR results were determined by two-tailed Student t test. Results are depicted as mean ± SEM. The symbols used to represent the P values were as follows; ns, p > 0.05; *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001; ****, p ≤ 0.0001.
Results
FAP is overexpressed in metastatic prostate cancer
Publicly available RNA-seq datasets were analyzed [25, 26] to quantify FAP expression between hormone-naïve primary prostate tumors and metastatic disease (Fig. 1A). Data analysis revealed a significant increase in FAP mRNA expression in metastatic disease compared to primary (P = 0.0003). Next, the dataset was stratified based on grade, treatment regimen, and location of metastasis to see if any further mRNA expression patterns could be defined (Fig. 1B). FAP expression was similar in primary tumor samples regardless of grade (Gleason score 6 – 9). In the metastatic tumor subtypes, treatment with second generation anti-androgens (enzalutamide or abiraterone) or chemotherapy (taxane) did not have a significant effect on FAP expression. Finally, we observed no difference in FAP mRNA expression among locations of metastasis (lymph node, bone, liver, other). To corroborate the FAP expression patterns found by RNA-seq, IHC analysis was performed on two tissue microarrays (TMA). We first analyzed a primary prostate adenocarcinoma TMA. No discernable staining for FAP was observed in any of the primary prostate tumor cores even across different Gleason scores (Fig. 1C). In a mCRPC TMA, we observed medium to strong IHC staining in metastatic lesions compared to normal prostate (Fig. 1D).
Figure 1.
FAP is overexpressed in metastatic prostate cancer compared to primary tumors. A, and B, RNA-seq analysis of FAP expression in primary tumor versus metastatic disease. Multiple raw FASTQ RNA-seq datasets were obtained via DbGAP and aligned and transcripts were quantified via uniform pipeline, enabling cross-experimental comparisons. Data are reported as FPKM. A, mRNA FAP expression was significantly increased in metastatic disease (n = 51) compared to primary tumors (n = 25). ***, P ≤ 0.001. B, Similar levels of FAP expression was observed across primary tumor grades, treatment history, and metastatic sites. C, Representative images of IHC staining for FAP in primary prostate cancer tumors. Magnification 40x. D, Representative images of IHC staining for FAP in normal prostate and several metastatic lesions. Magnification 40x.
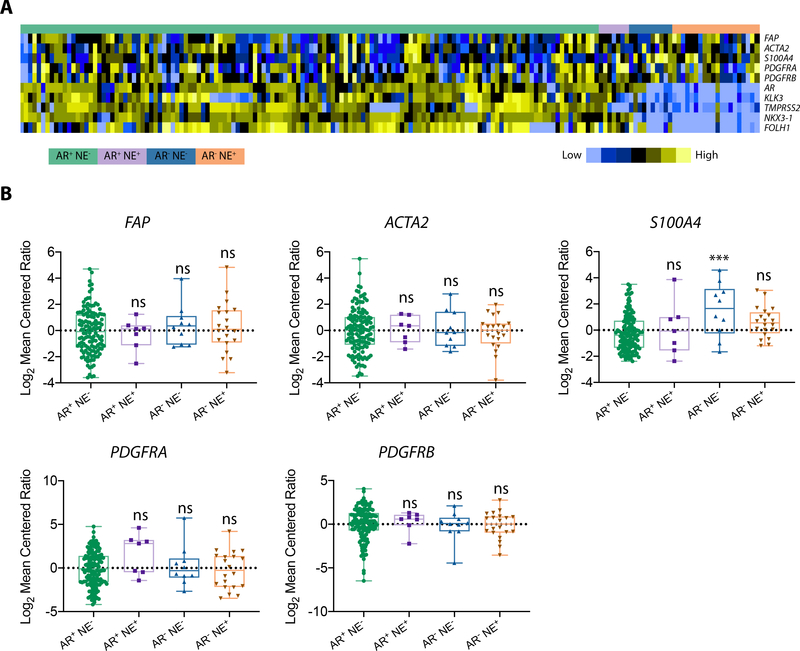
Further genetic analysis was performed to assess whether FAP overexpression was associated with specific metastatic prostate cancer gene signatures. We investigated FAP expression in the context of androgen receptor (AR) status and neuroendocrine (NE) differentiation using microarray data of 171 mCRPC tumors from 63 rapid autopsy subjects at the University of Washington (Fig. 2A.) [28, 31]. Quantitative analysis revealed that FAP gene expression was similar across all metastatic subtypes regardless of AR status or NE differentiation (Fig. 2B). The gene expression profiles of several CAF markers were also documented to have no significant difference among tumor genotypes. One exception being the AR−/NE− S100A4 population which was found to be significantly higher than AR+/NE− tumors (P = 0.001). Together, these analyses suggest that FAP expression is a characteristic of mCRPC regardless of genetic subtype, treatment regimen, or location of metastasis.
