Graphical abstract
Keywords: Haematococcus pluvialis, Phosphate concentration, Light stress, Astaxanthin, Biomass productivity, Specific light energy consumption
Highlights
-
•
Phosphate concentration and light stress notably affect astaxanthin productivity.
-
•
Combination of phosphate limitation and continuous light stress was most effective.
-
•
Combined stress increased cellular astaxanthin content up to 7 % by weight.
-
•
Specific light energy consumption was lowest under combined stress conditions.
Abstract
Nutrient composition and light stress significantly affect the productivity of astaxanthin in Haemotococcus pluvialis. Hence, the present study aimed to investigate the effect of initial phosphate concentration and two distinct light regimes on astaxanthin accumulation in H. pluvialis.
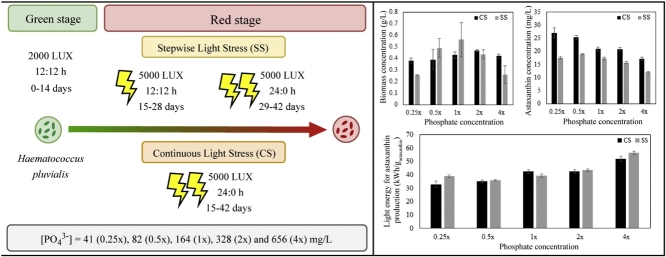
In the green stage, microalgae were cultivated in different initial phosphate concentrations under 2000 lx and a 12:12 h photoperiod. To initiate astaxanthin accumulation, an increased light intensity of 5000 lx was provided using two methods; (i) stepwise light stress, where a 12:12 h photoperiod was provided for 14 days, followed by 14 days of continuous illumination, and (ii) continuous illumination for 28 days.
Phosphate limitation and continuous light stress were favourable to enhance cellular astaxanthin accumulation, which reached 7% by weight. The highest astaxanthin concentration of 27.0 ± 1.9 mg/L and lowest specific light energy consumption of 32.9 ± 2.3 kW h/g astaxanthin were reported in cultures grown in 41 mg/L phosphate under continuous light stress.
1. Introduction
Astaxanthin is a high-value carotenoid pigment with potent antioxidant properties, enabling it to scavenge free radicals to protect cells from oxidative damage [1]. Consumption of astaxanthin as a dietary supplement imparts numerous health benefits to humans, including the improvement of eyesight, enhancement of skin health, prevention of cardiovascular disorders and amelioration of cellular function [[2], [3], [4]]. Furthermore, astaxanthin is used in therapeutic treatments due to its inherent anti-inflammatory/anti-tumoral properties as well as for the mitigation of Alzheimer’s and Parkinson’s diseases [5,6].
The green freshwater microalga Haematococcus pluvialis has been identified as the richest source of natural astaxanthin, being able to accumulate the pigment up to 1–5 % of its dry weight [7]. Astaxanthin derived from H. pluvialis has a market value of approximately $7000 per kg, and has been approved for human consumption by Food and Drug Administration (FDA) [8]. Therefore, H. pluvialis has been cultivated as a feedstock to produce natural astaxanthin for a wide range of applications in the food, pharmaceutical, nutraceutical and cosmetic industries [9].
In order to maximize the astaxanthin productivity of H. pluvialis, two-stage cultivation systems comprising of two separate photobioreactors are often employed. In the first stage (“green” stage), favourable culture conditions, i.e. optimum temperature, pH, irradiance level and media composition, are maintained to maximize biomass productivity [10]. The effective range of temperature for H. pluvialis has been reported as 25−33 °C, with higher temperatures inhibiting biomass production due to the negative effects of heat stress [9,11,12]. Similarly, H. pluvialis exhibits higher cell proliferation within the pH range of 7–8 [6,[13], [14], [15]], whilst deviations can affect the cell physiology and impede biomass growth. Furthermore, the effective range of light intensity for increased biomass production of H. pluvialis has been reported as 40–90 μmol m−2 s-1 [9,14,16]. However, light intensities lower than the optimum range can cause photolimitation, whereas higher intensities cause photoinhibition, thereby resulting in sub-optimal production of biomass [[17], [18], [19], [20]]. Among the multitude of parameters affecting microalgal growth, nutrient composition in the media is one of the most critical factors which determines biomass productivity and the duration of vegetative growth. Although nitrogen is often considered as the most crucial macronutrient for microalgal growth, phosphorus is also a critical element involved in the development of phospholipids and genetic materials, playing a key role in providing energy for cell division [21]. Tocquin et al. [22] demonstrated that a NO3-/PO43- ratio below 1 would maintain H. pluvialis cells in the vegetative state for a prolonged period, ensuring a high biomass density. Furthermore, Nahidian et al. [23] observed a 32 % increase in biomass productivity by employing Bold’s Basal Medium (BBM) modified with 3-fold higher phosphate concentration.
Upon synthesis of biomass, the second stage (“red” stage) is initiated by the provision of stress conditions such as high light intensities and nutrient starvation (i.e. nitrate and phosphate) to trigger the accumulation of astaxanthin in H. pluvialis [24]. However, the starvation of major nutrients and photoinhibition caused by high light intensities are detrimental to the vegetative growth of microalgae [25]. Nonetheless, it has been demonstrated that the limitation of phosphorus to induce astaxanthin biosynthesis is not as deleterious on the growth of H. pluvialis as compared to nitrogen limitation [24,26]. Harker et al. [26] observed that phosphorous starvation induced the accumulation of astaxanthin, whereas Boussiba and Vonshak [27] reported that high phosphate concentrations could also trigger astaxanthin synthesis. The reported contrarieties may be due to the difference in light availability which plays a significant role in the uptake and utilization of phosphorus in microalgal cells [28]. Thus, the influence of both macronutrients and light intensity should be taken into consideration when striving to enhance the productivity of biomass and astaxanthin from H. pluvialis.
Numerous studies have shown that the most significant factor which triggers astaxanthin accumulation in H. pluvialis is high levels of irradiance [18,29,30]. Nonetheless, excessive light can lead to photoinhibition caused by overloading of photosystems, as observed in numerous studies where high light stress resulted in a significant decrease in cell density [19,20]. Furthermore, the sudden shift from moderate light to continuous high light can affect the cell proliferation of H. pluvialis [31]. Thus, the detrimental effect of high light intensities on biomass density may consequently limit the overall astaxanthin concentration of cultures, despite increasing the relative proportion of astaxanthin in biomass. Additionally, if artificial lighting is employed for culture illumination, the energy intensity of the process would be increased, especially during the red stage where high light intensities and/or continuous illumination is required [32,33]. For instance, Blanken et al. [33] determined that supplying artificial lighting during the cultivation period can increase the production cost by $25.3 per kg of dry biomass whereas the preferred value is approximately $1.3.
Contrarily, astaxanthin accumulation in H. pluvialis via the use of stepwise light stress could facilitate the gradual transition of cultures into the red phase, limiting the adverse impact on biomass production [34,35]. This could essentially result in a higher final biomass concentration, albeit at a lower cellular content of astaxanthin. It is hypothesized that this method could also produce high concentrations of astaxanthin, since the lower cellular content may be compensated by the high biomass yield [13]. Furthermore, the use of stepwise light stress would lower the overall light energy consumption within a set cultivation period. Nonetheless, in order to determine the efficacy of this method for astaxanthin production, it is essential to evaluate the productivity and specific light energy consumption in comparison to the conventional process [36].
