Abstract
For centuries, attempts have been continuously made to artificially reconstitute counterparts of in vivo organs from their tissues or cells. Only in the recent decade has organoid technology as a whole technological field systematically emerged and been shown to play important roles in tissue engineering. Based on their self-organizing capacities, stem cells of versatile organs, both harvested and induced, can form 3D structures that are structurally and functionally similar to their in vivo counterparts. These organoid models provide a powerful platform for elucidating the development mechanisms, modeling diseases, and screening drug candidates. In this review, we will summarize the advances of this technology for generating various organoids of tissues from the three germ layers and discuss their drawbacks and prospects for tissue engineering.
Keywords: organoid, tissue engineering, 3D culture
Introduction
The limitations of both research models for organ development and donor sources for organ transplantation have prompted the early study of tissue engineering to generate the functional substitutes for in vivo organs. From 2500 years ago, people attempted to replace the missing teeth with artificial ones that were engraved from the tissues of oxen bones. Thereafter, many efforts were made to replace the other damaged tissues with some artificial structures. A specific organ contains various cell types that have their own functions and are regulated sophisticatedly by specific environmental factors, such as growth factors. In addition, the cells in all tissues are supported by extracellular matrices to interact and communicate with each other (Gumbiner, 1996). Collectively, the contributions from previous studies on tissue engineering can be primarily divided into two categories: many biomaterials have been gradually identified, and several kinds of three-dimensional matrices that mimic their extracellular matrices have been successfully created (Hollister, 2005; Bonzani et al., 2007; Moutos et al., 2007; Balakrishnan and Banerjee, 2011; Li et al., 2017; Nezakati et al., 2018). On the other hand, a large body of work has been made through the studies of stem cell biology aiming to generate the diversified cell types in vitro. Notably, pluripotent stem cell (PSC) lines, which resemble embryonic stem cells (ESCs) due to their robust proliferation and flexible differentiation into three embryonic cell layers, were successfully established from mice in 1981 and from humans in 1998 (Evans and Kaufman, 1981; Thomson et al., 1998). Then, PSC was reported to be induced from the fibroblasts of either mouse or human and were able to differentiate into different cell types (Takahashi and Yamanaka, 2006; Takahashi et al., 2007). However, the applications of stem cells, especially the adult stem cells, are still hindered by their limited maintenance and expansion in vitro. In recent years, with the elucidation of stem cell niches and the key signaling pathways that both support and regulate stem cells, organoid technology has shown dramatic advances and has facilitated tissue engineering remarkably.
Organoids are termed as the in vitro biological complexes with 3D structures that contain one or more cell types and that partially recapitulate the structure and function of their in vivo counterparts. The initial development of organoid technology can be traced back to the 1970s, when James G. Rheinwald and Howard Green generated the stratified squamous epithelial colonies, which provided a basis for generating the 3D structures in vitro. However, at that time, the used method was just straightforward by plating the primary human keratinocytes onto three T3 fibroblasts under some culture conditions (Rheinwald and Green, 1975). Later, the significant advances in technologies related to extracellular biology and suspension culture paved the way for the further development of organoid technology. By year of 2009, the group of Dr Hans Clevers reported that the intestinal organoid could be generated from a single intestinal stem cell without a mesenchymal niche (Sato et al., 2009). This achievement became a landmark in the history of organoid technology, which inspired the following productions of major 3D structures resembling many other organs in ectoderm, mesoderm, and endoderm (Pastrana, 2013; Lancaster and Knoblich, 2014; Clevers, 2016; Fatehullah et al., 2016; Kelava and Lancaster, 2016; Yin et al., 2016; Barkauskas et al., 2017; Bredenoord et al., 2017; Di Lullo and Kriegstein, 2017; Huch et al., 2017; Quadrato and Arlotta, 2017; Takebe et al., 2017; Rossi et al., 2018; Qian et al., 2019) and pushed ahead with the application of organoid technology in tissue engineering. Here, we will summarize the discoveries in organoid technology for tissue engineering and discuss the challenges as well as the promises for their expectable future developments.
Current organoid technologies
The ectoderm organoids
The ectoderm is the outermost layer of the embryo and finally generates the epidermis and nerve tissues including the brain and skin. To date, organoid systems for both the brain and skin have been established. The brain is the most complex organ in the body, which contains the cortex, brain stem, basal ganglia, and cerebellum. Among these regions, the cortex is the largest part and controls higher nervous activities. During human cortical neurogenesis, the neuroepithelial progenitors either proliferate symmetrically to amplify the pool of cortical progenitors or give rise to the radial glial (RG) progenitors. The RG progenitors reside in the ventricular zone (VZ) with other progenitors. The intermediate progenitors are enriched in the VZ. The neuron progenitors generate the diverse neurons. Both neurons and astrocytes constitute the cortical plate (CP) and are skirted by pioneer neurons, the Cajal–Retzius cells (Di Lullo and Kriegstein, 2017).
The generation of in vitro brains with functional brain compartments will significantly affect research on neural development and disease. Many efforts have been made to recapitulate specific human brain regions in vitro from ESCs or PSCs (Eiraku et al., 2008; Muguruma et al., 2010; Danjo et al., 2011; Eiraku and Sasai, 2012; Mariani et al., 2012). The groups of Andrew P. Jackson and Juergen A. Knoblich successfully generated the self-assembled cerebral organoid of the developing brain as a whole for the first time (Lancaster et al., 2013). Based on previous works, they cultured the human PSC (hPSC)-derived embryoid bodies in a bioreactor in order to form large and complex structures. In their culture system, the researchers used the Matrigel as the scaffold and the spinning bioreactor to enhance nutrient absorption, distinguishing their strategy from previous methods. These techniques were vital for complex brain organoid formation. With a fully established protocol, they finally obtained the cerebral organoids with discrete brain-like regions, including the specific region of the cortex. Moreover, the derived cortex region in the cerebral organoids was found to contain the progenitor populations that produced the mature cortical neuron subtypes. However, all those brain-like regions in cerebral organoids were incompletely differentiated, and the layers of cortex were incomplete and lack CPs. Thus, they were still the immature organoids for both brain and cortex.
To generate more mature brain organoids, scientists have successfully established a strategy for the generation of brain-region-specific organoids from human induced PSCs (iPSCs) or ESCs (hESCs) (Mariani et al., 2015; Jo et al., 2016; Qian et al., 2016; Gabriel et al., 2017; Lancaster et al., 2017; Zhou et al., 2017). In particular, the generated forebrain organoids recapitulated the cortical structures with distinct layers resembling the in vivo brain zones, including VZ, the inner and outer VZ (iSVZ and oSVZ), and the CP, at molecular, cellular, and structural levels (Qian et al., 2016). In addition, the application of biomaterial [poly (lactide-coglycolide) copolymer (PLGA) fiber] could facilitate the reproducible establishment of the brain organoids with the characteristic cortical tissue architecture including a polarized CP and radial units (Lancaster et al., 2017). Furthermore, the scientists fused the specific organoids of different brain regions to model interregional interactions. It was reported concurrently that the assemblies of integrated human forebrain spheroids could be generated through the fusion of hPSC-derived dorsal or ventral forebrain organoids and that these fused organoids could model the interregional migration of γ-aminobutyric-acid-releasing (GABAergic) neurons (Bagley et al., 2017; Birey et al., 2017; Xiang et al., 2017). Moreover, through the fusion of thalamic organoids and brain organoids, the research group of In-Hyun Park also observed the reciprocal projections (Xiang et al., 2019). In addition, a system to recapitulate the in vivo-like topography in brain organoids was established. In this system, the researchers first generated inducible Sonic Hedgehog (SHH)-expressing hPSC cell aggregates and seeded wild-type hPSCs on top of them to form chimeric spheroids. Therefore, an SHH protein gradient was triggered by inducible SHH-expressing hPSC cell aggregates to establish the in vivo-like topography for major forebrain subdivisions during the development of forebrain organoids (Cederquist et al., 2019).