Figure 2.
FAP expression is uniform across metastatic prostate cancer genomic subtypes. A, FAP expression was evaluated in metastatic prostate cancer tumors that displayed gene signatures signifying AR status and NE differentiation. B, FAP expression in mCRPC patient samples was similar across all genetic subtypes. Expression of several CAF markers is also shown (ACTA2, S100A4, PDGFRA, and PDGFRB). Data are represented as Log2 Mean Centered Ratio. ***, P ≤ 0.001. For A, and B, AR+ NE− (n = 134); AR+ NE+ (n = 7); AR− NE− (n = 10); AR− NE+ (n = 20).
PET/CT imaging of FAP in an engineered model
Previously, we demonstrated that IRDye-800CW-conjugated B12 IgG could potently and selectively detect FAP-expressing cells by near infrared optical imaging [24]. These results suggested further development of B12 IgG as a PET imaging probe labeled with the long-lived positron-emitting radioisotope [89Zr] (t1/2 = 3.7 days). We first evaluated [89Zr]Zr-B12 IgG for the targeted imaging of FAP-positive cells in mice bearing subcutaneous CWR-R1FAP xenografts. We previously engineered a human FAP-expressing prostate cancer cell line, CWR-R1FAP, to use for proof-of-concept studies [24]. Mice were injected with [89Zr]Zr-B12 IgG via tail vein and imaged by μPET/CT at 24, 48, 72, 96, and 144 hours post-injection (Fig. 3A). Analysis of 2D and 3D PET images revealed the distribution of [89Zr]Zr-B12 IgG. Initial imaging probe uptake at 24 hours was high in the FAP-positive tumors and liver. Over the time course, retention of [89Zr]Zr-B12 IgG signal in the tumor remained high while liver signal diminished. By 144 hours post-injection, probe uptake in the FAP-positive tumors had cleared. Additional mice bearing subcutaneous CWR-R1FAP xenografts were injected with the non-specific [89Zr]Zr-IC IgG via tail vein and imaged at 24, 48, and 72 hours post-injection (Fig. 3A). Although some uptake was observed in the FAP-positive tumors, the signal was much lower than [89Zr]Zr-B12 IgG administered tumors. Furthermore, the probe rapidly cleared from the tumors and almost no signal was visible by 72 hours suggesting that uptake was due to the enhanced permeability and retention effect rather than selective localization.
Figure 3.
[89Zr]Zr-B12 IgG can detect FAP-positive prostate cancer tumors by PET/CT imaging. A, Representative PET/CT images of mice bearing subcutaneous CWR-R1FAP xenografts (white arrowhead). Mice (n = 3 for experimental and control groups) received 3.7 MBq (25–50 μg, 7.3 μg/MBq) of [89Zr]Zr-B12 IgG or 3.7 MBq (25–50 μg, 8.7 μg/MBq) [89Zr]Zr-IC IgG via tail vein and were imaged at the indicated time points. B, Quantitative analysis of subcutaneous xenografts from mice in Fig. 3A (n = 3 for experimental and control groups per time point) revealed significantly higher [89Zr] uptake in the tumors of [89Zr]Zr-B12 IgG administered animals compared to the [89Zr]Zr-IC IgG at 24, 48, and 72 hours post-injection. Values represent mean ± SEM. **, P ≤ 0.01. C, Ex vivo biodistribution of [89Zr]Zr-B12 IgG in tissues of mice bearing subcutaneous CWR-R1FAP xenografts. Mice (n = 3 mice per tissue per time point) were injected with 1.15 MBq (5.7 μg, 6.6 μg/MBq) of [89Zr]Zr-B12 IgG via tail vein prior to sacrifice at the indicated time points. Values represent mean ± SEM. ***, P ≤ 0.001; ****, P ≤ 0.0001.
Quantitative analysis of [89Zr] uptake in the FAP-positive tumors was in concordance with the 2D and 3D imaging data (Fig. 3B). At 24 hours, the [89Zr] uptake by tumors in animals administered with [89Zr]Zr-B12 IgG was two-fold higher than [89Zr]Zr-IC IgG with %ID/cc values averaging 38.32 ± 3.58 versus 15.69 ± 0.33 (P = 0.003). The difference in tumor uptake of [89Zr]Zr-B12 IgG compared to [89Zr]Zr-IC IgG increased to three-fold by 72 hours. %ID/cc values averaged 30.24 ± 2.48 versus 9.83 ± 0.73 (P = 0.0013). An ex vivo biodistribution study was performed to evaluate probe localization to the tumors and secondary organs over time (Fig. 3C). [89Zr] uptake in the main clearance organs (liver and kidney) and the spleen, where IgGs are known to localize nonspecifically, was observed as anticipated. Distribution of [89Zr]Zr-B12 IgG was similar in all other organs at each timepoint, except for the tumors. At 24 hours, [89Zr] uptake by FAP-positive tumors was detected, however, the mean %ID/g value of [89Zr] uptake was three-fold higher at 72 hours (P < 0.0001). Uptake in the FAP-positive tumors remained significantly higher at 144 hours compared to 24 hours (P < 0.0001). These data further reflect the trends observed in the 2D and 3D imaging data.