Therefore, the effect of nutrient composition of the culture media and/or light stress conditions (i.e. – source of light, intensity and photoperiod) should be evaluated to determine potential strategies to lower energy consumption during the red stage and reduce the cost of production. Thus, the main objective of the present study was to evaluate the effect of initial phosphate concentration of nutrient media and light stress (provided either stepwise or continuously) on biomass productivity and astaxanthin accumulation in H. pluvialis. To this end, H. pluvialis was cultivated under various phosphate concentrations and subjected to two different regimes of light stress induction. In the first method, the cultures at the end of the green stage were subjected to stepwise stress, where the light intensity was increased from 2000 lx to 5000 lx to induce astaxanthin accumulation under a 12:12 h photoperiod for 14 days, followed by continuous illumination at 5000 lx for 14 more days. The second method was the conventional process where continuous illumination under a high light intensity (5000 lx) was provided for the whole duration of the red stage. Thence, biomass productivity, astaxanthin accumulation and major pigment profiles were compared for each method. Furthermore, light energy consumption under both stepwise and continuous stress conditions was assessed to determine the most effective combination (i.e. initial phosphate concentration and light regime) for enhancing the astaxanthin productivity of H. pluvialis.
2. Materials and methods
2.1. Microalgal strain and pre-culture conditions
H. pluvialis UTEX 2505 was procured from the Culture Collection of Algae, University of Texas at Austin, USA. The seed culture used in the present study was grown in a 2 L Erlenmeyer flask comprising of 1.5 L BBM, consisting of the following nutrient composition [37,38]; 250.00 mg/L NaNO3, 75.00 mg/L K2HPO4, 175.00 mg/L KH2PO4, 75.00 mg/L MgSO4·7H2O, 25.00 mg/L CaCl2·2H2O, 25.00 mg/L NaCl, 50.00 mg/L Na2EDTA·2H2O, 31.00 mg/L KOH, 11.42 mg/L H3BO3, 1.44 mg/L MnCl2.4H2O, 8.82 mg/L ZnSO4.7H2O, 0.71 mg/L MoO3.2H2O, 1.57 mg/L CuSO4.5H2O, 0.49 mg/L Co(NO3)2.6H2O, 4.98 mg/L FeSO4.7H2O. The seed culture was maintained at ambient temperature (30 ± 2 °C) under a light intensity of 2000 lx on a 12:12 h light/dark cycle for 14 days.
2.2. Cultivation conditions
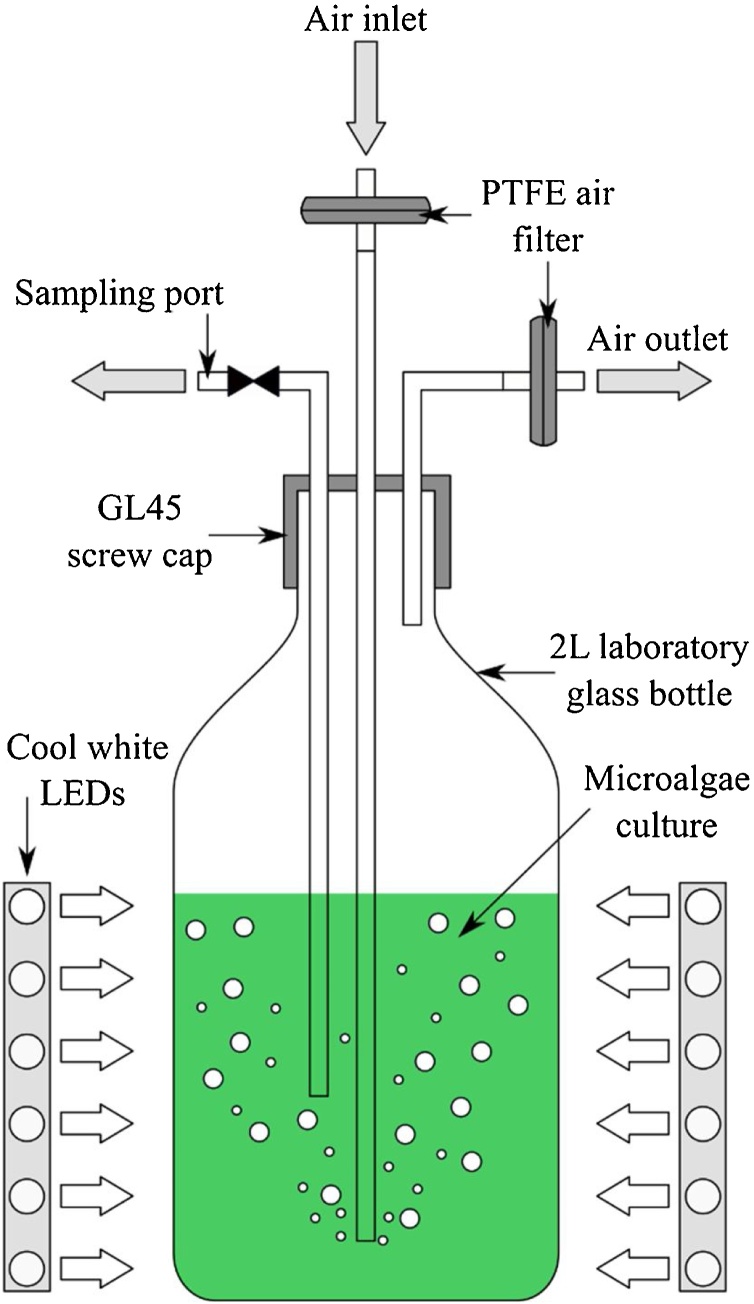
Growth experiments were performed in 2 L laboratory scale photobioreactors (Fig. 1), i.e. laboratory glass bottles (136 mm diameter) inclusive of 3-port GL45 screw caps (DURAN Screw Cap GL 45 with three ports, DWK Life Sciences GmbH, Germany) with a working volume of 1.8 L. Photobioreactors, accessories and growth media were sterilized by autoclaving at 121 °C for 20 min prior to microalgae cultivation. Microalgal cultures were aerated using atmospheric air, continuously provided through 0.22 μm polytetrafluoroethylene (PTFE) membranes at a flow rate of 1.5 L/min.
Fig. 1.
Schematic diagram of photobioreactor used for the cultivation of H. pluvialis. The photobioreactor consists of a 2 L glass bottle with GL45 screw cap embedded with ports for air inlet, air outlet and sampling. The air inlet and air outlet ports were equipped with 0.22 μm PTFE filters.
Microalgae was cultivated in batch mode for 42 days at ambient temperature (30 ± 2 °C) under artificial lighting provided by cool white LEDs (SMD 5050, Nationstar Optoelectronics Co., Ltd, China). Initially, an incident light intensity of 2000 lx was employed for vegetative growth of H. pluvialis for 14 days. Thereafter, light intensity was increased to 5000 lx to provide stress conditions for accumulation of astaxanthin; (i) by continuous illumination (CS) of cultures from day 15 onwards or (ii) by employing stepwise light stress (SS); 12:12 h light/dark cycle to induce a moderate level of stress from day 15 to day 28 followed by continuous illumination from day 29 to day 42. The light/dark regimes followed for illumination of microalgal cultures are summarized in Table 1. The incident light intensities of all cultures were measured using a photometer (LI-250A, LI-COR Biosciences, USA).
