Abstract
There is substantial evidence linking early-life stress (ELS) to negative health outcomes in adulthood, including addiction. However, the neurocognitive and behavioral mechanisms through which ELS increases these risks remain unclear. To address this gap in knowledge, we developed a novel instrumental learning paradigm to explore the effects of ELS on the balance of habitual versus goal-directed learning. Habits efficiently reproduce repetitive behaviors but are inflexible when reward contingencies related to those behaviors change. Persisting in performing a response after its outcome has been devalued is the hallmark of habitual behavior in instrumental learning. Participants with a history of higher ELS were significantly more likely to make habitual responses in this instrumental avoidance learning paradigm than individuals with a history of lower ELS. Logistic regression analysis showed that ELS is significantly related to habitual responding over and above the effects of retrospective socioeconomic status, trait and state anxiety, depression and recent levels of stress. Analysis of the differential impacts of the type of ELS suggested that these effects are largely driven by experiences of physical neglect.
Keywords: Avoidance, Early-Life Stress, Habit, Instrumental Learning, Socio-economic Status
1. Introduction
There is substantial evidence linking stress to poor physical and psychological health. Of particular interest is the role of stress during development. Stress during or immediately prior to sensitive periods of development during which there is accelerated neuroplasticity may embed the biological responses to stress into the cytoarchitecture of the brain in a way that is particularly resistant to change (Knudsen, 2004; for reviews see: Andersen, 2019; and Gee & Casey, 2015). Indeed, numerous studies demonstrate strong associations between early-life stress (ELS; e.g., loss events, child abuse or neglect) and negative health outcomes in adulthood; including increased risk of addiction (Anda et al., 2002), obesity (Williamson et al., 2002), diabetes (Kaufman et al., 2007), chronic obstructive pulmonary disease (Anda et al., 2008), heart disease (Dong et al., 2004), liver disease (Dong et al., 2003), sexually transmitted infection (Hillis, et al., 2000) and, ultimately, early mortality (Felitti, et al., 1998). A recent meta-analysis links higher ELS with a 6-fold risk of problematic drinking and a 10-fold risk of problematic drug use (Hughes et al., 2017). However, the neurocognitive and behavioral mechanisms through which ELS makes one at risk for these conditions is unclear.
Many of the health outcomes that are associated with stress are linked to the repeated performance of maladaptive behaviors (e.g., disordered eating or substance use). These behaviors persist in the face of the awareness that they are detrimental. Repeated behaviors that are insensitive to outcome contingencies are the features that define habits as opposed to goal-directed actions which adapt to changes in outcomes (Dickinson, 1985). Habits are an efficient way to perform routine actions without having to think about the consequences of those actions. Habits develop slowly with repeated successes in consistent environments. But habits can become maladaptive when they no longer align with an individual’s goals. We posit that an increased reliance on stimulus-response habits among individuals with ELS may explain some of these negative health effects. For example, many smokers continue to smoke even after negative health outcomes associated with smoking have begun to develop and in the face of the knowledge that smoking contributes to cancer, lung disease and premature death. Persistence in a behavior after outcomes related to that behavior are no longer valuable is the hallmark of habits. Accordingly, we believe that an over-reliance on habit learning may be a behavioral mechanism mediating the relationship between ELS and poor health outcomes during adulthood.
Typical experimental paradigms that assess habit within instrumental learning involve manipulating outcomes though a devaluation procedure—the making of a previously valuable outcome undesirable. A behavior is inferred to be habitual if it persists after devaluation or goal-directed if the behavior is modified as a result of the new outcome. Instrumental learning paradigms can either be appetitive or avoidant. In appetitive designs, the behavior is motivated through the accumulation of rewards (e.g., food) before devaluation (e.g., selective satiation, in which one food is consumed until it is no longer pleasurable) after which a habit test is performed. Production of the behavior associated with the no-longer-valuable food is indicative of a habit (see: Tricomi, et al., 2009). In avoidance designs, a behavior is learned to be performed in order to avoid an aversive outcome (e.g., shocks). After devaluation (e.g., the removal of one set of electrodes) producing the behavior associated with the devalued aversive outcome (e.g., shocks to the side where the electrodes are no longer attached) indicates a habit (Gillan, et al, 2014).
Different corticostriatal loops subserve the memory systems supporting goal-directed actions and stimulus response habits in instrumental learning (Yin & Knowlton, 2006). The associative network: the circuit formed by the caudate; associative pallidum; mediodorsal and ventral thalamus; and prefrontal and parietal association cortices governs goal-directed actions. As behavior becomes habitual, there is a shift from the associative corticostriatal loop to the sensorimotor network, which is the circuit formed by the putamen, motor pallidum, ventral thalamus and sensorimotor cortices. In rodent studies, lesions or inactivation of the dorsolateral striatum—the rodent homolog of the putamen—impaired the development of habits, resulting in a more goal-directed mode of behavioral control (Yin, Knowlton, & Balleine, 2004; Yin, Knowlton, & Balleine, 2006). Whereas, lesions or inactivation of the dorsomedial striatum—the rodent homolog of the caudate—have the opposite effect and result in a switch from goal-directed actions to habits (Yin et. al., 2005).
1.1. Stress and Instrumental learning
Multiple studies have demonstrated a link between stress and a shift in memory systems towards habits at the expense of goal-directed behavior. Experimentally induced stress before or after training in an instrumental task rendered participants’ behavior on a subsequent habit test insensitive to the change in the value of the food outcomes (Schwabe and Wolf, 2009 & 2010). In another study employing a spatial learning task, acute stress resulted in a shift towards stimulus-response strategies at the expense of spatial learning strategies (Schwabe et al, 2007; see: Wirz, Bogdanov, & Schwabe, 2018 for a review on the effects of stress on habitual behavior).
Chronic stress is similarly associated with an increased propensity for habits. Rats subjected to chronic stress became insensitive to changes in outcome value and resistant to changes in action-outcome contingency suggesting that stress biases behavioral strategies toward habit. The stressed rats displayed atrophy of medial prefrontal cortex and the associative striatum and hypertrophy of the sensorimotor striatum. (Dias-Ferreira et al, 2009)
Chronic stress in humans is associated with changes to both the caudate and putamen and a corresponding shift from goal-directed to habitual learning and behavior. In a study of Brazilian medical students, those studying for a major exam displayed greater habitual behavior during an appetitive task, with fMRI scans showing a shift in activation favoring the sensorimotor circuit over the associative circuit. Structural scans revealed atrophy of the medial prefrontal cortex and caudate and hypertrophy of the putamen versus controls—medical students not preparing for the exam. Follow-up scans taken after the students recovered from the exam showed the effects of chronic stress were transient (Soares et al, 2012). These morphological responses to stress have been partially replicated with MRIs of patients with occupational exhaustion syndrome—a stress disorder—which show enlarged amygdala volumes, and reduced caudate volumes compared to controls (Savic, Perski & Osika, 2018). These morphological differences were no longer present after a regimen of stress-reducing cognitive behavioral therapy on follow-up scans performed 1-to-2 years later.
Patterson, Craske & Knowlton (2019) developed an avoidance learning paradigm in which participants learned to make the correct key press in response to warning stimuli in order to avoid hearing screams in either their left or right ear. After a learning phase, participants were given a devaluation instruction informing them that one warning stimulus would be safe going forward requiring no response and its paired earphone was removed. Making one or more previously correct responses to the devalued stimulus during the habit test—performed in extinction—was defined as a habitual response. Participants also provided self-reported ratings of ELS, depression, trait and state anxiety and recent stress. In two studies examining the effects of training duration and distraction on habits, ELS was significantly associated with an increase in habitual responses. Prenatal exposure to stress is also linked to less flexible learning strategies (Sutherland, McDonald, & Savage, 2000; Schwabe, Bohbot, & Wolf, 2012).
