Abstract
Background:
Few studies have examined patient characteristics and treatment patterns among patients with dementia and agitation in the United States (US).
Objective:
To examine real-world treatment patterns and characteristics of patients with agitation related to dementia who were treated with antipsychotics in US residential care and community-based settings.
Methods:
This retrospective chart review collected US physician-level data from patients 55 to 90 years old initiated on an antipsychotic medication for the treatment of agitation related to dementia from January 2018 to May 2018. Clinical characteristics and treatment patterns were assessed overall and stratified by residential care and community-based settings.
Results:
A total of 313 participating physicians, 59.5% of whom were primary care physicians, abstracted 801 patient charts (residential care: n = 312; community-based: n = 489). Of patients with agitation who were initiated on an antipsychotic, most patients (74.5%) were initiated within 3 months of the onset of their studied agitation episode, and 62.8% experienced multiple agitation episodes before initiation. While non-pharmacological therapies are recommended first-line approach for agitation in dementia, use of non-pharmacological therapy before initiation of antipsychotics was reported for only 37.8% of patients in residential care and 21.3% in community-based settings.
Conclusion:
Most patients were initiated on an antipsychotic treatment after multiple episodes of agitation and largely without initial non-pharmacological therapy, suggesting that current treatment guideline recommendations for first-line non-pharmacological intervention may not be adequately followed in clinical practice. Understanding the clinical burden and treatment patterns among dementia patients with agitation is imperative for effective disease management.
INTRODUCTION
Dementia is a complex neurodegenerative disorder that affects 5.7 million individuals in the United States (US) and is one of the major causes of disability and dependence among the elderly [1]. Behavioral and psychological symptoms associated with dementia are frequent and likely occur in≥90% of patients with dementia over the course of their disease [2].
Agitation is among the most common behavioral symptoms associated with dementia and poses a major challenge for the treatment and management of patients [3, 4]. In 2015, the International Psychogeriatric Association developed a provisional consensus definition of agitation in patients with cognitive disorders [3]. Up until this time, there was no consensus definition of agitation in dementia, hampering the comparability of epidemiological and clinical studies. The prevalence of agitation in North America was recently reported to range from 5% to 86% in a systematic review of neuropsychiatric symptoms (NPS) in patients with dementia [5]. Similarly high agitation prevalence rates have been reported among individuals with dementia who live in residential care facilities (11% to 77%; up to 88% of institutionalized persons with early-onset dementia) [6, 7] and community-dwelling individuals (point-prevalence range: 18% to 62%; 32% to 40% with “clinically significant” agitation) [8, 9].
Agitation exacts a significant economic toll on individuals, families, and the healthcare system at large. A retrospective claims analysis of treatment patterns and healthcare utilization of patients with dementia with agitation and agitation-related symptoms [10] found people with agitation had a greater number of hospitalizations, hospital days, outpatient hospital/clinic visits, skilled nursing visits, and hospice visits compared with patients without agitation. Per-patient-per-year total healthcare costs also were significantly higher for patients with agitation versus without ($42,284 versus $32,640) as were inpatient, outpatient emergency department, and ambulatory costs.
Agitation also places a significant burden on patients with dementia and appears to increase with disease progression and disease severity [5]. Dementia patients with agitation have increased morbidity and mortality (including risk of suicidal ideation) [5] and faster cognitive and functional decline than dementia patients who do not exhibit agitation [11, 12]. Agitation is a leading predictor of which individuals with dementia will be transferred to residential care facilities [13–17]. Patients with dementia who exhibit agitation also place a substantial burden on caregivers, which can lead to reduced quality of life, depression, and anxiety [11, 18, 19]. The occurrence of agitation in dementia is also associated with increased odds of needing psychotropic medication, especially antipsychotics [20].
Various non-pharmacological and pharmacological strategies are used for the management of patients with dementia who exhibit agitation [21–23]. Historically, first- and second-generation antipsychotics have been used off-label as the first-line treatment of choice [24, 25]. However, there have been concerns about the risk of adverse events associated with antipsychotics, as well as concerns about their limited clinical efficacy in alleviating agitation [26]. In 2005, the U.S. Food and Drug Administration (FDA) issued an advisory warning after reports of a 60% to 70% increased risk of death associated with the use of atypical antipsychotics compared with placebo in older patients with dementia-related psychosis [27]. Safety warnings were added to the labels of all atypical antipsychotic drugs, and government agencies have since focused on limiting the use of antipsychotics in older adults [27]. In 2018, an international expert panel recommended non-pharmacological interventions, including person-centered care, environmental adaptation, caregiver training, and tailored activities as first-line approaches before any pharmacological treatments [21, 22]. Practice guidelines issued in 2016 by the American Psychiatric Association suggest that antipsychotics can be appropriate when symptoms of agitation or psychosis are severe, dangerous, and/or when symptoms cause significant distress to the patient [28]. However, at present, no antipsychotic agent is approved by the FDA for the treatment of agitation related to dementia [28].
Although antipsychotic treatments are recommended for patients with dementia and severe or recurring agitation, there is limited information published on current clinical practice regarding the use of antipsychotic treatments for the management of agitation related to dementia in real-world settings in the US [10]. The objective of this retrospective observational study was to examine real-world treatment patterns and characteristics of patients with agitation related to dementia who were treated with antipsychotics in residential care and community-based settings in the US.
METHODS
Data source
This retrospective observational study collected physician-level information using a short survey and patient-level information through medical chart abstraction conducted by physicians. Primary care physicians, geriatricians, neurologists, and psychiatrists were recruited via email from a large, geographically representative, and well-established International Organization for Standardization (ISO)-certified online panel. Participating physicians treating eligible patients were asked to provide clinical information, via an electronic case report form (eCRF) that was developed specifically for this study, on up to 10 randomly selected patients from their practice meeting the eligibility criteria. The data collection period spanned from January 2018 to May 2018. The first section of the eCRF included a screener section to determine the eligibility of the physician as well as a short physician survey. The remaining sections confirmed the eligibility of the patient for the study and collected patient-level clinical data. Data collected from physicians did not include any patient-identifying information, and the study was exempted from full review by the New England Institutional Review Board.
Study design and sample selection
This study aimed to collect information for approximately 800 patients initiated on an antipsychotic medication for the treatment of agitation related to dementia. The index date was defined as the initiation date of an antipsychotic for the treatment of agitation related to dementia/Alzheimer’s disease (AD). For patients who had received more than one antipsychotic for the treatment of their agitation before the chart abstraction date, the index date was the date of the last antipsychotic initiation in order to capture more contemporary data. Patients were observed until death or chart abstraction, whichever came first (see Supplementary Figure 1).
Physicians were eligible to participate in the survey if they were currently practicing medicine in the US, had treated at least 1 eligible patient with dementia, and had complete access to patients’ medical charts, including information on use of antipsychotic therapy.