Table 1.
Conditions employed for the cultivation of H. pluvialis under stepwise light stress (SS) and continuous light stress (CS).
| Green stage | Red stage | |||
|---|---|---|---|---|
| Time (days) | 0−14 | 15−28 | 29−42 | |
| Light intensity (LUX) | 2000 | 5000 | 5000 | |
| Photoperiod | SS | 12:12 h | 12:12 h | 24:0 h |
| CS | 12:12 h | 24:0 h | 24:0 h | |
To evaluate the effect of different initial phosphate concentrations on biomass productivity and astaxanthin accumulation, microalgae was cultivated in modified BBM with phosphate concentrations of 0.25x, 0.5x, 1x, 2x and 4x; where x is the phosphate concentration of standard BBM (164 mg/L).
2.3. Analytical methods
2.3.1. Determination of microalgal growth
H. pluvialis cultures were sampled at 48 h intervals under aseptic conditions for the determination of microalgal growth. Absorbance (A) of samples was measured at 680 nm and 750 nm using a UV–vis spectrophotometer (UV-1800, Shimadzu, Japan) and the biomass concentration was calculated using Eq. (1) [39].
| (1) |
where, A680 and A750 denote the absorbance of samples at 680 nm and 750 nm respectively.
After 42 days of cultivation, biomass was harvested via centrifugation (5804 R, Eppendorf, Germany) at 5000 rpm for 10 min and stored at −20 °C until analysis.
2.3.2. Determination of astaxanthin & chlorophyll contents
Pigment extraction was performed according to the procedure described by Yeguang et al. [40]. 5 ml of dimethyl sulfoxide (DMSO) (VWR International Ltd, United Kingdom) was added to 100 mg of biomass in a 15 ml centrifuge tube. The mixture was heat treated in a 70 °C water bath for 10 min with gentle shaking at regular intervals. Subsequently, the sample was centrifuged at 4000 g for 5 min and the supernatant was collected. The extraction procedure was repeated until the supernatant became colourless. The supernatant from each extraction was pooled and diluted to 25 ml with DMSO. The extracts were further diluted by 10 times with DMSO and absorbances were measured at 530 nm, 649.1 nm and 665.1 nm using a UV–vis spectrophotometer. mg
The concentration of astaxanthin (Ca) in the extracts was calculated using Eq. (2) [40] whereas the cellular astaxanthin content was calculated using Eq. (3).
| (2) |
| (3) |
where, A530 is the absorbance of the extracts at 530 nm.
Concentrations of chlorophyll-a (Ch-a) and chlorophyll-b (Ch-b) in the extracts were determined through Eqs. (4) and (5) respectively [41].
| (4) |
| (5) |
where, A649.1 and A665.1 denote the absorbance of extracts at 649.1 nm and 665.1 nm respectively.
2.4. Determination of specific light energy consumption in astaxanthin production
The total electrical energy consumption for illumination of cultures under SS and CS over the 42-day cultivation period was calculated by considering the rated power consumption of the cool white LED strips (2.16 W at 5000 lx) as well as the photoperiod and light intensity employed for each stage of growth. Thereafter, specific light energy consumption for astaxanthin production at the end of 42 days of cultivation was calculated using Eq. (6).
| (6) |
2.5. Statistical analysis
All experiments were performed in duplicate and results were expressed as mean value ± standard deviation. Two-way analysis of variance (ANOVA) was used to determine the relative influence of variables on responses, with p ≤ 0.05 deemed to be significant. Statistical analyses were conducted using Microsoft Excel 2016 (v16.0) software.
3. Results and discussion
H. pluvialis was cultivated in different initial phosphate concentrations under 2000 lx and 12:12 h photoperiod for 14 days during the green stage. Thereafter, the light intensity was increased to 5000 lx to initiate the red stage and induce the accumulation of astaxanthin using two distinct methods as described in Table 1. In the first method, light stress was provided in a stepwise manner (SS) to alleviate the negative effect on the growth of microalgae. In the second method, stress conditions were provided with continuous illumination (CS) to maximize the cellular content of astaxanthin despite the inhibitory effect on microalgal growth. Biomass productivity, astaxanthin accumulation and chlorophyll content under each phosphate concentration and light regime were evaluated.
3.1. Effect of phosphate concentration and light stress on biomass production
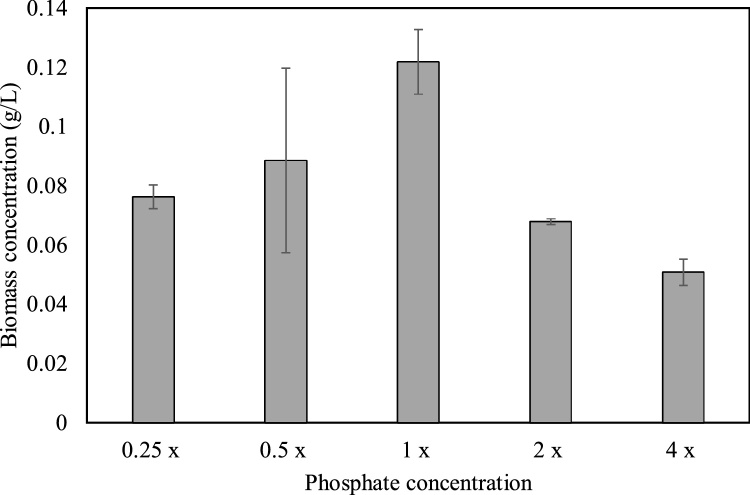
The results of the present study indicated that the initial phosphate concentration of the media had a significant impact on the biomass growth of cultures under both SS and CS (p < 0.05). As indicated in Fig. 2, the biomass concentration at the end of the green stage (after 14 days of cultivation) increased with increasing phosphate concentration up to 1x and decreased thereafter. Thus, the highest biomass concentration was observed in the 1x media (0.12 ± 0.01 g/L) whereas the lowest value was observed in the culture grown under 4x (0.05 ± 0.01 g/L). Accordingly, the initial phosphate concentration of the media had a significant influence on the biomass concentration in the green stage (p < 0.05).
Fig. 2.
Effect of initial phosphate concentration on biomass concentrations at the end of the green stage (14 days of cultivation). x denotes the concentration of phosphate in standard Bold’s Basal Medium.
In general, the biomass growth is lower in nutrient limited media as compared to nutrient replete media since macronutrients such as phosphorus are vital for the metabolism of microalgae [42,43]. Phosphorus improves the photosynthetic ability of microalgae, enhancing cell growth and proliferation [44]. Furthermore, phosphorous is crucial in the transfer of energy within cells, membrane development and biosynthesis of nucleic acids and phospholipids [45]. Thus, limited availability of phosphorous in the growth media would adversely affect cell growth, and energy otherwise used in cell division would be transferred to carotenogenesis [24,[46], [47], [48]]. Nonetheless, numerous studies have reported that high phosphate concentrations could also inhibit the growth of microalgae due to the phenomenon identified as phosphorus toxicity, as further evinced by results of the present study [49,50].
