Abstract
Background
In health disparities research, studies often fall short of their recruitment goals. Conducting a pilot feasibility study of recruitment in which data are collected systematically on recruitment processes can help investigators refine methods for the larger study. However, there are few guidelines for conducting pilot feasibility studies, and recruitment methods are seldom the focus. Feasibility indicators differ from traditional reports of recruitment results by focusing on the extent to which recruitment goals are met.
Methods
We present an organizing framework for assessing the feasibility of recruitment that includes eight steps, briefly: 1) specify recruitment goals; 2) specify recruitment processes; 3) establish a tracking system for each individual; 4) establish a tracking database for monitoring processes and results; 5) implement recruitment and track each individual’s progress; 6) summarize recruitment results; 7) calculate and interpret feasibility measures - were goals met; and 8) if goals were not met, utilize tracking data to modify methods for the larger study. We describe methods within each step, with added details for steps 2-5 (the specific processes). The framework draws from a small literature on recruitment feasibility with a focus on health disparities populations. The guidelines blend well-known methods of recruitment with additional information on calculating feasibility indicators.
Conclusions
These guidelines provide a first step in thinking systematically about recruitment feasibility, to advance the field of measuring feasibility. Feasibility indicators also can be used to track the effectiveness of innovative recruitment strategies as part of building the science of recruitment, especially in disparities populations.
Keywords: Feasibility Studies, Pilot Projects, Minority Group, Health Disparities, Recruitment, Minority Recruitment
Introduction
When recruitment goals are set for race/ethnicity or socioeconomic status, many studies fall short of achieving their recruitment numbers.1,2 This can result in underrepresentation of diverse population groups such that important research findings may not generalize to these groups, or in the most extreme cases, the study being terminated prematurely by governing boards. One strategy to prevent recruitment shortfalls is to test recruitment methods in a pilot feasibility study prior to implementing a larger study.3 Pilot studies are defined as “a small-scale test of methods and procedures to assess the feasibility/acceptability of an approach to be used in a larger scale study.”4 The aims are to field-test logistical aspects of the future study and to modify these methods accordingly for the larger study.5 Such a pilot study provides an opportunity to assess the relative success of candidate recruitment methods using quantifiable indicators.
Most pilot feasibility studies explore several methods and procedures such as randomization, delivery of an intervention, assessments and data collection, and estimation of group differences. Although recruitment can be one component,6 it seldom is the focus. In addition, there are remarkably few papers providing guidelines for conducting feasibility studies, or that specify indicators or measures that can help interpret results. Bowen and colleagues provide a framework for studying the feasibility of interventions, describing areas of focus (eg, acceptability of an intervention) and examples of feasibility questions and methods.7 However, no recruitment feasibility indicators are described. Also, while Orsmond and Cohn8 describe objectives and guiding questions of feasibility studies, including a section on recruitment with possible questions, they do not describe how to collect the data necessary to assess the feasibility of recruitment or indicate specific metrics to be used.
Therefore, there are relatively few guidelines for pilot study investigators to follow in order to obtain the necessary data to assess recruitment feasibility. Yet, failure to achieve enrollment targets in a large trial wastes resources and jeopardizes the ability to achieve study aims. The National Institute on Aging (NIA) has thus stated a need for tools to measure recruitment feasibility.2
There are guidelines for reporting results of recruitment in randomized trials. The Consolidated Standards of Reporting Trials (CONSORT) 2010 guidelines were developed to improve reporting of randomized controlled trials9 and include a familiar diagram for documenting the flow of participants through a trial, starting with the sampling frame and ending with the final number retained and analyzed, by treatment group. The process up to and including enrollment pertains to the recruitment phase, and it is standard practice to report the magnitude of loss at each step (eg, initial contact, eligibility screening) including reasons for non-participation.
However, recruitment feasibility measures differ from these standard recruitment reports in several ways. First, feasibility measures focus on the extent to which the goals of recruitment were met rather than simply on the recruitment rates. For example, a feasibility indicator is whether the recruitment methods yielded the desired sample composition (eg, age, racial/ethnic diversity) or an adequate sample size in the time allotted. Second, in a feasibility study, reasons for loss at each step are monitored through a tracking system and reported systematically. Third, if goals are not met, the tracking data can be used to identify the weak links in the process so that methods can be refined for a larger trial. Information on the reasons for that loss can be used to modify the strategies to improve the recruitment results for the larger study.
The purpose of this article is to provide an organizational framework to be used when examining recruitment feasibility, describe specific methods for each step, provide examples of indicators from the pilot feasibility literature, and describe how to interpret results to inform/modify methods to maximize recruitment for a larger study. The methods build on traditional, familiar recruitment strategies but add important steps relevant to feasibility assessment, specifically for recruitment strategies. Because our focus is on recruiting diverse populations, including those characterized by disparities in community-based or clinical settings, we emphasize methods for reaching these sometimes hard-to-reach population groups within these specific contexts. Nonetheless, because there are very few examples of recruitment feasibility indicators in diverse population groups, we rely on many examples from all groups since the indicators apply to all populations.
