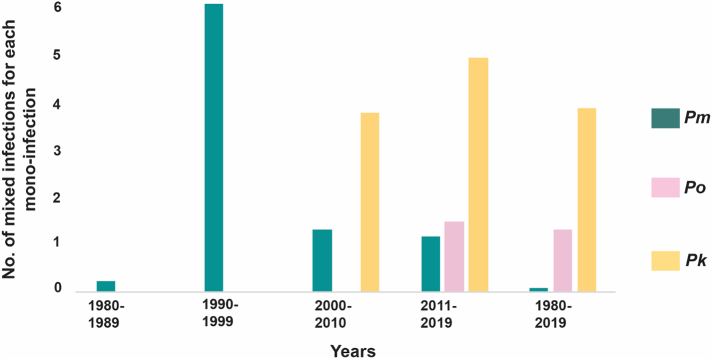Abstract
Background
Efforts for malaria elimination in India focus solely on the more prevalent human malaria parasites of Plasmodium falciparum (Pf) and Plasmodium vivax (Pv). The three non-Pf/Pv species - Plasmodium malariae (Pm), Plasmodium ovale (Po) and Plasmodium knowlesi (Pk) are seldom studied though they are often present as mixed infections with Pf/Pv and thus may be misdiagnosed. This study provides a comprehensive landscape of Pm, Po, and Pk infections from 1930 to 2020.
Methodology
We systematically searched for published literature on Pm, Po, and Pk in India from PubMed database and collated data from 35 studies. The data, starting from 1930, were mapped decade-wise across India. The prevalence of the three neglected Plasmodium species and their proportional contribution to reported Plasmodium mixed-infection were also calculated and analysed.
Principal findings
Amongst the three non-Pf/Pv species, Pm infections have been reported in greater numbers across India and were mostly mono-infections till 1980. From 1983 onwards, reports of Pm mixed infections with Pf/Pv started to emerge. In contrast, reports on occurrence of Po are still rare barring few mixed infection studies. Further, Pk mono- and mixed cases were first reported in 2004 in India and Pk now has been found reported from four Indian states.
Conclusion
This is the first account of country-wide assimilation of reported malaria parasite species data that covers Pm, Po, and Pk infection profiles from 1930 to 2020. This study illustrates the need to survey all 5 human malaria parasite species in India and to target them collectively during the malaria elimination phase.
Keywords: Neglected Plasmodium species, P. malariae, P. ovale, P. knowlesi, malaria
Abbreviations: ACT, Artemisinin-based combination therapy; CDC, Centres for Disease Control and Prevention; DBS, Dried Blood Spots; PCR, Polymerase Chain Reaction; RDT, Rapid Diagnostic Tests; IV, intravenous; G6PD, Glucose-6-Phosphate Dehydrogenase.; AS, Artesunate; SP, Sulfadoxine-Pyrimethamine; AL, Artemether-Lumefantrine; POC, Point of Care,; LAMP, Loop-mediated isothermal amplification.
Highlights
-
•
Current malaria elimination program in India focuses only on P. falciparum and P. vivax.
-
•
The 3 Plasmodium species- Pm, Po, and Pk are rarely studied and often misdiagnosed.
-
•
The overall prevalence of Pm is higher than Po and Pk in India.
-
•
Pm with Pf mixed infection contributes the highest proportion for the last three decades.
-
•
For malaria elimination, a complete coverage of all 5 Plasmodium species should be implemented.
1. Introduction
Malaria is a parasitic disease caused by various species of the protozoan Plasmodium that can spread to a susceptible mammalian host by female mosquito vectors belonging to Anopheles genus. In 2018, an estimated 228 million cases and ~ 0.40 million deaths from malaria were reported globally; 85% of malaria burden was in India and in 18 countries of Africa [1]. The Plasmodium species infecting humans are Plasmodium falciparum (Pf), Plasmodium vivax (Pv), Plasmodium malariae (Pm), Plasmodium ovale (Po), and Plasmodium knowlesi (Pk) either as single Plasmodium species (mono-infections) or with multiple species (mixed-infections) [2,3]. The most common causal agent for malaria in humans is Pf while the other four species (Pv, Pm, Po and Pk) are commonly termed as non-falciparum malaria agents. These five species differ from each other in their clinical manifestations, transmission dynamics, ability to elicit immune responses in hosts, and resistance patterns to antimalarial drugs [[4], [5], [6]], apart from the differences in their definitive hosts (invertebrate mosquito vectors) and secondary (mammalian) hosts. Whereas Pf (believed origin: avian parasite in humans and P. reichenowii in chimpanzees), Pv (believed origin: African great apes), Pm (believed origin: African great apes), and Po (believed origin: African great apes) have established themselves as human malaria parasites with the ability to sustain independent human-to-human transmission through anopheline mosquito vectors, Pk is unable to independently sustain human-to-human transmission in the absence of extra-human vertebrate hosts (long-tailed and pig-tailed macaques). The vector species transmitting most of the Pf and Pv malaria in India include An. culicifacies, An. dirus, An. fluviatilis, An. minimus, An. sundaicus and An. stephensi [7,8]. However, for the non-Pf/Pv malaria transmission in India, studies mentioning incriminating vector species are not conclusive but include An. culicifacies, An. fluviatilis, and An. varuna for Pm [9], An. stephensi, An. subpictus and An. dirus for Po [7,10] and An. dirus, An. sundaicus and An. latens for Pk [[11], [12], [13], [14], [15]].
Pf remains the most virulent form of the parasite and is responsible for most of the fatalities. Defying the norm of Pf being a threat to malaria elimination program, Pv is now equally considered a threat to elimination with the emergence of cases with severe clinical presentations and deaths [16]. In addition, the threat from Pv is due to the asymptomatic infections and clinical relapses attributed to Pv hypnozoites. According to the World Malaria Report 2019, India accounted for 47% of all Pv cases globally in 2018 [1]. The other three species are often neglected but their infections have been reported in India since several decades.
In terms of prevalence/occurrence, Pf and Pv dominate Pm in India [16]. Malaria due to Pm is known as quartan malaria since fever in such patients frequently occurs every fourth day [2]. The peak parasitemia in Pm infection is lower than that in Pf or Pv infections [9]. This is one of the main reasons that most Pm infections are either overlooked or are misidentified as mono-infections of Pf or Pv by microscopy; note that Pf has a tendency to predominate in mixed infections [4]. Pf is known to infect all ages/stages of RBCs; Pm preferably infects the older RBCs while Pv and Po invade younger RBCs [4,9]. Further, the intra-erythrocytic asexual developmental cycle of Pm is of 72 hours which might give more time to the human host to develop early immunity [9]. An interesting feature of Pm is its ability to persist in mammalian hosts for an extended period of time [9]. These key factors probably make Pm infections milder. Unlike Pm, Po causes tertian malaria in humans and is less dangerous than Pf [17]. Po consists of two distinct species - Po curtisi and Po wallikeri that occur globally [18]. Po cases in India are rarely reported as compared to Pm cases. Po, much like Pv, can cause relapse since its life cycle includes hypnozoites which can lay dormant in liver cells in human hosts. Although Po causes mild infections, few case studies indicate the potential of this particular species to cause severe disease and even death [19,20]. The fourth non-falciparum species, Pk, is known to infect macaques but it also has the capability to infect humans [3]. Pk is often misdiagnosed as Pm microscopically and has been proven to reach high parasitemia and to cause anemia in patients [21].
The three non-PfPv species (Pm, Po, and Pk) are thus either overlooked or misdiagnosed as either Pf or Pv and hence are clubbed as neglected Plasmodium species [22]. Despite scattered reports of these non-PfPv species in India, their true burden and distribution across the country remains elusive. There is also a lack of knowledge about Pm, Po and Pk vectors, infection rates and transmission dynamics. Further, the roles of each of Pm, Po, and Pk towards their specific clinical manifestations in mixed infections remains unclear. Knowing the burden and geographical distribution of these non-PfPv species singly or in combination across India will not only highlight their undermined importance but also help in planning dedicated studies towards estimating their real prevalence, understanding their eco-epidemiology, transmission dynamics, responsible vector species identification, vectorial capacity, and type of natural hosts (particularly in case of Pk where a zoonotic spillover is proposed). Information on these parameters will enrich our knowledge for species-specific interventions and will make us future-ready to plan malaria elimination.