Patients were eligible if they 1) were suspected of having or diagnosed as having AD or other forms of dementia; 2) received an antipsychotic medication for the treatment of agitation related to dementia; and 3) were 55 to 90 years old at the index date. Patients were excluded from the study if they received an antipsychotic medication for the treatment of a condition other than agitation related to dementia; if they had a history of HIV, AIDS, stroke, or transient ischemic attack before the index date; or if they had an unknown living accommodation setting (i.e., it was unknown whether the patient lived in residential care or community-based settings). The index date was required to occur at least 90 days before the chart abstraction date to allow sufficient follow-up time for the observation of outcomes and no more than 2 years before the chart abstraction date to capture outcomes in contemporary practice. Physicians were allowed to select an option “Unknown” if they had no information for a given component of the chart abstraction. The proportion of physicians indicating “Unknown” was assessed for each component. No patients were excluded from the sample due to data incompleteness.
Measures, outcomes, and statistical analyses
Physician characteristics included the number of patients with suspected or diagnosed dementia under their care, number of years in practice, primary specialty, and US census region of practice. Patient characteristics and outcomes included demographics, comorbidities, characteristics related to dementia and agitation episodes, treatment patterns, and adverse events. Descriptive statistics were used to summarize physician and patient characteristics and outcomes; means, standard deviations, and medians were used to summarize continuous variables, and frequencies and percentages were used to summarize categorical variables. Differences between residential care and community-based settings were tested using Wilcoxon rank-sum tests for continuous variables and chi-squared tests for categorical variables.
Among patients with available information on antipsychotic discontinuation status, time to discontinuation of the antipsychotic initiated on the index date was estimated using Kaplan-Meier survival analyses. Time to discontinuation was measured from the index date to the earlier date between the date of discontinuation or 90 days after the index date. Patients who did not discontinue the antipsychotic initiated on the index date or who died were censored as of the 90th day following the index date or date of death, whichever occurred first. Antipsychotic discontinuation rates (with 95% CIs) were estimated at 30, 60, and 90 days from the index date. Analyses were conducted among all patients meeting the selection criteria and were also stratified by living accommodation setting: residential care setting (i.e., intermediate form, dementia-specific facility, or long-term care) and community-based setting (i.e., patient’s private home).
A subgroup analysis among patients with dementia of the Alzheimer’s type was also conducted. Statistical analyses were conducted using R-Markdown software [29, 30].
RESULTS
Physician and patient characteristics
A total of 313 physicians participated in the study. Most physicians were primary care physicians (59.4%) and had been practicing for more than 10 years (70.9%). All US census regions were represented, with most practices in a suburban (51.9%) or urban (34.4%) setting (Supplementary Table 1).
Physicians provided data on a total of 801 patients, 312 in residential care settings and 489 in community-based settings. Baseline patient characteristics for all patients, residential care setting patients, and community-based patients are presented in Table 1. The mean patient age was 73.6 years and 49.3% were female. The mean duration of follow-up for all patients was 9.5 months. Hypertension, hyperlipidemia, and gastroesophageal reflux/peptic ulcer disease were the most common frequent medical comorbidities. The most frequent psychiatric comorbidities were anxiety, depression, and insomnia. Among both settings, at the index date, antidementia, antihypertensive, lipid-lowering, and antidepressant medications were the most frequently used medications exclusive of antipsychotics; hypnotics and antiepileptics were not frequently used (<5%).
Table 1.
Baseline patient demographics and characteristics at the index date among patients with agitation and dementia initiated on an antipsychotic in residential care and community-based settings
| Characteristic | All Patients | Residential Care Setting | Community-Based Setting | P |
| (N = 801) | (n = 312) | (n = 489) | ||
| Age (y), mean±SD | 73.6±8.1 | 75.0±7.4 | 72.6±8.4 | <0.01** |
| Female, n (%) | 395 (49.3) | 158 (50.6) | 237 (48.5) | 0.60 |
| Predominant race, n (%) | 0.09 | |||
| White or Caucasian | 589 (73.5) | 229 (73.4) | 360 (73.6) | |
| Asian | 61 (7.6) | 16 (5.1) | 45 (9.2) | |
| Black or African American | 137 (17.1) | 58 (18.6) | 79 (16.2) | |
| Other/unknown | 14 (1.7) | 9 (2.9) | 5 (1.0) | |
| Type(s) of insurance, n (%)* | ||||
| Commercial/private insurance | 211 (26.3) | 72 (23.1) | 139 (28.4) | 0.11 |
| Medicare | 534 (66.7) | 209 (67.0) | 325 (66.5) | 0.94 |
| Medicaid | 80 (10.0) | 39 (12.5) | 41 (8.4) | 0.08 |
| Dual eligibility (Medicare or Medicaid) | 107 (13.4) | 61 (19.6) | 46 (9.4) | <0.01** |
| Other/unknown | 30 (3.7) | 12 (3.8) | 18 (3.7) | 0.66 |
| Most frequent medical comorbidities, n (%) | ||||
| Hypertension | 519 (64.8) | 217 (69.6) | 302 (61.8) | <0.05** |
| Hyperlipidemia | 296 (37.0) | 122 (39.1) | 174 (35.6) | 0.35 |
| Gastroesophageal reflux disease or peptic ulcer disease | 175 (21.8) | 73 (23.4) | 102 (20.9) | 0.45 |
| Osteoarthritis | 153 (19.1) | 79 (25.3) | 74 (15.1) | <0.01** |
| Diabetes | 144 (18.0) | 69 (22.1) | 75 (15.3) | <0.05** |
| Most frequent psychiatric comorbidities, n (%) | ||||
| Anxiety | 243 (30.3) | 115 (36.9) | 128 (26.2) | <0.01** |
| Depression | 198 (24.7) | 74 (23.7) | 124 (25.4) | 0.66 |
| Insomnia | 142 (17.7) | 54 (17.3) | 88 (18.0) | 0.88 |
| Altered mental status | 73 (9.1) | 38 (12.2) | 35 (7.2) | <0.05** |
| Delirium | 44 (5.5) | 26 (8.3) | 18 (3.7) | <0.01** |
| Medication other than antipsychotics, n (%)* | ||||
| Antidementia | 457 (57.1) | 189 (60.6) | 268 (54.8) | 0.12 |
| Acetylcholinesterase inhibitor | 390 (48.7) | 159 (51.0) | 231 (47.2) | 0.34 |
| Memantine | 241 (30.1) | 115 (36.9) | 126 (25.8) | <0.01** |
| Antihypertensive | 364 (45.4) | 151 (48.4) | 213 (43.6) | 0.20 |
| Lipid-lowering | 257 (32.1) | 119 (38.1) | 138 (28.2) | <0.01** |
| Antidepressant | 191 (23.8) | 92 (29.5) | 99 (20.2) | <0.01** |
| Aspirin or NSAID | 148 (18.5) | 71 (22.8) | 77 (15.7) | <0.05** |
| Antidiabetic | 129 (16.1) | 61 (19.6) | 68 (13.9) | <0.05** |
| Anticoagulant | 36 (4.5) | 16 (5.1) | 20 (4.1) | 0.61 |
| Hypnotic | 17 (2.1) | 11 (3.5) | 6 (1.2) | 0.05 |
| Antiepileptic | 16 (2.0) | 9 (2.9) | 7 (1.4) | 0.24 |
| None | 52 (6.5) | 18 (5.8) | 34 (7.0) | 0.61 |
| Duration of follow-up (mo), mean±SD | 9.5±5.2 | 9.4±5.2 | 9.6±5.3 | 0.52 |
NSAID, non-steroidal anti-inflammatory drug; SD, standard deviation. *Categories are non-mutually exclusive. **Indicates statistical significance at the 5% level.
