Abstract
The coronavirus disease 2019 (COVID-19) pandemic represents a severe global health threat. Selenium (Se), as one of the essential trace elements in human body, is well known for its antioxidant and immunity-boosting capabilities that induce a strong antiviral effect. In response to the global pandemic, we highlight here the current status of Se in combating different viruses, as well as the potential application of nano-selenium (nanoSe) in combating COVID-19.
Keywords: COVID-19, Selenium, Viruses, Nano-selenium
Graphical Abstract
1. Introduction
Since December 2019, hospitals in Wuhan, Hubei province, China found several cases of pneumonia of unknown cause with a history of exposure to seafood markets. It was confirmed to be caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The subsequent coronavirus disease 2019 (COVID-19) pandemic has put the health of humans around the world under severe threat. Governments have taken measures to prevent the infection of COVID-19 by self-isolating, social distancing, wearing masks and monitoring temperature frequently. Researchers and medical workers are putting all their efforts into developing testing methods, antiviral drugs and vaccines to diagnose, treat and defend against COVID-19.
Coronaviruses have a cell membrane structure with three types of proteins on it: spike glycoprotein (S, Spike Protein), small envelope glycoprotein (E, Envelope Protein) and membrane glycoprotein (M, Membrane Protein), and a few types have hemagglutinin glycoprotein (HE protein, Haemaglutinin-esterase) [1]. The nucleic acid of coronavirus is a single stranded RNA, which is more likely to survive than DNA viruses [2].
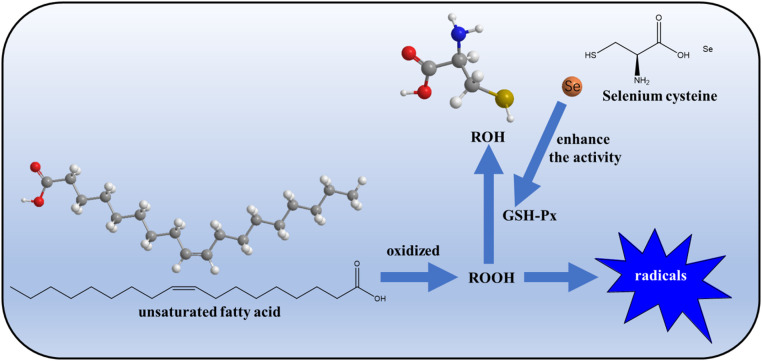
Selenium (Se) is one of the essential trace elements in animals and human beings. It can boost the immune function of the body by enhancing the role of T lymphocyte (T cells) and Natural Killer (NK) cells, which can kill tumor cells [3]. Se is present mainly in the form of selenoproteins, of which about 3% are involved in the synthesis of glutathione peroxidase (GSH-Px) [4]. If Se is deficient in the body, the content and activity of GSH-Px are also decreased, leading to decreased antioxidant capacity of cells and increased oxidative damage, as shown in Fig. 1 [5].
Fig. 1.
The antioxidant process of Se in organisms during normal metabolism.
Compared with other forms of Se, nano-selenium (nanoSe) has low toxicity, comparable bioavailability and high efficiency in preventing oxidative damage [6], [7], [8], [9]. NanoSe can efficiently scavenge free radicals at a concentration of less than 0.5 mM [9]. Huang et al. [9] found that nanoSe with smaller size (5–15 nm) has better ability to clear free radicals, which has been used in the treatment of many diseases including cancer, inflammatory diseases, liver fibrosis, and drug-induced toxicity [10], [11], [12], [13].
2. Se fights against different viruses
Se has long been found to be directly involved in fighting against different viruses [3], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23] like influenza virus, herpes simplex virus type 1 (HSV-1), hepatitis C virus (HCV), coxsackie virus, and human immunodeficiency virus (HIV) ( Table 1).
Table 1.