1.2. Types of ELS
While there is evidence that ELS is associated with higher rates of habitual responding, it is unknown whether this effect is due to specific types of ELS (Patterson et al., 2019). There is a growing body of evidence suggesting different types of childhood maltreatment have differential impacts on later outcomes. Disrupted caregiving may be more closely linked to impaired regulation of the stress response and recovery from stress (Cole et al., 2012); whereas physical trauma appears to be linked to exaggerated activation of stress response systems to acute stress (Kuhlman et al., 2015); and an unpredictable environment seems to result in more frequent activation of physiological stress systems and elevated diurnal cortisol and circulating inflammation (Laurent et al., 2014; for a review see: Kuhlman et al., 2018). In the Minnesota Longitudinal Study of Risk and Adaptation, which tracked first-born children of mothers below the poverty line from the third trimester prior to birth, physical and cognitive neglect, but not physical and sexual abuse was associated with negative health outcomes in middle adulthood (Johnson et al., 2017). Physical neglect, but not other forms of childhood maltreatment, is associated with cortisol and macrophage migration inhibitory factor (MIF; a cytokine known to be involved in HPA axis regulation) values at baseline assessment in a high-risk population of youths (ages 14 to 19) from disadvantaged backgrounds (low income; 78% exposed to alcohol, tobacco or drugs prenatally; Bick et al., 2015). Neglect predicts higher childhood BMI among socially disadvantaged children (Knutson, et al., 2010). A recent meta-analysis of studies of individuals with eating disorders reveals elevated prevalence of childhood emotional neglect (53.3%, 7 studies, N = 963) and physical neglect (45.4%, 6 studies, N = 665; Pignatelli, et al., 2017). Taken together, these studies suggest that physical neglect may be most predictive of negative health outcomes in adulthood compared to other types of ELS. Minimally, there is sufficient evidence to suspect that different types of stress impact development differently.
1.3. Covariates that may be associated with Habits
1.3.1. Depression
Depression is associated with enhanced aversive Pavlovian-to-instrumental transfer (Nord, et al., 2018); however, the extent to which depression may alter the ratio of goal-directed to habitual strategies during instrumental learning is unknown. The high rate of co-morbidity between depression and substance use disorder suggests that there may be a neural or cognitive mechanism in common between these disorders. As many as one third of people with depression reported a co-substance abuse disorder at some point (Regier, et al., 1990) and individuals who abuse alcohol have 3 to 4 times the prevalence of depression than individuals who do not (Kessler, et al., 1997). One potential cognitive mechanism that could explain this relationship is a preponderance of habit behavior.
1.3.2. Anxiety
Attentional control theory posits that anxiety impairs goal-directed, top-down cognition and facilitates stimulus driven bottom-up processing (Eysenck et al, 2007). Evidence for anxiety contributing to enhanced habitual behavior in instrumental learning comes from shock-avoidance instrumental learning studies of people with obsessive-compulsive disorder (OCD; Gillan et al, 2014; and Gillan et al, 2015). After controlling for negative affect, checking symptoms—an OCD symptom—were associated with bias toward habits in non-clinical college students (Snorrason et al., 2016). The scream avoidance instrumental learning paradigm provides conflicting evidence for a link between anxiety and increased habits; in which only one of two experiments found state anxiety to be associated with increased habits (Patterson et al., 2019).
1.3.3. Socio-economic status (SES)
Poverty during childhood is associated with decreased gray matter volume in areas associated with executive function (frontal and temporal cortexes; Lawson et al, 2013; Hanson et al, 2013) and the hippocampus (Yu et al., 2007) as well as diminished multi-modal cognitive functioning (Rosen et al., 2018) and academic achievement (Hair et al, 2015). Low SES is associated with impaired selective attention during a dichotic listening paradigm in which participants have to attend one of two simultaneously playing audio stimuli (Stevens, Lauinger, & Neville, 2009; Stevens et al, 2015). However, the effects of poverty on neurocognitive development are likely confounded by a higher prevalence of ELS among impoverished individuals and the protective effects of the resources available to people with high SES. In a study of 145 10-year-old children who had been tracked longitudinally since preschool, quality of parenting and exposure to ELS partially mediated the relationship between SES and hippocampal volume (Luby, Belden & Botteron, 2013).
1.3. Hypotheses
Based on the association between physical neglect early in life and health outcomes and the mediating effect of SES on neurocognitive effects of stress, we had the following a priori hypotheses:
ELS, generally, will be associated with increased habits and these effects will be greatest when ELS included physical neglect.
There will be interactions between SES and trait anxiety and SES and current stress such that the habit-increasing effects of trait anxiety and current stress are mitigated by high SES.
2. Materials and Methods
2.1. Participants
Participants were undergraduate students recruited from the University of California, Los Angeles (UCLA), psychology subject pool and received credit toward partial completion of course requirements. A total of 150 participants were recruited. 17 participants were excluded for failure to exceed chance level performance during the final two rounds of the of the learning phase of the study. Chance level performance was considered to fall below the upper limit of the 95% confidence interval around a binomial inverse cumulative distribution function with 40 independent trials each with a 50% probability of success (a raw score of 25 correct responses). An additional 11 participants were excluded for providing incomplete questionnaire data, yielding a sample size of 122 (101 women, 21 men), ranging in age from 18–35 (M = 20.62 , SD = 2.46). Study procedures were approved by the Institutional Review Board of the University of California, Los Angeles, and all participants provided written record of informed consent.
2.2. Instrumental avoidance learning task
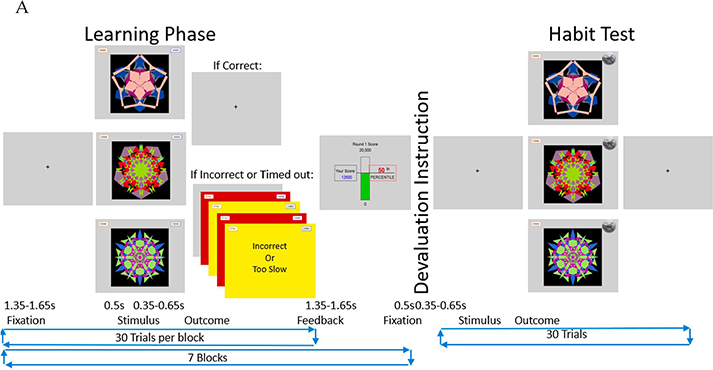
An instrumental avoidance learning task consisting of a learning phase, outcome devaluation instruction and an extinction phase was used (see Figure 1).
Figure 1.
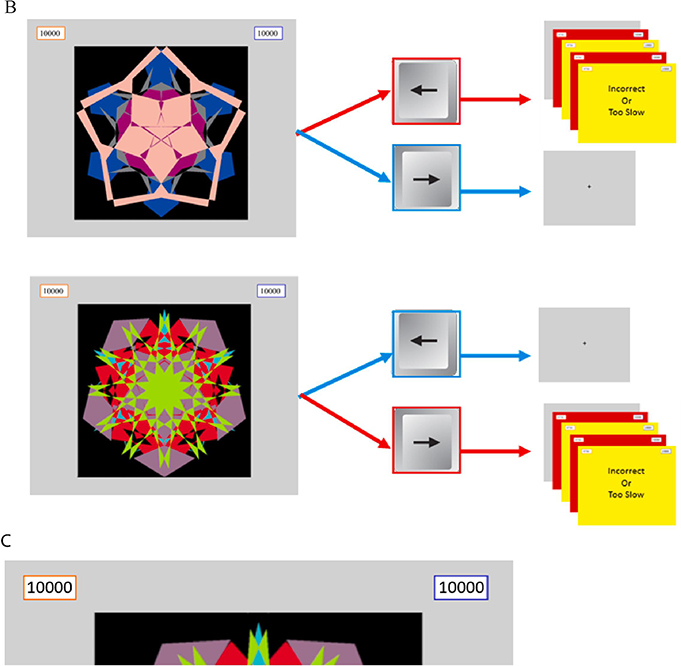
A: Task schematic: Participants performed 7 blocks of 30 trials in the learning phase then received a devaluation instruction in which they were informed one side would subsequently be safe—not requiring a response from its paired arrow key. After which participants performed the habit test in extinction. Pressing the formerly correct arrow key in response to the devalued stimulus is defined as a habit. Figure 1 B: Warning stimuli schematic: Participants learned to make avoidance responses to warning stimuli. If the correct response (blue) was made within .5s, no aversive outcome was delivered. Otherwise participants lost 750 points from the paired score and received feedback informing them they were incorrect or too slow (as appropriate) and red and yellow flashing screens. Figure 1 C shows a close-up image of the left and right scores.