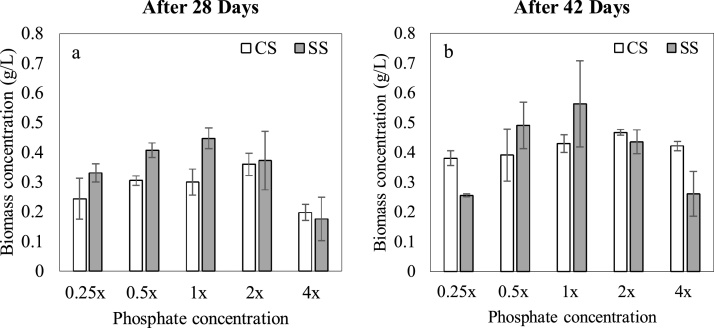
Upon 14 days of growth, the red stage was initiated by increasing the light intensity to 5000 lx and the stress conditions were induced using either CS or SS. The biomass concentration of all cultures subjected to SS showed a significant increase from day 14 to 28 under high irradiance as indicated in Fig. 3(a). This is further evinced by Supplementary Fig. 1, where all cultures under SS exhibited high growth rates as compared to the first 14 days. At the end of day 28, biomass concentrations of microalgal cultures provided with SS increased with phosphate concentration up to a maximum of 0.45 ± 0.04 g/L in the 1x culture (Fig. 3(a)). Further increment of phosphate concentration (i.e. 2x and 4x cultures) reduced biomass concentrations under SS, with the lowest values reported for the 4x culture at the end of 28 days. This is possibly due to the inhibitory effect of high phosphate concentrations on biomass growth [51].
Fig. 3.
Effect of stepwise light stress (SS) and continuous light stress (CS) on: (a) biomass concentration after 28 days, (b) biomass concentration after 42 days. x denotes the concentration of phosphate in standard Bold’s Basal Medium.
Thereafter, when continuous illumination was provided under SS (from day 29–42), biomass concentration of the 0.25x culture decreased from 0.33 ± 0.07 g/L to 0.26 ± 0.02 g/L as indicated in Fig. 3(a), (b). This could be due to cell death as a result of the combined effect of high light stress and nutrient depletion in the growth media [23]. Nonetheless, a slight increase in biomass concentration from 0.41 ± 0.02 g/L to 0.49 ± 0.07 g/L was observed in cultures under SS and 0.5x phosphate since nutrient limitation was not as severe as in 0.25 × . In contrast, biomass concentrations of 1x, 2x and 4x cultures under SS increased significantly from day 28 to 42, since the high initial phosphate concentrations enabled prolonged microalgal growth [23]. Accordingly, the highest final biomass concentration of 0.56 ± 0.14 g/L was observed in the 1x culture, whereas the lowest value was reported in 0.25x (0.26 ± 0.01 g/L).
As opposed to the cultures under SS, no distinctive variation was observed with phosphate concentration in the cultures subjected to CS upon transition from the green to the red stage. Biomass concentration of CS cultures increased from day 15–28 as indicated in Supplementary Fig. 1, even though a distinct pattern was not observed. Although the 1x culture showed the highest biomass concentration on day 14, the highest value on day 28 was exhibited by the 2x culture (0.36 ± 0.04 g/L). Furthermore, it was observed that biomass concentrations under CS were significantly lower than SS (p < 0.05) for all phosphate concentrations with the exception of 4x (Fig. 3(a)) at the end of 28 days, possibly due to the inhibitory effect on growth caused by continuous illumination; exposure to high light intensities under prolonged photoperiods exert damage to the photosynthetic apparatus of microalgae cells, inhibiting biomass growth [[52], [53], [54]]. Additionally, the growth of 4x culture under SS rapidly declined upon the provision of continuous illumination, despite the similar variation of biomass concentration under CS and SS up to day 28. This could have occurred due to the presence of vegetative cells which were sensitive to high light stress [55].
Moreover, biomass concentration under CS increased from day 29–42 for all phosphate concentrations; especially in the 4x culture where it increased remarkably from 0.20 ± 0.03 g/L to 0.42 ± 0.02 g/L. The highest final biomass concentration among cultures subjected to CS was observed in the 2x culture (0.47 ± 0.01 g/L), followed by 1x (0.43 ± 0.03 g/L). Furthermore, 2x and 4x cultures achieved higher final biomass concentrations under CS as compared to SS, indicating that CS had positively affected the biomass productivity under extreme phosphate conditions. Powell et al. [56] stated that high light availability induces rapid accumulation of acid-soluble polyphosphates (ASP), which is important for microalgal metabolism and synthesis of DNA and proteins [57]. However, high light intensities also trigger higher consumption of the synthesized ASP, which can be depleted in microalgal cells. Nonetheless, the higher availability of phosphorus in the media under CS would ensure the availability of ASP despite the high rates of consumption, which may explain the considerable growth in cultures with high phosphate concentrations under CS. On the contrary, it is plausible that the accumulation of ASP was lower under SS, thereby resulting in a lower biomass concentration, in spite of phosphate repletion. Therefore, it can be speculated that the net period of illumination supplied to a microalgal culture can affect its growth owing to differences in utilization of nutrients in the media.
Furthermore, according to Beck and Hall [51], high phosphate concentrations in photosynthetic organisms can negatively affect the uptake and metabolic activities involving several macro and micronutrients including potassium, ferrous and zinc; limiting cell growth as observed in the present study. Nonetheless, upon cell proliferation, phosphorous concentration in the media may be reduced to favourable values such that growth is increased with time as observed in 4x and 2x cultures under both CS and SS. Moreover, it was observed that biomass concentration of the 2x and 4x cultures under CS was higher than SS, possibly due to the effect of photophosphorylation [[58], [59], [60]]. Under this phenomenon, adenosine diphosphate (ADP) is converted to adenosine triphosphate (ATP) under the presence of light through the activation of photosystem II (PSII) [61]. Hence, it can be speculated that the higher net illumination period under CS causes more light energy to be transformed into chemical energy within the reaction centres of the cell. In contrast, SS conditions provide lower net illumination time, resulting in a lower amount of harvested light. This may have limited the biomass concentration of cultures exposed to SS as compared to CS, under phosphate replete conditions. Nevertheless, higher light availability (CS) may not always be beneficial for biomass growth, as evident from the lower biomass growth under 0.5x and 1x phosphate concentrations, where the effect of photoinhibition would have been more prominent. Therefore, it can be assumed that the availability of higher phosphate concentrations promoted the effect of photophosphorylation, which increased the biomass density of the culture despite the high light intensity.