Examples of Se against viruses.
| Viruses | Abbreviations | Reference |
|---|---|---|
| A/NWS/33 influenza virus | H1N1 | [14] |
| Herpes simplex virus type 1 | HSV-1 | [15] |
| Hepatitis C virus | HCV | [16] |
| Coxsackie virus | – | [17] |
| West Nile virus | WNV | [19] |
| Human papilloma virus | HPV | [3] |
| Epstein-Barr virus-early antigen | EBV-EA | [20] |
| Respiratory syncytial virus | RSV | [21] |
| Human immunodeficiency virus | HIV | [22] |
Cheng et al. [14] found that the moderate supplementation of Se could increase the levels of tumor necrosis factor-alpha (TNF-α) and interferon-gamma (IFN-γ) in vivo, thus improving the immune response against A/NWS/33 (H1N1) influenza virus. Wang et al. [15] found that Se combated HSV-1 in vitro by inhibiting the cytopathic effect and promoting cell apoptosis. Himoto et al. [16] found that the decreased level of Se in serum was in direct proportion to the severity of hepatic fibrosis, with Se deficiency associated with the aggravation of hepatic fibrosis in patients with chronic liver disease (CLD) related to HCV. Moreover, the Se serum level was negatively correlated with the homeostasis model for assessment of insulin resistance values (r = -0.304, p = 0.0338). So, Se deficiency seems to contribute to insulin resistance. Zhang et al. [17] found that an appropriate amount of Se could improve the activity of lipid peroxidase and reduce the production of lipid peroxidation products, thus lessening myocardial cell damage caused by coxsackie virus infection. Men et al. [18] found that, after treatment with Se, the cytotoxicity of polysaccharides from Caulerpa taxifolia was increased slightly but the activity was enhanced and the therapeutic index was increased making it a promising antiviral active substance. Verma et al. [19] found that adequate Se may be the key to protecting cells infected with West Nile virus (WNV) from virus-induced cell death. Cai et al. [3] showed that patients infected with human papilloma virus (HPV) had lower levels of average Se content in cervical tissue. Jian et al. [20] have shown that increased Se concentration in Se-enriched rice can inhibit the transformation of Epstein-Barr virus (EBV) into umbilical blood B lymphocytes and the early expression of Epstein-Barr virus-early antigen (EBV-EA) in Raji cells. Liu et al. [21] found that Se supplementation significantly increased the levels of Se and glutathione peroxidase in plasma and white blood cells, suggesting that Se could promote the recovery of acute lower respiratory tract infections caused by respiratory syncytial virus (RSV). Chen et al. [22] found that Se may play an important role in HIV replication, and supplementing Se could enhance the oxidative defense systems and improve the symptoms of acquired immunodeficiency syndrome (AIDS) patients. Cirelli et al. [23] found that the Se level of AIDS and AIDS related complex (ARC) patients was lower than that of healthy people, and the lower the Se level, the higher incidence rates.
Therefore, because Se can enhance the immune and antioxidant abilities of human body, it offers a basis for helping prevent and treat viral diseases. Sufficient levels of Se in the body can maintain human immune activity and normal redox regulation ability to resist the invasion of pathogens.
3. Using nano-selenium to fight against COVID-19?
Se has also been found to be directly associated with COVID-19. For example, it was found that the cure rate of COVID-19 patients in Enshi was 36.4%, while the overall cure rate in other cities in Hubei was 13.1% [24]. Studies found that Se levels in hair samples in Enshi were 3.13 ± 1.91 mg/kg for women and 2.21 ± 1.14 mg/kg for men [25], while Se levels in other parts of Hubei were only 0.55 mg/kg [26]. The intake of Se in Enshi was reported to be 550 μg/d in 2013 [25]. In contrast, Heilongjiang province in Northeast China had a high mortality rate of 2.4% of COVID-19 [24], compared with other provinces except Hubei, where it was reported to have an Se intake of only 16 μg/d in 2018 [27], and Se levels in hair in the Songnen Plain in Heilongjiang were only 0.26 mg/kg [26], [27]. These observations indicate that cure rates in cities outside Hubei are significantly correlated with Se intake levels. The higher the Se intake, the higher the cure rate of COVID-19. Furthermore, Moghaddam et al. [28] have found that Se levels in serum samples of surviving COVID-19 patients were higher than non-survivors ([Se] 53.3 ± 16.2 & 40.8 ± 8.1 µg/L, [SELENOP] 3.3 ± 1.3 & 2.1 ± 0.9 mg/L).
More importantly, Ebselen, an organic Se species, has been shown to inhibit COVID-19 by covalently binding to the COVID-19 virion Mpro through cell membranes [29]. Ebselen is not highly cytotoxic and has been shown to be safe in humans [30], [31], [32]. Ebselen is most effective at concentrations of 10 μM with COVID-19 infected Vero cells [29]. However, it was also reported that severe liver damage occurred in cases of COVID-19 [33]. On the other hand, Ebselen has been found to inhibit liver damage stimulated by a variety of chemicals, immune systems and microbes [34].