Methods
Our framework of eight specific steps for assessing recruitment feasibility is the foundation for the organization of this article (Figure 1). The eight steps in Figure 1 also provide a visual guide and summary of the entire process.
Figure 1. Organizing framework of steps to examine recruitment feasibility.
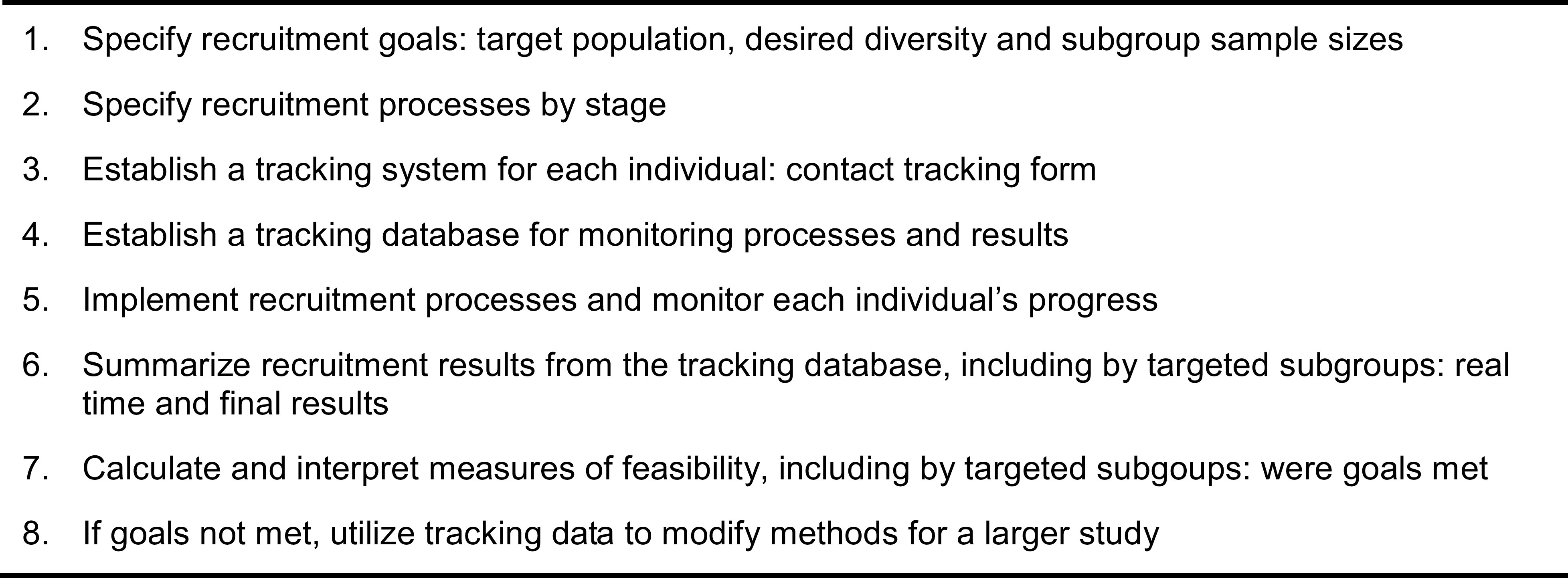
Step 1: Specify Recruitment Goals – Target Population, Desired Diversity, and Subgroup Sample Sizes
The process begins by specifying the recruitment goals for the pilot study, stated in terms of the target population, desired sample characteristics, and sample size. Specifying the target population requires identifying the inclusion criteria, eg, persons at risk of diabetes, sedentary older adults, or patient with breast cancer. Specifying the desired sample characteristics refers to the desired demographic distribution of the final sample (eg, 50% minority, 50% aged ≥65 years). For health disparities research, this typically includes a goal of sufficient representation of racial/ethnic minority groups or of those with lower socioeconomic status, groups known to be underrepresented in research. In a diabetes risk-reduction intervention delivered by a local public health department, the goal was to recruit primarily minority and underserved adults at risk of diabetes who lived within its geographic service area.10
The sample size goal can be stated for the overall sample, and if the sampling design calls for stratification by any characteristic (race/ethnic groups), targets within each stratum can be specified. In studies in which two or more groups will be compared (eg, comparing Latinos, Blacks, and Whites), the goal could be the equal representation of these groups.
Recruitment goals can also be stated within the context of estimated time frames for completing outreach, screening, recruitment, and enrollment, and the staff resources needed for this process. Although the pilot study sample will be much smaller than that planned for the larger study, one can test whether the study can recruit the target sample size in the requisite time period, or whether one can enroll a specific number of individuals per month (pace of recruitment). In studies recruiting participants to receive group sessions, goals may need to be stated in terms of pace of recruitment, eg, the number needed per month to be able to conduct appropriately sized groups in a timely manner.
Step 2: Specify Recruitment Processes by Stage
Once the target population and recruitment goals are determined, delineating the recruitment processes for the study involves laying out a flow chart of stages of outreach and recruitment, and the specific methods or protocol to be used for each one. We provide a list of possible recruitment processes by stage in Table 1, using as the example community-based studies in which there is no list of names of potential participants. The table focuses on the processes described below for steps 2-6. In Table 1, we distinguish two overarching phases: 1) outreach to find potential participants and describe the study, resulting in individuals providing contact information (the sampling frame); and 2) from the sampling frame, contact each individual, explain requirements, risks and benefits, answer questions, screen those interested, and enroll those who are eligible.