The above reported findings on in vitro models have shed light on brain development, function, and disease. However, the lack of an appropriate microenvironment, such as the vasculature, prevents these brain organoids from fully mimicking in vivo brain. To solve this problem, researchers have developed a vascularized and functional form of an in vivo model of human brain organoids (Mansour et al., 2018). After in vivo transplantation into the mouse adult brain, the human brain organoids could develop a functional vasculature system that could communicate with the host brain and showed various in vivo functional processes, including progressive neuronal differentiation and maturation, gliogenesis, integration of microglia, as well as growth of axons in the multiple regions of the host brain. Moreover, some organoid models of other brain regions, which contained several particular regions including the hippocampus (Sakaguchi et al., 2015), midbrain (Monzel et al., 2017), hypothalamus (Qian et al., 2018), cerebellum (Muguruma et al., 2015; Muguruma, 2018), pituitary (Zimmer et al., 2016), and retina (Eldred et al., 2018) were generated.
Another important derivative of ectoderm is the skin epidermis. Skin is composed of the epidermis and dermis. The dermis mainly originates from the mesoderm and produces other skin components, such as hair follicles (HFs), sweat glands, and nails (Fuchs, 2007). The generation of artificial skin substitutes has a long history, and the generated in vitro skin substitutes are presented as a skin-like bilayer by coculture of mesenchymal cells with epithelial cells either harvested or induced from PSCs (Zheng et al., 2005; Ehama et al., 2007; Oh et al., 2013; Sun et al., 2014; Gledhill et al., 2015). However, these models show limited potential in delineating the self-organization process, and organoid models have attracted increased attention. The Cheng-Ming Chuong group generated hair-bearing skin organoids from the dissociated single cells of neonatal mouse dorsal skin that provided a platform for a time-lapse analysis of cell behaviors and experimental manipulations (Lei et al., 2017). In addition, the Karl R. Koehler research team reported skin organoids with 3D culture from a homogeneous population of mouse PSCs (Lee et al., 2018a). These skin organoids could recapitulate fetal hair folliculogenesis by producing de novo HFs.
The organoid systems for ectoderm-derived tissues provide major insights into the development, function, and dysfunction of tissues and facilitate the establishment of organoid models for other tissues. In the near future, further studies may focus on the in vitro generation of more complex structures to recapitulate the interaction between neurological tissue and its microenvironment, such as the immune system.
The mesoderm organoids
During embryonic development, the mesoderm develops into the muscle, skin, gonad, and circulatory system including the heart, vasculature, blood, and kidney. Organoid models for kidney and blood vessels have been established.
As one of the most complex organs, the kidney develops from a ureteric bud (UB) and the adjacent intermediate mesoderm (IM). The IM further gives rise to metanephric mesenchyme (MM). MM can form nephrons, the structural and functional units of the kidney nephron. Each unit is composed of one renal corpuscle and one renal tubule. The first self-organizing kidney organoids with various key cellular populations were derived from hPSCs. This model was based on the findings that hESCs could differentiate into both UBs and MM, which further involved into generating nephrons after self-organization (Takasato et al., 2014). The research groups of Melissa H. Little and Joseph V. Bonventre concurrently reported that the self-assembled hPSC pellets could generate the kidney organoids that contained the nephrons associated with collecting duct networks surrounded by renal interstitium and endothelial cells through a designed process with step-by-step inductions of defined growth factors (Morizane et al., 2015; Takasato et al., 2015). In addition, the conditions including the soft extracellular environment, flow environment, and dynamic modulation of Wnt signaling were all known to be effective for the further induction of kidney organoid maturation (Garreta et al., 2019; Homan et al., 2019; Low et al., 2019). Moreover, the latter two conditions could be important for inducing the vascularization in hPSC-derived kidney organoids.
In addition to kidney organoids, other mesoderm organoids, such as blood vessel organoids, were generated (Wimmer et al., 2019). The group of Josef M. Penninger successfully developed a protocol to induce hPSCs into mesoderm cells that could be further induced to form blood vascular organoids. These blood vessel organoids were composed of both endothelial cells and pericytes, which self-assembled into cell complexes. Interconnected capillary networks enveloped by basement membranes were established in matured blood vascular organoids. After transplantation, blood vascular organoids could form a stable, perfused vascular tree, including arteries, arterioles, and venules. Upon exposure to hyperglycemia, these blood vessel organoids either cultured in vitro or transplanted in vivo could model the microvascular changes found in patients with diabetes and serve as a practical tool to identify regulators of diabetic vasculopathy.
Both the kidney and blood vessels are complex organs. The successfully generated organoid models further highlighted the powerful potential of organoid technology in tissue engineering, although optimization is needed for the generation of more mature and complex structures.
The definitive endoderm organoids
During embryonic development, the definitive endoderm gradually develops into the foregut, midgut, and hindgut (Zorn and Wells, 2009). The foregut further generates the thyroid, esophagus, trachea, stomach, lung, liver, biliary system, and pancreas (Zorn and Wells, 2009). The midgut gives rise to the small intestine, while the hindgut forms the colon. The organoid culture systems of many endoderm-derived organs including thyroid (Eldred et al., 2018), lung (Chen et al., 2017; Miller et al., 2018, 2019; Sachs et al., 2019), stomach (Barker et al., 2010; McCracken et al., 2014; Bartfeld et al., 2015), liver (Huch et al., 2015; Coll et al., 2018; Hu et al., 2018; Vyas et al., 2018; Artegiani et al., 2019), biliary system (Ogawa et al., 2015; Sampaziotis et al., 2015; Vyas et al., 2018), pancreas (Boj et al., 2015; Hohwieler et al., 2017), intestine (Sato et al., 2009; Spence et al., 2011), and colon (Yui et al., 2012; Crespo et al., 2017; Sugimoto et al., 2018) have been established successfully. Next, we will introduce these systems one by one according to their body locations from top to bottom.
Esophagus and lung organoids
The esophagus consists of a stratified squamous epithelium, muscle layers, and the enteric nervous system to sense stretch and control peristalsis. The keratinizing stratified epithelium could be generated as organoids in the ‘mini-gut’ medium (DeWard et al., 2014). Notably, two research groups simultaneously reported that they successfully generated the human esophageal organoids from hPSCs that contained the proliferative basal progenitors and the differentiated stratified squamous epithelium (Trisno et al., 2018; Zhang et al., 2018b). The establishment of human esophageal organoids is important for modeling human esophageal development and pathologies.
Lung respiratory trees and the adjacent cardiopulmonary vasculature precisely interact for gas exchange. During embryogenesis, the Nkx2.1-expressing (Nkx2.1+) cells in the foregut can form trachea and two primary lung buds. Then, each lung bud starts to branch and generates air sacs called alveoli which contribute to the function of gas exchange and the establishment of the airway in the proximal regions (Kotton and Morrisey, 2014). The lung organoid was reported to be generated from both adult stem cells and PSCs, either ESCs or hPSCs (Barkauskas et al., 2017). Lung basal cells, airway secretory club cells (previously known as Clara cells), and AEC2 cells can form lung organoid with varied differentiation capacities. Based on the activations of signaling pathways that direct lung development, the protocols to induce PSCs to differentiate into proximal and distal lung epithelial populations were successfully established (Huang et al., 2015b). The Jason R. Spence group successfully generated the human lung organoid similar to fetal lung (Dye et al., 2016; Miller et al., 2018, 2019). To further recapitulate the lung development, such as branching morphogenesis and proximodistal specification, the group of Hans–Willem Snoeck generated the lung bud organoid (LBO) from hPSCs, which contained both mesoderm and pulmonary endoderm. Each group of cells in the LBOs coordinately developed into branching airway and early alveolar structures following either in vivo transplantation or in vitro culture with Matrigel (Chen et al., 2017).