To the best knowledge of the authors, no evidence for direct and primary estimation of the burden of these species exists and no attempts to synthesize the available information from published data are known. Since on the whole research to estimate the burden of Pm, Po and Pk has been lacking in India, most of the information on these species is thought to be available as a secondary outcome of published research and/or case reports on Pf and Pv. Thus, the present study was done to establish a first of its kind synthesized database on the reported burden of Pm, Po and Pk in India from different studies published between 1930 and 2020. In the following sections, we will discuss in detail the species-specific distribution, prevalence, proportional contribution to reported Plasmodium mixed-infections, diagnosis and recommended treatment options/outcomes from published evidence of Pm, Po and Pk in India.
2. Methodology
2.1. Data sources and search strategy
A systematic literature analysis was conducted on human Plasmodium infections from Pm, Po and Pk. Data sources selected in this study were retrieved from PubMed. In addition, Google® was also explored to supplement the research articles which did not appear in PubMed. The search terms were “Plasmodium malariae” and/or “Plasmodium ovale”, and/or “Plasmodium knowlesi” with the “state/region name” and the country name - “India”. Some relevant articles were extracted from cross-referencing.
2.2. Inclusion and exclusion criteria
We included only reports of Pm, Po and Pk infections (either as mono- or mixed infections with any Plasmodium species) in humans (including case studies) with available full text. Case studies were only included when depicting the distribution of cases and were excluded for the calculation of either proportion or prevalence for the species. No time frame was specified while searching the databases.
2.3. Data extraction and analysis
The distribution of Pm, Po, and Pk as mono- and mixed infections (in various combinations with each other and with PfPv), reference year of data and name of the state/region/district were extracted from the reported studies and tabulated into a detailed district-level (where available) information spreadsheet (Supplementary tables 1 and 2). Each individual dataset was grouped into decadal data depending upon the availability for various years. Species-specific prevalence of Pm, Po, and Pk in India, in terms of mono- and mixed infections each, was estimated as a proportion of the total species-specific positive cases (as mono- & mixed infections) from the total number of persons screened for malaria for each decade and expressed as percentage. [Species-specific prevalence = (total number of species-specific cases / total number of persons screened for malaria) * 100]. The prevalence (%) in each decade was plotted as bar-graphs (Fig. 3a and b). The studies which did not specify the total number of persons screened for malaria were excluded for the estimation of prevalence. The prevalence of Pk could not be calculated as the total number of persons screened for malaria was not available. Details of the total number of people screened for malaria, the positive malaria cases and species distribution for respective geographical areas are available in supplementary table 1.
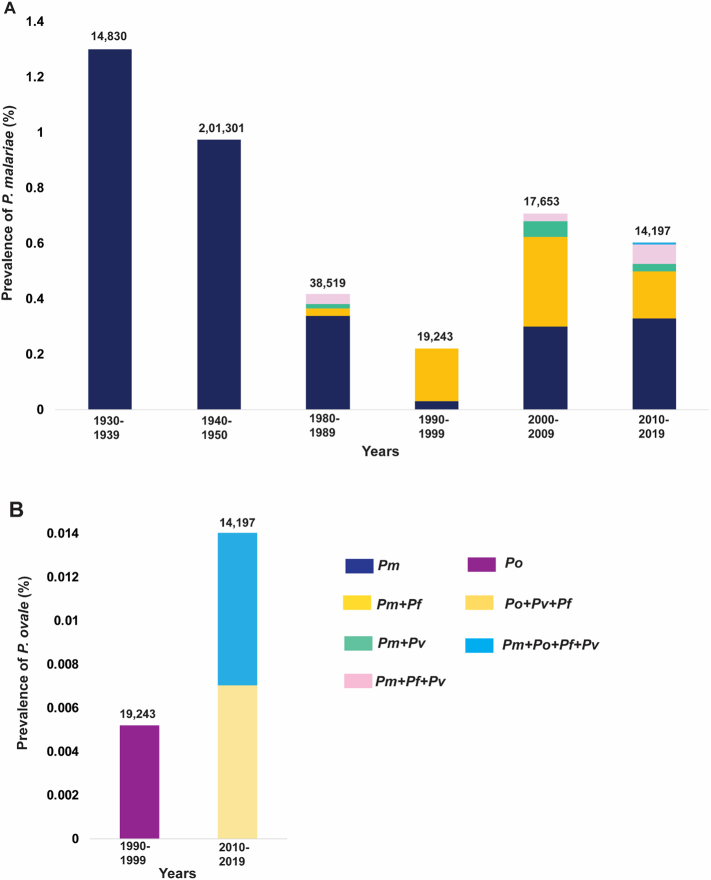
Fig. 3.
Graphical representation of prevalence. A) Pm cases either as mono- or mixed-infections (with Po, Pv and Pf) for the years 1930–2019 and, B) Po cases either as mono- or mixed-infections (with Pm, Pv and Pf) for the years 1990–2019 in India. The numbers at the top of each bar indicate the total number who were screened for malaria. The data time-frames (in decades) are specified at the bottom. Special note for Singh et al.: for the ease of description and uniformity, the data from 1930 to 1950 were split into that of two decades 1930–1939 and 1940–1950. The year 1950 was included in the previous decade (1940–1949) as there were no studies reporting Pm between 1951 and 1979. Where the data spanned across two decades and were not available separately for each of the two decades, such data were split equally into two decades in order to maintain uniformity.
The proportional contribution of different combinations of mixed-species in the total reported Plasmodium mixed infections was calculated by taking the percentage of the total number of different species-specific mixed-infections from the total number of mixed-infections (excluding PfPv) for a particular decade (Fig. 5) [Proportion of specific mixed-species combination = (total number of specific mixed-species combination / total number of mixed-species infections) * 100]. For calculating this, all the studies (excluding case studies) were included - even ones that did not specify the total number of persons screened for malaria (and hence were excluded from estimating prevalence). The results from PCR diagnosis were preferred over microscopy in calculating species-specific prevalence and proportions. All percentages in decimals are shown up to 2 decimals places by rounding-up to the next digit if more than five.
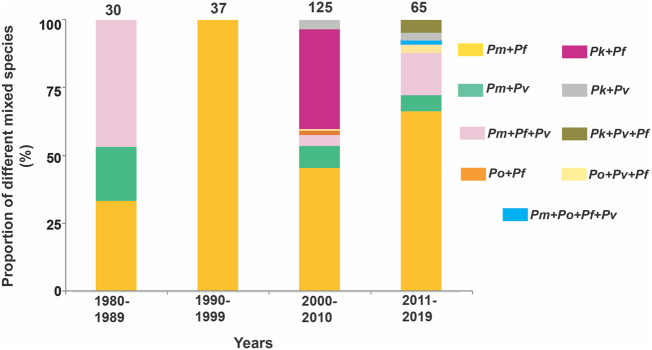
Fig. 5.
Proportion in mixed infections for the years 1980–2019: Data were not available for the years 1930–1979. The numbers at the top of each bar indicate total number of mixed species infections. The data timeframes (in decades) are specified at the bottom. The details of the total number of persons screened for malaria along with the positive malaria cases and species distribution for respective geographical areas are available in supplemenatry table 2.
3. Results
A total of 35 studies were included from 180 search hits retrieved from PubMed and Google® for Pm, Po, and Pk infections in India - spanning 1930 to 2020. For species-specific distribution and prevalence, these data were grouped into six decades: 1930–1939, 1940–1950, 1980–1989, 1990–1999, 2000–2009 and 2010–2019 based on the reference year of the data. Only scattered case reports of Pm/Po/Pk were found from 1951 to 1979 and hence these were not included in showing the decadal distribution. For proportional contribution of each species in Pm/Po/Pk mixed infections, the last two decades were modified to 2000–2010 and 2011–2019 as per the availability of data. The compiled data were used to map the distribution of occurrence of Pm/Po/Pk on India's map as per the above decadal intervals (Fig. 1a-f).