The ability of [89Zr]Zr-B12 IgG to image FAP-expressing cells in a complex microenvironment was investigated in mice bearing intratibial CWR-R1FAP xenografts. Following intratibial injection of luciferase-expressing CWR-R1FAP cells, bioluminescent imaging (BLI) was performed weekly to monitor tumor growth. Approximately five weeks post-injection, metastatic lesions in or around the bone had developed (Fig. 4A). Mice were injected with [89Zr]Zr-B12 IgG or [89Zr]Zr-IC IgG via tail vein and imaged 24 hours post-injection (Fig. 4A). In the [89Zr]Zr-B12 IgG administered animals, high probe uptake was observed in the FAP-positive leg tumor while no uptake was visible in the healthy leg. As shown in the 2D and 3D PET images, [89Zr]Zr-IC IgG signal in the FAP-positive leg tumor was considerably lower than [89Zr]Zr-B12 IgG at 24 hours post-injection (Fig. 4A). Insignificant nonspecific localization was also observed in the leg and spine of [89Zr]Zr-IC IgG administered animals. Quantification of [89Zr] uptake in the FAP-positive tumors reiterated these results. [89Zr] uptake in the animals administered with [89Zr]Zr-B12 IgG was almost five-fold higher than with [89Zr]Zr-IC IgG with %ID/cc values averaging 28.57 ± 3.23 versus 5.85 ± 0.58 (P = 0.0014).
Figure 4.
[89Zr]Zr-B12 IgG can detect FAP-positive metastatic prostate cancer tumors by PET/CT imaging. A, Representative PET/CT images of mice bearing intratibial CWR-R1FAP xenografts. Intratibial tumors were identified by BLI. Mice (n = 3 for experimental and control groups) received 3.8 MBq (25–50 μg, 10.6 μg/MBq) [89Zr]Zr-B12 IgG or 4.2 MBq (25–50 μg, 14.3 μg/MBq) [89Zr]Zr-IC IgG via tail vein and were imaged at 24 hours post injection. The tumor bearing leg (Tumor, T) was compared to the healthy leg (Healthy, H) in each animal. B, Quantitative analysis of intratibial xenografts from the mice in Fig. 4A (n = 2 for experimental and control groups) revealed significantly higher [89Zr] uptake in tumors treated with [89Zr]Zr-B12 IgG compared to the [89Zr]Zr-IC IgG. Values represent mean + SEM. **, P ≤ 0.01.
PET/CT imaging of FAP-expressing stroma
To develop a xenograft model that recapitulated the tumor microenvironment where FAP expression is localized to the stroma, and not cancer cells, we used the immortalized human prostate cancer stroma cell line, hPrCSC-44, in combination with the FAP null prostate cancer cell line DU145. hPrCSC-44 cells are cancer-associated fibroblasts that highly express FAP by flow cytometry as demonstrated by a PE conjugated commercial anti- human FAP mAb (Fig. 5A). The mouse xenograft model was developed by subcutaneous injection of hPrCSC-44 and DU145 cells (2:1 ratio; 2 × 106 : 1 × 106 cells; Fig. 5B). Mice bearing subcutaneous hPrCSC-44/DU145 xenografts were injected with [89Zr]Zr-B12 IgG or [89Zr]Zr-IC IgG and imaged serially at 24, 48, 72, 96, 120, and 144 hours post-injection (Fig. 5C). Analysis of the 2D and 3D imaging data revealed high uptake in the FAP-positive stroma tumors and liver at 24 hours in the animals administered [89Zr]Zr-B12 IgG. Retention of the imaging probe in the tumor and concordant loss of signal in the liver was also observed over time. By 144 hours post-injection, the [89Zr]Zr-B12 IgG signal was low but still detectable in the 2D PET images. In contrast, the animals administered [89Zr]Zr-IC IgG had extremely high uptake in the liver compared to the low uptake by the FAP-positive stroma tumors. Furthermore, [89Zr]Zr-IC IgG signal in the tumor had cleared by 72 hours. Quantification of [89Zr] uptake in the FAP-positive stroma tumors was significantly higher in the animals administered [89Zr]Zr-B12 IgG compared to [89Zr]Zr-IC IgG at 24, 48, 72, and 144 hours post-injection (Fig. 5D). Notably, tumor uptake of [89Zr]Zr-B12 IgG was four- to five-fold higher than [89Zr]Zr-IC IgG from 24 to 72 hours. Mean %ID/cc were 15.39 ± 0.93 (B12 IgG) versus 11.93 ± 2.38 (IC IgG) at 24 hours (P = 0.0007) and 34.13 ± 1.99 (B12 IgG) versus 6.12 ± 2.03 (IC IgG) at 72 hours (P = 0.0006).