3.2. The effect of phosphate concentration and light stress on chlorophyll content
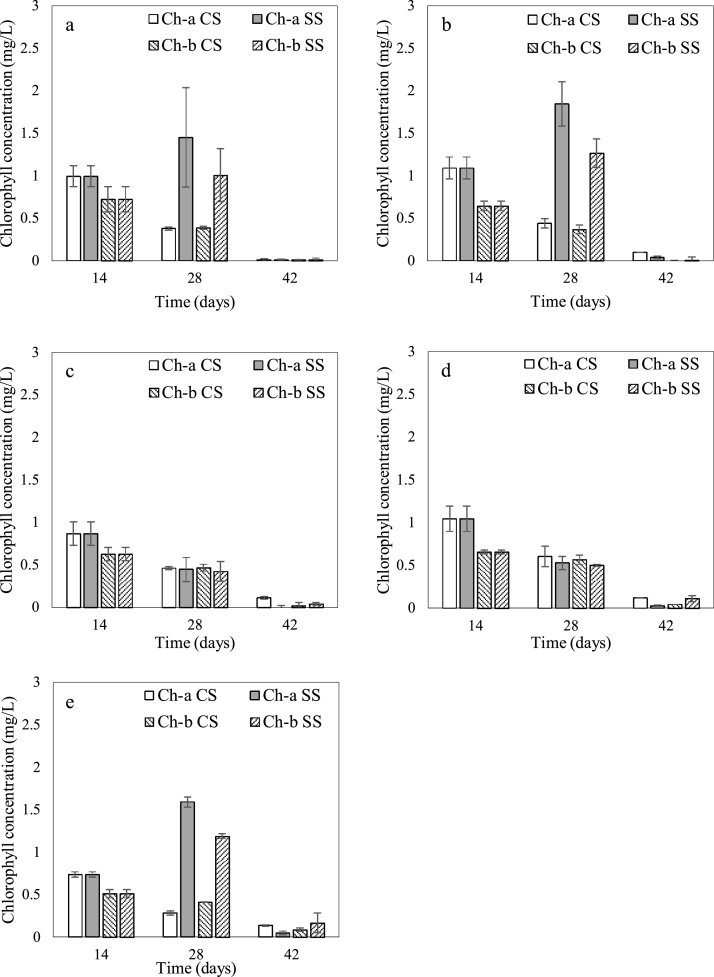
Comprehension of the pigment profile of harvested biomass would be advantageous during the production of microalgae-based astaxanthin, since the presence of contaminant pigments such as chlorophyll can lower the quality of the final extract [62]. Hence, in the present study, the effect of initial phosphate concentration on the major pigment profile on days 14, 28 and 42 was evaluated under SS and CS as represented in Fig. 4.
Fig. 4.
Variation of chlorophyll-a (Ch-a) and chlorophyll-b (Ch-b) concentrations under different initial phosphate concentrations (a) 0.25x, (b) 0.5x, (c) 1x, (d) 2x, (e) 4x (where x is the concentration of phosphate in standard Bold’s Basal Medium) and stepwise light stress (SS) or continuous light stress (CS). (Ch-a SS denotes chlorophyll-a content under stepwise stress; Ch-b SS denotes chlorophyll-b content under stepwise stress; Ch-a CS denotes chlorophyll-a content under continuous stress; Ch-b CS denotes chlorophyll-b content under continuous stress).
According to Fig. 4, the chlorophyll concentration increased from day 14 to 28 in 0.25x, 0.5x and 4x cultures subjected to SS. Nonetheless, chlorophyll levels of 1x and 2x declined under the high light intensity, which suggested a complex relationship between chlorophyll and phosphorus concentration under SS. According to the variation of chlorophyll content in 0.25x, 0.5x and 4x cultures under SS, it can be deduced that the cells were predominantly in the green stage within 14–28 days. On the contrary, the chlorophyll concentration in 1x and 2x cultures have declined from day 14 to 28, indicating that the cells were transitioning from the green stage to the red stage. At this intermediate stage, cells can be observed in a greenish orange colour resulting from the presence of both chlorophyll and carotenoids (primarily astaxanthin) [14,26]. Nonetheless, upon the supply of continuous light from day 29 onwards, both Ch-a and Ch-b decreased under all phosphate concentrations with the final chlorophyll concentrations (on day 42) approaching zero. At this stage, the carotenoids in the cell primarily comprise of astaxanthin (approximately 80–99 % of total carotenoids), resulting in a deep red colour [14].
As indicated by Fig. 4, both Ch-a and Ch-b concentrations of cultures grown under CS declined from day 14 onwards. Accordingly, statistical analysis indicated a significant difference between chlorophyll concentrations on day 14 and day 28 as well as between day 14 and day 42 (p < 0.05). This behaviour could be attributed to the higher light quantity incident on CS cultures owing to the longer net illumination time. Thus, at the final day of cultivation chlorophyll levels under all phosphate concentrations approached zero. In general, a marked decline in chlorophyll content was observed upon the provision of continuous light in cultures subjected to both SS and CS, indicating that continuous illumination resulted in the degradation of chlorophylls [63].
Results of the statistical analysis indicate that phosphate concentration imposed a significant impact on the concentrations of both Ch-a and Ch-b (p < 0.05). As depicted in Fig. 4, the highest Ch-a content was observed in the 0.5x culture (1.09 ± 0.13 mg/L) whereas 4x media exhibited the lowest value (0.74 ± 0.03 mg/L) at the end of the green stage (day 14). Ch-b levels were lower than Ch-a for all phosphate concentrations, with the highest and lowest concentrations of 0.72 ± 0.15 mg/L and 0.51 ± 0.05 mg/L observed in the 0.25x and 4x cultures respectively. Nahidian et al. [23] have reported a similar observation for phosphorous limited cultures, where chlorophyll concentration increased when phosphate content of the media was halved at a fixed nitrate concentration. Despite the lack of an obvious pattern, it was noteworthy that the chlorophyll content was lowest under the highest phosphate concentration. Nonetheless, the phosphorus toxicity at high concentrations could limit the biomass growth as discussed in Section 3.1, which also lowers the overall chlorophyll concentration of the culture [21].
The effects of light intensity and photoperiod on microalgal growth have been studied extensively [[64], [65], [66], [67], [68]]. In accordance, several researchers have observed that extensive increase of light intensity can decrease the chlorophyll content in green microalgae [[69], [70], [71]]. The results of the present study support these observations as evident by Fig. 4, where the decline in chlorophyll content of cultures upon prolonged exposure to the increased light intensity of 5000 lx is clearly demonstrated. In addition to the light intensity, the photoperiod also has a considerable effect on the pigment content of microalgae. As reported by George et al. [72], the chlorophyll concentration of Ankistrodesmus falcatus was higher in 12:12 h photoperiod than under continuous illumination. Accordingly, in the present study, the chlorophyll concentrations of 0.25x, 0.5x and 4x cultures which increased up to day 28 under SS, declined upon the provision of continuous light from day 29–42.
In the case of H. pluvialis, stress conditions including high light intensities and nutrient limitation has been found to increase the ratio of carotenoids to chlorophylls [73]. Aflalo et al. [74] stated that as H. pluvialis undergoes transition from the green vegetative stage to the red aplanospore stage, chlorophyll contents drop drastically. The ratio of carotenoids to chlorophylls is around 0.2 in the green stage and increases up to 2–9 in the red stage [14]. Similarly, as shown in Fig. 4, Ch-a and Ch-b declined upon the provision of light induced stress conditions whereas the astaxanthin content escalated considerably (Fig. 5(a), (b), (c) and (d)).