Prior to beginning the learning phase, participants were instructed: “The purpose of this research is to investigate how people learn to make responses to avoid unpleasant outcomes. During this experiment, you will start each round with two banks of 10,000 points, your task is to avoid losing as many points as possible. At the beginning of each trial, you will see a fractal image. Two of the fractal images you will see are warning stimuli, meaning that points will probably be deducted from one of your banks of points if you fail to press the correct key to avoid the loss of points. A third fractal image you will see is the safe stimulus, which indicates that no points will be lost during that trial. Now, you will see a demonstration of the three types of trials in the experiment. Press any key to continue.” Following the instructions, a demonstration of each trial type; left warning, right warning and safe was shown. The images used as warning and safe stimuli were counterbalanced across participants. After the demonstrations, the learning phase began.
The learning phase consisted of 7 blocks of 30 trials, 10 each for a warning stimulus indicating that the participant should press the right arrow key within 0.5 s or lose points from the right bank of points; a different warning stimulus indicating that the participant should press the left arrow key within 0.5 s or lose points from the left bank of points; and a safe stimulus that was never paired with the loss of points. Order of stimuli was randomized. Failure to make the correct response within 0.5 s to a warning stimulus resulted in the loss of 750 points from its paired bank followed by alternating red, yellow, red, then yellow screens each displayed for 0.1 s with the text “Incorrect” or “Too Slow” displayed in the center as appropriate. Each block started with an instruction to participants: “Round X will begin next. Please keep your fingers on the right and left arrow keys. When you are ready, press any key to being.) At the end of each block of 30 trials, a screen was displayed showing the participant’s total score for the block, and their “percentile rank.” Percentile ranks were given arbitrary values based on the participant’s performance rather than a comparison with the other participants (for specific values, see supplemental Table 1).
The devalued side was counterbalanced across participants. The outcome devaluation instruction to participants with the left side devalued was: “Congratulations, you have unlocked a bonus round! During the Bonus Round, Points in the Left Bank are now safe. You will be evaluated based on the accuracy of the responses you make with the Right Arrow Key. It is not necessary to make responses with the Left Arrow Key and you will no longer lose any points from the Left bank. Your only task is to avoid losing points from the right bank. Please keep your fingers on the Left and Right Arrow Keys. When you are ready, press any key to begin.” Subsequently, the points on the left side were shown superimposed over the image of a safe to indicate they were now safe.
The habit test consisted of 30 trials−−10 for each stimulus—performed in extinction, where incorrect responses were no longer penalized, but participants were not informed of this. Scores were shown on the screen throughout the trials but did not change regardless of the participant’s actual performance. Making one or more formerly correct responses to the devalued stimulus with its paired arrow key is indicative of habit learning.
2.3. Questionnaires
Participants were assessed for exposure to stress during their first 16 years of life using the Childhood Trauma Questionnaire Short Form (CTQ-SF; Bernstein et al., 2003), which is a 25-item questionnaire consisting of 5-item sub-scales for physical abuse (PA), physical neglect (PN), emotional abuse (EA), emotional neglect (EN) and sexual abuse (SA) with each question scored from 1 (never) to 5 (very often). Total Scores range from 25 (minimal experience of these stressors) to a maximum of 125 (frequent experience of all of these stressors).
Trait anxiety and state anxiety were assessed with the State-Trait Anxiety Inventory for Adults™ (STAI; Spielberger, 1989). The STAI is a 40-item questionnaire that attempts to differentiate between the temporary condition of state anxiety and the more general and long-standing quality of trait anxiety.
Retrospective subjective SES was assessed with a modified MacArthur Scale of Subjective Social Status (Adler et al., 2000). The MacArthur Scale of Subjective Social Status is a self-anchoring scale in which participants place themselves on a drawing of a ladder where the top step represents those in U.S. society who are best off in terms of income, education, and occupation and the bottom represents those who are worst off. The modifications to this scale consisted of asking the participant to score their immediate family instead of themselves individually and to do so retrospectively.
Perceived stress was assessed with the Perceived Stress Scale (PSS; Cohen & Williamson, 1988), which is a 10-item inventory designed to measure the degree to which respondents have felt their lives to be unpredictable, uncontrollable, and overloaded over the past month.
The Beck Depression Inventory-II (BDI; Beck et al., 1996), excluding the suicidality question, was used to assess symptoms of depression during the two weeks prior to testing.
2.4. Procedure
After providing informed consent, participants completed the experiment in private testing rooms on Apple iMac® computers under normal lighting conditions. The experiment was coded in MATLAB (Mathworks, 2018), using the Psychophysics Toolbox extensions (Brainard, 1997; Pelli, 1997; Kleiner et al, 2007). After completing the experiment participants responded to questionnaires on the computers.
2.5. Data analysis
All data were analyzed using IBM SPSS Statistics (Version 26) and MATLAB (Mathworks, 2019B). Participants were sorted into higher and lower early-life stress groups through a median split of CTQ-SF scores. Making one or more formerly correct responses to the devalued stimulus during the post-devaluation habit test with its paired arrow key is indicative of habit learning and was coded as one on a binarized outcome variable; making zero such responses was coded as zero. This is consistent with Patterson, Craske & Knowlton (2019); and was done to minimize the influence of participants who may have misunderstood or disregarded the devaluation instruction (and, accordingly, consistently responded to the devalued stimuli in a non-habitual manner) on the statistical analysis. Multivariate binary logistic regression was used to simultaneously assess the effects of CTQ-SF on habitual responding with covariates for trait anxiety, state anxiety, PSS, BDI, SES, and interactions between SES and PSS, BDI, trait anxiety and state anxiety. A separate multivariate binary logistic regression was used to simultaneously assess the effects of the different types of early-life stress (PA, PN, EA, EN, and SA) on habitual responding with covariates for trait anxiety, state anxiety, PSS, BDI, SES, and interactions between SES and PSS, BDI, trait anxiety and state anxiety. Dichotomous variables were dummy coded and continuous variables were mean-centered to reduce multicollinearity. A significance level of 0.05 was used for all statistical tests.
3. Results
Prevalence of ELS by type of stress and degree in the sample are reported in Table 1. Scores for ELS and covariates for lower and higher ELS participants are in Table 2. The mean CTQ-SF score was 36.1 and the median CTQ-SF score was 32. The lower ELS group had a mean CTQ-SF score of 27.8 (SD = 2.3) and the higher ELS group had a mean CTQ-SF score of 44.7 (SD = 12.5). The lower ELS group differed significantly from the higher ELS group on measures of depression, t(97.411) = 5.584, p < .001; trait anxiety, t(120) = 6.284, p < .001; state anxiety, t(120) = 4.559, p < .001; current stress, t(108.917) = 2.047, p = .043; and SES t(120) = 3.302, p = .001.