Liver and pancreatic organoids
The liver is comprised of many liver lobules and has many critical functions, such as bile acid secretion, drug metabolism, and ALB production, which are essential for food metabolism and detoxification. As the structural and functional unit of the liver, each liver lobule is composed of bile duct, a central vein, and a portal vein with hepatocytes arranged in linear cords between the capillary networks. Hepatocytes and bile duct cells are the main epithelial cell types in all liver lobules. Together, these two types of cells are responsible for major liver functions, while the hepatocytes especially have more other functions. During embryogenesis, both hepatocytes and bile duct cells are derived from the hepatoblasts in the foregut. In the adult liver, both cell types could replenish themselves, and more interestingly, they were found to convert into each other upon different particular liver injuries (Deng et al., 2018; Schaub et al., 2018). The first adult stem cell-derived liver organoid was generated from isolated Lgr5+ cells that were collected from the emerged cells around the bile duct after carbon tetrachloride (CCl4) treatment (Huch et al., 2013). These Lgr5+ cell-derived liver organoid can form both hepatocytes and bile duct cells after differentiation. Bipotential progenitor cells have been reported in adult human liver and could be isolated to generate liver organoids. These progenitor cell-derived liver organoids could differentiate into hepatocytes and cholangiocytes (Huch et al., 2015). To functionally mimic the in vivo liver organ, several groups also have successfully generated the mature hepatocyte-derived organoids (Hu et al., 2018; Zhang et al., 2018a). However, the proliferative capacities of all of these human mature hepatocyte-derived organoids are limited. To solve this problem, the Yunfang Wang research team generated liver organoids from hESCs that possessed a high proliferative ability and could generate functional hepatic organoids (Wang et al., 2019). To form complex structures and model liver development in vitro, the Shay Soker group plated fetal liver cells into acellular liver extracellular matrix scaffolds to form hepatobiliary organoids with both hepatocytes and bile duct structures (Vyas et al., 2018). Moreover, the 3D aggregates of PSC, human umbilical vein endothelial cells (HUVECs), and human mesenchymal stem cells (MSCs) could form a liver bud containing blood vessels (Takebe et al., 2013).
The pancreas consists of the exocrine structures, including duct cells and acinar cells, that secrete metabolism enzymes as well as the endocrine components known as islets composed of α, β, γ, and δ cells that control the blood sugar homeostasis. In the field of pancreatic tissue engineering, the organoid models of both normal and pathological exocrine duct structures were well established (Boj et al., 2015; Hohwieler et al., 2017). These organoids showed the characteristics of ductal cells and a strong proliferative capacity. In addition, one group reported that acinar cells could be dedifferentiated into progenitor-like cells by 3D suspension culture (Baldan et al., 2019). However, whether these progenitor-like cells can be differentiated back into acinar cells remains unclear. To date, the development of culture systems for endocrine components was still at an early stage. Based on the previous fundamental works on the successful generation of the functional islet cells from hPSCs (Pagliuca et al., 2014; Li et al., 2014a), the group of Jianhua Qin generated the islet-like organoids from hPSCs with an organ-on-a-chip platform (Tao et al., 2019). These islet-like organoids contained islet-specific α and β-like cells with favorable cell viability. However, these organoids required further maturation to fulfill the function of in vivo islets. In addition, Fanny Lebreton and colleagues found that the incorporation of human amniotic epithelial cells (hAECs) could promote to form the viable and functional organoids from human islets cells (Lebreton et al., 2019). In the near future, the pancreatic organoids will be expected to contain structural and functional units for both exocrine parts and endocrine components.
Stomach organoids
The human stomach can be divided into two parts, the corpus and the antrum, and the mouse stomach contains an additional forestomach region. Different regions are populated with different stem cell populations (Kim and Shivdasani, 2016). A single mouse Lgr5+ve cell located in the antrum region was shown to continuously expand for a long-term under the culture condition containing EGF, Noggin, and R-spondin1, and the expanded cells could form the organoids akin to mature pyloric epithelium (Barker et al., 2010). In addition to the establishment of mouse stomach organoids, the human stomach organoids were also successfully generated by the same group (Bartfeld et al., 2015). Notably, the cells of human stomach organoids could be further induced into some specific lineages of either gastric gland or gastric pit. In addition to adult stem cells, hPSC could be differentiated into stomach organoids (McCracken et al., 2014). These hPSC-derived human stomach organoids contained many specific gastric epithelial cells for primitive gastric gland- and pit-like domains, proliferative zones containing LGR5-expressing cells, and surface and antral mucous cells. In addition, these organoids also contained various gastric endocrine cells. These human gastric organoids provide a powerful platform to model gastric development and diseases, such as the identification of the signaling mechanisms that regulate gastric endocrine cell differentiation. Moreover, protocols for generating organoids containing both corpus- and antrum-specific cells from either ESCs or PSCs have been established (Noguchi and Kurisaki, 2017; Broda et al., 2019).
Small intestinal and colon organoids
In the adult small intestine, enterocytes, goblet cells, and enteroendocrine cells are all derived from transient amplifying progenitors in the crypt and migrate into the villi with a turnover of 5 days (Barker et al., 2007). The Hans Clevers group successfully identified the Lgr5+ cells as small intestinal stem cells and generated the first intestine organoid from the single Lgr5+ stem cell. The Lgr5+ stem cell-derived small intestinal organoid resembled the in vivo counterpart with a proliferative crypt and villi structure encompassing all differentiated cell types (Sato et al., 2009). On the other hand, the group of James M. Wells also successfully generated the characteristic small intestinal organoids from hPSCs through induction with a temporal series of growth factor according to the manipulations during embryonic small intestinal development (Spence et al., 2011). These hPSC-derived small intestine organoids could further mature after in vivo transplantation. To improve the in vitro culture conditions for the development of intestinal organoids, Nikolce Gjorevski and colleagues delineated the dynamic changes in the environment during organoid culture and produced the mechanical matrices to support organoid growth (Gjorevski et al., 2016). The researchers found that the high matrix stiffness significantly enhanced the small intestinal stem cells expansion, while a soft matrix promoted the both differentiation and the formation of small intestinal organoids.
Under specific conditions, a single Lgr5+ stem cell had the potential to form colon organoids that could integrate into the damaged colon in vivo and form the normal crypts with structural and functional characteristics (Yui et al., 2012). In addition to Lgr5+ stem cells as adult stem cells, the hPSCs could form colon organoids that recapitulated the characteristics of in vivo colons (Crespo et al., 2017; Sugimoto et al., 2018).
Prostate organoids
The prostate is a complex tubuloalveolar gland that secrets the fluids to nourish and protect germ cells. The prostate epithelium contains luminal cells, basal cells, and neuroendocrine cells. Luminal cells are located in the inner layer, while the basal cells and neuroendocrine cells reside in the outer layer. Both luminal cells and basal cells have potential to act as progenitor cells and to form prostate organoids (Valdez et al., 2012; Chua et al., 2014; Karthaus et al., 2014; Crowell et al., 2019). To date, all of the reported prostate organoids contain both luminal cells and basal cells with the characteristic locations. The prostate organoid model can serve as a powerful tool to study the behaviors of prostate cells in both normal and disease conditions. Zachary Richards and colleagues demonstrated that the organoid system could be utilized to study the interaction of stromal cells with epithelial cells (Richards et al., 2019). The organoids generated from different genetically engineered mouse models or patient tissue samples provide a platform for gene editing or drug treatment of cancer cells in vitro (Gao et al., 2014; Barros-Silva et al., 2018; Lawrence et al., 2018; Puca et al., 2018; Sandoval et al., 2018; Adams et al., 2019; Pappas et al., 2019; Zadra et al., 2019).
Organoid models for endoderm tissues have been extensively established, partially resulting from the developmental similarity between different endoderm organs. This similarity made it possible for the development of organoid culture conditions that could be suitable for other tissues.
Applications of the engineered organoids
Organoid models have been used to elucidate crucial scientific questions and mimic human diseases. Various previously generated organoids in vitro can recapitulate the in vivo organs to some extent, and these generated organoids can be utilized to model specific processes in dynamic development, various organ-specific diseases, human cancer, and drug testing.