Table 1. Examples of recruitment processes and tracking system data after establishing recruitment goals (step 1): community-based recruitment.
| Recruitment processes by stage (step 2) | Tracking system data elements (step 3) | ||||
| Stage | Description of processes | Product | Reasons for loss | ||
| Phase 1: Outreach to identify potential participants | |||||
| Identify sources of potential participants | Specify community-based locations/venues, sources of referral (clinics, doctors) | List of sources | NA | ||
| Conduct outreach | Hold informational meetings, events, post ads/flyers w/number to call | # of attendees, # of people talked to, type of event, location | NA | ||
| At events: explain study | Describe study, program, benefits, requirements, eligibility, invite to provide contact information | No contact information → | NA | ||
| Contact information ↓ | |||||
| Phase 2: Begins with sampling frame - persons with contact information (includes steps 4 and 5) | |||||
| Create list of names | Names and contact information of potential participants, provided at event or by calling in | # of people on list by source | NA | ||
| Attempt contact | Call, specify number of attempts | Not contacted → | Wrong number, voicemail, no answer, not available | ||
| Contacted ↓ | |||||
| Contact, explain study, invite for screening | Detailed description of study, program, benefits, eligibility, screening | Not interested → | Requires blood test, visit to inconvenient location | ||
| Agree to be screened ↓ | |||||
| Screen individual | Apply eligibility criteria (age, race/ethnicity, health conditions) | Ineligible → | Reasons for ineligibility | ||
| Eligible ↓ | |||||
| Explain specific requirements, schedule enrollment | More specific explanation, nature of randomization; answer questions | Not interested → | Inconvenient, not willing to be randomized | ||
| Still interested ↓ | |||||
| Complete enrollment | Complete consent and assessment; if relevant, randomize participant | Did not enroll → | No show, did not consent. | ||
| Enrolled ↓ | |||||
| Describe final sample (step 6) | Summarize demographics, CONSORT flow chart | NA | NA | ||
NA, not applicable; → indicates discontinuation of process; ↓ indicates continued to next stage.
Outreach begins by identifying sources of potential participants such as community venues (eg, community centers), and within these venues, reaching individuals through informational meetings or community events. At events, the study can be described to groups of individuals in a fair amount of detail, including benefits and requirements, and in the case of interventions, the program in which they would participate. Ads and flyers posted in the community can also reach potential individuals who can call in if interested, including via the internet. For example, a study of an internet-based advertising campaign to match men with prostate cancer to a clinical trial targeted geographic areas with higher proportions of minority, Spanish-speaking men to enhance reach to disparities populations.11
The sampling frame is the list of potential participants or study volunteers who provide contact information at the conclusion of these outreach strategies. The remaining stages involve making contact with each individual, explaining the study in more detail, answering questions, and inviting the person for screening. Screening can be done at that point, or an appointment can be scheduled if it requires a separate visit. If the outreach is via flyers and ads where interested individuals call a study contact person, a similar protocol can be specified for returning calls and explaining the study.
Studies in which patients are identified from health care settings require a slightly different set of steps. Although recruitment processes are similar, there are differences in how the sampling frame is created. Procedures for obtaining the names of eligible patients (eg, MD referral, patients visiting a primary care practice) are first specified. The result is an enumerated sampling frame, ie, a list of potential patients who meet preliminary eligibility criteria (eg, specific health condition, no comorbidities). Procedures need to be specified as to how to make initial contact (eg, by mail followed by telephone, by phone, or during a clinic visit), including a protocol for a maximum number of attempted contacts. Once an initial in-person contact is achieved, the stages are similar to phase 2 in Table 1 for community-based recruitment.
Specifying recruitment processes also includes deciding on who should be study recruiters. These individuals are trained to conduct outreach, relate to potential participants, explain the study, understand their concerns, and answer questions. For community-based recruitment, recruiters can be study staff or from the community (eg, community health workers can be hired and trained as recruiters).12 In clinical settings, clinical recruiters (eg, clinical research assistants, clinical research coordinators) are often responsible for recruitment. In some cases, interviewers may be the ones making initial contact if the sampling frame list includes addresses and phone numbers.13
In designing recruitment strategies, we recommend using multi-method approaches known to work well among diverse and vulnerable populations to optimize the potential yield at each stage. Determinants of effective recruitment in diverse populations (barriers and facilitators) have previously been identified. These fall within several categories, including characteristics of the study, the individuals being recruited, referring physicians, and study personnel, as well as the methods of recruitment.13-15 Based on known barriers and facilitators, a variety of tailored strategies have been developed and tested that maximize participation of diverse population groups.16 For example, strategies for recruiting older minority adults are summarized in a special supplement to The Gerontologist.17 Strategies have been described for recruiting specific subgroups such as African Americans18,19 and South Asians.20 Some disease-focused recruitment strategies have been recommended such as for Alzheimer’s disease21 and cancer research.22
Step 3: Establish a Tracking System for Each Individual – Contact Tracking Form
A tracking system provides a mechanism for monitoring the progression of each individual through the processes of recruitment, enrollment, and completion of all study requirements, including assessments. For this article, we discuss the tracking system only through enrollment.