Figure 5.
[89Zr]Zr-B12 IgG can detect FAP-positive tumor stroma by PET/CT imaging. A, Validation of membrane bound FAP expression in the hPrCSC-44 cell line by flow cytometry. hPrCSC-44 cells were stained using a commercial PE conjugated mAb (red line) and compared to an unstained control (blue line). B, Schematic of FAP-positive tumor stroma model. hPrCSC-44 and DU145 cells (2:1 ratio; 2 × 106 : 1 × 106 cells) were implanted subcutaneously in mice. Xenografts grew for five weeks before PET/CT imaging. C, Representative PET/CT images of mice bearing hPrCSC-44/DU145 subcutaneous xenografts (white arrowhead). Mice (n = 3 for experimental and control) received 4.6 MBq (25–50 μg, 6.7 μg/MBq) [89Zr]Zr-B12 IgG or 4.7 MBq (25–50 μg, 6.7 μg/MBq) [89Zr]Zr-IC IgG via tail vein and were imaged at the indicated time points. D, Quantitative analysis of subcutaneous xenografts from the mice in Fig. 5C (n = 3 for experimental and control groups per time point) revealed significantly higher [89Zr] uptake in the tumors of [89Zr]Zr-B12 IgG administered animals compared to the [89Zr]Zr-IC IgG at 48, 72, and 144 hours post-injection. Values represent mean ± SEM. **, P ≤ 0.01; ***, P ≤ 0.001. E, mRNA levels of human FAP in hPrCSC-44/DU145 (n = 3 technical and biological replicates) and FAP-negative (n = 3 technical and biological replicates) xenograft tissues were analyzed using quantitative RT-PCR. mRNA fold change is normalized to murine liver tissue. ***, P ≤ 0.001. F, Representative western blot detecting human FAP protein in hPrCSC-44/DU145 xenograft tissue using a commercial mAb.
To validate our FAP-positive tumor stroma model, tumors and livers from subcutaneous hPrCSC-44/DU145 xenografts administered [89Zr]Zr-B12 IgG were excised and frozen. FAP mRNA levels were analyzed from the frozen tissue samples and FAP-negative xenograft tissues by quantitative RT-PCR. Human FAP mRNA was detected in the hPrCSC-44/DU145 xenografts, but not the FAP-negative control when normalized to mouse liver tissue (P = 0.0006; Fig. 5E). Next, western blot using a commercial anti- human FAP mAb was performed to investigate FAP protein expression in the tissue samples. Human FAP was detected in all three hPrCSC-44/DU145 xenografts (Fig. 5F). Our tumor stroma model presents a more relevant tool for the evaluation of diagnostic and therapeutic agents for prostate cancer. Overall, these data validate [89Zr]Zr-B12 IgG as a probe for the targeted imaging of FAP-expressing stroma within the prostate tumor microenvironment.
Discussion
Nuclear imaging has proven invaluable at accelerating the development of diagnostics and therapeutics for cancer. New therapies are needed for mCRPC and the development of these agents is dependent on accurate imaging modalities to stage disease and evaluate patient response to therapy. Standard imaging modalities for prostate cancer possess various flaws and need improvement to drive therapeutic discovery. Current radiotracers lack selectivity for cancer tissue versus surrounding healthy tissue; have low sensitivity for small tumors and visceral metastatic lesions; and patients may be required to undergo multiple scans to assess all potential metastatic sites. These limitations urge the discovery and validation of new antigens in prostate cancer for targeted nuclear imaging and the subsequent development of innovative therapies. Antigens that can function as highly selective and sensitive imaging targets are key to the development of next-generation probes and the advancement of personalized medicine.
Imaging the tumor microenvironment with targeted probes is a key strategy to overcome current limitations given the essential role of the microenvironment in disease progression. Additionally, stromal cells have greater genomic stability than malignant cells which promotes retention of imaging targets [2, 32]. The tumor stroma can make up over 50% of the total tumor mass, or more, in cases of desmoplastic reaction. Therefore, targeting the tumor microenvironment could increase imaging sensitivity and have greater utility compared to current agents. Our group has previously shown that non-specific PET radiotracers have limited function in preclinical prostate cancer models [30]. CAFs are the major cell population in the tumor stroma and are critical to tumor growth beyond 1–2 mm indicating that CAF-targeted imaging could have improved sensitivity for small tumors [33]. In addition, reactive stroma formation occurs in both bone and visceral lesions, thus, imaging CAFs could expand tumor visualization for all metastatic sites. FAP is an attractive imaging target because of its upregulated expression in CAFs and localization to the tumor microenvironment of epithelial tumors. Therefore, FAP-targeted imaging could be used for heterogeneous tumor populations as a pan-cancer diagnostic and as a predictive biomarker for evaluating FAP-targeted therapies.