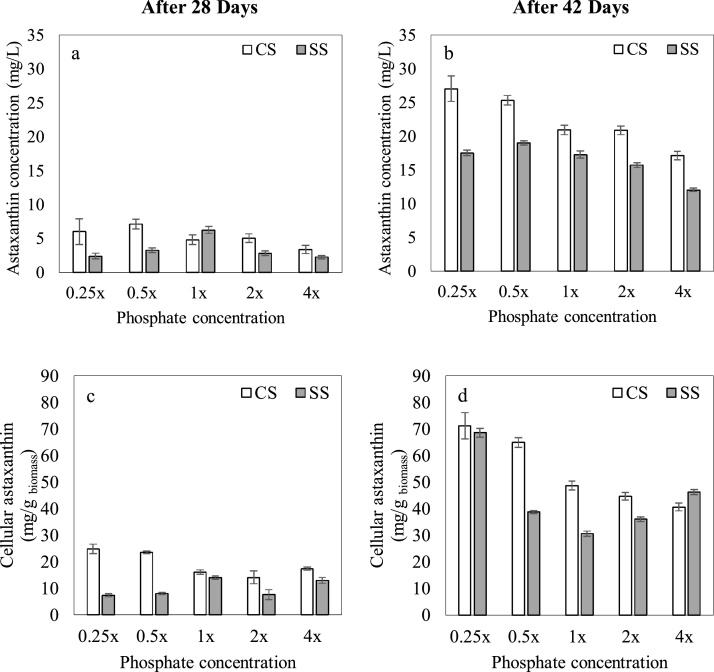
Fig. 5.
Effect of stepwise light stress (SS) and continuous light stress (CS) on: (a) astaxanthin concentration after 28 days, (b) astaxanthin concentration after 42 days, (c) cellular astaxanthin content after 28 days, and (d) cellular astaxanthin content after 42 days. x denotes the concentration of phosphate in standard Bold’s Basal Medium.
3.3. Effect of phosphate concentration and light stress on astaxanthin concentration
Fig. 5(a) and (b) represent the astaxanthin concentration of cultures on day 28 and day 42 respectively, whereas the variation of astaxanthin concentration upon provision of stress conditions is indicated in Supplementary Fig. 2.
Upon 28 days of cultivation, the astaxanthin concentration of cultures under SS increased significantly (p < 0.05) with increasing phosphate concentrations up to 1x (6.3 ± 0.3 mg/L) and declined thereafter. A similar pattern was observed for the variation of biomass concentration under SS, on day 28. Thus, it is evident that the astaxanthin concentration of cultures (at the end of 28 days of cultivation under SS) was more significantly affected by the biomass concentration of cultures as compared to the cellular astaxanthin content. This is further evinced by Fig. 5(c) where the cellular astaxanthin content under SS does not vary considerably with phosphate concentration.
On the contrary, the astaxanthin concentration of H. pluvialis cultivated under CS indicated no distinct pattern with the biomass concentration ((Figs. 3(a) and 5 (a)). Accordingly, the highest astaxanthin concentration was in the 0.5x culture due to its high cellular content of astaxanthin (Fig. 5(c)) despite not having the highest biomass concentration on day 28 under CS. The cellular content of astaxanthin on day 28 under CS was considerably higher than SS for both 0.25x and 0.5x cultures, possibly due to the combined effect of light stress and phosphorus limited media. Moreover, it was observed that the difference between the cellular content of astaxanthin in 1x, 2x and 4x cultures under CS and SS was not as prominent as in 0.25x and 0.5x cultures.
Astaxanthin concentration of all cultures increased from day 28 to 42, as evident in Fig. 5(a) and (b). The final astaxanthin concentrations of cultures under CS were significantly higher (p < 0.05) than SS for all phosphate concentrations. Moreover, it was observed that upon transition to continuous illumination, the cellular astaxanthin content of all cultures (CS and SS) increased significantly (p < 0.05). The final astaxanthin concentration of the cultures under CS increased with decreasing phosphate concentrations, with the maximum value of 27.0 ± 1.9 mg/L observed in 0.25 × . However, under SS, the final astaxanthin concentration was highest in the 0.5x culture (19.0 ± 0.3 mg/L) whilst 0.25x exhibited a lower value (17.5 ± 0.4 mg/L) due to the lower biomass concentration of the culture media. It was noteworthy that under both CS and SS, the final cellular astaxanthin contents in the 0.25x cultures were similar (71.2 ± 5.0 mg/g and 68.6 ± 1.6 mg/g respectively) despite the disparity on day 28. This is approximately 7 % by weight as compared to 4–5 % reported in the literature, indicating the phosphate depletion up to 0.25x coupled with high light intensity was a suitable strategy to increase astaxanthin productivity of H. pluvialis [[75], [76], [77]].
Stress conditions such as nutrient starvation, trigger the synthesis of carotenoids in microalgae, ensuring the survival of cells in nutrient depleted media [78]. According to Boussiba and Vonshak [27], phosphorus limitation triggers the synthesis of astaxanthin in H. pluvialis cells, similar to the effect of nitrogen depletion. Furthermore, it has been reported that the adverse effect of phosphorus limitation is not as significant as nitrogen limitation, which could be advantageous in simultaneously increasing both biomass and astaxanthin concentrations, thereby enhancing astaxanthin productivity [26]. Therefore, in the present study, it is evident that phosphorus limitation has served as a stress condition for astaxanthin accumulation, as higher concentrations of astaxanthin were observed in phosphorous limited media. The increased accumulation of astaxanthin can be identified as an adaptive mechanism to nutrient stress caused by low initial phosphate concentrations in the media, which continue to deplete with biomass growth [[79], [80], [81]].
Numerous studies have reported that the accumulation of astaxanthin in H. pluvialis is highly affected by light intensity, with vegetative cells rapidly transitioning into astaxanthin accumulating aplanospores upon the introduction of high light intensities and/or continuous illumination [66,82,83]. Therefore, high light intensity is considered as the main factor for the induction of astaxanthin biosynthesis [84]. It has been suggested that the generation of reactive oxygen species under high light intensities is the phenomenon which triggers the synthesis of astaxanthin in H. pluvialis [29,85]. Carotenoids such as astaxanthin protect photosynthetic organisms from damage caused by high irradiance through quenching of reactive oxygen and triplet chlorophyll [[86], [87], [88]]. According to Ben-Amotz et al. [89], carotenoids screen excessive blue light under high irradiation which can damage the antenna chlorophylls. Regardless of the exact mechanism which stimulates the biosynthesis of astaxanthin, it is evident that high light intensities and prolonged photoperiods are favourable for increasing the astaxanthin concentration of H. pluvialis cultures. The results of the present study further validate this by exhibiting higher astaxanthin accumulation under CS as compared to SS. In contrast to CS, the quantity of light provided for the SS cultures was lower, which yielded a lower concentration of astaxanthin as evident by the results of the present study. According to Kobayashi et al. [18], the quantity of light (light intensity × net illumination time) is the primary factor which triggers astaxanthin synthesis. Therefore, it can be assumed that under SS, the final astaxanthin concentration was lower owing to the shorter photoperiod employed during day 14 to day 28 in the second stage of the process. Although it was anticipated that SS would alleviate biomass losses due to severe light stress, the desired increment of overall astaxanthin concentration was not achieved. Similarly, Fabregas et al. [90] and Wayama et al. [91] remarked that continuous illumination is more favourable for astaxanthin formation as compared to the use of light/dark cycles. In accordance, lower astaxanthin content was achieved under SS condition as compared to CS during the present study, further evincing the effect of continuous illumination on astaxanthin synthesis.