A contact tracking form (paper or electronic) is created for each individual in the sampling frame to be used by recruiters to follow each individual through the entire process until a final disposition is made (eg, ineligible, enrolled). The form serves two purposes: 1) it can guide recruiters in the procedures or stages for recruiting individuals; and 2) it provides fields for recording the results of the process for each person at each stage. These two purposes are integrated on the form, eg, one section for each stage includes scripts and instructions to recruiters as well as fields for recording the results of that stage. For each stage, if an individual discontinues the process of recruitment (eg, is not interested, declines to be screened), the form can include a prompt to ask about the reason and fields for recording those reasons.
Examples of the types of codes that can be included on the contact tracking forms are shown in Table 1 (see step 3: tracking system data elements). In column 3, we refer to potential outcomes of each stage, which are the possible decision points for each individual at each stage (ie, are usually binary). In column 4, we provide examples of reasons for discontinuing. Anticipating potential reasons for not continuing at any stage provides a basis for creating structured response options on the tracking form (eg, health problems, not interested in research, do not want to provide biospecimens). Reasons for not continuing can also be obtained by asking open-ended questions and recording responses verbatim, to be coded later by investigators. Reasons also can be recorded by the recruiter based on impressions, observations, chart review data, or pre-screening (eg, later stage cognitive impairment precludes participation).
As an example, in the section on attempting initial contact by phone, the tracking form can include the protocol for the maximum number of attempted contacts with fields for recording the outcome of each attempt (eg, no answer, person not home). It can include whether the recruiter has spoken to the person, and if so, by which method (eg, telephone or in person), how many messages were left, the number of calls, and the times of the call. Within the section, the form includes fields for recording the final result of that stage (eg, never reached).
Step 4: Establish a Tracking Database for Monitoring Processes and Results
A tracking database is required for monitoring the entire process for everyone in the sampling frame. Data from each individual contact tracking form are entered for subsequent analysis and reporting. In community-based recruitment, each person is entered into the database when a contact tracking form is submitted by a recruiter. Thus, all descriptive information on each individual must be recorded on the form by the recruiter. In health care settings, each individual is usually entered into the database in advance of recruitment, along with contact information and any known descriptive variables, including those used for stratifying recruitment and/or reporting (eg, age, primary language, study site).
The Research Electronic Data Capture (REDCap) system is often used for creating such a database.23 REDCap is a password-protected and HIPAA-compliant data system. Names, contact information, and data collected from each tracking form can be entered directly into a REDCap database with a separate record for each individual. Subsequently, other study materials, such as surveys completed by the individual, may be logged in the same database under the participant’s individual record. The system also incorporates statistical package export facilities, which can then be utilized by researchers to track study progress, export data, and perform quantitative analyses using programs such as SAS.
Step 5: Implement Recruitment Processes and Monitor Each Individual’s Progress
Step 5 involves implementing all of these systems. In community-based recruitment, field recruiters conduct outreach events to contact potential individuals in identified locations. Once individual contact is made, the recruiter creates a contact tracking form for each individual. These forms are generally kept by recruiters as a “case load” of individuals in various stages of recruitment. Recruiters can routinely meet with a project director to review the cases. When a final disposition on an individual is clear and no more contact is planned (according to the protocol regarding the maximum number of contacts to be instigated), or a refusal is noted, the form is turned in for data entry into REDCap. In some cases, there may be reasons to consider keeping the person as active; for example, when a person is still thinking about it or consulting family members.
For studies in health care settings, the contact tracking form is initiated and maintained by the recruiter and usually includes the name and contact information, thus must be secured. The recruiter keeps one form for each individual until a final disposition (person declines or enrolls) is reached, and then submits it to the research staff for entry into the database. Alternatively, the tracking information can be entered in real-time in the data base system and updated until the final disposition is determined.
Step 6: Summarize Recruitment Results from the Tracking Database Including by Targeted Subgroups – Real Time and Final Results
Recruitment results can be calculated from the tracking database, including a description of the enrolled sample. At the end of the recruitment period, results from the REDCap tracking database are summarized using a CONSORT diagram, which includes the reasons for loss at every stage. Although those enrolled continue to be tracked throughout the study (through receipt of intervention and all assessments), the recruitment feasibility indicators require only the information through enrollment.
Recruitment results can be summarized on a regular basis to monitor recruitment in real time. Such reports early in the process can sometimes lead to immediate modifications to correct problems identified before finishing the pilot study. This can enable a review, for example of the yield of various outreach events to determine what is and is not working. Descriptive data can be tabulated and graphed by various groupings, eg, actual numbers vs (expected) monthly goals, or actual numbers by locations such as the county in which outreach is occurring.