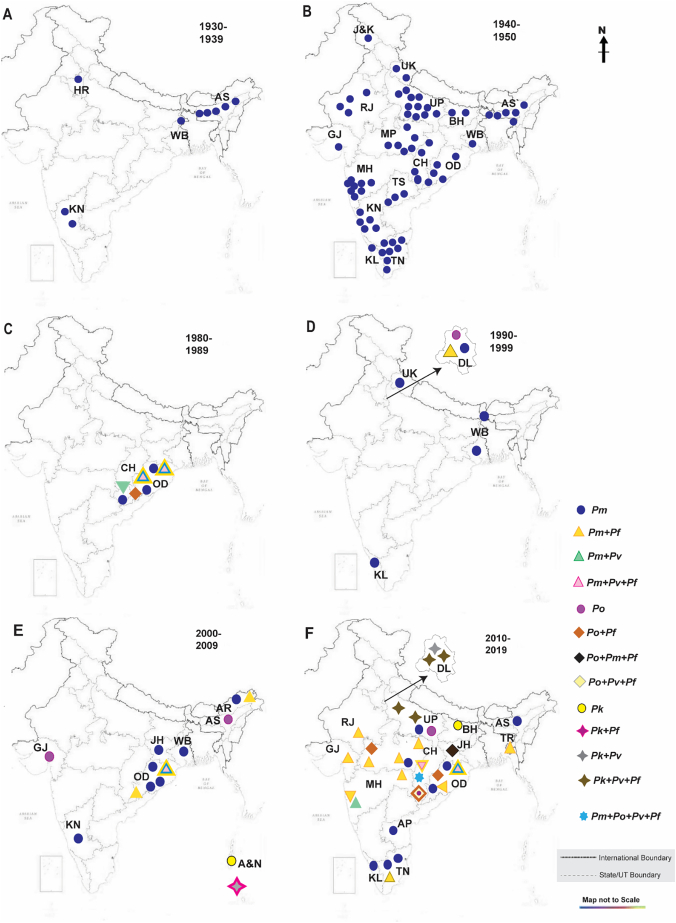
Fig. 1.
Geographical distribution for the years. A) 1930–1939; B) 1940–1950; C) 1980–1989, D) 1990–1999; E) 2000–2009; F) 2010–2019. P. malariae (Pm), P. ovale (Po) and P. knowlesi (Pk) mono-infections are denoted as circles; mixed infections are denoted as - triangle for Pm with Pf/Pv/Po; rhombus - for mixed infections with Po with Pf/Pv/Pm; four-point star - for mixed infections of Pk with Pf/Pv. 7-point star - for mixed infection with all four species i.e. Pm, Po, Pv and Pf.
Out of the 35 studies included in this paper, we found that in a little more than half (51%; N = 18; data reference years between 1930 and 2004) microscopy was used to diagnose malaria and to classify the attributing Plasmodium species. About 43% of the studies (N = 15; data reference year 2005 onwards) utilized both microscopy and PCR (nested and gene-specific) for malaria diagnosis and species confirmation. PCR alone was used to diagnose malaria and Plasmodium species in the rest ~6% studies included (N = 2).
3.1. Geographical distribution of Pm cases
We collated 24 reports for Pm from the 1930s to present. Of the three non-PfPv species, Pm cases have consistently been recorded in almost every decade since the 1930s (Fig. 1). However, from 1930 to 1975, only mono Backspace-infections of Pm were reported in various states of India (Fig. 1). Between 1930 and 1939, it was observed that Pm mono-infections were reported from four states of Assam, Haryana, Karnataka and West Bengal (Fig. 1a) [23]. However, from 1940 to 1950, the reported Pm infections were observed to be distributed throughout India (Fig. 1b) [23]. From 1960 to 1975, only a handful of case-studies reported mono-infections of Pm in Haryana, Karnataka, Kerala, and Tamil Nadu (not shown separately in figures) [24,25].
Reports of Pm mixed infection started to rise from the 1980s with the first case being reported from Sundargarh district of Odisha in 1988–1989 [26]. The mixed infections of Pm with Pf/Pv (Pm + Pf; Pm + Pv; Pm + Pv + Pf) were reported in Odisha's two main districts of Koraput and Sundargarh (Fig. 1c) [[26], [27], [28], [29]]. Along with Odisha, the Bastar district of Chhattisgarh (previously a part of Madhya Pradesh), reported Pm mono-infection and mixed infection (Pm + Pv) (Fig. 1c) [30]. From 1990 to 1999, case-studies for Pm infections were reported in three states namely – Kerala, Uttarakhand (previously a part of Uttar Pradesh) and West Bengal (Fig. 1d) [[31], [32], [33], [34]]. It is important to note that in Kerala, three cases found positive for Pm infection were earlier misidentified and reported as Pv cases (Fig. 1d) [34]. Pm mono-infection along with Pm + Pf mixed infection was also reported in Delhi in the same time period (Fig. 1d) [35].
Between 2000 and 2009, the cases for Pm infections were recorded in five different states of India namely - Arunachal Pradesh (Pm; Pm + Pf), Jharkhand (case study), Karnataka (case study from Bengaluru), multiple locations in Odisha (10 districts) (Pm; Pm + Pf; Pm + Pv; Pm + Pv + Pf) and West Bengal (Pm) (Fig. 1e; Supplementary table 1) [[36], [37], [38], [39], [40], [41]]. A study from Arunachal Pradesh was the first to report Pm cases from the North-Eastern part of India (Fig. 1e) [36]. For cases in Odisha, both diagnostic techniques were utilized (microscopy and PCR) to detect Pm cases and it was observed that microscopy significantly underestimated the frequency of both mono- and mixed Pm infections [39].
Multiple studies reported the mono- and mixed infections of Pm that were scattered in multiple states of India since 2010 but the prevalence of the same was low (Fig. 1f) [[42], [43], [44]]. The diagnosis of Pm cases in Assam was achieved by nested PCR technique although no cases were detected when diagnosis was performed microscopically – this re-confirms that microscopy alone is not sufficient for detection of all Pm infections [45]. A report of a unique combination of mixed infection of Pm with both Po and Pf (Pm + Po + Pf) emerged in Jharkhand in 2014 which was not reported anywhere earlier in India (Fig. 1f) [44]. Following the previous years' trend, Odisha reported Pm (mono- and mixed infections) cases in multiple districts of the state (Pm; Pm + Pf; Pm + Pv; Pm + Pv + Pf) (Fig. 1f) [44,46]. Bastar in Chhattisgarh appears to be one of the hotspots for these neglected Plasmodium infections as all cases of Pm and Po have been reported from this particular region in Chhattisgarh earlier as well [44,47,48]. Bastar is the first place in India where the report of a very rare case of mixed infection with four species (Pm, Po, Pv and Pf) emerged in 2015 [47]. However, no further studies have been reported for Pm infections from 2015. But the reported rise in Pm mixed infections in 2010–2015 suggests that Pm was able to infect humans at more regular intervals in combination with Pf - or more targeted studies were conducted to identify Pm infections - or possibly that a high number of studies reported Pm by chance.
3.2. Geographical distribution of Po and Pk infections
We collated 9 reports for Po infections from 1930 to date, starting in 1988 from Koraput district (Odisha) with three mixed infections of Po with Pf (Fig. 1c) [49]. The first report of Po mono-infection in north India was observed in Delhi in the year 1997 (Fig. 1d) [35]. Between 2000 and 2009, Po (mono-infections) were also reported in Assam and Gujarat (Fig. 1d) [50,51]. The Po + Pf pair has been recorded from Jharkhand, Madhya Pradesh and Odisha between 2010 and 2015 (Fig. 1f) [44,52,53]. Po sympatric species - Po curtisi and Po wallikeri - have been reported from Bastar (Chhattisgarh) along with a fatal case of cerebral malaria with Po + Pv + Pf (Fig. 1f) [52].
Only two studies have reported human Pk infections in India to date [54,55]. (Fig. 1f). Between 2004 and 2010, 53 Pk infections (mostly mixed with Pf (46) and Pv (4)) were reported from the Andaman and Nicobar Islands predominantly from Car Nicobar and Port Blair (Fig. 1f) [54]. The other study published in 2020 (samples collection was done from 2011 to 2018), the occurrence of Pk infections was reported from Bihar, Delhi and Uttar Pradesh (Fig. 1f) [55].
The distribution of the number of non-PfPv infections (mono- and mixed-infections) across the identified six decades (Fig. 2) shows rise in Pm mixed infections from the 1980s, while Po mixed and Pk mixed infections were reported between 2011 and 2019 and 2000–2010 respectively (Fig. 2). The reported number of mixed infections for Pm was greater than Pm mono-infections from 1990 onwards. For the combined six decades (1930–2019), the number of Pm mono-infections seems to be considerable higher than Pm mixed infections while mixed infections for both Po and Pk dominated over their corresponding mono-infections.