Characteristics
Table 2 presents the baseline patient characteristics among patients with agitation and dementia initiated on an antipsychotic in residential care and community-based settings. Most patients had dementia of the Alzheimer’s type (83.0%) and a moderate (64.5%) severity of dementia. Severe dementia was reported for 17.6% of patients in residential care settings and 7.2% of patients in community-based settings (p < 0.01). The Mini-Mental State Examination was the most commonly used tool to assess dementia severity (46.4%), yet no formal criteria were used for 46.2% of patients. The time between the first symptoms of dementia and the index date was reported for 58.2% of the sample, with a mean of 22.8 months (median: 17.0 months).
Table 2.
Baseline patient characteristics at the index date among patients with agitation and dementia initiated on an antipsychotic in residential care and community-based settings
| Characteristic | All Patients | Residential Care | Community-Based | P |
| (N = 801) | Setting (n = 312) | Setting (n = 489) | ||
| Type of dementia, n (%)* | ||||
| AD | 665 (83.0) | 254 (81.4) | 411 (84.0) | 0.38 |
| Mixed dementia | 135 (16.9) | 62 (19.9) | 73 (14.9) | 0.08 |
| Frontotemporal dementia | 44 (5.5) | 19 (6.1) | 25 (5.1) | 0.67 |
| Other† | 3 (0.4) | 3 (1.0) | 0 (0.0) | 0.11 |
| Time between first symptoms of dementia/AD and the index date (mo), mean±SD | 22.8±20.8 | 22.3±20.9 | 23.0±20.7 | 0.75 |
| Unknown, n (%) | 322 (40.2) | 134 (42.9) | 188 (38.4) | 0.23 |
| Severity of dementia/AD, n (%) | <0.01** | |||
| Mild | 176 (22.0) | 47 (15.1) | 129 (26.4) | |
| Moderate | 517 (64.5) | 205 (65.7) | 312 (63.8) | |
| Severe | 90 (11.2) | 55 (17.6) | 35 (7.2) | |
| Unknown | 18 (2.2) | 5 (1.6) | 13 (2.7) | |
| Criteria used to rate the severity of the patient’s dementia/AD, n (%)‡ | 0.76 | |||
| Mini-Mental State Examination score | 363 (46.4) | 139 (45.3) | 224 (47.1) | |
| Clinical dementia rating scale | 29 (3.7) | 14 (4.6) | 15 (3.2) | |
| Other clinical evaluating method§ | 29 (3.7) | 11 (3.6) | 18 (3.8) | |
| No formal criteria used for rating | 362 (46.2) | 143 (46.6) | 219 (46.0) | |
| Severity of agitation, n (%) | <0.01** | |||
| Mild | 201 (25.1) | 59 (18.9) | 142 (29.0) | |
| Moderate | 522 (65.2) | 210 (67.3) | 312 (63.8) | |
| Severe | 62 (7.7) | 36 (11.5) | 26 (5.3) | |
| Unknown | 16 (2.0) | 7 (2.2) | 9 (1.8) | |
| Criteria used to rate the severity of the agitation symptoms, n (%)¶ | 0.06 | |||
| NPI score | 33 (4.2) | 9 (3.0) | 24 (5.0) | |
| NPI-NH score | 12 (1.5) | 7 (2.3) | 5 (1.0) | |
| CMAI score | 41 (5.2) | 23 (7.5) | 18 (3.8) | |
| Other clinical evaluation¶¶ | 32 (4.1) | 13 (4.3) | 19 (4.0) | |
| No formal criteria used for rating | 667 (85.0) | 253 (83.0) | 414 (86.3) | |
| Patient has an informal caregiver, n (%) | <0.01** | |||
| Yes | 588 (73.4) | 178 (57.1) | 410 (83.8) | |
| No | 191 (23.8) | 126 (40.4) | 65 (13.3) | |
| Unknown | 22 (2.7) | 8 (2.6) | 14 (2.9) | |
| Caregiver relationship, n (%)†† | <0.01** | |||
| Spouse | 308 (52.4) | 58 (32.6) | 250 (61.0) | |
| Sibling | 47 (8.0) | 22 (12.4) | 25 (6.1) | |
| Child | 207 (35.2) | 88 (49.4) | 119 (29.0) | |
| Friend | 17 (2.9) | 8 (4.5) | 9 (2.2) | |
| Other/unknown | 9 (1.5) | 2 (1.1) | 7 (1.7) |
AD, Alzheimer’s disease; CMAI, Cohen-Mansfield Agitation Inventory; NPI, Neuropsychiatric Inventory; NPI-NH, Neuropsychiatric Inventory-Nursing Home version; SD, standard deviation. *Categories are non-mutually exclusive. **Indicates statistical significance at the 5% level. †Other reported types of dementia included Parkinson’s disease, Pick’s disease, and alcohol induced. ‡Evaluated among patients with known severity of dementia/AD. §Other reported clinical evaluating methods included Brief Interview for Mental Status (BIMS), Functional Assessment Staging Test (FAST), Montreal Cognitive Assessment (MOCA), neuropsychiatric testing, Mini-Cog©, Saint Louis University Mental Status Examination (SLUMS) score, and physician assessment. ¶Evaluated among patients with known severity of agitation. ¶¶Other reported clinical evaluation included physician assessment. ††Evaluated among patients with an informal caregiver.
Severity of agitation in the sample was reported to be moderate (65.2%) for most patients. Among patients in residential care and community-based settings, severe agitation was reported in 11.5% and 5.3% of patients, respectively (p < 0.01). Most physicians (85.0%) reported no use of formal criteria to rate the severity of agitation in their patients. A version of the Neuropsychiatric Inventory (NPI; either the NPI or NPI-Nursing Home version) was reported for rating agitation severity among 5.7% of physicians, and 5.2% reported using the Cohen-Mansfield Agitation Inventory (CMAI). Overall, 73.4% of patients were reported to have an informal caregiver (57.1% in residential care and 83.8% in community-based settings, p < 0.01). In residential care settings, the patient’s caregiver was a spouse for 32.6% and a child for 49.4% of the patients; in community-based settings, these proportions were 61.0% and 29.0%, respectively (p < 0.01).