Enrollment rates are a necessary first step toward calculating feasibility indicators. There are several ways to calculate enrollment rates, eg, rates of loss/continuation using various denominators (eg, percent enrolled of the sampling frame or of those who were contacted). The yield (percent of those contacted who enrolled) is the most commonly reported rate.24 However, enrollment in relation to other denominators can be useful, such as the percent of the sampling frame enrolled or the percent of those eligible that enrolled.
Rates can be calculated and reported for the total sample and by any stratification variables that were part of the design such as race/ethnicity. For example, recruiting for a one-time telephone survey of diverse general medicine patients, response rates were reported separately by race/ethnicity and language strata.13 These stratified results are useful when assessing whether goals were met within strata (step 7).
Step 7: Calculate and Interpret Measures of Feasibility, Including by Targeted Subgroups – Were Goals Met?
Step 7 is to calculate feasibility measures, which involves interpreting the recruitment results from Step 6 in relation to recruitment goals to indicate whether the results met, exceeded, or fell short of the targets. Regarding enrollment goals, actual vs expected recruitment can be plotted in Excel or other spreadsheet software that produces graphic displays. More often than not, studies fall short of their recruitment goals in a specific time frame.25-29 Occasionally, studies achieve their goal30 or even surpass the goal31 suggesting that the recruitment methods were successful.
Efficiency
The feasibility question is whether the sample size goal in the larger study can be met within the anticipated time, budget, and staffing. Efficiency refers to the amount of time and resources needed to enroll the final sample in the pilot study,4,25 which can be extrapolated to the larger study. The cost to recruit participants is one useful efficiency measure in planning a larger study, especially when reported by different strategies for identifying potential participants. One study tracked costs of recruitment in a study of a heart disease self-management program and reported how the average cost of recruiting and enrolling one participant varied depending on the recruitment method.24
Pace of Recruitment
The key feasibility question is, “Is the pace of recruitment sufficient for a larger study within a specific budget and study period?” The desired pace of recruitment is established during study design and can refer to the target number to be screened or enrolled per month.4 For example, a feasibility study of an internet-delivered cognitive behavioral therapy for persons with spinal cord injury reported recruiting on average 5 patients per month.32 A study of a physical activity intervention in cancer patients recruited 1.6 patients per month (over 16 months).29 The pace can be assessed also for a specific phase of recruitment, eg, average time delay from screening to enrollment.4.The pace can be a limiting factor in seeking patients with a rare condition or unusual situation, thus recruitment strategies need to identify the most limiting resource. For example, in a study of lucidity in dementia in which the goal is to document brief episodes of sudden, unexpected return of ability to communicate, the events may be fleeting or rare; thus, the feasibility study may focus on the number of observations, staff reports and time required per incident identified.
Results by Source of Referral, Outreach and Recruitment Method, or Study Site (for Multisite Trials)
All of the above recruitment feasibility measures and rates can be calculated separately by sources of referral or outreach venues such as community-based vs hospital- or clinic-based.6,10,24 This enables investigators to identify sources and methods that yielded the most participants or the lowest cost, thus focusing on methods with the highest efficiency for the main study. In the recruitment feasibility study for a heart disease self-management program (introduced above), Ramsay and colleagues reported costs per enrollee by recruitment method, noting that that the lowest cost was from flyers, word of mouth, and referral from community partners, and the highest cost from health fairs.24 In the same study, the highest yield (percent enrolled by number contacted) was from information sessions and flyers.
Results can be used to plan modifications to recruitment methods. For example, in a physical activity trial for cancer patients that relied on referrals from 21 oncologists, 76% of those recruited were referred by a single oncologist, one who was more engaged in promoting physical activity than the others. Investigators concluded that more efforts to encourage involvement of oncologists would have helped.29 Similarly, in a weight-loss trial for African American families, those recruited via culturally relevant ads, events, or word-of-mouth were almost twice as likely to enroll as those contacted from non-culturally relevant settings.33 A bilingual, low-literacy internet-based outreach campaign examined the feasibility of using alternative wording of ads to help men select a clinical trial, which allowed selection of ads that were most effective in getting men to provide contact information.11
The benefits of engaging stakeholders, a recommended strategy for recruiting underrepresented minorities, can also be tracked. In a study to recruit vulnerable adults into a diabetes risk reduction intervention, nearly half attending a screening event heard about it through community partners and professionals, indicating that engaging community stakeholders was more effective than outreach events held by study staff.10 This finding is consistent with the that of Ramsay and colleagues, that referrals from community partners had the lowest cost per enrollee.19
Sample Characteristics Results
Describing the final sample in sufficient detail in terms of demographics and other characteristics can determine how well the sample reflects the sampling frame or desired sample characteristics. For examining feasibility, these results are interpreted in light of the goals, whether the study recruited the desired diversity, or whether the final sample is representative of the desired sample.24,30 In the diabetes risk-reduction study aiming to enroll lower SES and minority adults from the health department service area (described above), investigators surpassed their goals, as the study overrepresented Latinos and African Americans and individuals with low levels of education compared to local census data.10
Step 8: If Goals Not Met, Utilize Tracking Data to Modify Methods for a Larger Study