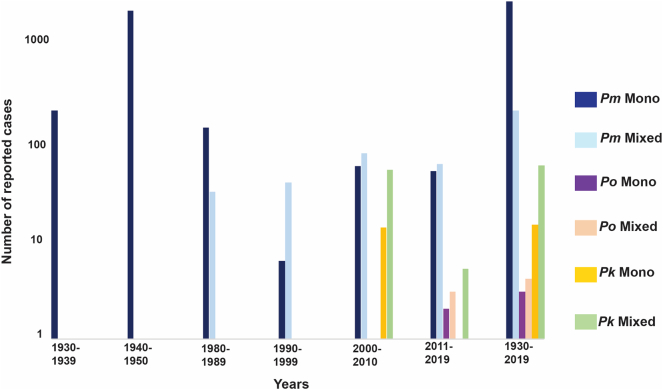
Fig. 2.
Distribution of non-PfPv infections. The number of reported cases for both mono- and mixed Pm, Po, and Pk from the 1930s have been plotted to depict trends. Pm mono-infections prevailed over mixed infections from 1930 to 1989 but from 1990s mixed infections dominated. For both Po and Pk, mixed infections have prevailed over the years. For the combined 6 decades (1930–2019), Pm mono-infections dominated over mixed infections but mixed infections for Po and Pk dominated over their mono-infections. Y-axis depicts the number of reported cases while x-axis depicts decades for respective reported cases.
3.3. Variations in parasite species-specific prevalence
We then analysed species-specific prevalence of mono- and mixed infections across six decades in India (Fig. 3a). A higher prevalence of Pm mono-infection was observed during 1930–1939 (1.3%) as compared to that in the following decade (0.9%). Moreover, Pm in the form of both mono- and mixed infection was observed to be more prevalent during 2000–2009 [0.68% (Pm - 0.3%, Pm + Pf - 0.3%, Pm + Pv - 0.06%, Pm + Pf + Pv - 0.03%)] followed by 2010–2019 [0.57% (Pm - 0.3%, Pm + Pf - 0.2%, Pm + Pv - 0.03%, Pm + Pf + Pv - 0.07%, Pm + Po + Pv + Pf - 0.01%)] and 1980–1989 [0.33% (Pm - 0.3%, Pm + Pf - 0.03%, Pm + Pv - 0.02%, Pm + Pf + Pv - 0.04%)]. Furthermore, during 1990–1999 Pm was reported with only Pf in the form of mixed infections (0.2%) and rest as mono-infection (0.03%) (Fig. 3a).
It has been reported that Pm mono-infections were scattered in 14 regions/states of India between 1930 and 1950 (Fig. 3a) [23]. The highest prevalence in that period for Pm cases was recorded in West Bengal (1.8%), followed by current state of Telangana (1.4%), Assam (1.4%), Tamil Nadu (1.2%) and Maharashtra (1.1%) (Supplementary table 1) [23]. Reports of mixed infections of Pm with Pf/Pv started to emerge from 1981 with very low prevalence from the two neighbouring states - Chhattisgarh (Pm + Pv - 0.1%) and Odisha (Pm + Pf - 0.02%; Pm + Pv - 0.01%; Pm + Pv + Pf - 0.04%) (Fig. 3a; Supplementary table 1) [[26], [27], [28], [29], [30]]. Pm mixed infection with Pf was reported in Delhi with 1.2% prevalence in 1997 (Supplementary table 1) [35]. Pm cases have been persistent in Odisha and have been recorded every decade from the 1980s. From 2000 to 2009, only three states reported a low prevalence of Pm mono and/or mixed infections – Arunachal Pradesh, Odisha and West Bengal [36,[39], [40], [41]]. Different combinations of mixed infections were observed in Odisha (Pm + Pf - 0.5%, Pm + Pv - 0.1%, and Pm + Pv + Pf - 0.04%) and Arunachal Pradesh (Pm + Pf - 0.05%) whereas West Bengal reported only Pm mono-infections (Fig. 3a; Supplementary table 1) [36,39,40]. The highest prevalence of Pm + Pf (22%) was observed in 2008 and was distributed in 8 different districts of Odisha (Supplementary table 1) [39].
Between 2010 and 2015, Pm + Pv + Pf mixed-species infections occurred at a prevalence of 0.1%. In addition, a rare case with four species infection was observed in Chhattisgarh (Pm + Po + Pv + Pf - 0.01%) (Fig. 3a; Supplemenatry table 1) [47]. Pm mixed infections were reported in six states namely - Assam (2012; Pm + Pf-0.8%), Chhattisgarh (2015; Pm + Pf - 0.04%; Pm + Pv + Pf - 0.1%), Madhya Pradesh (2012; Pm + Pf - 0.4%), Maharashtra (2012; Pm + Pf - 0.4%; Pm + Pv - 0.4%), Odisha (2014; Pm + Pf - 0.3%; Pm + Pv - 0.2%; Pm + Pv + Pf - 0.3%), and Tamil Nadu (2012; Pm + Pf - 0.2%) (Fig. 3a) [42,[45], [46], [47], [48]]. Pm mono-infections were also observed in the same time period in five states - Assam (1%), Andhra Pradesh (0.8%), Madhya Pradesh (1%), Odisha (0.6%), and Tamil Nadu (0.8%) (Supplemenatry table 1) [42,45,46,48]. Pm mono- and mixed infections therefore show their persistent nature in various states of India with varying prevalence. However, no reports of Pm monoand/or mixed infection cases have been noted after 2015. This may stem from the use of microscopy and RDTs for diagnosis as former will tend to miss or misidentify non-PfPv infections while the latter cannot detect these neglected Plasmodium parasites.
The overall prevalence of Po has been low compared to that of Pm. Po mono-infection was detected in Delhi between 1992 and 1997 but at low prevalence of 0.03% (Fig. 3b) [35]. Alarmingly to date only Bastar district in Chhattisgarh has reported the prevalence of a unique mixed combination of Po (Po + Pv + Pf - 0.01%) and a rare quadruple mixed species infection (Pm + Po + Pv + Pf - 0.01%) in the year 2015 (Fig. 3b; Supplemenatry table 1) [47]. Such cases depict the complexity of emerging mixed Plasmodium infections and their threat to elimination of malaria if the focus remains only on Pv and Pf surveillance.
We have also analysed the dominance of mixed over mono-infections expressed in terms of number of mixed infections per mono-infection for each species across the identified decades (Fig. 4). Mixed infections consistently dominate for each species (except between 1980 and 1989) (Fig. 4). For each mono-infection of Pm and Po, just a little over 1 mixed infection was reported except for Pm in 1980–1989 (less than one mixed infection for each mono-infection) and in 1990–1999 (more than 6 mixed infections for each mono-infection) was reported (Fig. 4). Strikingly, the dominance of mixed over mono-infections was greater for Pk wherein between 3 and 5 mixed infections were always reported for each mono-infection. Overall, mixed infections dominated for Po and Pk (Fig. 4).
Fig. 4.
Distribution of mono- or mixed infections. The dominance of mixed over mono-infections was calculated by taking the ratio for mixed over mono-infections for each Pm, Po, and Pk cases from the 1980s decade.
3.4. Proportional contributions in mixed Plasmodium infections
Amongst all mixed infections, Pm + Pf has contributed the highest proportion for the last three decades (Figs. 1, 6). Reports of Pm and Po co-infection started to emerge in 1980s (Fig. 1) when all 30 mixed infections had Pm as a component. The triple infection Pm + Pv + Pf comprised ~47% of the total followed by Pm + Pf (33%) and Pm + Pv (20%) in 1980s. Between 1990 and 1999, of 37 mixed infections, all were Pm + Pf while from 2000 onwards diverse combinations of mixed-infections involving Pm/Po/Pk were reported which included all five Plasmodium species i.e. Pm, Po, Pk, Pv, and Pf (Fig. 5). Between 2000 and 2010, almost half (46%) of the total 125 mixed infections had Pm + Pf. Pk with either Pf (37%) or Pv (~4%) were also observed for the first time in Andaman & Nicobar between 2004 and 2010 (Fig. 5; Supplemenatry table 2) [54]. Between 2000 and 2010, Pm + Pv and Pm + Pv + Pf contributed 8% and 4% respectively to the total mixed infections reported. Po as mixed with Pf and with both Pv and Pf was also reported for the first time in Bastar (Chhattisgarh) and contributed ~1.6% and 0.8% of the total mixed infections respectively (Fig. 5; Supplementary table 2) [53]. In the most recent decade (2010–2019), a total of 22 studies have reported 65 mixed infection cases and the majority of mixed infections involved Pm and Pf (66%). The reported proportion of Pm + Pv + Pf mixed infections was ~4 times to that in the previous decade (Fig. 5). In the same period, mixed infection of Po was again reported along with a rare quadruple infection (1.5%) in which all four species infected a patient (Pm + Po + Pv + Pf) (Fig. 5) [47]. Interestingly, mixed infections of Pk with Pv alone (3%) and both Pf and Pv (4.6%) were also reported from 2011 to 2019 (Fig. 5; Supplementary table 2) [55]. The details for the proportional contribution of Plasmodium mixed species are provided in supplmentary table 2.