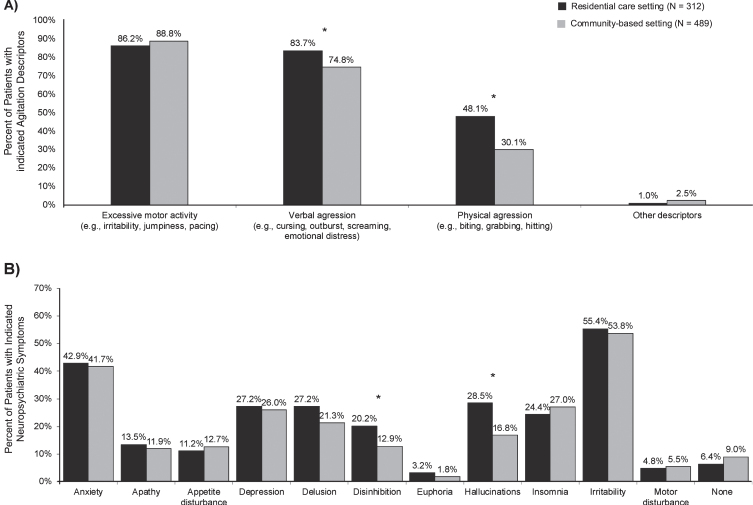
Neuropsychiatric symptoms at the index date for patients with dementia in residential care and community-based settings are presented in Fig. 1. Excessive motor activity and verbal aggression were the most common agitation descriptors leading to initiation of antipsychotics in both settings (residential care setting: 86.2% and 83.7%, respectively; community-based setting: 88.8% and 74.8%, respectively). Physical aggression was reported in 48.1% of patients in residential care settings and in 30.1% of patients in community-based settings (p < 0.01). Other NPS most frequently reported included irritability (residential care setting = 55.4%; community-based setting = 53.8%; p = 0.70) and anxiety (residential care setting = 42.9%; community-based setting = 41.7; p = 0.79).
Fig. 1.
Neuropsychiatric symptoms at the index date for patients with agitation and dementia initiated on an antipsychotic in residential care and community-based settings. A) Agitation descriptors leading to initiation of an antipsychotic. B) Other neuropsychiatric symptoms. *Indicates statistical significance at the 5% level.
Treatment patterns
Agitation episodes
Table 3 describes characteristics of agitation episodes among patients with dementia who were initiated on an antipsychotic in residential care and community-based settings. In most patients, the time from the beginning of the studied agitation episode to the index date was 1–3 months (overall = 40.8%; residential care setting = 42%; community-based setting = 40.1%) and 33.7% of patients were initiated on an antipsychotic within 1 month (residential care setting = 31.4%; community-based setting = 35.2%) (p = 0.85). Most patients (overall = 62.8%; residential care setting = 61.2%; community-based setting = 63.8%; p = 0.69) had multiple episodes of agitation during the course of their dementia. For most patients, the time from the beginning of the first agitation episode to the index date was less than 6 months (overall = 64.9%; residential care setting = 64.1%; community-based setting = 65.4%; p < 0.05).
Table 3.
Characteristics of agitation episodes among patients with agitation and dementia initiated on an antipsychotic in residential care and community-based settings
| Agitation Characteristic | All Patients | Residential Care Setting | Community-Based Setting | P |
| (N = 801) | (n = 312) | (n = 489) | ||
| Time from beginning of the studied agitation episode to index date, n (%) | 0.85 | |||
| Less than 1 month | 270 (33.7) | 98 (31.4) | 172 (35.2) | |
| 1–3 months | 327 (40.8) | 131 (42.0) | 196 (40.1) | |
| 4–6 months | 128 (16.0) | 55 (17.6) | 73 (14.9) | |
| 7–12 months | 24 (3.0) | 9 (2.9) | 15 (3.1) | |
| More than 12 months | 8 (1.0) | 3 (1.0) | 5 (1.0) | |
| Unknown | 44 (5.5) | 16 (5.1) | 28 (5.7) | |
| Experienced multiple episodes of agitation over the course of their dementia, n (%) | 0.69 | |||
| Yes | 503 (62.8) | 191 (61.2) | 312 (63.8) | |
| No | 230 (28.7) | 95 (30.4) | 135 (27.6) | |
| Unknown | 68 (8.5) | 26 (8.3) | 42 (8.6) | |
| Time from beginning of the first agitation episode to index date, n (%) | <0.05** | |||
| Less than 6 months | 520 (64.9) | 200 (64.1) | 320 (65.4) | |
| 6–11 months | 144 (18.0) | 50 (16.0) | 94 (19.2) | |
| More than 12 months | 51 (6.4) | 29 (9.3) | 22 (4.5) | |
| Unknown | 86 (10.7) | 33 (10.6) | 53 (10.8) |
**Indicates statistical significance at the 5% level.
Treatment patterns
Table 4 presents the non-pharmacological and pharmacological (antipsychotic) treatment patterns among patients with dementia and agitation who were initiated on an antipsychotic in residential care and community-based settings. Most patients did not receive formal non-pharmacological therapy (i.e., sensory interventions, active therapy/structured activities, complementary alternative medicine, or psychological therapy) for their agitation symptoms before initiation of antipsychotics (overall = 60.0%; residential care setting = 51.9%; and community-based setting = 65.2%; p = 0.01). Nearly all (87.1%) patients had received a single antipsychotic agent for the treatment of agitation as of their index date. A smaller proportion of patients received 2 (9.0%) or 3 or more (3.9%) antipsychotic agents.
Table 4.