Table 1:
Prevalence of ELS in sample
| CTQ-SF Total | 25 | 26–35 | 36–45 | 46–55 | 56+ |
| 10.7 % | 52.4 % | 18.9 % | 9 % | 9 % | |
| Subscale Score | 5 | 6–10 | 11–15 | 16–20 | 21–25 |
| PA | 64.8 % | 27.8 % | 7.4 % | 0 % | 0 % |
| PN | 48.4 % | 41.8 % | 9.8 % | 0 % | 0 % |
| EA | 27 % | 50.9 % | 13.1 % | 6.5 % | 2.5 % |
| EN | 29.5 % | 43.5 % | 15.5 % | 9.9 % | 1.6 % |
| SA | 82.8 % | 11.5 % | 3.2 % | 2.5 % | 0 % |
Percentage of participants reporting Early-Life Stress (ELS) broken down by type and degree of ELS. Scores on each item range from 1 (“never”) to 5 (“Very Often”). CTQ-SF, Childhood Trauma Questionnaire – Short Form, PN, Physical Neglect, EA, Emotional Abuse, EN, Emotional Neglect, PA, Physical Abuse, SA, Sexual Abuse (Bernstein et al., 2003).
Table 2:
Characteristics of lower and higher ELS groups
| Low ELS | High ELS | |
|---|---|---|
| CTQ – SF | 27.8 (2.3) | 44.7 (12.5) |
| Depression | 6.6 (7.3) | 16.6 (11.8) |
| State Anxiety | 35.4 (6.1) | 40.9 (7.2) |
| Trait Anxiety | 37.9 (9.7) | 50.0 (11.5) |
| PSS | 22.3 (3.6) | 23.9 (4.8) |
| SES | 4.2 (1.7) | 5.3 (1.9) |
| Age | 20.8 (2.9) | 20.4 (1.9) |
| PN | 5.5 (1.2) | 8.1 (2.8) |
| EA | 5.9 (1.1) | 10.9 (4.7) |
| EN | 5.9 (1.2) | 11.9 (4.7) |
| PA | 5.3 (0.9) | 7.2 (2.9) |
| SA | 5.2 (0.9) | 6.6 (3.4) |
Mean (SD) scores on questionnaire measures for participants grouped by reported level of ELS. CTQ-SF, Childhood Trauma Questionnaire – Short Form, PN, Physical Neglect, EA, Emotional Abuse, EN, Emotional Neglect, PA, Physical Abuse, SA, Sexual Abuse (Bernstein et al., 2003); Depression, Beck Depression Inventory-II (Beck et al., 1996); State and Trait Anxiety, State-Trait Anxiety Inventory (Spielberger, 1983); PSS, Perceived Stress Scale (Cohen et al., 1983); and SES, McArthur Scale of Subjective Social Status (Adler et al, 2000).
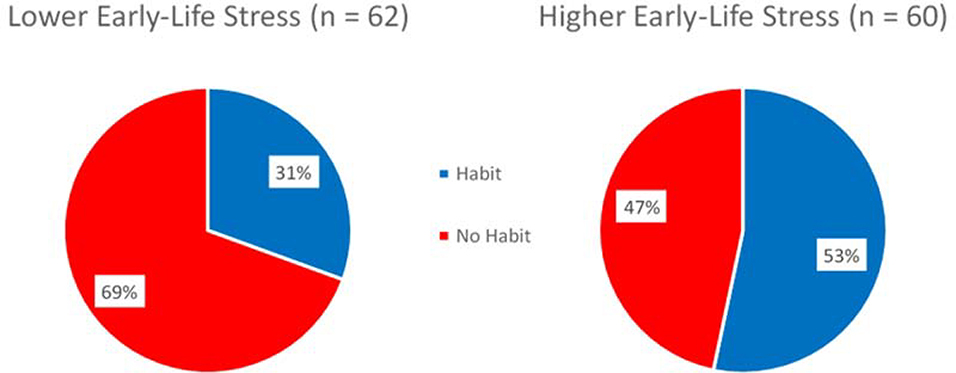
Fifty three percent of higher ELS individuals (above the median CTQ value) performed one or more habit responses during the habit tests whereas only 30 percent of lower ELS individuals did (see Figure 2). A chi-square test of independence was performed to examine the relation between ELS and habits. The relation between these variables was significant with a medium effect size, χ2 (1, N = 122) = 6.452, p =.011, φ = Cramer’s V = .23. Individuals with higher ELS were more likely to make habit responses during the habit test than were individuals with lower ELS.
Figure 2.
Pie charts depicting the proportion of higher and lower ELS individuals who performed one or more habit responses during the habit test. Individuals with higher ELS were more likely to make habit responses during the habit test than were individuals with lower ELS.
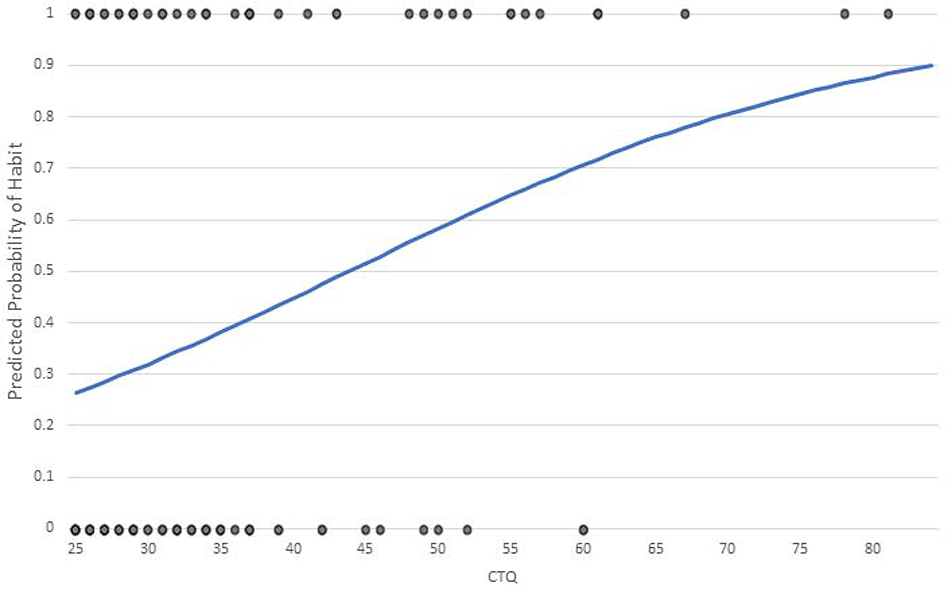
We tested for the effects of ELS on habit responses during the habit test by conducting a binary logistic regression analysis on responding (with the formerly correct response key) to the devalued warning stimulus during the post-devaluation habit test. Participants’ responses were binned into zero responses to the devalued stimulus (no habit) or one or more responses to the devalued stimulus (habit). Pressing the key associated with the other warning stimulus during the presentation of the devalued warning stimulus was treated as a non-habit response. This analysis tested the effects of ELS by using CTQ-SF as a continuous predictor variable while statistically controlling for the effects of trait anxiety, state anxiety, PSS, BDI, SES, and interactions between SES and PSS, BDI, trait anxiety and state anxiety. The results of this analysis are in Table 3. Consistent with our hypothesis that ELS will be associated with greater habitual behavior, CTQ-SF was found to be a significantly positive predictor of habit responding, B = 0.054, p = 0.034, and eB = 1.056. For every one-point increase in CTQ-SF score, the expected odds of performing a habit response are increased by 5.6% over and above the effects of the other variables in the model. Figure 3 shows the probability of making a habit response for levels of ELS holding all other variables constant at their means.
Table 3.