3.5. Treatments and outcomes
The treatment plan for malaria relies on the specific diagnosis of the Plasmodium species followed by recommended chemotherapy. Chloroquine may be administered in conventional doses for Pm, Po, and Pk at 10 mg base/kg body weight for uncomplicated cases [56]. The Government of India's recommended treatment plan for Po is the same as for Pv as both species tend to form hypnozoites and hence use chloroquine (25 mg/kg body weight for three days) along with radical treatment with primaquine (15 mg daily for 14 days; total 3.5 mg base/kg body weight) [57]. Although Pm is reported to be sensitive to chloroquine, Government of India recommends that Pm should be treated in the same way as Pf. For mixed infections of Pm with Pf/Pv/Po, the focus should be on the species known to have a higher virulence or fatality - hence the treatment for mixed infections involving Pm/Po/Pk is generally recommended to be the same as Pf treatment i.e. artemisinin combination therapies (AS+SP; artesunate 4 mg/kg body weight for 3 days; sulfadoxine 25 mg/kg body weight; and pyrimethamine 1.25 mg/kg body weight) should be administered in all India except the Northeast [57]. In such areas with documented failure rates of more than 10% with sulfadoxine-pyrimethamine, artemether combined with lumefantrine (AL) is the treatment of choice for Pf malaria (artemether 80 mg + lumefantrine 480 mg for more than 34 kg weight) [57]. Although, most malaria mixed infection cases recover there have been a few reports of fatal outcomes from Pm + Pf.
Two studies have reported Pk infections from India and showed its distribution along with Pf and/or Pv [54,55]. Pk mono-infections are likely susceptible to chloroquine while the co-infecting species Pf might be resistant to chloroquine in India. For this reason, Pk infections with Pf + Pv are given ACTs. Fatalities related to Pk infections have been reported from Malaysian region earlier and has been considered as potentially life-threatening in a few cases [21].
For the treatment of Po infections, all the cases reported from Indian states since 1980s are being prescribed chloroquine with mostly successful treatment outcomes. To prevent relapse due to the ability of Po to form hypnozoites, primaquine has to be co-administered with chloroquine in all G6PD sufficient children and adults except infants and pregnant ladies. A few instances of severe Po infections, either mono- or mixed infections have been reported. In 1988, a case report from Odisha showed the infection of a 7-month-old male with Pv + Po mixed infection. This patient was given 50 mg of chloroquine, but within two months patient was found to be positive again with Po + Pv + Pf mixed infection [49]. A fatal case of Po infection was also reported in Delhi in 1997 wherein a 60-year old female who was hospitalised in a comatose state died within 48 hours of starting quinine-sulphate infusion [35]. Another case of severe disease due to Po was reported in 2015 in a 75-year-old male in Uttar Pradesh. The patient presented with Po infection with complicated jaundice, hypotension, thrombocytopenia and acute renal failure [58]. The patient was administered IV artesunate, ceftriaxone and antipyretics and discharged on primaquine for 14 days [58]. An interesting case of a 12-year-old boy with rare quadruple mixed species infection (Pm + Po + Pv + Pf) was reported in Chhattisgarh [47]. In this case, the bivalent Pf + Pv RDT showed the presence of Pf infection only. The microscopist was unable to identify other Plasmodium species and the patient was given oral AS + SP. The fever of the patient did not reduce, and the patient was then given IV Quinine. However, the patient soon left the hospital and the exact outcome could not be determined [47]. This particular case of misdiagnosis emphasises the importance of utilizing PCR-based diagnosis techniques for non-PfPv species. Misdiagnosis poses severe health concerns and can lead to delayed treatment/parasite clearance.
4. Discussion
The Government of India has developed a framework to eliminate malaria from India by the end of 2030 [59]. This program focuses on eliminating the burden of malaria due to Pf and Pv in India. However, no light is shed upon the burden and/or elimination of the non-Pf/Pv malaria agents (Pm, Po, and Pk). Malaria in India is predominantly caused by Pf and Pv with gradually declining peaks in reported cases from 1976 (~6.4 million cases), 1996 (~3 million cases) and 2015 (~1.1 million cases) [59]. Despite this, there has been a conspicuous increase in the proportional contribution of Pf malaria as compared to the Pv malaria in India: 12% in 1976, 39% in 1996 and 67% in 2015, as reported by the National Vector-Borne Disease Control Program (NVBDCP) of the Government of India [59]. The NVBDCP however does not track malaria cases due to Pm, Po and Pk and hence data on non-PfPv infections in India are available only in published case reports and other studies, and that too usually as a secondary outcome. This study, therefore, puts together and elaborates on the published data from all possible resources on the occurrence, distribution and prevalence of neglected Plasmodium spp. in India over nearly a century (1930–2020). The current study shows that Pm and Po infections (though less when compared to Pv and Pf) have been reported since 1930 and the 1980s respectively followed by that of Pk from 2004 onwards. This work thus emphasises the significance and need of tracking malaria infections due to the neglected parasites - Pm, Po, and Pk.
Infection by Pm is shown to be more prevalent than Po or Pk in India. Pm is reported to survive in a single host for decades and this might be advantageous to its long-term survival, particularly in absence of symptoms in the host [60,61]. Persistence of Pm in infecting humans presents an opportunity for sustained transmission of malaria [60,61]. The highest number of reported studies for mixed infections of Pm seems to be concentrated in Odisha while for Po mixed infections, Bastar (Chhattisgarh) has the highest number of reports. So, it could be proposed that both Odisha and Bastar (Chhattisgarh) may be considered as potential hotspots for Pm and Po infections in India respectively. While human Pk infections are more common in neighbouring south-east Asian countries like Malaysia, only 2 studies have reported Pk infections in India from four states namely- Andaman & Nicobar, Bihar, Delhi, and Uttar Pradesh (2004–2018) [54,55]. It is to be noted that Pk infections were previously misdiagnosed as Pm as they appear to be morphologically similar and it was not until molecular detection methods (like PCR) were used that Pk infections became common in South-East Asia [62]. This information on potential Pm/Po/Pk hotspots could serve a vital role for the decision-making bodies to focus on development of policies on the targeted detection, diagnosis, and treatment of neglected Plasmodium species in India. It is worthwhile to note that non-PfPv mixed infections were reported more after 1980s for Pm and after the year 2000 for Pk and Po mixed infections. This could be due to many factors including more emphasis on non-PfPv species detection, better technology (PCR) to detect mixed infections (especially those involving non-PfPv species), and misdiagnosis of species by microscopy particularly between Pv and Pm. However, it has to be noted that the relative abundance of reported/published prevalence of non-PfPv species in certain areas is most likely to be due to a higher number of studies conducted and therefore reported due to a higher established burden of Pf and Pv in these areas (Odisha, Chhattisgarh, Madhya Pradesh). A similar discrepancy could be clearly interpreted from the difference in reported Pm occurrence between 1930s and 1940s. It is evident from the data in supplementary tables that both decades, starting 1930 and 1940, differ in the number of studies reported within the respective time brackets. Whereas 1930s reported only 3 studies (involving 3 states; with 14,830 blood smears examined), 1940s reported 14 studies from 14 states wherein 2,10,301 blood smears were examined. Thus, it is clear that the wider reported distribution of Pm during 1940–1950 is due to huge increase in the number of reporting studies, and Fig. 1 shows only the occurrence of Pm across India. Further, it is important to note that the prevalence of Pm is similar in 1930s (1.3%) and 1940s (0.97%) and hence it could be said that the burden of Pm is similar between the two decades but the reporting is different. This again highlights the need for adding these non-PfPv species in routine surveillance, independent of existing burden of Pf and Pv malaria, to gauge the real burden of neglected Plasmodium species in India and the reasons thereof. In addition, since most of the PCR-based Plasmodium-species research is focussed in areas with relatively better access to health care and health seeking behaviour, these neglected Plasmodium species may be left undetected in remote and hard-to-reach areas with ongoing malaria transmission. A further layer of complexity in detecting and understanding the real burden of these neglected Plasmodium infections in humans is added by a relative lack of entomological studies to identify the local vectors that transmit these parasites to humans. This is particularly true for Pk infections as reported from Andaman and Nicobar Islands where An. sundaicus has been specifically reported to carry Pk parasites [63].