Non-pharmacological and pharmacological treatment patterns among patients with agitation and dementia initiated on an antipsychotic in residential care and community-based settings
| All Patients | Residential Care Setting | Community-Based Setting | P | |
| (N = 801) | (n = 312) | (n = 489) | ||
| Non-pharmacological therapy for agitation before index date | ||||
| Use of non-pharmacological therapy, n (%) | <0.01** | |||
| Yes | 222 (27.7) | 118 (37.8) | 104 (21.3) | |
| No | 481 (60.0) | 162 (51.9) | 319 (65.2) | |
| Unknown | 98 (12.2) | 32 (10.3) | 66 (13.5) | |
| Duration of non-pharmacological therapy, n (%)* | <0.01** | |||
| Less than 1 month | 43 (19.4) | 30 (25.4) | 13 (12.5) | |
| 1–3 months | 83 (37.4) | 46 (39.0) | 37 (35.6) | |
| 4–6 months | 32 (14.4) | 16 (13.6) | 16 (15.4) | |
| 7–12 months | 14 (6.3) | 8 (6.8) | 6 (5.8) | |
| More than 12 months | 8 (3.6) | 6 (5.1) | 2 (1.9) | |
| Ongoing | 25 (11.3) | 6 (5.1) | 19 (18.3) | |
| Unknown | 7 (7.7) | 6 (5.1) | 11 (10.6) | |
| Type(s) of non-pharmacological therapy used if applicable, n (%)*, † | ||||
| Sensory interventions | 116 (52.3) | 71 (60.2) | 45 (43.3) | <0.01** |
| Active therapy/structured activities | 137 (61.7) | 80 (67.8) | 57 (54.8) | <0.01** |
| Complementary alternative medicine | 39 (17.6) | 27 (22.9) | 12 (11.5) | <0.01** |
| Psychological therapy | 84 (37.8) | 50 (42.4) | 34 (32.7) | <0.01** |
| Other | 1 (0.5) | 0 (0.0) | 1 (1.0) | <0.01** |
| Antipsychotic treatment for agitation | ||||
| No. of different antipsychotic agents received, n (%)‡ | <0.05** | |||
| One | 698 (87.1) | 257 (82.4) | 441 (90.2) | |
| Two | 72 (9.0) | 37 (11.9) | 35 (7.2) | |
| Three or more | 31 (3.9) | 18 (5.8) | 13 (2.7) | |
| First antipsychotic agent received among those who received 2 or more antipsychotic agents, n (%) | 0.73 | |||
| Quetiapine | 20 (19.4) | 8 (14.5) | 12 (25.0) | |
| Haloperidol | 17 (16.5) | 10 (18.2) | 7 (14.6) | |
| Risperidone | 14 (13.6) | 8 (14.5) | 6 (12.5) | |
| Aripiprazole | 13 (12.6) | 8 (14.5) | 5 (10.4) | |
| Olanzapine | 6 (5.8) | 4 (7.3) | 2 (4.2) | |
| Ziprasidone | 4 (3.9) | 3 (5.5) | 1 (2.1) | |
| Other/unknown | 29 (28.2) | 14 (25.5) | 15 (31.3) | |
| Second antipsychotic agent received among those who received 3 or more antipsychotic agents, n (%) | 0.21 | |||
| Quetiapine | 2 (6.5) | 0 (0) | 2 (15.4) | |
| Haloperidol | 2 (6.5) | 1 (5.6) | 1 (7.7) | |
| Risperidone | 3 (9.7) | 3 (16.7) | 0 (0) | |
| Aripiprazole | 2 (6.5) | 1 (5.6) | 1 (7.7) | |
| Olanzapine | 3 (9.7) | 3 (16.7) | 0 (0) | |
| Ziprasidone | 1 (3.2) | 1 (5.6) | 0 (0) | |
| Other/unknown | 18 (58.1) | 9 (50) | 9 (69.2) |
SD, standard deviation. *Evaluated among patients using non-pharmacological therapy: sensory (e.g., music therapy, light therapy, pet therapy, multisensory stimulation), active therapy/structured activities (e.g., dancing, exercise, art therapy, social interaction), complimentary alternative medicine (e.g., aromatherapy, reflexology, massage), and psychological therapy (e.g., validation therapy, cognitive behavioral therapy, relaxation training). **Indicates statistical significance at the 5% level. †Categories are non-mutually exclusive. ‡Number of different antipsychotic agents received including the antipsychotic agent initiated on the index date. Of note, a patient may have received different agents in combination (i.e., not necessarily over the course of separate lines of therapy). In addition, a patient may have received the same agent more than once over the course of separate lines of therapy.
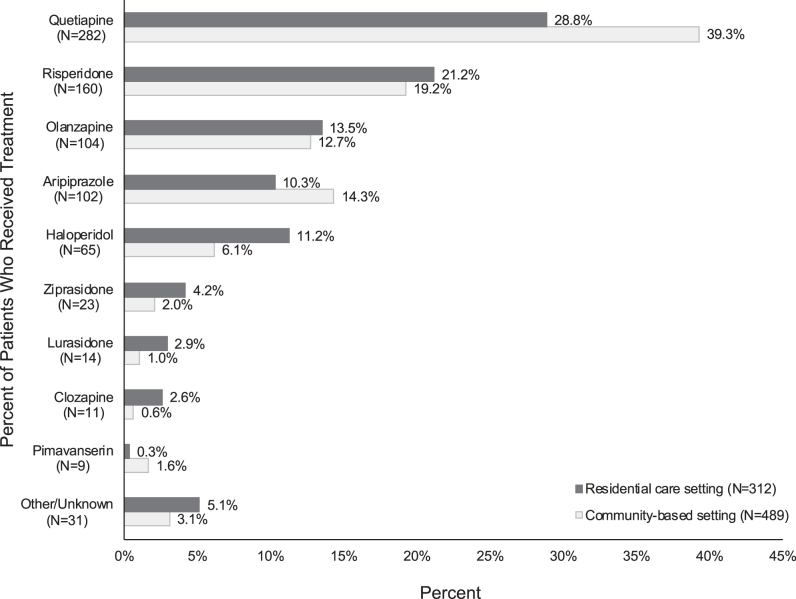
The most common antipsychotic treatments initiated on the index date for the treatment of agitation related to dementia/AD were quetiapine (35.2%), risperidone (20.0%), and olanzapine (13.0%) (Fig. 2).
Fig. 2.
Antipsychotic treatment received for the treatment of agitation related to dementia/Alzheimer’s disease among patients with agitation and dementia initiated on an antipsychotic in residential care and community-based settings on the index date.* †. *Antipsychotic agents received on the index date were statistically different between residential care and community-based setting at the 5% level. †Other antipsychotic agents initiated on the index date included paliperidone (0.7%), olanzapine and fluoxetine (0.7%), aripiprazole lauroxil (0.6%), asenapine (0.4%), brexpiprazole (0.4%), chlorpromazine (0.4%), cariprazine (0.2%), loxapine (0.1%), and pimozide (0.1%). The index treatment was unknown for 1 patient (0.1%).
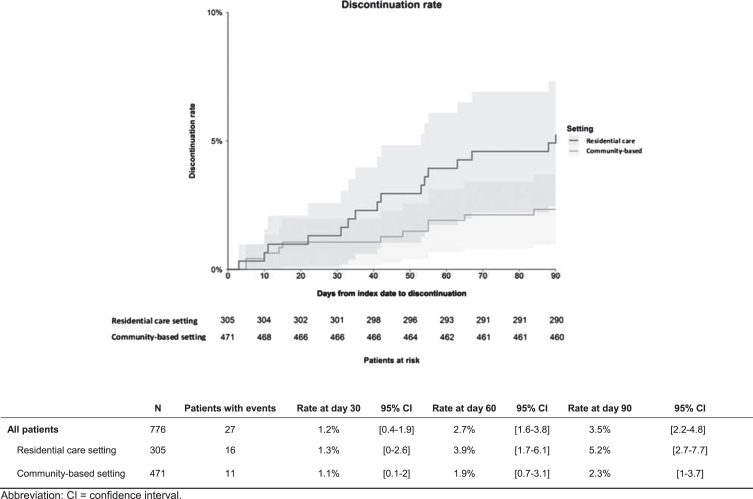
Antipsychotic discontinuation rates overall were significantly higher among patients with dementia and agitation in the residential care setting versus the community-based setting (log rank, p < 0.05). However, there was no significant difference in antipsychotic discontinuation rates at 30, 60, and 90 days (Fig. 3).
Fig. 3.
Antipsychotic discontinuation rate among patients with agitation and dementia initiated on an antipsychotic in residential care and community-based settings.