The NIA advocates using feasibility study results to inform refinement of outreach and recruitment strategies.2 If enrollment rates or the pace of recruitment are insufficient, or the targeted diversity is not achieved, the final step is to consider whether recruitment strategies can be reengineered to improve results. Results from Step 7 can be used to identify the weak points, ie, where in the process the most people were lost to recruitment, and the reasons for loss at those points. For example, if too many people were lost because of ineligibility, inclusion criteria could possibly be broadened, eg, extend the age range.2 In a study where recruitment fell short, the exclusion criterion for systolic blood pressure was too stringent, thus investigators lowered the inclusion cutoff to be able to enroll the requisite sample in the larger study.26
Other examples illustrate this point. In one study, recruiters reported that the consent form was too complicated and not understood, resulting in modifications to simplify consent procedures for the larger study; other recruitment feasibility results prompted the authors to simplify inclusion criteria and extend recruitment time.28 In a pilot feasibility study on managing comorbidity of colorectal cancer patients, investigators recruited 72 patients (the goal was 124).27 Because patients were only eligible prior to surgery and recruiters reported that many patients were too overwhelmed to join the study, investigators expanded eligibility criteria to include patients post-operatively, and lengthened the duration of recruitment from 9 months to 12 months. Similarly, in a study of Latinas with breast cancer, the eligibility window was expanded from 3 months post diagnosis to 12 months, because of the burden on recruiting hospitals to identify women within the first three months of diagnosis.34
If an initial sampling frame yields insufficient numbers of study participants, additional sources/study sites may need to be found. In a feasibility study of biomarkers for cerebral amyloid angiopathy, patients were identified from a single specialist center.35 However, most of these (78.5%) were ineligible (co-morbid cognitive impairment, failure to meet imaging criteria), thus investigators suggested utilizing centralized, multi-center databases to identify more potentially eligible patients. In the above-mentioned study of Latinas with breast cancer, an insufficient number of patients were being referred due to staff turnover and unfilled positions at the hospitals, which required establishing relationships with other providers to achieve a stable stream of referrals.34 Similarly, in a pilot study to identify racial/ethnic differences in the gut microbiome of patients with colorectal cancer, insufficient numbers of African American patients were being identified at the study site defined as the study clinic’s catchment area. The study was later expanded to include an additional site that served a more diverse patient panel.
Discussion
As noted by the NIA, building an applied science of recruitment involves developing and testing strategies before they are widely implemented, and making results available for new studies.2 What distinguishes recruitment feasibility studies from general recruitment results is that assessing feasibility requires more precise delineation of the specific steps in the process. With each step, it is important to anticipate and code reasons for loss of individuals. This added detail is needed to enable pilot investigators to know the specific reasons for losses at each step to help them plan and increase efficiency of recruitment in a larger study. In traditional CONSORT charts, many of these steps are lumped together, obscuring some points where losses may have occurred.
Some have suggested that feasibility indicators should be stated in terms of “clear quantitative benchmarks” or “progression criteria” by which results are judged. Thus, if recruitment goals do not achieve an a priori goal, the possibility of a larger study is rejected. For example, a pilot study of a weight management program for fathers was not considered feasible for progression to a full scale RCT based on prespecified recruitment criteria that were not met.25 For purposes of testing the feasibility of methods to reach diverse populations, we recommend using feasibility results to inform modifications for a larger trial (Step 8). One study’s experiences illustrate the value of this. The investigators set “progression criteria” for a full-scale trial as a goal of recruiting 50% of the target sample; however, the pilot study recruited 44%, which would have precluded moving forward. They determined that the main reason for declining was the time commitment required by the intervention. Based on this knowledge and their willingness to reduce the burden on respondents, they decided to proceed and modify the time commitment requirement.36
We found only a few examples of recruitment feasibility studies in health disparities or diverse populations. Thus, most of our examples are from studies of less diverse populations. We encourage more investigators to include recruitment feasibility in their pilot studies of disparities populations to increase availability of evidence of these strategies.
Conclusion
There is an extensive literature on the science of inclusion and on recruitment; however, the focus of this article has been specifically on recruitment feasibility indicators. The framework presented here is a “first generation” effort to establish guidelines and steps to explore the feasibility of recruitment in a systematic fashion. As such, examples of how other investigators tackled the problem, when synthesized into a framework is an important first step. As more studies of recruitment feasibility are published, more details about effective indicators can be identified.
Although many pilot feasibility studies include recruitment as one part, it is seldom featured. Perhaps others will build on these ideas to advance the field. Feasibility indicators can also be used to track innovative recruitment strategies as part of adding to the existing science of recruitment among diverse groups and increasing the representativeness of studies.
Acknowledgments
This work was supported in part by National Institutes of Health (NIH)/National Institute for Aging (NIA) grant 2R13AG023033-16. In addition, the authors disclose receipt of financial support for the research, authorship, and/or publication of this article: Dr. Nápoles was supported by the Division of Intramural Research, National Institute on Minority Health and Health Disparities. Dr. Teresi is funded from a collaborative effort of three National Institute on Aging Centers: Alzheimer’s Disease - RCMAR Center (Columbia University, grant number 1P30AG059303, Manly, Luchsinger); the Edward R. Roybal Translational Research Center (Cornell University, grant number 5P30AG022845, Reid, Pillemer and Wethington) and the Measurement Methods and Analysis Core of a Claude D. Pepper Older Americans Independence Center (Mount Sinai 1P30AG028741, Siu). We are grateful to Dr. Steven P. Wallace for his insightful comments on this manuscript.