One of the major restrictions in comparing the data for the neglected species includes the type of diagnostic techniques (microscopy and PCR) used to detect these infections as the limits of detection are different in both techniques. PCR is ~50–100 times more sensitive than microscopy [[64], [65], [66], [67], [68]] for the detection of Plasmodium (particularly of low density infections). PCR is also a robust tool for species identification (particularly in case of mixed-species infections) and for estimating parasite densities. This therefore contributes to the difference in reported prevalence of different Plasmodium species, either singly or as mixed infections, before and after the introduction of PCR in mid-1990s. Thus, an increase in species-specific prevalence of infections (particularly for non-PfPv species) post-PCR period may be a false increase as more infections were being detected by PCR.
On the other hand, microscopy has its own limitations not because of the technique but due to a lack of skilled manpower particularly to differentiate various Plasmodium species. Hence microscopy cannot be recommended as a routine point-of-care (POC) diagnostics for the three neglected Plasmodium spp. The use of artificial intelligence (AI) coupled with microscopy may be harnessed to compensate for the dependence on skilled workforce, but its use is still in infancy even for detection of Pf and Pv [69,70]. A pentavalent pan-Plasmodium RDT is the only practical solution worth exploring as a POC screening/diagnostic technique. In addition, a POC PCR or its modification like the Loop-mediated isothermal amplification (LAMP) could be another solution feasible if coupled to the pan-Plasmodium RDT in order to improve its sensitivity and specificity [71,72]. Alternatively, current bivalent Pf/Pv RDTs could be used as a screening tool coupled with traditional lab-based confirmatory PCR. The dual use of RDT/PCR for non-Pf and non-Pv infections can be exploited so that if the bivalent RDTs suggest a non-Pf/Pv infection, PCR can then be done to identify the other three neglected species. The problem could still persist if Pf/Pv is also present with either Pm/Po/Pk as reported in many studies. Therefore, a robust solution for community-wide screening for all 5 Plasmodium species would be a pan-Plasmodium pentavalent RDT.
Further, for countries like India, it would be worthwhile to explore the incorporation of making and archiving dried blood spots (DBS) along with a microscopic blood smear in the National malaria control programs. This is expected to generate a repository from which a population representative sub-sample (proportional to the population size) could be examined intermittently to screen for Pm/Po/Pk infections along with for sub-microscopic Pf/Pv across India. Special emphasis is required for detecting Pk infections in India not only in humans but also in suspected vectors and natural hosts. For Pk transmission, there is the presence of suitable vectors (including An. dirus, An. sundaicus and An. latens), pigtailed macaques and susceptible human hosts in India, especially in North-Eastern states (that neighbour the Pk endemic regions of South-East Asia) and in Andaman and Nicobar Islands (that harbour the parasite) [[11], [12], [13], [14], [15]]. Thus, to conclude, data on neglected (non-PfPv) infections in India is meagre and is required for species-specific therapeutic purposes, for disease prevention, to enhance our understanding of disease epidemiology, to address transmission dynamics and to inform malaria control strategies. The need and type of preventive measures will depend upon the real burden of these species, their specific vector bionomics and their parasite biology. More importantly, in light of India's commitment of malaria elimination by 2030, a ‘complete’ coverage must include all human malaria parasites species.
Role of the funding source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author statement
Rini Chaturvedi: Data acquisition, analysis and interpretation, drafting the manuscript and editing the manuscript critically. Nimita Deora.: Data acquisition, analysis, and interpretation, editing the manuscript critically. Deepam Bhandari- Data collection and analysis, drafting the manuscript. Suhel Parvez: drafting and editing the manuscript critically. Abhinav Sinha: Supervision, Data analysis and interpretation, editing the manuscript critically. Amit Sharma: Original idea, conceptually designing the work, data interpretation, drafting the manuscript and revising it critically.
Declaration of Competing Interest
The authors declare no conflict of interests.
Acknowledgements
We thank GR Alex and Rai Sharma for invaluable insights. AS is supported by funds from DBT, MMV, NIH and DST's JC Bose fellowship.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.onehlt.2020.100190.
Contributor Information
Abhinav Sinha, Email: aspsm2003@yahoo.co.in.
Amit Sharma, Email: directornimr@gmail.com.
Appendix A. Supplementary data
Supplementary figure
Supplementary table 1 and 2
References
- 1.World Malaria Report . World Health Organization Press 2019; Geneva: 2019. https://www.who.int/publications-detail/world-malaria-report-2019 [Google Scholar]
- 2.Garnham P. The myth of quartan malaria. R. Soc. Trop. Med. Hyg. 1981;75 doi: 10.1016/0035-9203(81)90229-7. [DOI] [PubMed] [Google Scholar]
- 3.Singh B., Sung L.K., Matusop A., Radhakrishnan A., Shamsul S.S.G., Cox-Singh J., Thomas A., Conway D.J. A large focus of naturally acquired Plasmodium knowlesi infections in human beings. Lancet. 2004 doi: 10.1016/S0140-6736(04)15836-4. [DOI] [PubMed] [Google Scholar]
- 4.McKenzie F.E., Bossert W.H. Multispecies Plasmodium infections of humans. J. Parasitol. 1999;85:12–18. doi: 10.2307/3285692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davidson G., Chua T.H., Cook A., Speldewinde P., Weinstein P. Defining the ecological and evolutionary drivers of Plasmodium knowlesi transmission within a multi-scale framework. Malar. J. 2019 doi: 10.1186/s12936-019-2693-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh N., Chand S.K., Bharti P.K., Singh M.P., Chand G., Mishra A.K., Shukla M.M., Mahulia M.M., Sharma R.K. Dynamics of forest malaria transmission in Balaghat district, Madhya Pradesh, India. PLoS One. 2013;8 doi: 10.1371/journal.pone.0073730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh P., Lingala M.A.L., Sarkar S., Dhiman R.C. Mapping of malaria vectors at District Level in India: Changing scenario and identified gaps. Vector-Borne Zoonotic Dis. 2017 doi: 10.1089/vbz.2016.2018. [DOI] [PubMed] [Google Scholar]
- 8.Dash A.P., Adak T., Raghavendra K., Singh O.P. The biology and control of malaria vectors in India. Curr. Sci. 2007;92:10. [Google Scholar]
- 9.Collins W.E., Jeffery G.M. Plasmodium malariae: Parasite and disease. Clin. Microbiol. Rev. 2007:579–592. doi: 10.1128/CMR.00027-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins W.E., Jeffery G.M. Plasmodium ovale: Parasite and disease. Clin. Microbiol. Rev. 2005 doi: 10.1128/CMR.18.3.570-581.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kantele A., Jokiranta T.S. Review of cases with the emerging fifth human malaria parasite, Plasmodium knowlesi. Clin. Infect. Dis. 2011:1356–1362. doi: 10.1093/cid/cir180. [DOI] [PubMed] [Google Scholar]