The most frequent reasons for discontinuation of the antipsychotic treatment initiated on the index date among patients (n = 16) in the residential care setting were insufficient response (62.5%) and deterioration of patient’s quality of life (31.3%). In contrast, among patients in the community-based setting (n = 11), non-severe adverse events (27.3%), and deterioration of patient’s functional status (18.2%) were the most frequent reasons for antipsychotic discontinuation.
Thirty-five percent of patients experienced adverse events after their index date and 82.7% experienced an adverse event within the first 90 days following the index date (data not shown). The most frequently reported adverse events were sedation (13.2%), somnolence (10.5%), dizziness (8.7%), falls (7.7%), and weight gain (6.1%).
Results remained similar in the sensitivity analysis examining the subgroup of patients with dementia of the Alzheimer’s type (665/801 = 83.0%) versus patients with mixed, frontotemporal, or other dementia (data not shown).
DISCUSSION
This study identified novel information from US clinical practice regarding real-world treatment patterns and clinical characteristics of patients with agitation related to dementia who were treated with antipsychotics in community-based and residential care settings.
Non-pharmacological interventions, including person-centered care, environmental adaptation, caregiver training, and tailored activities, are currently recommended as first-line approaches before pharmacological treatment for the management of behavioral and psychological symptoms in AD [21, 22]. However, the current study suggests that these recommendations may not be adequately followed in real-world practice. Less than 30% of patients with dementia received non-pharmacological therapy for agitation symptoms before the index date, as documented in medical charts. This figure is consistent with a US retrospective review of the medical records of 123 nursing home residents that found that 41.9% of patients with dementia and NPS, including agitation, did not receive any non-pharmacological therapy, although over 90% received a pharmacological treatment for their NPS [31].
Overall, the use of non-pharmacological therapies in the current study was low among both care settings. This may be attributable to a lack of training for physicians and caregivers, limited resources, and limited access to specialized staff to administer individualized person-centered interventions as well as a need for faster relief of agitation symptoms to enhance patient and caregiver safety. Non-pharmacological therapies were used more frequently in residential care settings versus community-based settings. In residential care settings, use of formal non-pharmacological therapies was reported in approximately 40% of patients. The use of formal non-pharmacological therapies was particularly low among patients in community-based settings, where fewer than 1 in 4 reportedly received such treatments.
In a recent review article that included 74 studies examining non-pharmacological management of behavioral symptoms in patients with dementia, most interventions were shown to be implemented in residential care facilities (n = 63, 82%) as opposed to the community (n = 13, 17%) [32]. In residential care settings, multiple healthcare professionals (e.g., physicians, nurses, psychologists) are typically involved in a patient’s treatment [33]. In this setting, there is probably greater access to training in use of non-pharmacological treatment options to treat agitation and more integration of non-pharmacological therapy into the facilities’ treatment protocols than in the community-based setting.
In the current study, most patients (87.1%) were initiated on an antipsychotic for the first time at the index date. A numerically higher, but not statistically significant, proportion of patients in residential care settings continued to receive the index antipsychotic for more than 90 days compared with patients in community-based settings. This finding suggests that efficacy and safety monitoring of antipsychotics may be more active in the residential care setting than in the community-based setting. We note that the overall discontinuation rate of antipsychotics was significantly higher in the residential care setting compared with the community-based setting; however, when limited to only individuals who discontinued antipsychotics within 90 days after treatment initiation (n = 27), statistical significance was not reached. Finally, while most patients in the study started and were maintained on their index antipsychotic, some patients historically received as many as 2 or more antipsychotics for agitation, reinforcing the need for new and effective treatments.
Among patients with dementia who were initiated on an antipsychotic, about 3 of 4 patients were initiated within 3 months after the onset of their studied agitation episode. While 62.8% of patients experienced multiple agitation episodes before initiation, nearly two thirds were initiated on an antipsychotic treatment within 6 months of their first agitation episode. Notably, alternative pharmacological treatments for agitation or comorbid NPS [34, 35] were present at the index date; these alternative treatments were given to less than 1 in 4 patients who were treated with an antidepressant and to less than 1 in 20 patients who were treated with an antiepileptic or hypnotic in addition to the antipsychotic. It is nonetheless possible that physicians prescribing antiepileptic drugs (e.g., gabapentin) for reasons such as the treatment of pain or anxiety did not report use of these antiepileptics. Accordingly, the use of antiepileptics could be higher than reported in the current study. Furthermore, based on the rates of NPS (e.g., irritability, anxiety, and insomnia) observed on the index date, a more widespread use of psychotropic drugs would have been expected, raising the broader question of whether antipsychotics were initiated to treat other NPS along with agitation [10].
Over 90% of patients initiated on an antipsychotic in the current study were reported to have mild-to-moderate symptoms of agitation. However, patients’ level of distress was not captured in this study; therefore, it is conceivable that some patients with mild-to-moderate symptoms may have experienced a level of distress sufficient for clinicians to justify the use of antipsychotics. The American Psychological Association guidelines recommend quantitative measurement to assess the type, frequency, severity, pattern, and timing of agitation symptoms [28]. However, in current study, 85% of patients were not evaluated with standardized instruments for the severity of their agitation symptoms. Lack of use of standardized instruments such as the NPI or CMAI rating scales may have resulted in inconsistent or inaccurate evaluation of symptom severity for some patients. Although rating scales can more accurately define and measure NPS than clinical judgment alone, it remains unclear whether routine use of rating scales in real-world practice leads to improved outcomes.
Overall, the most common antipsychotic treatments initiated on the index date for the treatment of agitation were quetiapine, risperidone, and olanzapine. However, haloperidol was used more frequently in the residential care setting than in the community-based setting (11.2% versus 6.1%, respectively). Because of the risk profile of haloperidol [36], it is somewhat surprising that > 10% of patients in the residential care setting were treated with this drug, although higher use might be attributed to the lower cost of haloperidol. Higher use of haloperidol in residential care settings versus community-based settings has been reported previously [37]. Quetiapine and aripiprazole were used more often in community-based settings than in residential care settings (quetiapine: 39.3% versus 28.8%, respectively; aripiprazole: 14.3% versus 10.3%, respectively). A possible explanation is that these agents might be a more favorable treatment choice in higher functioning patients. Aripiprazole and quetiapine might also be used for the treatment of major depressive disorder in community-based settings, although the rates of major depressive disorder in the current study were low. Other studies have reported higher use of quetiapine in the residential care setting than in community-based settings [37, 38]. Further investigation is needed to understand the precise reason for differences in the patterns of use of these antipsychotics among community-based and residential care settings.
In this real-world study, the clinical management of agitation in patients with dementia is further complicated by the presence of psychiatric and medical comorbidities. The most frequent psychiatric comorbidities were anxiety, depression, and insomnia. Patients in the current study were frequently being treated for medical conditions such as hypertension, hyperlipidemia, and diabetes. At the index date, antidementia, antihypertensive, lipid-lowering, and antidepressant medications were the most frequently used medications exclusive of antipsychotics. Comorbid conditions and their associated treatments among patients with agitation symptoms and dementia require careful monitoring during antipsychotic treatment.