The major concern of using Se to fight against different viruses is its toxicity at high doses. Therefore, low toxicity Se is highly desirable to achieve an equivalent antiviral capability. NanoSe has been proposed for the treatment of different diseases like cancers [35] and Huntington's disease [36]. NanoSe has also been found to be less toxic than other Se compounds. For example, the median lethal dose (LD50) of nanoSe (113.0 mg/kg bw) in mice is seven times lower than that of selenite (15.7 mg/kg bw), and four times lower than that of organic Se like SeMet (25.6 mg/kg bw) [37], [38]. NanoSe can also effectively increase the persistence of cytokine-induced killer (CIK) cells in peripheral blood in the body. For example, by combining nanoSe and CIK cells, more NK cells can be induced to infiltrate the tumor, which triggers a powerful immune response for effective cancer immunotherapy [39]. Liu et al. [40] have also found that selenocystine promotes cancer immunotherapy based on NK cells. Hu et al. [41] have shown that nanoSe can enhance the anti-tumor cytotoxicity of Vγ9Vδ2 T cells with excellent anti-tumor activity. NanoSe also has broad clinical prospects in immunotherapy. NanoSe can be metabolized into selenocystine, thereby regulating the expression of a variety of proteins and other metabolisms in CIK and tumor cells, helping to promote CIK therapy [39]. Besides, functionalized nanoSe maybe developed to improve their efficiency in combating SARS-CoV-2 [42]. NanoSe has also been found to have good biocompatibility [42], [43], [44], [45], and can thus be used as an antiviral drug carrier. Therefore, considering these merits of nanoSe, it is promising in combating COVID-19. However, there are still very few anti-viral experiments using nanoSe against COVID-19. More studies are needed to confirm the role of Se and especially nanoSe in COVID-19 patients.
4. Summary and outlooks
Se has long been found to fight against different viruses and has also been found to be correlated with positive outcomes for COVID-19 patients. Antiviral effect against SARS-CoV-2 has been confirmed using Ebselen. Considering the low toxicity, antioxidant and immunity-boosting capabilities and other merits of nanoSe, it is desirable and reasonable to investigate further its use in the fight against SARS-CoV-2 and improve the health outcomes of COVID-19 patients. We hope that Se, and especially nanoSe, can play an important role in fighting against COVID-19 in the near future.
CRediT authorship contribution statement
Yu-Feng Li, Yong-Liang Yu and Chunying Chen conceptualized the manuscript. Lina He, Jiating Zhao, Liming Wang, Quancheng Liu and Yuqin Fan drafted the manuscript. All the authors read and approved the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors are grateful for the financial support from the Ministry of Science and Technology of China (2016YFA0201600), National Natural Science Foundation of China (11975247, 11475496), and Guizhou Department of Science and Technology (No. QKH-2016–2804).
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Footnotes
In memory of Professor Wenjie Yang (1964–2020) at China CDC for her dedicated study on selenium.