In this study, we reveal FAP as a candidate for the targeted imaging and treatment of mCRPC. Genomic and IHC analyses demonstrate FAP expression in metastatic disease across genomic subtypes, treatment regimens, and metastatic sites. Our FAP-targeted nuclear imaging probe, [89Zr]Zr-B12 IgG, demonstrated excellent selectivity and sensitivity toward FAP-expressing cells in several preclinical models of prostate cancer. This was documented by high tumor uptake at 24 hours post-injection and long-term retention of [89Zr]Zr-B12 IgG signal compared to the [89Zr]Zr-IC IgG (Fig. 3A; Fig. 4A; Fig. 5C). The ex vivo biodistribution study highlighted the promising pharmacokinetics of [89Zr]Zr-B12 IgG (Fig. 3C). We observed blood clearance by 24 hours and [89Zr]Zr-B12 IgG uptake in the tumor was higher than any other organ by 72 hours. [89Zr]Zr-B12 IgG also showed utility in the intratibial and prostate tumor stroma xenograft models further suggesting its high sensitivity in advanced models (Fig. 4; Fig. 5). In addition, we introduce a clinically relevant model of the prostate tumor microenvironment to help advance the development of FAP-targeted diagnostic and therapeutic agents for mCRPC. FAP is highly expressed by CAFs in the tumor stroma, but not cancer cells, and models that reflect this will be critical for continued studies of FAP and prostate cancer and could improve translation of targeted probes into the clinic. This model could be further improved with the development of a humanized mouse to evaluate [89Zr]Zr-B12 IgG imaging in more advanced prostate tumor microenvironments.
Other FAP-targeted approaches have been developed for theranostic use in oncology. The first generation anti-FAP mAb, F19, and its humanized version, sibrotuzumab, were labeled with 131I and used for SPECT imaging of patients with metastatic cancer [34, 35]. Both were shown to have slow clearance and no reported internalization, which limits their function for imaging or therapy. Furthermore, the humanized antibody caused an immune response in patients and sibrotuzumab was withdrawn from clinical trials. The 177Lu-labeled mAbs ESC11 and ESC14 were used for SPECT/CT imaging in xenograft models and tested for therapeutic efficacy in a single radioimmunotherapy study [36]. No data has been published from patient trials, thus, translational potential cannot be assessed. DOTA coupled, FAP-targeted enzyme inhibitors (FAPI) were labeled with 68Ga for PET/CT imaging and 90Y for radioimmunotherapy in metastatic cancer patients [37–39]. A limited study was done in mCRPC patients that lacked PSMA expression, but more work is needed to evaluate the radiotracers utility throughout metastatic disease. Additionally, FAPIs have short biological half-lives, a key determinant for isotope selection, which limits their theranostic applications. A monocloncal antibody is better suited for therapeutic development given the higher therapeutic benefit from prolonged exposure to cytotoxic agents. A FAP-targeted peptide, FAP-2286, is also being developed for peptide-targeted radionuclide therapy by Clovis Oncology, but so far no data has been published [40]. While these studies demonstrate the utility of FAP-targeted imaging, they focus on applications in breast, colorectal, and lung cancers. To date, almost no work has been published on FAP-targeted imaging in prostate cancer. Although PSMA-targeted imaging probes have been used for prostate cancer, variable expression in metastatic tumors and difficulty detecting visceral lesions limits their utility [41]. There remains a need for a selective and sensitive imaging probe that can be widely used in mCRPC patients. FAP-targeted imaging probes could overcome current limitations given the genomic stability of the tumor microenvironment, the essential role of CAFs in tumor progression, and the localized overexpression of FAP in the prostate tumor stroma.