Discrepancies between treatment guidelines and clinical practice, in addition to a high prevalence of psychiatric and medical comorbidities, were observed in this chart analysis among dementia patients with agitation. These issues coupled with the absence of safe and effective FDA-approved pharmacological treatments and the lack of guideline recommendations for the optimal sequence of interventions (i.e., when to add or switch non-pharmacological to or from non-pharmacological therapy) adds to the challenge of optimally treating agitation in patients with dementia.
Several ongoing clinical trials are currently evaluating the efficacy of various potential therapies for the treatment of agitation related to dementia, including serotonin reuptake inhibitors, cholinesterase inhibitors, synthetic cannabinoids, glutamate receptor modulators, and antipsychotics [39]. In light of the unmet needs of patients with dementia and agitation, these potential new therapies hold the promise of alleviating the burden of agitation symptoms on affected patients, their caregivers, and society as a whole.
This study is subject to several limitations. Information captured by the eCRF was limited to information available in the patients’ medical records held by the physicians participating in the study. Therefore, information on health care services received outside the physician’s care setting that were not recorded in the medical chart was not captured, including non-pharmacological therapies received in a residential care setting or adverse events for which the patient sought care elsewhere. Similarly, medical and psychiatric history may be incomplete if clinical information before the patient’s first visit to the responding physician was not recorded in the medical chart. For example, time since first symptoms of dementia was unknown for 40.2% of the sample, which could explain the discordance between the high proportion of patients reported with moderate-to-severe dementia and the relatively short time (less than 2 years) reported since the onset of symptoms of dementia. In addition, historical administration of other psychotropic drugs either for comorbid NPS such as depression or anxiety or for agitation may have not been included. Furthermore, by study design, patients switching to another antipsychotic after the index date were not captured because the index date was the date of the most recent initiation of an antipsychotic, and no information on titration or dose reduction was collected. The population in this study is younger than what might be expected of a dementia cohort. But this was a population of patients treated with antipsychotics, which could also explain why patients are relatively young (mean age: 73.6 years) for a dementia cohort. Finally, recruitment for this online panel-based chart review was dependent on voluntary participation of physicians who selected eligible charts; although physicians were instructed to randomly select patients who met the inclusion criteria, selection bias may exist, and the sample may not be representative of the overall population with AD taking antipsychotics for the management of their agitation symptoms.
Conclusions
This real-world analysis provides novel information regarding US treatment patterns and clinical characteristics of patients with agitation related to dementia who were treated with antipsychotics in residential care and community-based settings. Of patients with agitation who were initiated on an antipsychotic, most were initiated within 3 months after the onset of their studied agitation episode, with many experiencing multiple agitation episodes before initiation. Most patients were initiated on an antipsychotic after multiple episodes of agitation and largely without initial non-pharmacological therapy, suggesting that current treatment guideline recommendations for first-line non-pharmacological intervention may not be adequately followed in clinical practice. This study helps to fill a gap in the current understanding of the burden of agitation in dementia. Effectively understanding the clinical burden and treatment patterns among dementia patients with agitation is imperative for effective disease management.
Supplementary Material
ACKNOWLEDGMENTS
Medical writing assistance was provided by Sara Kaffashian (formerly Analysis Group, Inc.), Susan Bartko-Winters, PhD (Global Outcomes Group), and Emily Kuhl, PhD (Global Outcomes Group). Editorial support was provided by Esther Tazartes, MS (Global Outcomes Group).
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/20-0127r2).
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-200127.
REFERENCES
- [1].Alzheimer’s Association, AD Facts and Figures, https://www.alz.org/alzheimers-dementia/facts-figures, Accessed on September 23, 2018.
- [2]. Preuss UW, Wong JW, Koller G (2016) Treatment of behavioral and psychological symptoms of dementia: A systematic review, Psychiatr Pol 50, 679–715. [DOI] [PubMed] [Google Scholar]
- [3]. Cummings J, Mintzer J, Brodaty H, Sano M, Banerjee S, Devanand DP, Gauthier S, Howard R, Lanctot K, Lyketsos CG, Peskind E, Porsteinsson AP, Reich E, Sampaio C, Steffens D, Wortmann M, Zhong K (2015) Agitation in cognitive disorders: International Psychogeriatric Association provisional consensus clinical and research definition, Int Psychogeriatr 27, 7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Howard RJ (2016) Disentangling the treatment of agitation in Alzheimer’s disease, Am J Psychiatry 173, 441–443. [DOI] [PubMed] [Google Scholar]
- [5]. Anatchkova M, Brooks A, Swett L, Hartry A, Duffy RA, Baker RA, Hammer-Helmich L, Sanon Aigbogun M (2019) Agitation in patients with dementia: A systematic review of epidemiology and association with severity and course. Int Psychogeriatr, doi: 10.1017/S1041610218001898 [DOI] [PubMed]
- [6]. Mulders AJ, Fick IW, Bor H, Verhey FR, Zuidema SU, Koopmans RT (2016) Prevalence and correlates of neuropsychiatric symptoms in nursing home patients With young-onset dementia: The BEYOnD Study, J Am Med Dir Assoc 17, 495–500. [DOI] [PubMed] [Google Scholar]
- [7]. Selbaek G, Engedal K, Bergh S (2013) The prevalence and course of neuropsychiatric symptoms in nursing home patients with dementia: A systematic review, J Am Med Dir Assoc 14, 161–169. [DOI] [PubMed] [Google Scholar]
- [8]. Borsje P, Wetzels RB, Lucassen PL, Pot AM, Koopmans RT (2015) The course of neuropsychiatric symptoms in community-dwelling patients with dementia: A systematic review, Int Psychogeriatr 27, 385–405. [DOI] [PubMed] [Google Scholar]
- [9]. Livingston G, Barber J, Marston L, Stringer A, Panca M, Hunter R, Cooper C, Laybourne A, La Frenais F, Reeves S, Manela M, Lambe K, Banerjee S, Rapaport P (2019) Clinical and cost-effectiveness of the Managing Agitation and Raising Quality of Life (MARQUE) intervention for agitation in people with dementia in care homes: A single-blind, cluster-randomised controlled trial, Lancet Psychiatry 6, 293–304. [DOI] [PubMed] [Google Scholar]