Results of binary logistic regression analysis showing the effect of CTQ-SF score (CTQ) on responding to the devalued stimulus.
| Variable | B | S.E. | Wald | Sig. | Exp(B) |
|---|---|---|---|---|---|
| CTQ | 0.054 | 0.026 | 4.490 | 0.034 | 1.056 |
| Depression | 0.042 | 0.037 | 1.242 | 0.265 | 1.042 |
| State Anxiety | 0.064 | 0.053 | 1.432 | 0.231 | 1.066 |
| Trait Anxiety | −0.002 | 0.040 | 0.003 | 0.953 | 0.998 |
| Current Stress | −0.092 | 0.060 | 2.304 | 0.129 | 0.912 |
| Subjective SES | −0.194 | 0.131 | 2.190 | 0.139 | 0.823 |
| Age | 0.045 | 0.088 | 0.258 | 0.611 | 1.046 |
| Gender (Male vs Female) | 0.276 | 0.585 | 0.222 | 0.637 | 1.318 |
| SES by Current Stress | −0.081 | 0.045 | 3.292 | 0.070 | 0.922 |
| SES by Trait Anxiety | 0.001 | 0.023 | 0.001 | 0.973 | 1.001 |
| SES by State Anxiety | 0.007 | 0.032 | 0.040 | 0.841 | 1.007 |
| SES by Depression | 0.018 | 0.023 | 0.641 | 0.423 | 1.018 |
| Constant | −0.649 | 0.533 | 1.481 | 0.224 | 0.523 |
Figure 3.
shows the effects of ELS on the predicted probability of making one or more habit responses during the habit test holding the other variables in the model constant at their means.
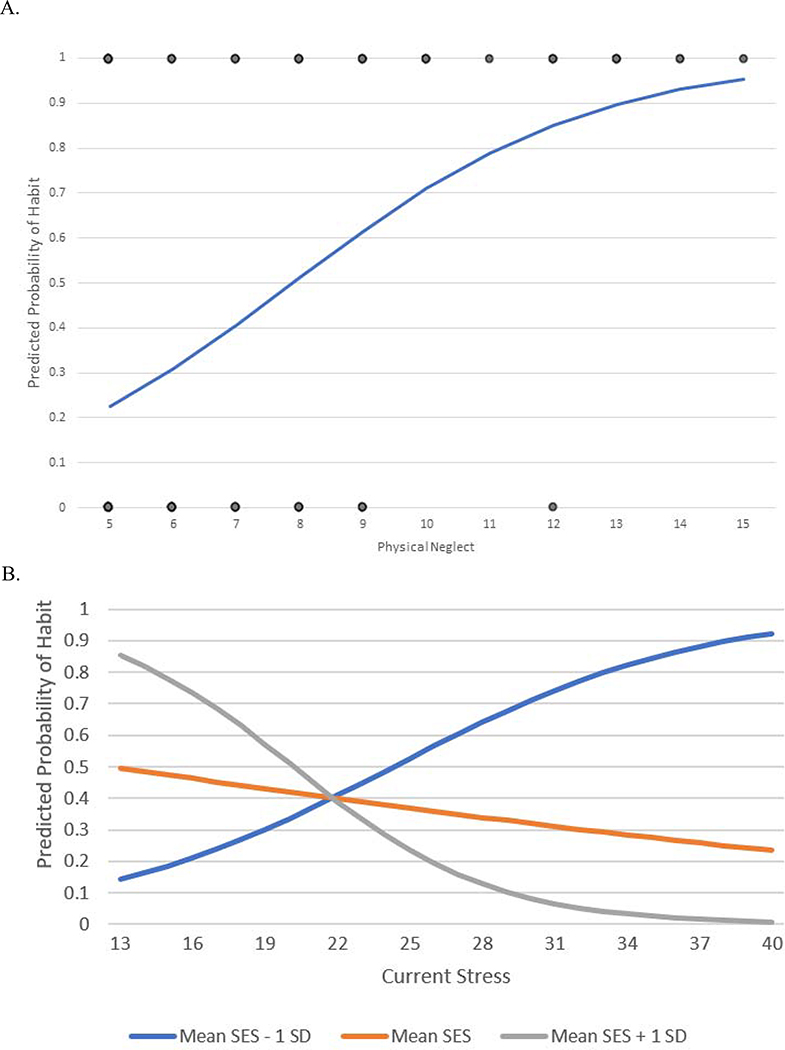
Because we hypothesized that different types of ELS would differentially impact habits, we conducted a separate binary logistic regression analysis on habit responses to the devalued stimulus during the habit test assessing the effects of the subscales of ELS (PN, PA, EN, EA and SA) while statistically controlling for depression, perceived stress, trait anxiety, state anxiety, gender, age, SES, and interactions between SES and depression, perceived stress, trait anxiety and state anxiety. The results of this analysis are in Table 4. Consistent with our hypothesis that PN will be the type of ELS most strongly associated with greater habitual behavior, PN was found to be a significantly positive predictor of habit responding, B = 0.425, p = 0.001, and eB = 1.529. For every one-point increase in PN score, the expected odds of performing a habit response are increased by 52.9% over and above the effects of the other variables in the model. The interaction between current stress and SES was also found to be significant, p = 0.034. Figure 4a shows the probability of making a habit response for levels of PN, holding all other variables constant at their means. Figure 4b shows the probability of making a habit response for levels of current stress at mean SES, mean SES minus one standard deviation and mean SES plus one standard deviation while holding all other variables in the model constant at their means.
Table 4.
Results of binary logistic regression analysis showing the effect of different types of reported early-life stress on responding to the devalued stimulus.
| Variable | B | S.E. | Wald | Sig. | Exp(B) |
|---|---|---|---|---|---|
| Depression | 0.058 | 0.041 | 2.045 | 0.153 | 1.060 |
| State Anxiety | 0.071 | 0.061 | 1.335 | 0.248 | 1.074 |
| Trait Anxiety | −0.006 | 0.045 | 0.017 | 0.896 | 0.994 |
| Current Stress | −0.043 | 0.066 | 0.423 | 0.515 | 0.958 |
| SES | −0.133 | 0.137 | 0.940 | 0.332 | 0.875 |
| Age | 0.017 | 0.105 | 0.027 | 0.870 | 1.017 |
| Gender | 0.417 | 0.647 | 0.416 | 0.519 | 1.518 |
| SES by Current Stress | −0.106 | 0.050 | 4.493 | 0.034 | 0.899 |
| SES by Trait Anxiety | 0.001 | 0.026 | 0.001 | 0.970 | 1.001 |
| SES by State Anxiety | 0.007 | 0.036 | 0.040 | 0.841 | 1.007 |
| SES by Depression | 0.025 | 0.024 | 1.035 | 0.309 | 1.025 |
| Physical Neglect | 0.425 | 0.131 | 10.527 | 0.001 | 1.529 |
| Emotional Abuse | −0.193 | 0.115 | 2.827 | 0.093 | 0.824 |
| Emotional Neglect | 0.080 | 0.081 | 0.964 | 0.326 | 1.083 |
| Physical Abuse | 0.156 | 0.131 | 1.425 | 0.233 | 1.169 |
| Sexual Abuse | 0.071 | 0.126 | 0.318 | 0.573 | 1.073 |
| Constant | −0.801 | 0.586 | 1.865 | 0.172 | 0.449 |
Figure 4.
A shows the effects of Physical Neglect on the predicted probability of making one or more habit responses during the habit test holding the other variables in the model constant at their means. Figure 4B shows the effects of current stress on the predicted probability of making one or more habit responses during the habit test at mean SES, mean SES minus one standard deviation and mean SES plus one standard deviation while holding all other variables in the model constant at their means.