- 12.Prakash A., Walton C., Bhattacharyya D.R., Loughlin S.O., Mohapatra P.K., Mahanta J. Molecular characterization and species identification of the Anopheles dirus and An. minimus complexes in north-east India using r-DNA ITS-2. Acta Trop. 2006;100:156–161. doi: 10.1016/j.actatropica.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Mewara A., Sehgal R. Guest commentary: Plasmodium knowlesi-need to diagnose in India. Trop. Parasitol. 2017:2–4. doi: 10.4103/2229-5070.202287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Subbarao S.K. Centenary celebrations article: Plasmodium knowlesi: from macaque monkeys to humans in south-East Asia and the risk of its spread in India. J. Parasit. Dis. 2011:87–93. doi: 10.1007/s12639-011-0085-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parija S., Giri S. Emerging protozoal pathogens in India: how prepared are we to face the threat? Trop. Parasitol. 2012;2:13–19. doi: 10.4103/2229-5070.97233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghosh S.K., Rahi M. Malaria elimination in India - the way forward. J. Vector Borne Dis. 2019 doi: 10.4103/0972-9062.257771. [DOI] [PubMed] [Google Scholar]
- 17.McMillan B., Kelly A. Ovale malaria in eastern New Guinea. Trop. Geogr. Med. 1967;19:172–176. [PubMed] [Google Scholar]
- 18.Sutherland C.J., Tanomsing N., Nolder D., Oguike M., Jennison C., Pukrittayakamee S., Dolecek C., Hien T.T., do Rosário V.E., Arez A.P., Pinto J., Michon P., Escalante A.A., Nosten F., Burke M., Lee R., Blaze M., Otto T.D., Barnwell J.W., Pain A., Williams J., White N.J., Day N.P.J., Snounou G., Lockhart P.J., Chiodini P.L., Imwong M., Polley S.D. Two nonrecombining sympatric forms of the human malaria parasite plasmodium ovale occur globally. J. Infect. Dis. 2010 doi: 10.1086/652240. [DOI] [PubMed] [Google Scholar]
- 19.Facer C.A., Rouse D. Spontaneous splenic rupture due to Plasmodium ovale malaria. Lancet. 1991 doi: 10.1016/0140-6736(91)91562-9. [DOI] [PubMed] [Google Scholar]
- 20.Lau Y.L., Lee W.C., Tan L.H., Kamarulzaman A., Syed Omar S.F., Fong M.Y., Cheong F.W., Mahmud R. Acute respiratory distress syndrome and acute renal failure from Plasmodium ovale infection with fatal outcome. Malar. J. 2013 doi: 10.1186/1475-2875-12-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cox-Singh J., Davis T.M.E., Lee K.-S., Shamsul S.S.G., Matusop A., Ratnam S., Rahman H.A., Conway D.J., Singh B. Plasmodium knowlesi malaria in humans is widely distributed and potentially life threatening. Clin. Infect. Dis. 2008 doi: 10.1086/524888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hotez P.J., Aksoy S., Brindley P.J., Kamhawi S. What constitutes a neglected tropical disease? PLoS Negl. Trop. Dis. 2020 doi: 10.1371/journal.pntd.0008001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.SINGH J., KRISHNASWAMI A.K., RAMAKRISHNAN S.P. The distribution of human Plasmodia in India. Indian J. Malariol. 1952;6:415–433. [PubMed] [Google Scholar]
- 24.Roy G., Chakrapani K.P., Zacharias J. Plasmodium malariae in Kerala and tamilnadu states of India (1960-75) Trans. R. Soc. Trop. Med. Hyg. 1977 doi: 10.1016/0035-9203(77)90053-0. [DOI] [PubMed] [Google Scholar]
- 25.Midha N., Grover P.S., Sood V.P., Chugh T.D. Focal outbreak of Plasmodium malariae infection in Rohtak District (Haryana) Indian J. Pathol. Microbiol. 1979;22:377–380. [PubMed] [Google Scholar]
- 26.Yadav R.S., Sharma V.P., Ghosh S.K., Kumar A. Quartan malaria--an investigation on the incidence of Plasmodium malariae in Bisra PHC, District Sundargarh, Orissa. Indian J. Malariol. 1990;27:85–94. [PubMed] [Google Scholar]
- 27.Ghosh S.K., Yadav R.S. Naturally acquired concomitant infections of bancroftian filariasis and human plasmodia in Orissa. Indian J. Malariol. 1995;32:32–36. [PubMed] [Google Scholar]
- 28.Das L.D., Mohapatra S.S.S., Jambulingam P., Gunasekaran K., Pani S.P., Das P.K. Malaria and other common ailments among upper Bonda tribals in Koraput district, Orissa, Indian. J. Med. Res. - Sect. A Infect. Dis. 1989;89:334–339. [PubMed] [Google Scholar]
- 29.Rajagopalan P.K., Das P.K., Pani S.P., Jambulingam P., Mohapatra S.S.S., Gunasekaran K., Das L.K. Parasitological aspects of malaria persistence in Koraput district Orissa, India. Indian J. Med. Res. - Sect. A Infect. Dis. 1990;91:44–51. [PubMed] [Google Scholar]
- 30.Panda R., Verma K.V., Rahman S.J. Present status of Plasmodium malariae infection in Bastar District (M.P.) J. Commun. Dis. 1990;22:185–190. [PubMed] [Google Scholar]
- 31.Shukla R.P., Kohli V.K. Plasmodium malariae - A case report from District Nainital, Uttar Pradesh. Indian J. Malariol. 1998;35:39–40. [PubMed] [Google Scholar]
- 32.Mandal B., Mitra N.K., Mukhopadhyay A.K., Mukherjee H., Hati A.K. Emerging Plasmodium falciparum in an endemic area in Calcutta. J. Indian Med. Assoc. 1998;96:328–329. [PubMed] [Google Scholar]
- 33.Das S., Malakar P., Saha G.K., Dasgupta B., Hati A.K. A case of Plasmodium malariae infection in the Dooars region of West Bengal, India. Indian J. Malariol. 1996;33:159–160. [PubMed] [Google Scholar]
- 34.Charan J., Dass M., Jayakumary S. Plasmodium Malariae - a report of three cases. J. Commun. Dis. 2000;32:237–239. [PubMed] [Google Scholar]
- 35.Singh S., Singh N., Handa R. Tumor necrosis factor-alpha in patients with malaria. Indian J. Malariol. 2000;37:27–33. [PubMed] [Google Scholar]
- 36.Mohapatra Pradyumna Kishore, Prakash Anil, Bhattacharyya Dibya Ranjan. Detection & molecular confirmation of a focus of Plasmodium malariae in Arunachal Pradesh, India. Indian J. Med. Res. 2008;128:52–56. [PubMed] [Google Scholar]
- 37.Singh R.K., Pandey P.L. Detection of two cases of Plasmodium malariae in Jharkhand. Indian J. Malariol. 2002;39:48–49. [PubMed] [Google Scholar]
- 38.Sitalakshmi S., Srikrishna A., Damodar P. Plasmodium malariae malaria - A case report. J. Indian Med. Assoc. 2005;103:547–550. [PubMed] [Google Scholar]
- 39.Dhangadamajhi G., Kar S.K., Ranjit M.R. High prevalence and gender bias in distribution of Plasmodium malariae infection in central east-coast India. Trop. Biomed. 2009;26:326–333. [PubMed] [Google Scholar]
- 40.Sahu S.S., Gunasekaran K., Vanamail P., Jambulingam P. Persistent foci of falciparum malaria among tribes over two decades in Koraput district of Odisha State, India. Malar. J. 2013 doi: 10.1186/1475-2875-12-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sur D., von Seidlein L., Manna B., Dutta S., Deb A.K., Sarkar B.L., Kanungo S., Deen J.L., Ali M., Kim D.R., Gupta V.K., Ochiai R.L., Tsuzuki A., Acosta C.J., Clemens J.D., Bhattacharya S.K. The malaria and typhoid fever burden in the slums of Kolkata, India: data from a prospective community-based study. Trans. R. Soc. Trop. Med. Hyg. 2006 doi: 10.1016/j.trstmh.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 42.Haanshuus C.G., Chandy S., Manoharan A., Vivek R., Mathai D., Xena D., Singh A., Langeland N., Blomberg B., Vasanthan G., Sitaram U., Appasamy J., Nesaraj J., Henry A., Patil S., Alvarez-Uria G., Armstrong L., Mørch K. A high malaria prevalence identified by PCR among patients with acute undifferentiated fever in India. PLoS One. 2016 doi: 10.1371/journal.pone.0158816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bharti P.K., Chand S.K., Singh M.P., Mishra S., Shukla M.M., Singh R., Singh N. Emergence of a new focus of Plasmodium malariae in forest villages of district Balaghat, Central India: Implications for the diagnosis of malaria and its control. Trop. Med. Int. Heal. 2013 doi: 10.1111/tmi.12005. [DOI] [PubMed] [Google Scholar]