- [10]. Aigbogun MS, Stellhorn R, Hartry A, Baker RA, Fillit H (2019) Treatment patterns and burden of behavioral disturbances in patients with dementia in the United States: A claims database analysis, BMC Neurol 19, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Maust DT, Kim HM, Seyfried LS, Chiang C, Kavanagh J, Schneider LS, Kales HC (2015) Antipsychotics, other psychotropics, and the risk of death in patients with dementia: Number needed to harm, JAMA Psychiatry 72, 438–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Peters ME, Schwartz S, Han D, Rabins PV, Steinberg M, Tschanz JT, Lyketsos CG (2015) Neuropsychiatric symptoms as predictors of progression to severe Alzheimer’s dementia and death: The Cache County Dementia Progression Study, Am J Psychiatry 172, 460–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Gaugler JE, Yu F, Krichbaum K, Wyman JF (2009) Predictors of nursing home admission for persons with dementia, Med Care 47, 191–198. [DOI] [PubMed] [Google Scholar]
- [14]. Gauthier S, Loft H, Cummings J (2008) Improvement in behavioural symptoms in patients with moderate to severe Alzheimer’s disease by memantine: A pooled data analysis, Int J Geriatr Psychiatry 23, 537–545. [DOI] [PubMed] [Google Scholar]
- [15]. Knapp M, Chua KC, Broadbent M, Chang CK, Fernandez JL, Milea D, Romeo R, Lovestone S, Spencer M, Thompson G, Stewart R, Hayes RD (2016) Predictors of care home and hospital admissions and their costs for older people with Alzheimer’s disease: Findings from a large London case register, BMJ Open 6, e013591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Yaffe K, Fox P, Newcomer R, Sands L, Lindquist K, Dane K, Covinsky KE (2002) Patient and caregiver characteristics and nursing home placement in patients with dementia, JAMA 287, 2090–2097. [DOI] [PubMed] [Google Scholar]
- [17]. Cloutier M, Gauthier-Loiselle M, Gagnon-Sanschagrin P, Guerin A, Hartry A, Baker RA, Duffy R, Gwin K, Aigbogun MS (2019) Institutionalization risk and costs associated with agitation in Alzheimer’s disease, Alzheimers Dement (NY) 5, 851–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Brodaty H, Donkin M (2009) Family caregivers of people with dementia, Dialogues Clin Neurosci 11, 217–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Mohamed S, Rosenheck R, Lyketsos CG, Schneider LS (2010) Caregiver burden in Alzheimer disease: Cross-sectional and longitudinal patient correlates, Am J Geriatr Psychiatry 18, 917–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Maust DT, Langa KM, Blow FC, Kales HC (2017) Psychotropic use and associated neuropsychiatric symptoms among patients with dementia in the USA, Int J Geriatr Psychiatry 32, 164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Wolf MU, Goldberg Y, Freedman M (2018) Aggression and agitation in dementia, Continuum (Minneap Minn) 24, 783–803. [DOI] [PubMed] [Google Scholar]
- [22]. Kales HC, Lyketsos CG, Miller EM, Ballard C (2018) Management of behavioral and psychological symptoms in people with Alzheimer’s disease: An international Delphi consensus, Int Psychogeriatr 31, 83–90. [DOI] [PubMed] [Google Scholar]
- [23]. Wang G, Albayrak A, van der Cammen TJ (2019) A systematic review of non-pharmacological interventions for BPSD in nursing home residents with dementia: From a perspective of ergonomics, Int Psychogeriatr 31, 1137–1149. [DOI] [PubMed] [Google Scholar]
- [24]. De Deyn PP, Drenth AF, Kremer BP, Oude Voshaar RC, Van Dam D (2013) Aripiprazole in the treatment of Alzheimer’s disease, Expert Opin Pharmacother 14, 459–474. [DOI] [PubMed] [Google Scholar]
- [25]. Creese B, Da Silva MV, Johar I, Ballard C (2018) The modern role of antipsychotics for the treatment of agitation and psychosis in Alzheimer’s disease, Expert Rev Neurother 18, 461–467. [DOI] [PubMed] [Google Scholar]
- [26]. Marcinkowska M, Sniecikowska J, Fajkis N, Pasko P, Franczyk W, Kolaczkowski M (2020) Management of dementia-related psychosis, agitation and aggression: A review of the pharmacology and clinical effects of potential drug candidates, CNS Drugs 34, 243–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].US Food and Drug Administration, FDA Public Health Advisory: Deaths With Antipsychotics in Elderly Patients With Behavioral Disturbances, http://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm053171, Accessed on July 4, 2019.
- [28]. Reus VI, Fochtmann LJ, Eyler AE, Hilty DM, Horvitz-Lennon M, Jibson MD, Lopez OL, Mahoney J, Pasic J, Tan ZS, Wills CD, Rhoads R, Yager J (2016) The American Psychiatric Association Practice Guideline on the Use of Antipsychotics to Treat Agitation or Psychosis in Patients With Dementia, Am J Psychiatry 173, 543–546. [DOI] [PubMed] [Google Scholar]
- [29].R Core Team, A language and environment for statistical computing. https://www.R-project.org/.
- [30]. Allaire JJ, Horner J, Marti V, Porte N, Markdown: ‘Markdown’ Rendering for R. R package version 0.8. https://CRAN.R-project.org/package=markdown.
- [31]. Kverno KS, Rabins PV, Blass DM, Hicks KL, Black BS (2008) Prevalence and treatment of neuropsychiatric symptoms in advanced dementia, J Gerontol Nurs 34, 8–15; quiz 16-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32]. Caspar S, Davis ED, Douziech A, Scott DR (2018) Nonpharmacological management of behavioral and psychological symptoms of dementia: What works, in what circumstances, and why? Innov Aging 2, igy001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Zucchella C, Sinforiani E, Tamburin S, Federico A, Mantovani E, Bernini S, Casale R, Bartolo M (2018) The multidisciplinary approach to Alzheimer’s disease and dementia. A narrative review of non-pharmacological treatment, Front Neurol 9, 1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34]. Porsteinsson AP, Antonsdottir IM (2017) An update on the advancements in the treatment of agitation in Alzheimer’s disease, Expert Opin Pharmacother 18, 611–620. [DOI] [PubMed] [Google Scholar]
- [35]. Liu CS, Ruthirakuhan M, Chau SA, Herrmann N, Carvalho AF, Lanctôt KL (2016) Pharmacological management of agitation and aggression in Alzheimer’s disease: A review of current and novel treatments, Curr Alzheimer Res 13, 1134–1144. [DOI] [PubMed] [Google Scholar]
- [36]. Steinberg M, Lyketsos CG (2012) Atypical antipsychotic use in patients with dementia: Managing safety concerns, Am J Psychiatry 169, 900–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37]. Gruber-Baldini AL, Stuart B, Zuckerman IH, Simoni-Wastila L, Miller R (2007) Treatment of dementia in community-dwelling and institutionalized medicare beneficiaries, J Am Geriatr Soc 55, 1508–1516. [DOI] [PubMed] [Google Scholar]
- [38]. Hessmann P, Dodel R, Baum E, Muller MJ, Paschke G, Kis B, Zeidler J, Klora M, Reese JP, Balzer-Geldsetzer M (2018) Antipsychotic treatment of community-dwelling and institutionalised patients with dementia in Germany, Int J Psychiatry Clin Pract 22, 232–239. [DOI] [PubMed] [Google Scholar]
- [39].NIH, US National Library of Medicine, Clinicaltrials.gov, The effect of cannabis on dementia related agitation and aggression NCT03328676, Last updated April 23, 2019, https://clinicaltrials.gov/ct2/show/NCT03328676?cond=agitation+dementia&rank=1, Accessed on February 3, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.