References
- 1.Schoeman D.F.B. Coronavirus envelope protein: current knowledge. Virol. J. 2019;16:69. doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seah I., Su X., Lingam G. Revisiting the dangers of the coronavirus in the ophthalmology practice. Eye. 2020;34:1155–1157. doi: 10.1038/s41433-020-0790-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai H., Guan T., Guo L., Weng K. Detection and clinical significance of selenium content in cervical tissue infected with human papilloma virus. J. Path Biol. 2006;1(6):410–411. [Google Scholar]
- 4.Seyler R.W., Jr., Olson J.W., Maier R.J. Superoxide dismutase-deficient mutants of Helicobacter Pylori are hypersensitive to oxidative stress and defective in host colonization. Infect. Immun. 2001;69:4034–4040. doi: 10.1128/IAI.69.6.4034-4040.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinbrenner H., Al-Quraishy S., Dkhil M.A., Wunderlich F., Sies H. Dietary selenium in adjuvant therapy of viral and bacterial infections. Adv. Nutr. 2015;6:73–82. doi: 10.3945/an.114.007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hosnedlova B., Kepinska M., Skalickova S., Fernandez C., Ruttkay-Nedecky B., Peng Q., Baron M., Melcova M., Opatrilova R., Zidkova J., Bjørklund G., Sochor J., Kizek R. Nano-selenium and its nanomedicine applications: a critical review. Int. J. Nanomed. 2018;13:2107–2128. doi: 10.2147/IJN.S157541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J., Wang X., Xu T. Elemental selenium at nano size (Nano-Se) as a potential chemopreventive agent with reduced risk of selenium toxicity: comparison with se-methylselenocysteine in mice. Toxicol. Sci. 2008;101:22–31. doi: 10.1093/toxsci/kfm221. [DOI] [PubMed] [Google Scholar]
- 8.Khurana A., Tekula S., Saifi M.A., Venkatesh P., Godugu C. Therapeutic applications of selenium nanoparticles. Biomed. Pharmacother. 2019;111:802–812. doi: 10.1016/j.biopha.2018.12.146. [DOI] [PubMed] [Google Scholar]
- 9.Huang B., Zhang J., Hou J., Chen C. Free radical scavenging efficiency of Nano-Se in vitro. Free Rad. Biol. Med. 2003;35:805–813. doi: 10.1016/s0891-5849(03)00428-3. [DOI] [PubMed] [Google Scholar]
- 10.Huang Y., He L., Liu W., Fan C., Zheng W., Wong Y.S., Chen T. Selective cellular uptake and induction of apoptosis of cancer-targeted selenium nanoparticles. Biomaterials. 2013;34:7106–7116. doi: 10.1016/j.biomaterials.2013.04.067. [DOI] [PubMed] [Google Scholar]
- 11.Kumar G.S., Kulkarni A., Khurana A., Kaur J., Tikoo K. Selenium nanoparticles involve HSP-70 and SIRT1 in preventing the progression of type 1 diabetic nephropathy. Chem. Biol. Interact. 2014;223:125–133. doi: 10.1016/j.cbi.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 12.Wang H., Wei W., Zhang S.Y., Shen Y.X., Yue L., Wang N.P., Xu S.Y. Melatonin-selenium nanoparticles inhibit oxidative stress and protect against hepatic injury induced by Bacillus Calmette-Guerin/lipopolysaccharide in mice. J. Pineal Res. 2005;39:156–163. doi: 10.1111/j.1600-079X.2005.00231.x. [DOI] [PubMed] [Google Scholar]
- 13.Li Y., Li X., Wong Y.S., Chen T., Zhang H., Liu C., Zheng W. The reversal of cisplatin-induced nephrotoxicity by selenium nanoparticles functionalized with 11-mercapto-1-undecanol by inhibition of ROS-mediated apoptosis. Biomaterials. 2011;32:9068–9076. doi: 10.1016/j.biomaterials.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Cheng Y., Wang S., Yao H., Fan J., Lu B. Protection from H1N1 Influenza Virus infections in mice by supplementation with selenium. Progr. Modern. Biomed. 2015;15:3220–3227. [Google Scholar]
- 15.Wang A., Yu K., Zou W., Song K. Anti-herpes simplex virus type 1 activity of trace element selenium in vitro. J. Nanchang Univ. 2012;52(9):1–4. [Google Scholar]
- 16.Himoto T., Yoneyama H., Kurokohchi K., Inukai M., Masugata H., Goda F., Haba R., Watababe S., Kubota S., Senda S., Masaki T. Selenium deficiency is associated with insulin resistance in patients with hepatitis C virus-related chronic liver disease. Nutr. Res. 2011;31:829–835. doi: 10.1016/j.nutres.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 17.Zhang F., Wang H., Zhao L., Duan Y., Gao Y. Effect of selenium on lipid peroxidation of coxsackie venereal myocarditis in mice. Chin. J. Appl. Physiol. 2009;26(1):70–71. [PubMed] [Google Scholar]