Anahita Amiri and colleagues reported that the hPSC-derived cerebral cortical organoids could model the dynamic process of human cortical development and be utilized to depict the transcriptomic and epigenomic change during cortical development. In addition, these hPSC-derived cerebral cortical organoids were used to analyze the differentially active genes and enhancers during the transition of stem cells to progenitors (Amiri et al., 2018). Indeed, organoid technology has been benefited from the development of molecular analyses, which will help further elucidate the molecular mechanisms conversely. In addition, organoid technology can be utilized in studies on other organ diseases. Many achievements utilizing these technologies have been reported. For example, brain organoids could be utilized to model ZIKV infection and psychiatric diseases (Mariani et al., 2015; Qian et al., 2016; Birey et al., 2017; Gabriel et al., 2017). Kidney organoids were generated for personalized disease modeling such as BK virus infection, cystic fibrosis, and polycystic kidney disease (Morizane et al., 2015; Cruz et al., 2017; Low et al., 2019; Schutgens et al., 2019). Blood vessel organoids played a role in modeling diabetic vasculopathy (Wimmer et al., 2019); gastric organoids provided a platform for Helicobacter pylori infection (Wimmer et al., 2019). Lung organoids could be infected with respiratory syncytial virus to model lung disease (Chen et al., 2017). Liver organoids were proven to be a powerful tool to study liver steatohepatitis (Artegiani et al., 2019; Ouchi et al., 2019). The application of chemical probes in 3D gastric organoids could help elucidate the key transition from the mitotic spindle to the central spindle before anaphase onset (Liu et al., 2020).
Moreover, organoid technology could be applied to cancer tissue engineering, including modeling the tumor formation process or generating tumor biobanks. The combination of CRISPR–Cas9-mediated gene editing and organoid technology facilitates the investigation of potential driver genes in tumor formation with in vitro models, which has shed light on brain (Bian et al., 2018), gastrointestinal (Li et al., 2014b), and liver cancer (Artegiani et al., 2019). Undoubtedly, this model will benefit from high-throughput screening of target genes involved in the tumor process and contribute to deciphering the dynamic changes during tumor formation and progression. In addition, organoid banks for glioblastoma (Jacob et al., 2019), breast cancer (Sachs et al., 2018), liver cancer (Broutier et al., 2017), pancreatic cancer (Boj et al., 2015; Huang et al., 2015a; Roe et al., 2017; Tiriac et al., 2018), prostate cancer (Gao et al., 2014), colorectal cancer (van de Wetering et al., 2015; Weeber et al., 2015; Crespo et al., 2017; Bolhaqueiro et al., 2019), bladder cancer (Lee et al., 2018b), endometrial cancer (Boretto et al., 2019), rectal cancer (Ganesh et al., 2019; Yao et al., 2020), ovarian cancer (Kopper et al., 2019), and esophageal cancer (Li et al., 2018; Kijima et al., 2019) have been established. Inter- and intratumoral heterogeneity is present, and this heterogeneity emphasizes the importance of precision medicine. Organoid biobanks from patient tissues offer the opportunity to test drugs for individual patients and help illustrate the genetic and epigenetic landscape of numerous cancer samples to advance the mechanistic understanding of tumors.
In the near future, we predict that organoid technology will play increasingly important roles in modeling and delineating the processes during the normal organ development or under its diseases conditions.
Drawbacks for organoid technology
Although organoid technology shows the strong potential in tissue engineering, further developments are needed to precisely and efficiently generate in vitro organ counterparts. At present, there are still many drawbacks in this field, including the limited maturity and cell diversity of the currently generated in vitro organoids when considered to replace the in vivo organs, the unsuitable size of generated organoids for organ transplantation, and the poor reproducibility for massive production and the deficiency of the vascular, nervous, and immune system to recapitulate the in vivo tissue interaction.
Currently, all organoids generated under in vitro conditions cannot fully recapitulate organs on both cell types and cell maturity. It is still difficult to fully mimic in vivo situations under in vitro conditions. In part, the limitation is because the development process is dynamic and the needed growth factors are released in a pulse and concentration-dependent manner for the commitments of different cell types. However, the difficulties could be overcome to attain the long-term goals of generating the mature organoids for all different organs. Some strides have already been made in the study of brain organoids. For example, release has been successfully established by the aggregation of inducible SHH-expressing hPSCs. Under this condition, the concentrations of SHH can be controlled in a distance-dependent manner (Cederquist et al., 2019). This system can be regarded as the signaling center to induce the self-organization along the dorso-ventral and antero-posterior positional axes. In addition to the achievement of the signaling center, the organoids of individual brain parts could be generated separately then combined finally as fused brain organoids to form more mature and more complex brain organoids (Bagley et al., 2017; Birey et al., 2017; Xiang et al., 2017, 2019). Moreover, with advances in the development of development biology and molecular biology, recapitulation of any elaborate process in vitro could be possible in the future.
Presently, many organoids are derived from the iPSCs. iPSCs that are incompletely differentiated have the potential to form tumors such as teratomas. Generally, iPSCs can generate many byproducts, such as mesenchymal cells, during the induction process. Furthermore, most iPSC-derived tissues are still at the fetal stage and are less mature than adult tissues. Therefore, the identification and purification of the adult stem cells to generate various organoids is still an indispensable strategy for generating organoids at the present times.
More importantly, most currently generated organoids have a limited size partially due to the lack of blood vessels and vascular structures for adequate nutrient absorption. The current method to solve this problem is to coculture with the HUVECs that can be derived from iPSCs (Takebe et al., 2013). However, such a method needs to be tested broadly and proven during the processes of generating the organoids for all of organs. One concern is that the incorporation of HUVECs may result in immunological rejection.
Moreover, the conditions for organoid cultures are still not fully optimized, which can result in a varied degree of reproducibility even though under the same conditions. This phenomenon was discussed in a study comparing the cell populations of individual brain organoid generated by different methods, which revealed that the organoids were only highly reproducible under one condition but varied substantially in other conditions (Velasco et al., 2019). Thus, it is necessary to identify the determinants for generating the reproducible organoids, which can be generally used to generate the reproducible organoids for all organs. This is a fundamental requirement for in vitro organoids to provide source of organ transplantation.
Finally, the biological activities in organs usually require the crosstalk between parenchymal cells and immune cells or even cells of the peripheral nervous system. However, most of the currently generated organoids still lack of cells of both the immune system and peripheral nervous system. This existing deficiency hinders organoids from completely reflecting the fully natural characteristics of their in vivo counterparts for modeling both developmental and pathological processes.
Prospects for organoid technology
In the future, the above limitations will be gradually resolved, and the organoid cultures will potentially be facilitated by the application of 3D bioprinting. As a new and potentially promising technology, 3D bioprinting enables the fabrication of biomaterials according to designed structures. These structures are joined by hydrogels that have many advantages in properties, including printability, crosslinking, biocompatibility, and controllability (Karthaus et al., 2014; Crowell et al., 2019). Studies have shown that 3D bioprinting technology can print the complex and well-separated collagen scaffolds (Kang et al., 2016; Lee et al., 2019). In addition, other reported findings demonstrated that different cell types or subtypes could be arranged in surroundings with different cell matrices (Engler et al., 2006; Pek et al., 2010; Huang et al., 2017; Brusatin et al., 2018). In combinations of 3D bioprinting technology, the designed structures with more cellular-specific and well-separated properties will be printed for applications in organoid technology, which should be more suitable to support the growth and maturation of different cell types and to maintain their overall cell diversities. Moreover, 3D bioprinting can produce the multiple scales of vasculatures for improving the nutrient absorption and enlarging the size of organoids (Lee et al., 2019). Ideally, the generated organoid can be expected to become an in vitro functional organ with different parenchymal cell types, blood vessels, and the nervous and immune systems under some particular conditions.