Together, our data support [89Zr]Zr-B12 IgG as an ideal candidate for the noninvasive PET/CT imaging of mCRPC by targeting FAP. In addition to the antibody’s favorable pharmacokinetics and binding affinity, B12 IgG is internalized by FAP-expressing cells as indicated by the high probe uptake, tumor retention, and previous in vitro characterization studies [26]. Antibody internalization is a key property that permits the retention of imaging signal and efficacy of potential immunoconjugate therapies. Thus, these properties warrant further development of B12 IgG for translation into the clinic as a theranostic platform technology. Antibody-chelator conjugation chemistry facilitates the exchange of nuclides that can be used for either diagnostic or therapeutic purposes. Therefore, B12 IgG can be evaluated as a radioimmunotherapy agent. Clinical studies with 177Lu- or 225Ac-PSMA targeted mAbs are currently ongoing and suggest that development of other radioimmunotherapies could be effective against mCRPC [42, 43]. B12 IgG could also be used to create a library of antibody drug conjugates (ADC) for targeted delivery of cytotoxic agents. Finally, combination therapies that target both CAFs and cancer cells are shown to have a synergistic effect [44, 45]. B12 IgG may be used as part of a combination therapy to target the tumor microenvironment and advance therapeutic strategies to improve patient outcomes.
Translational Statement.
Targeted imaging probes for measuring disease burden and therapeutic response are dependent on the detection of unique antigens expressed by the tumor. Men who fail standard-of-care therapies and progress to metastatic castration resistant prostate cancer (mCRPC) are left with few therapeutic options. The current imaging standards for prostate cancer are limited which hinders the development of new and urgently needed therapies. Our study shows fibroblast activation protein alpha (FAP) is a relevant target for the PET/CT imaging of mCRPC. FAP is emerging as the next pan-cancer target given its upregulated expression in cancer-associated fibroblasts and localization to the tumor microenvironment. Our FAP-targeted, radiolabeled probe was validated in preclinical imaging studies and demonstrated to be highly selective for FAP-expressing tumors. This study suggests [89Zr]Zr-B12 IgG is an ideal candidate for noninvasive PET/CT imaging in mCRPC and more importantly it represents a platform technology for the development of theranostics targeting FAP.
Acknowledgements
This work was supported by NIH/NCI R01 CA237272 (to A.M. LeBeau), NIH/NCI R01 CA233562 (to A.M. LeBeau), a 2018 Prostate Cancer Foundation Challenge Award (to A.M. LeBeau), a 2013 Prostate Cancer Foundation Young Investigator Award (to A.M. LeBeau), and NIH/NCI T32 CA009138 (to H.M. Hintz). The authors acknowledge the University Imaging Centers at the University of Minnesota (Minneapolis, MN) for their technical advice and support of various imaging equipment. In particular, the authors thank Jason Mitchell for his PET/CT imaging assistance. We thank the University of Minnesota’s Research Animal Resources staff for assisting with research animal maintenance and include a special thanks to Jason Austin for his help with injections and Yingming Li for her expertise with the IT model. The authors are grateful to Colleen Forster and the rest of the Biorepository and Laboratory Services Division at the University of Minnesota (Minneapolis, MN) for their histology and pathology support.
Footnotes
Disclosure of Potential Conflict of Interest: AM LeBeau and HM Hintz are listed as inventors on pending patent number 16/778,977.
References
- 1.Valkenburg KC, De Groot AE, and Pienta KJ. Targeting the tumour stroma to improve cancer therapy. Nat Rev Clin Oncol 2018;15:366–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen X and Song E. Turning foes to friends: Targeting cancer-associated fibroblasts. Nat Rev Drug Discov 2019;18:99–115. [DOI] [PubMed] [Google Scholar]
- 3.Scanlan MJ, Raj BK, Calvo B, Garin-Chesa P, Sanz-Moncasi MP, Healey JH, et al. Molecular cloning of fibroblast activation protein alpha, a member of the serine protease family selectively expressed in stromal fibroblasts of epithelial cancers. Proc Natl Acad Sci U S A 1994;91:5657–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu F, Qi L, Liu B, Liu J, Zhang H, Che D, et al. Fibroblast activation protein overexpression and clinical implications in solid tumors: A meta-analysis. PLoS One 2015;10:e0116683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tuxhorn JA, Ayala GE, Smith MJ, Smith VC, Dang TD, and Rowley DR. Reactive stroma in human prostate cancer: Induction of myofibroblast phenotype and extracellular matrix remodeling. Clin Cancer Res 2002;8:2912–23. [PubMed] [Google Scholar]