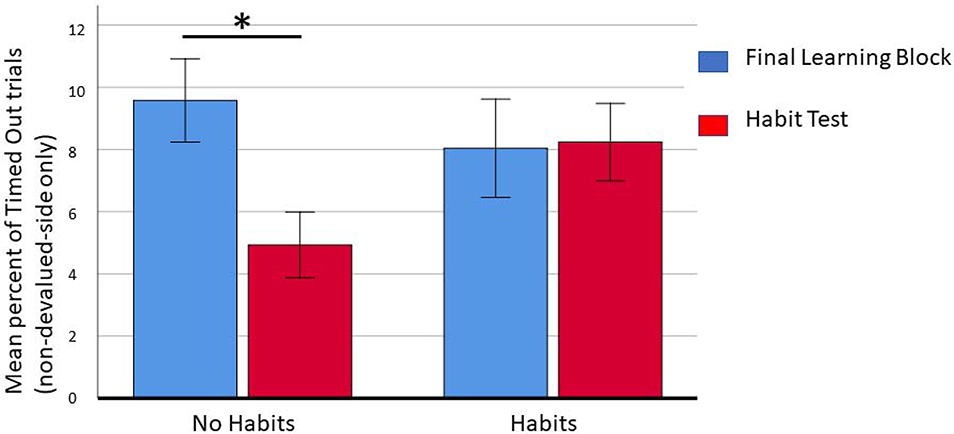
If there is no response cost associated with performing the habitual behavior, then it seems that subjects might continue pressing the key associated with the devalued outcome regardless of the nature of the association learned. We hypothesize that individuals who stop responding to the devalued stimulus will receive a performance boost for the non-devalued side by keying into the non-devalued warning stimulus and ignoring the devalued warning stimulus. This boost should be reflected in a lower rate of non-devalued trials ending as timed-out during the habit test than the last practice block. We would not expect to see this improvement for individuals who persist with the habit response. This forgone advantage would represent a response cost associated with performing the habitual behavior. A two-way repeated measures analysis of variance (ANOVA) revealed a significant interaction between habitual performance and change in the rate of non-devalued trials ending in timed-out, F(1, 1) = 4.08, p = .046. A post hoc paired sample t-test indicated that the average number of timed-out trials to the non-devalued stimulus was significantly lower during the habit test (M = 0.49 SD = .81) than the final learning block (M = .96, SD = 1.2) for individuals who did not make a habit response during the habit test; t(70) = 3.07, p = .003. Figure 5 shows the mean rate of timed-out trials to the non-devalued warning stimulus during the final learning block and the habit test for individuals who did and did not perform a habit response. Thus, a cost is indeed incurred by habitual responding, and those subjects who were able to reduce monitoring of the devalued stimulus performed better on the non-devalued response.
Figure 5.
shows the mean rate of timed-out trials to the non-devalued warning stimulus during the final learning block and the habit test for individuals who did and did not perform a habit response. The difference is only significant for individuals who did not perform a habitual response during the habit test.
4. Discussion
We observed evidence of enhanced avoidance habits in adults with a self-reported history of ELS during a novel instrumental avoidance learning paradigm. As hypothesized, analysis of the different types of ELS suggests that these effects are largely due to a history of physical neglect during development. These results have implications for understanding how ELS increases the risk of addiction during adulthood. Furthermore, we provide evidence suggesting that the relationship between ELS and enhanced habit acquisition during instrumental learning reported in Patterson, Craske & Knowlton (2019) generalizes across avoidance procedures using different types of negative stimuli.
The deleterious consequences of neglect during childhood have received less attention than those of physical or sexual abuse (National Scientific Council on the Developing Child, 2012). The absence of responsive care is processed as a serious threat to well-being, and may reflect a home situation that is unsanitary with uncertain access to food. This type of environment likely induces chronic activation of the stress response during development (Schreier et al., 2020). The present results are consistent with literature showing an association between habitual responding and chronic stress, and suggest that chronic stress effects in childhood persist into adulthood. It can be argued that under stressful situations, reliance on habits is adaptive in that it results in reliable, rapid responding that frees up cognitive resources for other tasks (Wirz et al., 2018). However, an overreliance on habit can lead to reduced behavioral flexibility and predisposition to addiction and other pathological behaviors.
Another implication of this experiment pertains to the role of SES. An abundance of resources may mitigate the effects of perceived stress on neurocognitive variables. While developmental research reliably finds robust negative effects of low SES on cognitive functioning (Lawson, Hook & Farah, 2017), our results suggest that these studies may be confounded by the effects of ELS and the increased risk for trauma among lower SES individuals. In addition, our results suggest that in individuals with low SES backgrounds, current stress becomes associated with habitual responding.
4.1. Limitations
While the results from this study have implications for understanding how ELS increases the risk of addiction, it does not explicitly tie an increased prevalence of habits to poorer health or riskier health behaviors. Future work should do so by assessing habits and health behaviors and outcomes in a representative sample.
A major limitation of our sample is that we did not have a sufficient number of men to adequately assess sex differences in the effect of ELS and other variables on habitual behavior. Work in rodent models suggests that the development of habits in instrumental learning is facilitated in females compared to males (Schoenberg et al., 2019), and that ELS affects neurodevelopment differently in males and females (Kunzler et al., 2015). There is extensive evidence of physiological sex differences in response to ELS (Heim & Nemeroff, 1999), and thus it is unclear if the present results would be obtained in men.
It is also important to note that the UCLA psychology subject pool is not a representative sample of the greater population of people, perhaps limiting the generalizability of findings from this population. Our use of a sample of university students is likely to select for resiliency among individuals most exposed to ELS, which suggests that this study may understate the effects of ELS on a bias towards habits in instrumental avoidance learning compared to a representative sample.
Finally, the limitation of retrospective surveys of early-life experiences and of early autobiographical memories means we cannot know precisely when during development people experienced ELS. This ambiguity prevents us from being able to test the theorized role of sensitive periods of development. Longitudinal studies tracking people through development into adulthood may provide a more granular assay of ELS and allow testing of these sensitive periods.
4.2. Future Directions
Future work should attempt to determine if the increased prevalence of habits among individuals exposed to ELS mediates the relationship between ELS and negative health outcomes such as addiction or compulsive eating, or if these relationships are independent. Additionally, an area of future work suggested by the present study would be to see if individuals exposed to ELS display increased habitual responding in appetitive instrumental learning paradigms or if this relationship is unique to avoidance learning.
Supplementary Material
A history of early-life stress was associated with an increased rate of making habitual responses on an avoidance learning task.
Experiencing physical neglect was the type of childhood adversity most closely associated with habitual responding.
A history of early-life stress was related to habitual responding even when controlling for effects of socioeconomic status and state and trait anxiety.
These results suggest a neurocognitive mechanism by which early-life stress predisposes individuals to addictive behaviors in adulthood.