Conclusion
The rapid development of organoid technology in tissue engineering has occurred in the last decade. Organoid culture systems have been established for almost all organs derived from three germ layers to recapitulate their in vivo counterparts (Figure 1), even though their maturity and complexity are varied. The generated organoids will contribute to the future research on the mechanisms of organ development and supply new promising opportunities for disease modeling and drug screening. However, the current organoid technology still has many weaknesses and limitations in maturity, reproducibility, and complexity, because the presently generated organoids still cannot fully recapitulate in vivo organ development. Through a deeper understanding of stem cell biology and developmental biology as well as the upcoming progress in material technology such as 3D printing, we will be able to prospect that the new organoid technology will promote the development of tissue engineering and facilitate both fundamental and clinical research by fully modeling the in vivo counterparts structurally and functionally for each in vivo organ.
Figure 1.
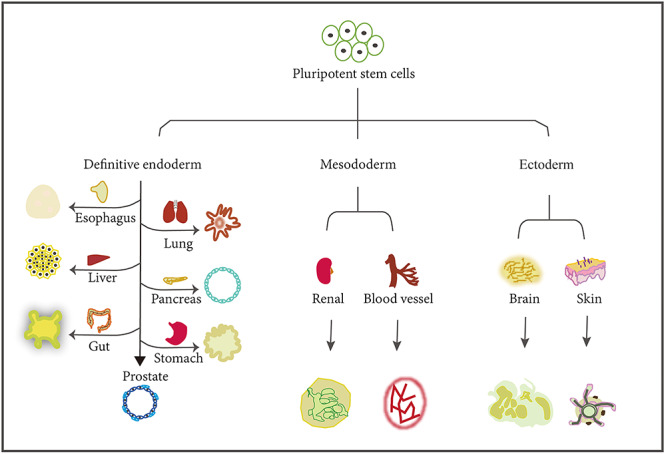
The versatile organoids generated from hPSCs. The organoids for organs in the three germ layers can be derived from iPSCs. PSCs could be induced to form the three germ layers, including the definitive endoderm, mesoderm, and ectoderm. The three germ layers could be further differentiated into various organs, such as the esophagus, lung, liver, pancreas, gut, stomach, and prostate for definitive endoderm, renal and blood vessels for mesoderm, and brain and skin for ectoderm.
Funding
This study was supported by grants from the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA16020905 and XDB19000000), the National Key Research and Development Program of China (2017YFA0505500 and 2018ZX10302207-00*-001), the Basic Frontier Science Research Program of Chinese Academy of Sciences (ZDBS-LY-SM015), and the National Natural Science Foundation of China (81830054 and 81772723).
Conflict of interest: none declared.
References
- Adams, E.J., Karthaus, W.R., Hoover, E., et al. (2019). FOXA1 mutations alter pioneering activity, differentiation and prostate cancer phenotypes. Nature 571, 408–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiri, A., Coppola, G., Scuderi, S., et al. (2018). Transcriptome and epigenome landscape of human cortical development modeled in organoids. Science 362, eaat6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artegiani, B., van Voorthuijsen, L., Lindeboom, R.G.H., et al. (2019). Probing the tumor suppressor function of BAP1 in CRISPR-engineered human liver organoids. Cell Stem Cell 24, 927–943.e6. [DOI] [PubMed] [Google Scholar]
- Bagley, J.A., Reumann, D., Bian, S., et al. (2017). Fused cerebral organoids model interactions between brain regions. Nat. Methods 14, 743–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakrishnan, B., and Banerjee, R. (2011). Biopolymer-based hydrogels for cartilage tissue engineering. Chem. Rev. 111, 4453–4474. [DOI] [PubMed] [Google Scholar]
- Baldan, J., Houbracken, I., Rooman, I., et al. (2019). Adult human pancreatic acinar cells dedifferentiate into an embryonic progenitor-like state in 3D suspension culture. Sci. Rep. 9, 4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkauskas, C.E., Chung, M.I., Fioret, B., et al. (2017). Lung organoids: current uses and future promise. Development 144, 986–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker, N., Huch, M., Kujala, P., et al. (2010). Lgr5+ve stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 6, 25–36. [DOI] [PubMed] [Google Scholar]
- Barker, N., van Es, J.H., Kuipers, J., et al. (2007). Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007. [DOI] [PubMed] [Google Scholar]
- Barros-Silva, J.D., Linn, D.E., Steiner, I., et al. (2018). Single-cell analysis identifies LY6D as a marker linking castration-resistant prostate luminal cells to prostate progenitors and cancer. Cell Rep. 25, 3504–3518.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartfeld, S., Bayram, T., van de Wetering, M., et al. (2015). In vitro expansion of human gastric epithelial stem cells and their responses to bacterial infection. Gastroenterology 148, 126–136.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian, S., Repic, M., Guo, Z., et al. (2018). Genetically engineered cerebral organoids model brain tumor formation. Nat. Methods 15, 631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birey, F., Andersen, J., Makinson, C.D., et al. (2017). Assembly of functionally integrated human forebrain spheroids. Nature 545, 54–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boj, S.F., Hwang, C.I., Baker, L.A., et al. (2015). Organoid models of human and mouse ductal pancreatic cancer. Cell 160, 324–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolhaqueiro, A.C.F., Ponsioen, B., Bakker, B., et al. (2019). Ongoing chromosomal instability and karyotype evolution in human colorectal cancer organoids. Nat. Genet. 51, 824–834. [DOI] [PubMed] [Google Scholar]
- Bonzani, I.C., Adhikari, R., Houshyar, S., et al. (2007). Synthesis of two-component injectable polyurethanes for bone tissue engineering. Biomaterials 28, 423–433. [DOI] [PubMed] [Google Scholar]
- Boretto, M., Maenhoudt, N., Luo, X., et al. (2019). Patient-derived organoids from endometrial disease capture clinical heterogeneity and are amenable to drug screening. Nat. Cell Biol. 21, 1041–1051. [DOI] [PubMed] [Google Scholar]
- Bredenoord, A.L., Clevers, H., and Knoblich, J.A. (2017). Human tissues in a dish: the research and ethical implications of organoid technology. Science 355, 6322. [DOI] [PubMed] [Google Scholar]
- Broda, T.R., McCracken, K.W., and Wells, J.M. (2019). Generation of human antral and fundic gastric organoids from pluripotent stem cells. Nat. Protoc. 14, 28–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broutier, L., Mastrogiovanni, G., Verstegen, M.M., et al. (2017). Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat. Med. 23, 1424–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusatin, G., Panciera, T., Gandin, A., et al. (2018). Biomaterials and engineered microenvironments to control YAP/TAZ-dependent cell behaviour. Nat. Mater. 17, 1063–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cederquist, G.Y., Asciolla, J.J., Tchieu, J., et al. (2019). Specification of positional identity in forebrain organoids. Nat. Biotechnol. 37, 436–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y.W., Huang, S.X., de Carvalho, A., et al. (2017). A three-dimensional model of human lung development and disease from pluripotent stem cells. Nat. Cell Biol. 19, 542–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua, C.W., Shibata, M., Lei, M., et al. (2014). Single luminal epithelial progenitors can generate prostate organoids in culture. Nat. Cell Biol. 16, 951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers, H. (2016). Modeling development and disease with organoids. Cell 165, 1586–1597. [DOI] [PubMed] [Google Scholar]
- Coll, M., Perea, L., Boon, R., et al. (2018). Generation of hepatic stellate cells from human pluripotent stem cells enables in vitro modeling of liver fibrosis. Cell Stem Cell 23, 101–113.e7. [DOI] [PubMed] [Google Scholar]
- Crespo, M., Vilar, E., Tsai, S.Y., et al. (2017). Colonic organoids derived from human induced pluripotent stem cells for modeling colorectal cancer and drug testing. Nat. Med. 23, 878–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell, P.D., Fox, J.J., Hashimoto, T., et al. (2019). Expansion of luminal progenitor cells in the aging mouse and human prostate. Cell Rep. 28, 1499–1510.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz, N.M., Song, X., Czerniecki, S.M., et al. (2017). Organoid cystogenesis reveals a critical role of microenvironment in human polycystic kidney disease. Nat. Mater. 16, 1112–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danjo, T., Eiraku, M., Muguruma, K., et al. (2011). Subregional specification of embryonic stem cell-derived ventral telencephalic tissues by timed and combinatory treatment with extrinsic signals. J. Neurosci. 31, 1919–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, X., Zhang, X., Li, W., et al. (2018). Chronic liver injury induces conversion of biliary epithelial cells into hepatocytes. Cell Stem Cell 23, 114–122.e3. [DOI] [PubMed] [Google Scholar]