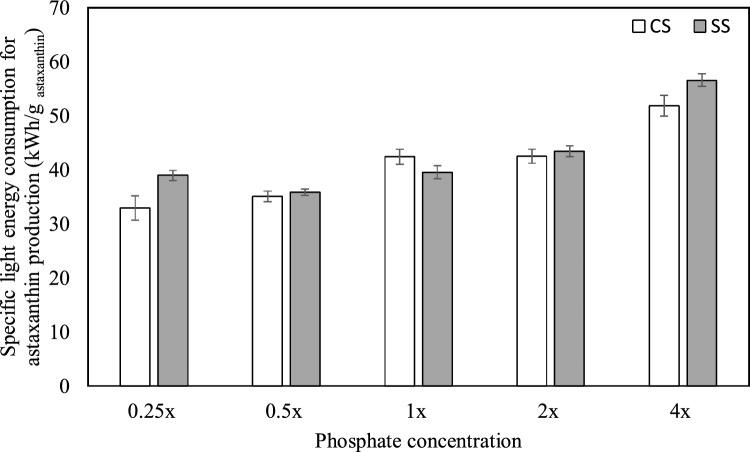
3.4. Specific light energy consumption for astaxanthin production
Carotenogenesis is triggered in H. pluvialis upon exposure to higher light intensities and/or prolonged photoperiods [14]. Hence, it is vital to assess the energy consumption for lighting in order to identify the most effective strategy for astaxanthin production in H. pluvialis. The total consumption of light energy over the 42-day cultivation period was lower under SS (1.23 kW h) as compared to CS (1.60 kW h) due to the difference in the photoperiods during the red stage.
According to Fig. 6, the lowest specific light energy consumption (i.e. – light energy required for the production of 1 g of astaxanthin) of 32.9 ± 2.3 kW h/g astaxanthin was observed in the 0.25x culture under CS. Despite the higher light energy consumption over the 42-day cultivation period, the specific light energy consumption was generally lower in cultures grown under CS in comparison to SS at corresponding phosphate concentrations due to the higher astaxanthin content of CS cultures. A notable exception to this pattern was observed in 1x media, where the specific energy consumption was lower in the SS culture (39.6 ± 1.2 kW h/g astaxanthin) than in the corresponding culture grown under CS (42.4 ± 1.4 kW h/g astaxanthin). This is because there is no considerable difference in astaxanthin concentration of 1x cultures under SS and CS (17.3 ± 0.6 mg/L and 21.0 ± 0.7 mg/L respectively) as compared to other phosphate concentrations.
Fig. 6.
Specific light energy consumption for astaxanthin production under different initial phosphate concentrations and stepwise light stress (SS) or continuous light stress (CS). x denotes the concentration of phosphate in standard Bold’s Basal Medium.
Furthermore, the lowest specific light energy consumption under SS was 35.9 ± 0.6 kW h/g astaxanthin in the 0.5x culture, which is approximately 9% higher than the lowest value reported in the current study. Hence, through further optimization of astaxanthin productivity in phosphate limited cultures under SS, there is potential to lower the specific light energy consumption to be on par with phosphate limited CS cultures.
Nonetheless, the light source, photobioreactor design and growth conditions employed have a crucial role in determining the specific light energy consumption in astaxanthin production. Wong et al. [66] employed red LEDs, blue LEDs and white plasma lights to analyze the effect of different light sources and photoperiods on the astaxanthin accumulation of H. pluvialis cultivated in 6 L lab-scale photobioreactors. The lowest specific light energy consumption of approximately 46.11 kW h/g astaxanthin was observed in cultures subjected to continuous illumination with red LEDs. Pérez-López et al. [92] have reported specific light energy consumption of 68.38 kW h/g astaxanthin during the cultivation of H. pluvialis in a 15 L tubular airlift photobioreactor illuminated with 36 W fluorescent bulbs. The values thus reported are markedly higher than the lowest specific light energy consumption observed in the present study (32.9 ± 2.3 kW h/g astaxanthin). Therefore, it is evident that the specific light energy consumption could be further reduced by optimization of microalgae cultivation.
Moreover, the most suitable combination of initial phosphate concentration and light regime should be selected on the basis of a comprehensive techno-economic analysis of the entire process, as the specific energy consumption for the downstream processing steps of harvesting, drying, cell disruption and astaxanthin extraction would vary with the biomass concentration of cultures and the cellular astaxanthin content [6].
4. Conclusions
In the present study, the highest astaxanthin productivity was exhibited by cultures grown under phosphate limitation (41 mg/L; 0.25x of the standard BBM scale) and subjected to CS. The high product yields were achieved due to the superior cellular content of astaxanthin which approached values as high as 7 % by weight, thereby compensating for the comparatively lower biomass productivity under the aforementioned conditions. Furthermore, the specific light energy consumption for astaxanthin production was the lowest (32.9 ± 2.3 kW h/g astaxanthin) in phosphate-limited cultures (0.25x) subjected to CS. In this context, it is evident that the combined stress of phosphate limitation and CS was the most effective combination used in this study to enhance the productivity of astaxanthin in H. pluvialis. Thus, it is plausible that energy-efficient production of natural astaxanthin could be performed through the utilization of phosphate limitation and CS. Moreover, results further indicated that SS could be optimized to lower the specific energy consumption to be competitive with CS, ultimately reducing the overall energy consumption. Hence, future studies should aim to further improve media composition and light regime to achieve a compromise between biomass production, astaxanthin accumulation and light energy consumption. Nonetheless, comprehensive techno-economic feasibility studies and life cycle assessment of both alternatives in the pilot-scale (inclusive of downstream processes) is essential to identify the most effective technique of improving astaxanthin productivity.