Acknowledgements:
The authors acknowledge support from NIH/NIDA 5R01DA045716 to BJK and fellowship support to ALG from NIH/NICHD 5T32HD091059
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler NE, Epel ES, Castellazzo G, Ickovics, & J. R. (2000). Relationship of subjective and objective social status with psychological and physiological functioning: Preliminary data in healthy, White women. Health Psychology, 19, 586–592. [DOI] [PubMed] [Google Scholar]
- Anda RF, Brown DW, Dube SR, Bremner JD, Felitti VJ, & Giles GH (2008). Adverse childhood experiences and chronic obstructive pulmonary disease in adults. American Journal of Preventive Medicine, 34(5), 396–0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anda RF, Whitfield CL, Felitti VJ, Chapman D, Edawards VJ, Dube SR, & Williamson DF (2002). Adverse childhood experiences, alcoholic parents, and later risk of alcoholism and depression. Psychiatric Services, 53(8), 1001–1009. [DOI] [PubMed] [Google Scholar]
- Andersen SL (2019). Stress, sensitive periods, and substance abuse. Neurobiology of Stress, 10, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JM, Bryant KG, & Chandler LJ (2019). Inactivation of ventral hippocampus projections promotes sensitivity to changes in contingency. Learning & Memory, 26, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, and Brown GK (1996). Beck Depression Inventory: Second Edition Manual. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Stokes J, Handelsman L, Medrano M, Desmond D, & Zule W (2003). Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse & Neglect, 27, 169–190. [DOI] [PubMed] [Google Scholar]
- Bick J, Nguyen V, Leng L, Piecychna M, Crowley MJ, Bucala R, Mayes LC, & Grigorenko EL (2014). Preliminary associations between childhood neglect, MIF and cortisol: Potential pathway to long-term disease risk. Developmental Psychobiology, 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke CH, Harrell CS, & Neigh GN (2012). Stress-induced sex differences: Adaptations mediated by the glucocorticoid receptor. Hormones and Behavior, 62(3), 210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH (1997). The Psychophysics Toolbox, Spatial Vision 10:433–436. [PubMed] [Google Scholar]
- Cho YT, Ernst M, & Fudge JL (2013). Cortico-amygdala-striatal circuits are organized as hierarchical subsystems through the primate amydgala. Journal of Neuroscience, 33(35), 14017–140030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, & Williamson G (1988). Perceived stress in a probability sample of the United States In Spacapan S & Oskamp S (Eds.), The social psychology of health: Claremont Symposium on applied social psychology. Newbury Park, CA: Sage. [Google Scholar]
- Cole SW, Conti G, Arevalo JMG, Ruggiero AM, Heckman JJ, Suomi SJ. (2012). Transcriptional modulation of the developing immune system by early life social adversity. Proceedings of the National Academy of Science, 109, 20578–20583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Muir JL, Balleine BW (2001). The role of the nucleus accumbens in instrumental conditioning: Evidence of a functional dissociation between accumbens core and shell. The Journal of Neuroscience, 21(9), 3251–3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias-Ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, Cerqueira JJ, Costa RM, Sousa N (2009). Chronic stress causes frontostriatal reorganization and affects decision-making. Science, 325, 621–625. [DOI] [PubMed] [Google Scholar]
- Dickinson A (1985). Actions and habits: the development of behavioural autonomy. Philosophical Transactions of the Royal Society B: Biological Sciences, 308, 67–78. [Google Scholar]
- Dong M, Giles. WH, Felitti VJ, Dube SR, Williams JE, Chapman DP, & Anda RF (2004). Insights into causal pathways for ischemic heart disease: Adverse childhood experiences study. Circulation, 110(13), 1761–1766. [DOI] [PubMed] [Google Scholar]
- Dong M, Dube SR, Felitti VJ, Giles WH, & Anda RF (2003). Adverse childhood experiences, obesity and liver disease: New insights into the causal pathway. Archines of Internal Medicine, 163(16), 1949–1956. [DOI] [PubMed] [Google Scholar]
- Eysenck MW, Derakshan N, Santos R, & Calvo MG (2007). Anxiety and cognitive performance: Attentional control theory. Emotion, 7(2), 336–53. [DOI] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, & Marks JS (1998). Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The adverse childhood experiences (ACE) study. American Journal of Preventive Medicine, 14(4), 245–258. [DOI] [PubMed] [Google Scholar]
- Gee DG, & Casey BJ (2015). The impact of developmental timing for stress and recovery. Neurobiology of Stress, 1, 184–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillan CM, Morein-Zamir S, Urcelay GP, Sule A, Voon V, Apergis-Schoute AM, Fineberg NA, Sahakian BJ, & Robbins TW (2014). Enhanced avoidance habits in obsessive-compulsive disorder. Biological Psychiatry, 75, 631–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff B, Gee DG, Telzer EH, Humpherys KL, Gabard-Durnam L, Flannery J, & Tottenham N (2013). Reduced nucleus accumbens reactivity and adolescent depression following early-life stress. Neuroscience, 249, 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hair NL, Hanson JL, Wolfe BL, Pollak SD (2015). Association of Child Poverty, Brain Development, and Academic Achievement. JAMA Pediatrics, 169(9), 822–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Hair N, Shen DG, Shi F, Gilmore JH, Wolfe BL, & Pollak SD (2013) Family Poverty Affects the Rate of Human Infant Brain Growth. PLoS ONE, 8(12): e80954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis SD, Anda RF, Felitti VJ, Nordenberg D, & Marchbanks PA (2000). Adverse childhood experiences and sexually transmitted diseases in men and women: A retrospective study. Pediatrics, 106(1). [DOI] [PubMed] [Google Scholar]
- Hughes K, Bellis MA, Hardcastle KA, Sethi D, Butchart A, Mikton C, Jones L, & Dunne MP (2017). The effects of multiple adverse childhood experiences on health: A systematic review and meta-analysis. The Lancet Public Health, 2(8), e356–e366. [DOI] [PubMed] [Google Scholar]
- Johnson WF, Huelsnitz CO, Carlson EA, Roisman GI, Englund MM, Miller GE, & Simpson JA (2017). Childhood abuse and neglect and physical health at midlife: Prospective, longitudinal evidence. Development and Psychopathology, 29, 1936–1946. [DOI] [PubMed] [Google Scholar]
- Kaufman D, Banerji MA, Shorman I, Smith ELP, Copland JD, Rosenblum LA, & Kral JG (2007) Early-life stress and the development of obesity and insulin resistance in juvenile bonnet macaques. Diabetes, 56(5), 1382–1386. [DOI] [PubMed] [Google Scholar]
- Kessler KS, Gatz M, Gardner CO, & Pedersen NL (1996). The epidemiology of co-occuring addictive and mental disorders. The American Journal of Orthopsychiatry, 66, 17–31. [DOI] [PubMed] [Google Scholar]
- Kleiner M, Brainard D, Pelli D, 2007, “What’s new in Psychtoolbox-3?” Perception 36 ECVP Abstract Supplement. [Google Scholar]
- Knudsen EI (2004). Sensitive periods in the development of the brain and behavior. Journal of Cognitive Neuroscience, 16(8), 1412–1425. [DOI] [PubMed] [Google Scholar]
- Knutson JF, Taber SM, Murray AJ, Valles NL, & Koeppl G (2010). The role of care neglect and supervisory neglect in childhood obesity in a disadvantaged sample. Journal of Pediatric Psychology, 35, 523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman KR, Chiang JJ, Horn S, & Bower JE (2018). Developmental psychoneuroendocrine and psychoneuroimmune pathways from childhood aversity to disease. Neuroscience & Biobehavioral Reviews, 80, 166–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman KR, Geiss EG, Vargas I, Lopez-Duran NL. (2015). Differential associations between childhood trauma subtypes and adolescent HPA-axis functioning. Psychoneuroendocrinology, 54, 103–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunzler Jan & Braun Katharina & Bock Joerg. (2013). Early life stress and sex-specific sensitivity of the catecholaminergic systems in prefrontal and limbic regions of Octodon degus. Brain structure & Function, 220, 861–8. [DOI] [PubMed] [Google Scholar]
- Laurent HK, Neiderhiser JM, Natsuaki MN, Shaw DS, Fisher PA, Reiss D, Leve LD. (2014) Stress system development from age 4.5 to 6: Family environment predictors and adjustment implications of HPA activity stability versus change. Developmental Psychobiology, 56, 340–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson GM, Duda JT, Avants BB, Wu J, & Farah MJ (2013). Associations between children’s socioeconomic status and prefrontal cortical thickness. Developmental Science, 16(5), 641–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson GM, Hook CJ, & Farah MJ (2017). A meta-analysis of the relationship between socioeconomic status and executive function performance among children. Developmental Science, 21(2), 1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lex A & Hauber W (2008). Dopamine D1 and D2 receptors in the nucleus accumbens core and shell mediate Pavlovian-instrumental transfer. Learning & Memory. 15, 483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovibond PF. (1983). Facilitation of instrumental behavior by a Pavlovian appetitive conditioned stimulus. Journal of Experimental Psychology Animal Behavior Processes, 9, 225–247. [PubMed] [Google Scholar]