- DeWard, A.D., Cramer, J., and Lagasse, E. (2014). Cellular heterogeneity in the mouse esophagus implicates the presence of a nonquiescent epithelial stem cell population. Cell Rep. 9, 701–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lullo, E., and Kriegstein, A.R. (2017). The use of brain organoids to investigate neural development and disease. Nat. Rev. Neurosci. 18, 573–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye, B.R., Dedhia, P.H., Miller, A.J., et al. (2016). A bioengineered niche promotes in vivo engraftment and maturation of pluripotent stem cell derived human lung organoids. eLife 5, e19732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehama, R., Ishimatsu-Tsuji, Y., Iriyama, S., et al. (2007). Hair follicle regeneration using grafted rodent and human cells. J. Invest. Dermatol. 127, 2106–2115. [DOI] [PubMed] [Google Scholar]
- Eiraku, M., and Sasai, Y. (2012). Self-formation of layered neural structures in three-dimensional culture of ES cells. Curr. Opin. Neurobiol. 22, 768–777. [DOI] [PubMed] [Google Scholar]
- Eiraku, M., Watanabe, K., Matsuo-Takasaki, M., et al. (2008). Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell 3, 519–532. [DOI] [PubMed] [Google Scholar]
- Eldred, K.C., Hadyniak, S.E., Hussey, K.A., et al. (2018). Thyroid hormone signaling specifies cone subtypes in human retinal organoids. Science 362, 6411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler, A.J., Sen, S., Sweeney, H.L., et al. (2006). Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689. [DOI] [PubMed] [Google Scholar]
- Evans, M.J., and Kaufman, M.H. (1981). Establishment in culture of pluripotential cells from mouse embryos. Nature 292, 154–156. [DOI] [PubMed] [Google Scholar]
- Fatehullah, A., Tan, S.H., and Barker, N. (2016). Organoids as an in vitro model of human development and disease. Nat. Cell Biol. 18, 246–254. [DOI] [PubMed] [Google Scholar]
- Fuchs, E. (2007). Scratching the surface of skin development. Nature 445, 834–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel, E., Ramani, A., Karow, U., et al. (2017). Recent Zika virus isolates induce premature differentiation of neural progenitors in human brain organoids. Cell Stem Cell 20, 397–406.e5. [DOI] [PubMed] [Google Scholar]
- Ganesh, K., Wu, C., O'Rourke, K.P., et al. (2019). A rectal cancer organoid platform to study individual responses to chemoradiation. Nat. Med. 25, 1607–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, D., Vela, I., Sboner, A., et al. (2014). Organoid cultures derived from patients with advanced prostate cancer. Cell 159, 176–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garreta, E., Prado, P., Tarantino, C., et al. (2019). Fine tuning the extracellular environment accelerates the derivation of kidney organoids from human pluripotent stem cells. Nat. Mater. 18, 397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjorevski, N., Sachs, N., Manfrin, A., et al. (2016). Designer matrices for intestinal stem cell and organoid culture. Nature 539, 560–564. [DOI] [PubMed] [Google Scholar]
- Gledhill, K., Guo, Z., Umegaki-Arao, N., et al. (2015). Melanin transfer in human 3D skin equivalents generated exclusively from induced pluripotent stem cells. PLoS One 10, e0136713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner, B.M. (1996). Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell 84, 345–357. [DOI] [PubMed] [Google Scholar]
- Hohwieler, M., Illing, A., Hermann, P.C., et al. (2017). Human pluripotent stem cell-derived acinar/ductal organoids generate human pancreas upon orthotopic transplantation and allow disease modelling. Gut 66, 473–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollister, S.J. (2005). Porous scaffold design for tissue engineering. Nat. Mater. 4, 518–524. [DOI] [PubMed] [Google Scholar]
- Homan, K.A., Gupta, N., Kroll, K.T., et al. (2019). Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat. Methods 16, 255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, H., Gehart, H., Artegiani, B., et al. (2018). Long-term expansion of functional mouse and human hepatocytes as 3D organoids. Cell 175, 1591–1606.e19. [DOI] [PubMed] [Google Scholar]
- Huang, G., Li, F., Zhao, X., et al. (2017). Functional and biomimetic materials for engineering of the three-dimensional cell microenvironment. Chem. Rev. 117, 12764–12850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, L., Holtzinger, A., Jagan, I., et al. (2015a). Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell- and patient-derived tumor organoids. Nat. Med. 21, 1364–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, S.X., Green, M.D., de Carvalho, A.T., et al. (2015b). The in vitro generation of lung and airway progenitor cells from human pluripotent stem cells. Nat. Protoc. 10, 413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huch, M., Dorrell, C., Boj, S.F., et al. (2013). In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature 494, 247–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huch, M., Gehart, H., van Boxtel, R., et al. (2015). Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell 160, 299–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huch, M., Knoblich, J.A., Lutolf, M.P., et al. (2017). The hope and the hype of organoid research. Development 144, 938–941. [DOI] [PubMed] [Google Scholar]
- Jacob, F., Salinas, R.D., Zhang, D.Y., et al. (2019). A patient-derived glioblastoma organoid model and biobank recapitulates inter- and intra-tumoral heterogeneity. Cell 180, 188–204.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo, J., Xiao, Y., Sun, A.X., et al. (2016). Midbrain-like organoids from human pluripotent stem cells contain functional dopaminergic and neuromelanin-producing neurons. Cell Stem Cell 19, 248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, H.W., Lee, S.J., Ko, I.K., et al. (2016). A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 34, 312–319. [DOI] [PubMed] [Google Scholar]
- Karthaus, W.R., Iaquinta, P.J., Drost, J., et al. (2014). Identification of multipotent luminal progenitor cells in human prostate organoid cultures. Cell 159, 163–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelava, I., and Lancaster, M.A. (2016). Stem cell models of human brain development. Cell Stem Cell 18, 736–748. [DOI] [PubMed] [Google Scholar]
- Kijima, T., Nakagawa, H., Shimonosono, M., et al. (2019). Three-dimensional organoids reveal therapy resistance of esophageal and oropharyngeal squamous cell carcinoma cells. Cell. Mol. Gastroenterol. Hepatol. 7, 73–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, T.H., and Shivdasani, R.A. (2016). Stomach development, stem cells and disease. Development 143, 554–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopper, O., de Witte, C.J., Lohmussaar, K., et al. (2019). An organoid platform for ovarian cancer captures intra- and interpatient heterogeneity. Nat. Med. 25, 838–849. [DOI] [PubMed] [Google Scholar]
- Kotton, D.N., and Morrisey, E.E. (2014). Lung regeneration: mechanisms, applications and emerging stem cell populations. Nat. Med. 20, 822–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster, M.A., Corsini, N.S., Wolfinger, S., et al. (2017). Guided self-organization and cortical plate formation in human brain organoids. Nat. Biotechnol. 35, 659–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster, M.A., and Knoblich, J.A. (2014). Organogenesis in a dish: modeling development and disease using organoid technologies. Science 345, 1247125. [DOI] [PubMed] [Google Scholar]