- 18.Men X., Xu W., Zhu X., Ma W. Extraction, selenium-nanoparticle preparation and anti-virus bioactivity determination of polysaccharides from Caulerpa taxifolia. J. Chin. Med. Mater. 2009;32(12):1891–1894. [PubMed] [Google Scholar]
- 19.Verma S., Molina Y., Lo Y.Y., Cropp B., Nakano C., Yanagihara R., Nerurkar V.R. In vitro effects of selenium deficiency on West Nile virus replication and cytopathogenicity. Virol. J. 2008;5:66. doi: 10.1186/1743-422X-5-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jian S., Mei C., Liang Y., Li D., Chen Q. Influence of selenium-rich rice transformation of umbilical blood B lymphocytes by Epstein-Barr virus and Epstein-Barr virus early antigen expression. Chin. J. Cancer. 2003;22(1):26–29. [PubMed] [Google Scholar]
- 21.Liu X., Yin S., Li G., Gao H., Fang H. Effects of selenium supplement on acute lower respiratory tract infection caused by respiratory syncytial virus. Chin. J. Prev. Med. 1997;31(6):358–361. [PubMed] [Google Scholar]
- 22.Chen C., Zhou J., Xu H. New progress in the research on the relationship between Selenium and AIDS. Prog. Biochem. Biophys. 1997;24(4):327–330. [Google Scholar]
- 23.Cirelli A., Ciardi M., de Simone C., Sorice F., Giordano R., Ciaralli L., Costantini S. Serum selenium concentration and disease progress in patients with HIV infection. Clin. Biochem. 1991;24:211–214. doi: 10.1016/0009-9120(91)90601-a. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J., Taylor E.W., Bennett K., Saad R., Rayman M.P. Association between regional selenium status and reported outcome of COVID-19 cases in China. Am. J. Clin. Nutr. 2020;111:1297–1299. doi: 10.1093/ajcn/nqaa095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang Y., Wang Q., Gao J., Lin Z., Bañuelos G., Yuan L., Yin X. Daily dietary selenium intake in a high selenium area of Enshi, China. Nutrients. 2013;5:700–710. doi: 10.3390/nu5030700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li S., Banuelos G.S., Wu L., Shi W. The changing selenium nutritional status of Chinese residents. Nutrients. 2014;6:1103–1114. doi: 10.3390/nu6031103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dinh Q.T.C.Z., Huang J., Tran T.A.T., Wang D., Yang W., Zhou F., Wang M., Yu D., Liang D. Selenium distribution in the Chinese environment and its relationship with human health: a review. Environ. Int. 2018;112:294–309. doi: 10.1016/j.envint.2017.12.035. [DOI] [PubMed] [Google Scholar]
- 28.Moghaddam A., Heller R.A., Sun Q., Seelig J., Cherkezov A., Seibert L., Hackler J., Seemann P., Diegmann J., Pilz M., Bachmann M., Minich W.B., Schomburg L. Selenium deficiency is associated with mortality risk from COVID-19. Nutrients. 2020;12:2098. doi: 10.3390/nu12072098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin Z., Du X., Xu Y., Deng Y., Liu M., Zhao Y., Zhang B., Li X., Zhang L., Peng C., Duan Y., Yu J., Wang L., Yang K., Liu F., Jiang R., Yang X., You T., Liu X., Yang X., Bai F., Liu H., Liu X., Guddat L.W., Xu W., Xiao G., Qin C., Shi Z., Jiang H., Rao Z., Yang H. Structure of M(pro) from SARS-CoV-2 and discovery of its inhibitors. Nature. 2020;582:289–293. doi: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- 30.Lynch E., Kil J. Development of Ebselen, a glutathione peroxidase mimic, for the prevention and treatment of noise-induced hearing loss. Semin. Hearing. 2009;30:47–55. [Google Scholar]
- 31.Kil J., Lobarinas E., Spankovich C., Griffiths S.K., Antonelli P.J., Lynch E.D., Le Prell C.G. Safety and efficacy of Ebselen for the prevention of noise-induced hearing loss: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2017;390:969–979. doi: 10.1016/S0140-6736(17)31791-9. [DOI] [PubMed] [Google Scholar]