- Luby J, Belden A, & Botteron K (2013). The effects of Poverty on Childhood Brain Development: The mediating effects of caregiving and stressful life events. JAMA Pediatrics, 167(12), 1135–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATLAB. (2018). version 9.5 (R2018b). Natick, Massachusetts: The MathWorks Inc. [Google Scholar]
- Mehta MA, Golembo NI, Nosarti C, Colvert E, Mota A, Williams SCR, Rutter M, & Songua-Barke EJS (2009). Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: The English and Romanian adoptees study pilot. Journal of Child Psychology and Psychiatry, 50(8), 943–951. [DOI] [PubMed] [Google Scholar]
- Murschall A & Hauber W Inactivation of the ventral tegmental area abolished the general excitatory influence of Pavlovian cues on instrumental performance. Learning & Memory, 13, 123–126. [DOI] [PubMed] [Google Scholar]
- National Scientific Council on the Developing Child (2012). The Science of Neglect: The Persistent Absence of Responsive Care Disrupts the Developing Brain: Working Paper 12. http://www.developingchild.harvard.edu
- Nesvag R, Knudsen GP, Bakken IJ, Hoye A, Ystrom E, Suren P, Reneflot A, Stoltenberg C, & Reichborn-Kjennerud T (2015). Substance use disorders in schizophrenia, biolar disorder, and depression illness: A registry-based study. Social Psychiatry and Psychiatric Epidemiology, 50(8), 1267–76. [DOI] [PubMed] [Google Scholar]
- Nord CL, Lawson RP, Huys QJM, Pilling S, & Roiser JP (2018). Depression is associated with enhanced aversive Pavlovian control over instrumental behavior. Scientific Reports, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson TK, Craske MG, & Knowlton BJ (2019). Enhanced avoidance habits in relation to history of early-life stress. Frontiers in Psychology, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelli DG (1997) The Video Toolbox software for visual psychophysics: Transforming numbers into movies, Spatial Vision 10:437–442. [PubMed] [Google Scholar]
- Pignatelli AM, Wampers M, Loriedo C, Biondi M, & Vanderlinden J (2017). Childhood neglect in eating disorders: A systematic review and meta-analysis. Journal of Trauma & Dissociation, 18, 100–115. [DOI] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, & Goodwin FK (1990). Comorbidity of mental disorders with alcohol and other drug abuse: Results from the Epidemiologic Catchment Area (ECA) study. Journal of the American Medical Association, 264, 2511–2518. [PubMed] [Google Scholar]
- Rosen MS, Sheridan MA, Sambrook K, Meltzoff AN, & McLaughlin KA (2018). Socioeconomic disparities in academic achievement: A multi-modal investigation of neural mechanisms in children and adolescents. Neuroimage;173 :298–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savic I, Perski A, & Osika W (2018). MRI shows that exhaustion syndrome due to chronic occupational stress is associated with partially reversible cerebral changes. Cerebral Cortex, 28(3), 894–906. [DOI] [PubMed] [Google Scholar]
- Schoenberg HL, Sola EX, Seyller E, Kelberman M, & Toufexis DJ (2019). Female rates express habitual behavior earlier in operant training than males. Behavioral Neuroscience, 133(1), 110–120. [DOI] [PubMed] [Google Scholar]
- Schreier HMC, Kuras YI, McInnis CM, Thoma MV, St. Pierre DG, Hanlin L, Chen X, Wang D, Goldblatt D, & Rohleder N (2020). Childhood physical neglect is associated with exaggerated systemic and intracellular inflammatory responses to repeated psychosocial lstress in adulthood. Frontiers in Psychiatry, 11:504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L, Bohbot VD, and Wolf OT (2012). Prenatal stress changes learning strategies in adulthood. Hippocampus, 22(11), 2136–2143. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Oitzl MS, Philippsen C, Richter S, Bohringer A, Wippich W, & Schachinger H (2007). Stress modulates the use of spatial verus stimulus-response learning strategies in humans. Learning & Memory 14(1–2), 109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L, and Wolf OT (2009). Stress prompts habit behavior in humans. Journal of Neuroscience, 29, 7191–7198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L, and Wolf OT (2010). Socially evaluated cold pressor stress after instrumental learning favors habits over goal-directed action. Psychoneuroendocrinology 35, 977–986. [DOI] [PubMed] [Google Scholar]
- Shirayama Y & Chaki S (2006). Neurochemistry of the nucleus accumbens and its relevance to depression and antidepressant action in rodents. Curr Neuropharmacol, 4(4), 277–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snorrason I, Lee HJ, de Wit S, & Woods DW (2016). Are nonclinical obsessive-compulsive symptoms associated with bias towards habits? Psychiatry Research, 241, 221–223. [DOI] [PubMed] [Google Scholar]
- Soares JM, Sampaio A, Ferreira LM, Santos NC, Marques F, Palha JA, Cerqueira JJ, & Sousa N (2012). Stress-induced changes in human decision-making are reversible. Translational Psychiatry. 2(7), 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD (1989). State-Trait Anxiety Inventory: Bibliography (2nd ed.). Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Stevens C, Lauinger B, & Neville H (2009). Differences in the neural mechanisms of selective attention in children from different socioeconomic backgrounds: An event-related brain potential study. Developmental Science, 12(4), 634–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C, Paulsen D, Yasen A, & Neville H (2015). Atypical auditory refractory periods in children from lower socio-economic status backgrounds: ERP evidence for a role of selective attention. International Journal of Psychophysiology, 95, 156–166. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, McDonald RJ, and Savage DD (2000). Prenatal exposure to moderate levels of ethanol can have long-lasting effects on learning and memory in adult offspring. Psychobiology, 28, 532–539. [DOI] [PubMed] [Google Scholar]
- Talmi D, Seymour B, Dayan P & Dolan RJ (2008). Human Pavlovian-instrumental transfer. Journal of Neuroscience, 28, 360–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Quinn BT, McCarry TW, Nurse M, Gilhooly T, Milner A, Galvan A, Davidson MC, Eigsti IM, Thoman KM, Freed P, Booma ES, Gunnar M, Altemus M, Aronson J, & Casey BJ (2010). Prolonged institutional rearing is associated with atypically larger amygdala volume and difficulties in emotion regulation. Developmental Science, 13(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapold MA, & Overmier JB (1972). The second learning process in instrumental learning In Black AH & Prokasy WF (Eds.), Classical conditioning II: Current research and theory. New York, NY: Appleton-Century-Crofts. [Google Scholar]
- Tricomi E, Balleine BW, and O’Doherty JP (2009). A specific role for posterior dorsolateral striatum in human habit learning. European Journal of Neuroscience, 29, 2225–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson DF, Thompson TJ, Anda RF, Dietz WH, & Felitti V (2002) Body weight and obesity in adults and self-reported abuse in childhood. International Journal of Obesity, 26, 1075–1082. [DOI] [PubMed] [Google Scholar]
- Wirz L, Bogdanov M, & Schwabe L (2018). Habits under stress: Mechanistic insights across different types of learning. Current Opinion in Behavioral Science, 20, 9–16. [Google Scholar]
- Yin HH, & Knowlton BJ (2006). The role of the basal ganglia in habit formation. Nature Reviews, 7, 464–476. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, and Balleine BW (2004). Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. European Journal of Neuroscience, 19, 181–189. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, and Balleine BW (2006). Inactivation of dorsolateral striatum enhances sensitivity to changes in the action–outcome contingency in instrumental conditioning. Behavioural Brain Research, 166, 189–196. [DOI] [PubMed] [Google Scholar]
- Yin HH, Ostlund SB, & Balleine BW (2008). Reward-guided learning beyond dopamine in the nucleus accumbes: The integrative functions of cortico-basal ganglia networks. European Journal of Neuroscience, 28(8)m 1437–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Ostlund SB, Knowlton BJ, & Balleine BW (2005). The role of the dorsomedial striatum in instrumental conditioning. European Journal of Neuroscience, 22(2), 513–523. [DOI] [PubMed] [Google Scholar]
- Youssef M, Atsak P, Cardenas J, Kosmidis S, Leonardo ED, & Dranovsky A (2019). Early life stress delays hippocampal development and diminishes the adult stem cell pool in mice. Scientific Reports, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Daugherty AM, Anderson DM, Nishimura M, Brush D, Hardwick A, Lacey W, Raz S, & Ofen N (2017). Socioeconomic status and hippocampal volume in children and young adults. Developmental Science, 21(3), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.