- Lancaster, M.A., Renner, M., Martin, C.A., et al. (2013). Cerebral organoids model human brain development and microcephaly. Nature 501, 373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence, M.G., Obinata, D., Sandhu, S., et al. (2018). Patient-derived models of abiraterone- and enzalutamide-resistant prostate cancer reveal sensitivity to ribosome-directed therapy. Eur. Urol. 74, 562–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebreton, F., Lavallard, V., Bellofatto, K., et al. (2019). Insulin-producing organoids engineered from islet and amniotic epithelial cells to treat diabetes. Nat. Commun. 10, 4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, A., Hudson, A.R., Shiwarski, D.J., et al. (2019). 3D bioprinting of collagen to rebuild components of the human heart. Science 365, 482–487. [DOI] [PubMed] [Google Scholar]
- Lee, J., Bscke, R., Tang, P.C., et al. (2018a). Hair follicle development in mouse pluripotent stem cell-derived skin organoids. Cell Rep. 22, 242–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S.H., Hu, W., Matulay, J.T., et al. (2018b). Tumor evolution and drug response in patient-derived organoid models of bladder cancer. Cell 173, 515–528.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei, M., Schumacher, L.J., Lai, Y.C., et al. (2017). Self-organization process in newborn skin organoid formation inspires strategy to restore hair regeneration of adult cells. Proc. Natl Acad. Sci. USA 114, E7101–E7110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W., Cavelti-Weder, C., Zhang, Y., et al. (2014a). Long-term persistence and development of induced pancreatic β cells generated by lineage conversion of acinar cells. Nat. Biotechnol. 32, 1223–1230. [DOI] [PubMed] [Google Scholar]
- Li, X., Francies, H.E., Secrier, M., et al. (2018). Organoid cultures recapitulate esophageal adenocarcinoma heterogeneity providing a model for clonality studies and precision therapeutics. Nat. Commun. 9, 2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X., Nadauld, L., Ootani, A., et al. (2014b). Oncogenic transformation of diverse gastrointestinal tissues in primary organoid culture. Nat. Med. 20, 769–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y., Xiao, Y., and Liu, C. (2017). The horizon of materiobiology: a perspective on material-guided cell behaviors and tissue engineering. Chem. Rev. 117, 4376–4421. [DOI] [PubMed] [Google Scholar]
- Liu, X., Xu, L., Li, J., et al. (2020). Mitotic motor CENP-E cooperates with PRC1 in temporal control of central spindle assembly. J. Mol. Cell Biol. doi: 10.1093/jmcb/mjz051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low, J.H., Li, P., Chew, E.G.Y., et al. (2019). Generation of human PSC-derived kidney organoids with patterned nephron segments and a de novo vascular network. Cell Stem Cell 25, 373–387.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour, A.A., Goncalves, J.T., Bloyd, C.W., et al. (2018). An in vivo model of functional and vascularized human brain organoids. Nat. Biotechnol. 36, 432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani, J., Coppola, G., Zhang, P., et al. (2015). FOXG1-dependent dysregulation of GABA/glutamate neuron differentiation in autism spectrum disorders. Cell 162, 375–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani, J., Simonini, M.V., Palejev, D., et al. (2012). Modeling human cortical development in vitro using induced pluripotent stem cells. Proc. Natl Acad. Sci. USA 109, 12770–12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken, K.W., Cata, E.M., Crawford, C.M., et al. (2014). Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature 516, 400–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, A.J., Dye, B.R., Ferrer-Torres, D., et al. (2019). Generation of lung organoids from human pluripotent stem cells in vitro. Nat. Protoc. 14, 518–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, A.J., Hill, D.R., Nagy, M.S., et al. (2018). In vitro induction and in vivo engraftment of lung bud tip progenitor cells derived from human pluripotent stem cells. Stem Cell Rep. 10, 101–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzel, A.S., Smits, L.M., Hemmer, K., et al. (2017). Derivation of human midbrain-specific organoids from neuroepithelial stem cells. Stem Cell Rep. 8, 1144–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morizane, R., Lam, A.Q., Freedman, B.S., et al. (2015). Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat. Biotechnol. 33, 1193–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutos, F.T., Freed, L.E., and Guilak, F. (2007). A biomimetic three-dimensional woven composite scaffold for functional tissue engineering of cartilage. Nat. Mater. 6, 162–167. [DOI] [PubMed] [Google Scholar]
- Muguruma, K. (2018). Self-organized cerebellar tissue from human pluripotent stem cells and disease modeling with patient-derived iPSCs. Cerebellum 17, 37–41. [DOI] [PubMed] [Google Scholar]
- Muguruma, K., Nishiyama, A., Kawakami, H., et al. (2015). Self-organization of polarized cerebellar tissue in 3D culture of human pluripotent stem cells. Cell Rep. 10, 537–550. [DOI] [PubMed] [Google Scholar]
- Muguruma, K., Nishiyama, A., Ono, Y., et al. (2010). Ontogeny-recapitulating generation and tissue integration of ES cell-derived Purkinje cells. Nat. Neurosci. 13, 1171–1180. [DOI] [PubMed] [Google Scholar]
- Nezakati, T., Seifalian, A., Tan, A., et al. (2018). Conductive polymers: opportunities and challenges in biomedical applications. Chem. Rev. 118, 6766–6843. [DOI] [PubMed] [Google Scholar]
- Noguchi, T.-A.K., and Kurisaki, A. (2017). Formation of stomach tissue by organoid culture using mouse embryonic stem cells In: Tsuji, T. (ed). Organ Regeneration: 3D Stem Cell Culture & Manipulation. New York, NY: Springer, 217–228. [DOI] [PubMed] [Google Scholar]
- Ogawa, M., Ogawa, S., Bear, C.E., et al. (2015). Directed differentiation of cholangiocytes from human pluripotent stem cells. Nat. Biotechnol. 33, 853–861. [DOI] [PubMed] [Google Scholar]
- Oh, J.W., Hsi, T.C., Guerrero-Juarez, C.F., et al. (2013). Organotypic skin culture. J. Invest. Dermatol. 133, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchi, R., Togo, S., Kimura, M., et al. (2019). Modeling steatohepatitis in humans with pluripotent stem cell-derived organoids. Cell Metab. 30, 374–384.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliuca, F.W., Millman, J.R., Gurtler, M., et al. (2014). Generation of functional human pancreatic β cells in vitro. Cell 159, 428–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas, K.J., Choi, D., Sawyers, C.L., et al. (2019). Prostate organoid cultures as tools to translate genotypes and mutational profiles to pharmacological responses. J. Vis. Exp. e60346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastrana, E. (2013). Stem cells: the developing human brain--modeled in a dish. Nat. Methods 10, 929. [DOI] [PubMed] [Google Scholar]
- Pek, Y.S., Wan, A.C., and Ying, J.Y. (2010). The effect of matrix stiffness on mesenchymal stem cell differentiation in a 3D thixotropic gel. Biomaterials 31, 385–391. [DOI] [PubMed] [Google Scholar]
- Puca, L., Bareja, R., Prandi, D., et al. (2018). Patient derived organoids to model rare prostate cancer phenotypes. Nat. Commun. 9, 2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian, X., Jacob, F., Song, M.M., et al. (2018). Generation of human brain region-specific organoids using a miniaturized spinning bioreactor. Nat. Protoc. 13, 565–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian, X., Nguyen, H.N., Song, M.M., et al. (2016). Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell 165, 1238–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian, X., Song, H., and Ming, G.L. (2019). Brain organoids: advances, applications and challenges. Development 146, pii: dev166074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadrato, G., and Arlotta, P. (2017). Present and future of modeling human brain development in 3D organoids. Curr. Opin. Cell Biol. 49, 47–52. [DOI] [PubMed] [Google Scholar]
- Rheinwald, J.G., and Green, H. (1975). Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell 6, 331–343. [DOI] [PubMed] [Google Scholar]
- Richards, Z., McCray, T., Marsili, J., et al. (2019). Prostate stroma increases the viability and maintains the branching phenotype of human prostate organoids. iScience 12, 304–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe, J.S., Hwang, C.I., Somerville, T.D.D., et al. (2017). Enhancer reprogramming promotes pancreatic cancer metastasis. Cell 170, 875–888.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi, G., Manfrin, A., and Lutolf, M.P. (2018). Progress and potential in organoid research. Nat. Rev. Genet. 19, 671–687. [DOI] [PubMed] [Google Scholar]