- 32.Masaki C., Sharpley A.L., Cooper C.M., Godlewska B.R., Singh N., Vasudevan S.R., Harmer C.J., Churchill G.C., Sharp T., Rogers R.D., Cowen P.J. Effects of the potential lithium-mimetic, Ebselen, on impulsivity and emotional processing. Psychopharmacology. 2016;233:2655–2661. doi: 10.1007/s00213-016-4319-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feng G., Zheng K.I., Yan Q.Q., Rios R.S., Targher G., Byrne C.D., Poucke S.V., Liu W.Y., Zheng M.H. COVID-19 and liver dysfunction: current insights and emergent therapeutic strategies. J. Clin. Trans. Hepatol. 2020;8:18–24. doi: 10.14218/JCTH.2020.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kono H., Arteel G.E., Rusyn I., Sies H., Thurman R.G. Ebselen prevents early alcohol-induced liver injury in rats. Free Radic. Biol. Med. 2001;30(4):403–411. doi: 10.1016/s0891-5849(00)00490-1. [DOI] [PubMed] [Google Scholar]
- 35.Pi J., Jiang J., Cai H., Yang F., Jin H., Yang P., Cai J., Chen Z.W. GE11 peptide conjugated selenium nanoparticles for EGFR targeted oridonin delivery to achieve enhanced anticancer efficacy by inhibiting EGFR-mediated PI3K/AKT and Ras/Raf/MEK/ERK pathways. Drug Deliv. 2017;24:1549–1564. doi: 10.1080/10717544.2017.1386729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cong W., Bai R., Y.-F. Li, Wang L., Chen C. Selenium nanoparticles as an efficient nanomedicine for the therapy of Huntington’s Disease. ACS Appl. Mater. Interfaces. 2019;11:34725–34735. doi: 10.1021/acsami.9b12319. [DOI] [PubMed] [Google Scholar]
- 37.Wang H., Zhang J., Yu H. Elemental selenium at nano size possesses lower toxicity without compromising the fundamental effect on selenoenzymes: comparison with selenomethionine in mice. Free Radic. Biol. Med. 2007;42:1524–1533. doi: 10.1016/j.freeradbiomed.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 38.Zhang J., Gao X., Zhang L., Bao Y. Biological effects of a nano red elemental selenium. BioFactors. 2001;15:27–38. doi: 10.1002/biof.5520150103. [DOI] [PubMed] [Google Scholar]
- 39.Liu T., Xu L., He L., Zhao J., Zhang Z., Chen Q., Chen T. Selenium nanoparticles regulates selenoprotein to boost cytokine-induced killer cells-based cancer immunotherapy. Nano Today. 2020;35 [Google Scholar]
- 40.Liu C., Lai H., Chen T. Boosting natural killer cell-based cancer immunotherapy with selenocystine/transforming growth factor-beta inhibitor-encapsulated nanoemulsion. ACS Nano. 2020;14:11067–11082. doi: 10.1021/acsnano.9b10103. [DOI] [PubMed] [Google Scholar]
- 41.Hu Y., Liu T., Li J., Mai F., Li J., Chen Y., Jing Y., Dong X., Lin L., He J., Xu Y., Shan C., Hao J., Yin Z., Chen T., Wu Y. Selenium nanoparticles as new strategy to potentiate gammadelta T cell anti-tumor cytotoxicity through upregulation of tubulin-alpha acetylation. Biomaterials. 2019;222 doi: 10.1016/j.biomaterials.2019.119397. [DOI] [PubMed] [Google Scholar]
- 42.Yang Y., Xie Q., Zhao Z., He L., Chan L., Liu Y., Chen Y., Bai M., Pan T., Qu Y., Ling L., Chen T. Functionalized selenium nanosystem as radiation sensitizer of I125 seeds for precise cancer therapy. ACS Appl. Mater. Interfaces. 2017;9:25857–25869. doi: 10.1021/acsami.7b07167. [DOI] [PubMed] [Google Scholar]
- 43.Huang J., Huang W., Zhang Z., Lin X., Lin H., Peng L., Chen T. Highly uniform synthesis of selenium nanoparticles with egfr targeting and tumor microenvironment-responsive ability for simultaneous diagnosis and therapy of nasopharyngeal carcinoma. ACS Appl. Mater. Interfaces. 2019;11:11177–11193. doi: 10.1021/acsami.8b22678. [DOI] [PubMed] [Google Scholar]
- 44.Li T., Pan S., Gao S., Xiang W., Sun C., Cao W., Xu H. Diselenide-pemetrexed assemblies for combined cancer immuno-, radio-, and chemotherapies. Angew. Chem. Int. Ed. 2020;59:2700–2704. doi: 10.1002/anie.201914453. [DOI] [PubMed] [Google Scholar]
- 45.Gao S., Li T., Guo Y., Sun C., Xianyu B., Xu H. Selenium-containing nanoparticles combine the NK cells mediated immunotherapy with radiotherapy and chemotherapy. Adv. Mater. 2020;32 doi: 10.1002/adma.201907568. [DOI] [PubMed] [Google Scholar]