- 6.Hayward SW, Wang Y, Cao M, Hom YK, Zhang B, Grossfeld GD, et al. Malignant transformation in a nontumorigenic human prostatic epithelial cell line. Cancer Res 2001;61:8135–42. [PubMed] [Google Scholar]
- 7.Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, and Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res 1999;59:5002–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tuxhorn JA, Mcalhany SJ, Dang TD, Ayala GE, and Rowley DR. Stromal cells promote angiogenesis and growth of human prostate tumors in a differential reactive stroma (drs) xenograft model. Cancer Res 2002;62:3298–307. [PubMed] [Google Scholar]
- 9.Giannoni E, Bianchini F, Masieri L, Serni S, Torre E, Calorini L, et al. Reciprocal activation of prostate cancer cells and cancer-associated fibroblasts stimulates epithelial-mesenchymal transition and cancer stemness. Cancer Res 2010;70:6945–56. [DOI] [PubMed] [Google Scholar]
- 10.Klarmann GJ, Hurt EM, Mathews LA, Zhang X, Duhagon MA, Mistree T, et al. Invasive prostate cancer cells are tumor initiating cells that have a stem cell-like genomic signature. Clin Exp Metastasis 2009;26:433–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gleave ME, Hsieh JT, Von Eschenbach AC, and Chung LW. Prostate and bone fibroblasts induce human prostate cancer growth in vivo: Implications for bidirectional tumor-stromal cell interaction in prostate carcinoma growth and metastasis. J Urol 1992;147:1151–9. [DOI] [PubMed] [Google Scholar]
- 12.Chung LW, Hsieh CL, Law A, Sung SY, Gardner TA, Egawa M, et al. New targets for therapy in prostate cancer: Modulation of stromal-epithelial interactions. Urology 2003;62:44–54. [DOI] [PubMed] [Google Scholar]
- 13.Kaminski A, Hahne JC, Haddouti El M, Florin A, Wellmann A, and Wernert N. Tumour-stroma interactions between metastatic prostate cancer cells and fibroblasts. Int J Mol Med 2006;18:941–50. [PubMed] [Google Scholar]
- 14.Langsteger W, Rezaee A, Pirich C, and Beheshti M. 18f-naf-pet/ct and 99mtc-mdp bone scintigraphy in the detection of bone metastases in prostate cancer. Seminars in Nuclear Medicine 2016;46:491–501. [DOI] [PubMed] [Google Scholar]
- 15.Wondergem M, Van Der Zant FM, Knol RJJ, Burgers AMG, Bos SD, De Jong IJ, et al. 99mtc-hdp bone scintigraphy and 18f-sodiumfluoride pet/ct in primary staging of patients with prostate cancer. World Journal of Urology 2018;36:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phelps ME. Positron emission tomography provides molecular imaging of biological processes. Proceedings of the National Academy of Sciences 2000;97:9226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Zuckier LS, and Ghesani NV. Dominant uptake of fatty acid over glucose by prostate cells: A potential new diagnostic and therapeutic approach. Anticancer Res 2010;30:369–74. [PubMed] [Google Scholar]
- 18.Zhu A, Lee D, and Shim H. Metabolic positron emission tomography imaging in cancer detection and therapy response. Semin Oncol 2011;38:55–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wondergem M, Van Der Zant FM, Knol RJJ, Burgers AMG, Bos SD, De Jong IJ, et al. (99m)tc-hdp bone scintigraphy and (18)f-sodiumfluoride pet/ct in primary staging of patients with prostate cancer. World J Urol 2018;36:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindenberg ML, Turkbey B, Mena E, and Choyke PL. Imaging locally advanced, recurrent, and metastatic prostate cancer: A review. JAMA Oncol 2017;3:1415–22. [DOI] [PubMed] [Google Scholar]
- 21.Umbehr MH, Muntener M, Hany T, Sulser T, and Bachmann LM. The role of 11c-choline and 18f-fluorocholine positron emission tomography (pet) and pet/ct in prostate cancer: A systematic review and meta-analysis. Eur Urol 2013;64:106–17. [DOI] [PubMed] [Google Scholar]
- 22.Turkbey B, Mena E, Shih J, Pinto PA, Merino MJ, Lindenberg ML, et al. Localized prostate cancer detection with 18f facbc pet/ct: Comparison with mr imaging and histopathologic analysis. Radiology 2014;270:849–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tosoian JJ, Gorin MA, Rowe SP, Andreas D, Szabo Z, Pienta KJ, et al. Correlation of psma-targeted (18)f-dcfpyl pet/ct findings with immunohistochemical and genomic data in a patient with metastatic neuroendocrine prostate cancer. Clin Genitourin Cancer 2017;15:e65–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hintz HM, Cowan A, Shapovalova M, and Lebeau A. Development of a cross-reactive monoclonal antibody for detecting the tumor stroma. Bioconjug Chem 2019; [DOI] [PubMed] [Google Scholar]