- Sachs, N., de Ligt, J., Kopper, O., et al. (2018). A living biobank of breast cancer organoids captures disease heterogeneity. Cell 172, 373–386.e10. [DOI] [PubMed] [Google Scholar]
- Sachs, N., Papaspyropoulos, A., Zomer-van Ommen, D.D., et al. (2019). Long-term expanding human airway organoids for disease modeling. EMBO J. 38, e100300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi, H., Kadoshima, T., Soen, M., et al. (2015). Generation of functional hippocampal neurons from self-organizing human embryonic stem cell-derived dorsomedial telencephalic tissue. Nat. Commun. 6, 8896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaziotis, F., de Brito, M.C., Madrigal, P., et al. (2015). Cholangiocytes derived from human induced pluripotent stem cells for disease modeling and drug validation. Nat. Biotechnol. 33, 845–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval, G.J., Pulice, J.L., Pakula, H., et al. (2018). Binding of TMPRSS2-ERG to BAF chromatin remodeling complexes mediates prostate oncogenesis. Mol. Cell 71, 554–566.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, T., Vries, R.G., Snippert, H.J., et al. (2009). Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265. [DOI] [PubMed] [Google Scholar]
- Schaub, J.R., Huppert, K.A., Kurial, S.N.T., et al. (2018). De novo formation of the biliary system by TGFβ-mediated hepatocyte transdifferentiation. Nature 557, 247–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutgens, F., Rookmaaker, M.B., Margaritis, T., et al. (2019). Tubuloids derived from human adult kidney and urine for personalized disease modeling. Nat. Biotechnol. 37, 303–313. [DOI] [PubMed] [Google Scholar]
- Spence, J.R., Mayhew, C.N., Rankin, S.A., et al. (2011). Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470, 105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto, S., Ohta, Y., Fujii, M., et al. (2018). Reconstruction of the human colon epithelium in vivo. Cell Stem Cell 22, 171–176.e5. [DOI] [PubMed] [Google Scholar]
- Sun, B.K., Siprashvili, Z., and Khavari, P.A. (2014). Advances in skin grafting and treatment of cutaneous wounds. Science 346, 941–945. [DOI] [PubMed] [Google Scholar]
- Takahashi, K., Tanabe, K., Ohnuki, M., et al. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872. [DOI] [PubMed] [Google Scholar]
- Takahashi, K., and Yamanaka, S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676. [DOI] [PubMed] [Google Scholar]
- Takasato, M., Er, P.X., Becroft, M., et al. (2014). Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney. Nat. Cell Biol. 16, 118–126. [DOI] [PubMed] [Google Scholar]
- Takasato, M., Er, P.X., Chiu, H.S., et al. (2015). Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 526, 564–568. [DOI] [PubMed] [Google Scholar]
- Takebe, T., Sekine, K., Enomura, M., et al. (2013). Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 499, 481–484. [DOI] [PubMed] [Google Scholar]
- Takebe, T., Zhang, B., and Radisic, M. (2017). Synergistic engineering: organoids meet organs-on-a-Chip. Cell Stem Cell 21, 297–300. [DOI] [PubMed] [Google Scholar]
- Tao, T., Wang, Y., Chen, W., et al. (2019). Engineering human islet organoids from iPSCs using an organ-on-chip platform. Lab Chip 19, 948–958. [DOI] [PubMed] [Google Scholar]
- Thomson, J.A., Itskovitz-Eldor, J., Shapiro, S.S., et al. (1998). Embryonic stem cell lines derived from human blastocysts. Science 282, 1145. [DOI] [PubMed] [Google Scholar]
- Tiriac, H., Belleau, P., Engle, D.D., et al. (2018). Organoid profiling identifies common responders to chemotherapy in pancreatic cancer. Cancer Discov. 8, 1112–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trisno, S.L., Philo, K.E.D., McCracken, K.W., et al. (2018). Esophageal organoids from human pluripotent stem cells delineate Sox2 functions during esophageal specification. Cell Stem Cell 23, 501–515.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez, J.M., Zhang, L., Su, Q., et al. (2012). Notch and TGFβ form a reciprocal positive regulatory loop that suppresses murine prostate basal stem/progenitor cell activity. Cell Stem Cell 11, 676–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco, S., Kedaigle, A.J., Simmons, S.K., et al. (2019). Individual brain organoids reproducibly form cell diversity of the human cerebral cortex. Nature 570, 523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas, D., Baptista, P.M., Brovold, M., et al. (2018). Self-assembled liver organoids recapitulate hepatobiliary organogenesis in vitro. Hepatology 67, 750–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S., Wang, X., Tan, Z., et al. (2019). Human ESC-derived expandable hepatic organoids enable therapeutic liver repopulation and pathophysiological modeling of alcoholic liver injury. Cell Res. 29, 1009–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeber, F., van de Wetering, M., Hoogstraat, M., et al. (2015). Preserved genetic diversity in organoids cultured from biopsies of human colorectal cancer metastases. Proc. Natl Acad. Sci. USA 112, 13308–13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Wetering, M., Francies, H.E., Francis, J.M., et al. (2015). Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 161, 933–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer, R.A., Leopoldi, A., Aichinger, M., et al. (2019). Human blood vessel organoids as a model of diabetic vasculopathy. Nature 565, 505–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang, Y., Tanaka, Y., Cakir, B., et al. (2019). hESC-derived thalamic organoids form reciprocal projections when fused with cortical organoids. Cell Stem Cell 24, 487–497.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang, Y., Tanaka, Y., Patterson, B., et al. (2017). Fusion of regionally specified hPSC-derived organoids models human brain development and interneuron migration. Cell Stem Cell 21, 383–398.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, Y., Xu, X., Yang, L., et al. (2020). Patient-derived organoids predict chemoradiation responses of locally advanced rectal cancer. Cell Stem Cell 26, 17–26.e6. [DOI] [PubMed] [Google Scholar]
- Yin, X., Mead, B.E., Safaee, H., et al. (2016). Engineering stem cell organoids. Cell Stem Cell 18, 25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yui, S., Nakamura, T., Sato, T., et al. (2012). Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5+ stem cell. Nat. Med. 18, 618–623. [DOI] [PubMed] [Google Scholar]
- Zadra, G., Ribeiro, C.F., Chetta, P., et al. (2019). Inhibition of de novo lipogenesis targets androgen receptor signaling in castration-resistant prostate cancer. Proc. Natl Acad. Sci. USA 116, 631–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, K., Zhang, L., Liu, W., et al. (2018a). In vitro expansion of primary human hepatocytes with efficient liver repopulation capacity. Cell Stem Cell 23, 806–819.e4. [DOI] [PubMed] [Google Scholar]
- Zhang, Y., Yang, Y., Jiang, M., et al. (2018b). 3D modeling of esophageal development using human PSC-derived basal progenitors reveals a critical role for notch signaling. Cell Stem Cell 23, 516–529.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, Y., Du, X., Wang, W., et al. (2005). Organogenesis from dissociated cells: generation of mature cycling hair follicles from skin-derived cells. J. Invest. Dermatol. 124, 867–876. [DOI] [PubMed] [Google Scholar]
- Zhou, T., Tan, L., Cederquist, G.Y., et al. (2017). High-content screening in hPSC-neural progenitors identifies drug candidates that inhibit Zika virus infection in fetal-like organoids and adult brain. Cell Stem Cell 21, 274–283.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer, B., Piao, J., Ramnarine, K., et al. (2016). Derivation of diverse hormone-releasing pituitary cells from human pluripotent stem cells. Stem Cell Rep. 6, 858–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorn, A.M., and Wells, J.M. (2009). Vertebrate endoderm development and organ formation. Annu. Rev. Cell Dev. Biol. 25, 221–251. [DOI] [PMC free article] [PubMed] [Google Scholar]