- 44.Krishna S., Bharti P.K., Chandel H.S., Ahmad A., Kumar R., Singh P.P., Singh M.P., Singh N. Detection of mixed infections with plasmodium spp. by PCR, India, 2014. Emerg. Infect. Dis. 2015 doi: 10.3201/eid2110.150678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dhiman S., Goswami D., Kumar D., Rabha B., Sharma D.K., Bhola R.K., Baruah I., Veer V. Nested PCR detection of Plasmodium malariae from microscopy confirmed P. falciparum samples in endemic area of NE india. Folia Parasitol. (Praha) 2013 doi: 10.14411/fp.2013.041. [DOI] [PubMed] [Google Scholar]
- 46.Pati P., Rana R.K., Khuntia H.K., Bal M.S., Ranjit M.R. The prevalence of P. Malariae in Odisha, India. Trop. Biomed. 2017;34:607–614. [PubMed] [Google Scholar]
- 47.Krishna S., Bhandari S., Bharti P.K., Basak S., Singh N. A rare case of quadruple malaria infection from the highly malaria-endemic area of Bastar, Chhattisgarh, India. PLoS Negl. Trop. Dis. 2017 doi: 10.1371/journal.pntd.0005558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krishna S., Yadav A., Bhandari S., Vishwakarma A.K., Bharti P.K., Mandavi P.L., Bahgel P., Basak S., Sharma R.K., Singh N. Prevalence of malaria in two highly endemic Community Health Centers in the Bastar district, Chhattisgarh showing mixed infections with Plasmodium species. Sci. Rep. 2017 doi: 10.1038/s41598-017-16974-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jambulingam P., Mohapatra S.S.S., Das L.K., Das P.K., Rajagopalan P.K. Detection of Plasmodium ovale in Koraput district, Orissa state. Indian J. Med. Res. - Sect. A Infect. Dis. 1989;89:115–116. [PubMed] [Google Scholar]
- 50.Prakash A., Mohapatra P.K., Bhattacharyya D.R., Goswami B.K., Mahanta J. Plasmodium ovale: First case report from Assam, India. Curr. Sci. 2003;84:1187–1188. [Google Scholar]
- 51.Marathe A., Date V., Shah H.N., Tripathi J.R. Plasmodium ovale - A case report from Gujarat. J. Vector Borne Dis. 2006;43:206–208. [PubMed] [Google Scholar]
- 52.Chaturvedi N., Bhandari S., Bharti P.K., Basak S.K., Singh M.P., Singh N. Sympatric distribution of Plasmodium ovale curtisi and P. ovale wallikeri in India: Implication for the diagnosis of malaria and its control. Trans. R. Soc. Trop. Med. Hyg. 2015;109:352–354. doi: 10.1093/trstmh/trv015. [DOI] [PubMed] [Google Scholar]
- 53.Singh R., Jain V., Singh P.P., Bharti P.K., Thomas T., Basak S., Singh N. First report of detection and molecular confirmation of Plasmodium ovale from severe malaria cases in central India. Trop. Med. Int. Heal. 2013 doi: 10.1111/tmi.12184. [DOI] [PubMed] [Google Scholar]
- 54.Tyagi R.K., Das M.K., Singh S.S., Sharma Y.D. Discordance in drug resistance-associated mutation patterns in marker genes of plasmodium falciparum and plasmodium knowlesi during coinfections. J. Antimicrob. Chemother. 2013 doi: 10.1093/jac/dks508. [DOI] [PubMed] [Google Scholar]
- 55.Kaur C., Pramanik A., Kumari K., Mandage R., Dinda A.K., Sankar J., Bagga A., Agarwal S.K., Sinha A., Singh G., Acharya P. Renal detection of Plasmodium falciparum, Plasmodium vivax and Plasmodium knowlesi in malaria associated acute kidney injury: A retrospective case-control study. BMC Res. Notes. 2020 doi: 10.1186/s13104-020-4900-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dutta T., Vadivelan M. Recent advances in the management of Plasmodium knowlesi infection. Trop. Parasitol. 2014;4:31–34. doi: 10.4103/2229-5070.129158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.NVBDCP, Operational Manual for Malaria Elimination in India 2016, 2016. file:///C:/Users/USER/Desktop/Operational-Manual-Malaria-2016-Version-1.pdf.
- 58.Tomar L.R., Giri S., Bauddh N.K., Jhamb R. Complicated malaria: a rare presentation of Plasmodium ovale. Trop. Dr. 2015;45:140–142. doi: 10.1177/0049475515571989. [DOI] [PubMed] [Google Scholar]
- 59.NVBDCP, National Framework for Malaria Elimination in India, 2016. http://nvbdcp.gov.in/Doc/National-framework-for-malaria-elimination-in-India-2016–2030.pdf.
- 60.DUGGAN A.J., SHUTE P.G. Quartan malaria relapsing after thireteen years. J. Trop. Med. Hyg. 1961;64:20–21. [PubMed] [Google Scholar]
- 61.Sutherland C.J. Persistent parasitism: the adaptive biology of malariae and ovale malaria. Trends Parasitol. 2016 doi: 10.1016/j.pt.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 62.Singh B., Daneshvar C. Human infections and detection of plasmodium knowlesi. Clin. Microbiol. Rev. 2013 doi: 10.1128/CMR.00079-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vidhya P.T., Sunish I.P., Maile A., Zahid A.K. Anopheles sundaicus mosquitoes as vector for plasmodium knowlesi, andaman and Nicobar Islands, India. Emerg. Infect. Dis. 2019;25:817–820. doi: 10.3201/eid2504.181668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mathison B.A., Pritt B.S. Update on malaria diagnostics and test utilization. J. Clin. Microbiol. 2017:2009–2017. doi: 10.1128/JCM.02562-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Berzosa P., De Lucio A., Romay-Barja M., Herrador Z., González V., García L., Fernández-Martínez A., Santana-Morales M., Ncogo P., Valladares B., Riloha M., Benito A. Comparison of three diagnostic methods (microscopy, RDT, and PCR) for the detection of malaria parasites in representative samples from Equatorial Guinea 11 Medical and Health Sciences 1108 Medical Microbiology. Malar. J. 2018;17:333. doi: 10.1186/s12936-018-2481-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Krampa F., Aniweh Y., Awandare G., Kanyong P. Recent Progress in the development of diagnostic tests for malaria. Diagnostics. 2017;7:54. doi: 10.3390/diagnostics7030054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mfuh K.O., Achonduh-Atijegbe O.A., Bekindaka O.N., Esemu L.F., Mbakop C.D., Gandhi K., Leke R.G.F., Taylor D.W., Nerurkar V.R. A comparison of thick-film microscopy, rapid diagnostic test, and polymerase chain reaction for accurate diagnosis of Plasmodium falciparum malaria. Malar. J. 2019;18:73. doi: 10.1186/s12936-019-2711-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zimmerman P.A., Howes R.E. Malaria diagnosis for malaria elimination. Curr. Opin. Infect. Dis. 2015:446–454. doi: 10.1097/QCO.0000000000000191. [DOI] [PubMed] [Google Scholar]
- 69.Linder N., Turkki R., Walliander M., Mårtensson A., Diwan V., Rahtu E., Pietikäinen M., Lundin M., Lundin J. A malaria diagnostic tool based on computer vision screening and visualization of Plasmodium falciparum candidate areas in digitized blood smears. PLoS One. 2014;9 doi: 10.1371/journal.pone.0104855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Park H.S., Rinehart M.T., Walzer K.A., Ashley Chi J.T., Wax A. Automated Detection of P. falciparum using machine learning algorithms with quantitative phase images of unstained cells. PLoS One. 2016;11:e0163045. doi: 10.1371/journal.pone.0163045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tambo M., Mwinga M., Mumbengegwi D.R. Loop-mediated isothermal amplification (LAMP) and polymerase chain reaction (PCR) as quality assurance tools for rapid diagnostic test (RDT) malaria diagnosis in northern Namibia. PLoS One. 2018;13 doi: 10.1371/journal.pone.0206848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mohon A.N., Lee L.D.Y., Bayih A.G., Folefoc A., Guelig D., Burton R.A., LaBarre P., Chan W., Meatherall B., Pillai D.R. NINA-LAMP compared to microscopy, RDT, and nested PCR for the detection of imported malaria. Diagn. Microbiol. Infect. Dis. 2016;85:149–153. doi: 10.1016/j.diagmicrobio.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure
Supplementary table 1 and 2