- 25.Pflueger D, Terry S, Sboner A, Habegger L, Esgueva R, Lin PC, et al. Discovery of non-ets gene fusions in human prostate cancer using next-generation rna sequencing. Genome Res 2011;21:56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robinson D, Van Allen EM, Wu YM, Schultz N, Lonigro RJ, Mosquera JM, et al. Integrative clinical genomics of advanced prostate cancer. Cell 2015;161:1215–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhanvadia RR, Vanopstall C, Brechka H, Barashi NS, Gillard M, Mcauley EM, et al. Meis1 and meis2 expression and prostate cancer progression: A role for hoxb13 binding partners in metastatic disease. Clin Cancer Res 2018;24:3668–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar A, Coleman I, Morrissey C, Zhang X, True LD, Gulati R, et al. Substantial interindividual and limited intraindividual genomic diversity among tumors from men with metastatic prostate cancer. Nat Med 2016;22:369–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeglis BM and Lewis JS. The bioconjugation and radiosynthesis of 89zr-dfo-labeled antibodies. J Vis Exp 2015; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glumac PM, Gallant JP, Shapovalova M, Li Y, Murugan P, Gupta S, et al. Exploitation of cd133 for the targeted imaging of lethal prostate cancer. Clin Cancer Res 2020;26:1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beltran H, Prandi D, Mosquera JM, Benelli M, Puca L, Cyrta J, et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat Med 2016;22:298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pidsley R, Lawrence MG, Zotenko E, Niranjan B, Statham A, Song J, et al. Enduring epigenetic landmarks define the cancer microenvironment. Genome Res 2018;28:625–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tillmanns J, Hoffmann D, Habbaba Y, Schmitto JD, Sedding D, Fraccarollo D, et al. Fibroblast activation protein alpha expression identifies activated fibroblasts after myocardial infarction. J Mol Cell Cardiol 2015;87:194–203. [DOI] [PubMed] [Google Scholar]
- 34.Welt S, Divgi CR, Scott AM, Garin-Chesa P, Finn RD, Graham M, et al. Antibody targeting in metastatic colon cancer: A phase i study of monoclonal antibody f19 against a cell-surface protein of reactive tumor stromal fibroblasts. J Clin Oncol 1994;12:1193–203. [DOI] [PubMed] [Google Scholar]
- 35.Scott AM, Wiseman G, Welt S, Adjei A, Lee FT, Hopkins W, et al. A phase i dose-escalation study of sibrotuzumab in patients with advanced or metastatic fibroblast activation protein-positive cancer. Clin Cancer Res 2003;9:1639–47. [PubMed] [Google Scholar]
- 36.Fischer E, Chaitanya K, Wuest T, Wadle A, Scott AM, Van Den Broek M, et al. Radioimmunotherapy of fibroblast activation protein positive tumors by rapidly internalizing antibodies. Clin Cancer Res 2012;18:6208–18. [DOI] [PubMed] [Google Scholar]
- 37.Loktev A, Lindner T, Mier W, Debus J, Altmann A, Jager D, et al. A tumor-imaging method targeting cancer-associated fibroblasts. J Nucl Med 2018;59:1423–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lindner T, Loktev A, Altmann A, Giesel F, Kratochwil C, Debus J, et al. Development of quinoline-based theranostic ligands for the targeting of fibroblast activation protein. J Nucl Med 2018;59:1415–22. [DOI] [PubMed] [Google Scholar]
- 39.Kratochwil C, Flechsig P, Lindner T, Abderrahim L, Altmann A, Mier W, et al. (68)ga-fapi pet/ct: Tracer uptake in 28 different kinds of cancer. J Nucl Med 2019;60:801–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Calais J Fap: The next billion dollar nuclear theranostics target? J Nucl Med 2020;61:163–5. [DOI] [PubMed] [Google Scholar]
- 41.Pandit-Taskar N, O’donoghue JA, Durack JC, Lyashchenko SK, Cheal SM, Beylergil V, et al. A phase i/ii study for analytic validation of 89zr-j591 immunopet as a molecular imaging agent for metastatic prostate cancer. Clin Cancer Res 2015;21:5277–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hofman MS, Emmett L, Violet J, A YZ, Lawrence NJ, Stockler M, et al. Therap: A randomized phase 2 trial of (177) lu-psma-617 theranostic treatment vs cabazitaxel in progressive metastatic castration-resistant prostate cancer (clinical trial protocol anzup 1603). BJU Int 2019;124 Suppl 1:5–13. [DOI] [PubMed] [Google Scholar]
- 43.Bal C, Yadav M, Ballal S, Tripathi M, and Sahoo R. Clinical experience on 225ac-psma-617 targeted alpha therapy in metastatic castration resistant prostate cancer patients: Safety and efficacy results. J Nucl Med 2019;60:462-. [Google Scholar]
- 44.Kakarla S, Chow KK, Mata M, Shaffer DR, Song XT, Wu MF, et al. Antitumor effects of chimeric receptor engineered human t cells directed to tumor stroma. Mol Ther 2013;21:1611–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Loeffler M, Kruger JA, Niethammer AG, and Reisfeld RA. Targeting tumor-associated fibroblasts improves cancer chemotherapy by increasing intratumoral drug uptake. J Clin Invest 2006;116:1955–62. [DOI] [PMC free article] [PubMed] [Google Scholar]