References
- 1.Oh SS, Galanter J, Thakur N, et al. . Diversity in clinical and biomedical research: a promise yet to be fulfilled. PLoS Med. 2015;12(12):e1001918. 10.1371/journal.pmed.1001918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Institute on Aging (NIA) Together We Make the Difference: National Strategy For Recruitment And Participation In Alzheimer’s And Related Dementias Clinical Research. Washington, DC: NIA. October 2018. Last accessed September 2, 2020 from https://www.nia.nih.gov/research/recruitment-strategy [DOI] [PMC free article] [PubMed]
- 3.Leon AC, Davis LL, Kraemer HC. The role and interpretation of pilot studies in clinical research. J Psychiatr Res. 2011;45(5):626-629. 10.1016/j.jpsychires.2010.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Center for Complementary and Integrative Health Uses and Misuses of Pilot studies. NCCIH Research Blog. 2017. Last accessed September 2, 2020 from: https://www.nccih.nih.gov/research/blog/uses-and-misuses-of-pilot-studies.
- 5.Kistin C, Silverstein M. Pilot studies: a critical but potentially misused component of interventional research. JAMA. 2015;314(15):1561-1562. 10.1001/jama.2015.10962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shafayat A, Csipke E, Bradshaw L, et al. . Promoting Independence in Dementia (PRIDE): protocol for a feasibility randomised controlled trial. Trials. 2019;20(1):709. 10.1186/s13063-019-3838-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowen DJ, Kreuter M, Spring B, et al. . How we design feasibility studies. Am J Prev Med. 2009;36(5):452-457. 10.1016/j.amepre.2009.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orsmond GI, Cohn ES. The distinctive features of a feasibility study: objectives and guiding questions. OTJR (Thorofare, NJ). 2015;35(3):169-177. 10.1177/1539449215578649 [DOI] [PubMed] [Google Scholar]
- 9.Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340(mar23 1):c869. https://doi. org/ 10.1136/bmj.c869 PMID:20332511 [DOI] [PMC free article] [PubMed]
- 10.Santoyo-Olsson J, Cabrera J, Freyre R, et al. . An innovative multiphased strategy to recruit underserved adults into a randomized trial of a community-based diabetes risk reduction program. Gerontologist. 2011;51(suppl 1):S82-S93. 10.1093/geront/gnr026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaplan CP, Siegel A, Leykin Y, et al. . A bilingual, Internet-based, targeted advertising campaign for prostate cancer clinical trials: assessing the feasibility, acceptability, and efficacy of a novel recruitment strategy. Contemp Clin Trials Commun. 2018;12:60-67. 10.1016/j.conctc.2018.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santoyo-Olsson J, Stewart AL, Samayoa C, et al. . Translating a stress management intervention for rural Latina breast cancer survivors: The Nuevo Amanecer-II. PLoS One. 2019;14(10):e0224068. 10.1371/journal.pone.0224068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nápoles-Springer AM, Santoyo J, Stewart AL. Recruiting ethnically diverse general internal medicine patients for a telephone survey on physician-patient communication. J Gen Intern Med. 2005;20(5):438-443. 10.1111/j.1525-1497.2005.0078.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sussman AL, Cordova C, Burge MR. A comprehensive approach to community recruitment for clinical and translational research. J Clin Transl Sci. 2018;2(4):249-252. 10.1017/cts.2018.324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmotzer GL. Barriers and facilitators to participation of minorities in clinical trials. Ethn Dis. 2012;22(2):226-230. [PubMed] [Google Scholar]
- 16.Nápoles A, Cook E, Ginossar T, Knight KD, Ford ME. Applying a conceptual framework to maximize the participation of diverse populations in cancer clinical trials. Adv Cancer Res. 2017;133:77-94. 10.1016/bs.acr.2016.08.004 10.1016/bs.acr.2016.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nápoles AM, Chadiha LA; Resource Centers for Minority Aging Research . Advancing the science of recruitment and retention of ethnically diverse populations. Gerontologist. 2011;51(suppl 1):S142-S146. 10.1093/geront/gnr019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barrett NJ, Ingraham KL, Vann Hawkins T, Moorman PG. Engaging African Americans in research: the recruiter’s perspective. Ethn Dis. 2017;27(4):453-462. 10.18865/ed.27.4.453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramsay JE, Hogan CK, Janevic MR, Courser RR, Allgood KL, Connell CM. Comparison of recruitment strategies for engaging older minority adults: results from Take Heart. J Gerontol A Biol Sci Med Sci. 2020;75(5):922-928. 10.1093/gerona/glz112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanaya AM, Chang A, Schembri M, et al. . Recruitment and retention of US South Asians for an epidemiologic cohort: experience from the MASALA study. J Clin Transl Sci. 2019;3(2-3):97-104. 10.1017/cts.2019.371 10.1017/cts.2019.371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilmore-Bykovskyi AL, Jin Y, Gleason C, et al. . Recruitment and retention of underrepresented populations in Alzheimer’s disease research: A systematic review. Alzheimers Dement (N Y). 2019;5(1):751-770. 10.1016/j.trci.2019.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Regnante JM, Richie N, Fashoyin-Aje L, et al. . Operational strategies in US cancer centers of excellence that support the successful accrual of racial and ethnic minorities in clinical trials. Contemp Clin Trials Commun. 2020;17:100532. https://doi.org/ 10.1016/j conctc.2020.100532 PMID:32055746 [DOI] [PMC free article] [PubMed]
- 23.Patridge EF, Bardyn TP. Research Electronic Data Capture (REDCap). J Med Libr Assoc. 2018;106(1):142-144. 10.5195/JMLA.2018.319 [DOI] [Google Scholar]
- 24.Yin Z, Lesser J, Paiva KA, et al. . Using mobile health tools to engage rural underserved individuals in a diabetes education program in south Texas: feasibility study. JMIR Mhealth Uhealth. 2020;8(3):e16683. 10.2196/16683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griffin T, Sun Y, Sidhu M, et al. . Healthy Dads, Healthy Kids UK, a weight management programme for fathers: feasibility RCT. BMJ Open. 2019;9(12):e033534. 10.1136/bmjopen-2019-033534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ravenell J, Leighton-Herrmann E, Abel-Bey A, et al. . Tailored approaches to stroke health education (TASHE): study protocol for a randomized controlled trial. Trials. 2015;16(1):176. 10.1186/s13063-015-0703-4 10.1186/s13063-015-0703-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Signal V, Jackson C, Signal L, et al. . Improving management of comorbidity in patients with colorectal cancer using comprehensive medical assessment: a pilot study. BMC Cancer. 2020;20(1):50. 10.1186/s12885-020-6526-z 10.1186/s12885-020-6526-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koffman J, Yorganci E, Murtagh F, et al. The AMBER care bundle for hospital inpatients with uncertain recovery nearing the end of life: the ImproveCare feasibility cluster RCT. Health Technol Assess. 2019;23(55):1- 150. https://doi.org/ 10.3310/hta23550 PMID:31594555 [DOI] [PMC free article] [PubMed]
- 29.Lion A, Backes A, Duhem C, et al. Motivational interviewing to increase physical activity behavior in cancer patients: a pilot randomized controlled trial. Integr Cancer Ther. 2020;19(online). https://doi.org/ 10.1177/1534735420914973 PMID:32202163 [DOI] [PMC free article] [PubMed]
- 30.Law H, Izon E, Au-Yeung K, et al. Combined individual and family therapy in comparison to treatment as usual for people at-risk of psychosis: A feasibility study (IF CBT): Trial rationale, methodology and baseline characteristics. Early Interv Psychiatry. 2019;eip.12922. https://doi.org/ 10.1111/eip.12922 PMID:31876397 [DOI] [PubMed]
- 31.Katz Sand I, Benn EKT, Fabian M, et al. . Randomized-controlled trial of a modified Mediterranean dietary program for multiple sclerosis: A pilot study. Mult Scler Relat Disord. 2019;36:101403. 10.1016/j.msard.2019.101403 [DOI] [PubMed] [Google Scholar]
- 32.Mehta S, Hadjistavropoulos H, Nugent M, Karin E, Titov N, Dear BF. Guided internet-delivered cognitive-behaviour therapy for persons with spinal cord injury: a feasibility trial. Spinal Cord. 2020;58(5):544-552. 10.1038/s41393-019-0398-6 [DOI] [PubMed] [Google Scholar]
- 33.Huffman LE, Wilson DK, Kitzman-Ulrich H, Lyerly JE, Gause HM, Resnicow K. Huffman LE, Wilson DK, Kitzman-Ulrich H, Lyerly JE, Gause HM, Resnicow K. Associations between culturally relevant recruitment strategies and participant interest, enrollment and generalizability in a weight-loss intervention for African American families. Ethn Dis. 2016;26(3):295-304. 10.18865/ed.26.3.295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nápoles AM, Santoyo-Olsson J, Ortiz C, et al. . Randomized controlled trial of Nuevo Amanecer: a peer-delivered stress management intervention for Spanish-speaking Latinas with breast cancer. Clin Trials. 2014;11(2):230-238. 10.1177/1740774514521906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Banerjee G, Werring DJ. Feasibility of clinical trial recruitment for cerebral amyloid angiopathy: A specialist single centre experience. J Neurol Sci. 2020;409:116580. 10.1016/j.jns.2019.116580 [DOI] [PubMed] [Google Scholar]
- 36.Artom M, Czuber-Dochan W, Sturt J, Proudfoot H, Roberts D, Norton C. Cognitive-behavioural therapy for the management of inflammatory bowel disease-fatigue: a feasibility randomised controlled trial. Pilot Feasibility Stud. 2019;5(1):145. 10.1186/s40814-019-0538-y 10.1186/s40814-019-0538-y [DOI] [PMC free article] [PubMed] [Google Scholar]