CRediT authorship contribution statement
Vinoj Chamilka Liyanaarachchi: Formal analysis, Investigation, Writing - original draft. Gannoru Kankanamalage Sanuji Hasara Nishshanka: Formal analysis, Writing - review & editing. Rankoth Gedara Malith Malsha Premaratne: Formal analysis, Writing - review & editing. Thilini Udayangani Ariyadasa: Conceptualization, Methodology, Supervision, Writing - review & editing, Project administration, Funding acquisition. Pemaththu Hewa Viraj Nimarshana: Conceptualization, Visualization, Writing - review & editing. Anushree Malik: Writing - review & editing, Formal analysis.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgement
This work was supported by the Ministry of Science, Technology and Research, Sri Lanka [grant number: MSTR/TRD/AGR/3/2/18]; and open access publishing support was provided by the Senate Research Committee, University of Moratuwa, Sri Lanka.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.btre.2020.e00538.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Gong M., Bassi A. Carotenoids from microalgae: a review of recent developments. Biotechnol. Adv. 2016;34:1396–1412. doi: 10.1016/j.biotechadv.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Guerin M., Huntley M.E., Olaizola M. Haematococcus astaxanthin: applications for human health and nutrition. Trends Biotechnol. 2003;21:210–216. doi: 10.1016/S0167-7799(03)00078-7. [DOI] [PubMed] [Google Scholar]
- 3.Higuera-Ciapara I., Félix-Valenzuela L., Goycoolea F.M. Astaxanthin: a review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006;46:185–196. doi: 10.1080/10408690590957188. [DOI] [PubMed] [Google Scholar]
- 4.Davinelli S., Nielsen M., Scapagnini G. Astaxanthin in skin health, repair, and disease: a comprehensive review. Nutrients. 2018;10:522. doi: 10.3390/nu10040522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rammuni M.N., Ariyadasa T.U., Nimarshana P.H.V., Attalage R.A. Comparative assessment on the extraction of carotenoids from microalgal sources: astaxanthin from H. pluvialis and β-carotene from D. salina. Food Chem. 2019;277:128–134. doi: 10.1016/j.foodchem.2018.10.066. [DOI] [PubMed] [Google Scholar]
- 6.Panis G., Carreon J.R. Commercial astaxanthin production derived by green alga Haematococcus pluvialis: a microalgae process model and a techno-economic assessment all through production line. Algal Res. 2016;18:175–190. doi: 10.1016/j.algal.2016.06.007. [DOI] [Google Scholar]
- 7.Wan M., Hou D., Li Y., Fan J., Huang J., Liang S., Wang W., Pan R., Wang J., Li S. The effective photoinduction of Haematococcus pluvialis for accumulating astaxanthin with attached cultivation. Bioresour. Technol. 2014;163:26–32. doi: 10.1016/j.biortech.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 8.Hu J., Nagarajan D., Zhang Q., Chang J.S., Lee D.J. Heterotrophic cultivation of microalgae for pigment production: a review. Biotechnol. Adv. 2018;36:54–67. doi: 10.1016/j.biotechadv.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Khoo K.S., Lee S.Y., Ooi C.W., Fu X., Miao X., Ling T.C., Show P.L. Recent advances in biorefinery of astaxanthin from Haematococcus pluvialis. Bioresour. Technol. 2019;288 doi: 10.1016/j.biortech.2019.121606. [DOI] [PubMed] [Google Scholar]
- 10.García-Malea M.C., Acién F.G., Del Río E., Fernández J.M., Cerón M.C., Guerrero M.G., Molina-Grima E. Production of astaxanthin by Haematococcus pluvialis: taking the one-step system outdoors. Biotechnol. Bioeng. 2009;102:651–657. doi: 10.1002/bit.22076. [DOI] [PubMed] [Google Scholar]
- 11.Hong M.E., Hwang S.K., Chang W.S., Kim B.W., Lee J., Sim S.J. Enhanced autotrophic astaxanthin production from Haematococcus pluvialis under high temperature via heat stress-driven Haber–Weiss reaction. Appl. Microbiol. Biotechnol. 2015;99:5203–5215. doi: 10.1007/s00253-015-6440-5. [DOI] [PubMed] [Google Scholar]
- 12.Wan M., Zhang J., Hou D., Fan J., Li Y., Huang J., Wang J. The effect of temperature on cell growth and astaxanthin accumulation of Haematococcus pluvialis during a light – dark cyclic cultivation. Bioresour. Technol. 2014;167:276–283. doi: 10.1016/j.biortech.2014.06.030. [DOI] [PubMed] [Google Scholar]
- 13.Sarada R., Tripathi U., Ravishankar G. Influence of stress on astaxanthin production in Haematococcus pluvialis grown under different culture conditions. Process Biochem. 2002;37:623–627. doi: 10.1016/S0032-9592(01)00246-1. [DOI] [Google Scholar]
- 14.Shah M.M.R., Liang Y., Cheng J.J., Daroch M. Astaxanthin-producing green microalga Haematococcus pluvialis: from single cell to high value commercial products. Front. Plant Sci. 2016;7:1–28. doi: 10.3389/fpls.2016.00531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hwang S.-W., Il Choi H., Sim S.J. Acidic cultivation of Haematococcus pluvialis for improved astaxanthin production in the presence of a lethal fungus. Bioresour. Technol. 2019;278:138–144. doi: 10.1016/j.biortech.2019.01.080. [DOI] [PubMed] [Google Scholar]
- 16.Park J.C., Choi S.P., Hong M.-E., Sim S.J. Enhanced astaxanthin production from microalga, Haematococcus pluvialis by two-stage perfusion culture with stepwise light irradiation. Bioprocess Biosyst. Eng. 2014;37:2039–2047. doi: 10.1007/s00449-014-1180-y. [DOI] [PubMed] [Google Scholar]
- 17.Zhang L., Su F., Zhang C., Gong F., Liu J. Changes of photosynthetic behaviors and photoprotection during cell transformation and astaxanthin accumulation in haematococcus pluvialis grown outdoors in tubular photobioreactors. Int. J. Mol. Sci. 2017;18:1–14. doi: 10.3390/ijms18010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobayashi M., Kakizono T., Nishio N., Nagai S. Effects of light intensity, light quality, and illumination cycle on astaxanthin formation in a green alga, Haematococcus pluvialis. J. Ferment. Bioeng. 1992;74:61–63. doi: 10.1016/0922-338X(92)90271-U. [DOI] [Google Scholar]
- 19.Wang J., Han D., Sommerfeld M.R., Lu C., Hu Q. Effect of initial biomass density on growth and astaxanthin production of Haematococcus pluvialis in an outdoor photobioreactor. J. Appl. Phycol. 2013;25:253–260. doi: 10.1007/s10811-012-9859-4. [DOI] [Google Scholar]
- 20.Gu W., Li H., Zhao P., Yu R., Pan G., Gao S., Xie X., Huang A., He L., Wang G. Quantitative proteomic analysis of thylakoid from two microalgae (Haematococcus pluvialis and Dunaliella salina) reveals two different high light-responsive strategies. Sci. Rep. 2014;4 doi: 10.1038/srep06661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu L., Li Q., Yan G., Zhou D., Crittenden J.C. Hormesis effects of phosphorus on the viability of Chlorella regularis cells under nitrogen limitation. Biotechnol. Biofuels. 2019;12:121. doi: 10.1186/s13068-019-1458-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tocquin P., Fratamico A., Franck F. Screening for a low-cost Haematococcus pluvialis medium reveals an unexpected impact of a low N/P ratio on vegetative growth. J. Appl. Phycol. 2012;24:365–373. doi: 10.1007/s10811-011-9771-3. [DOI] [Google Scholar]
- 23.Nahidian B., Ghanati F., Shahbazi M., Soltani N. Effect of nutrients on the growth and physiological features of newly isolated Haematococcus pluvialis TMU. Bioresour. Technol. 2018;255:229–237. doi: 10.1016/j.biortech.2018.01.130. [DOI] [PubMed] [Google Scholar]
- 24.Minhas A.K., Hodgson P., Barrow C.J., Adholeya A. A review on the assessment of stress conditions for simultaneous production of microalgal lipids and carotenoids. Front. Microbiol. 2016;7 doi: 10.3389/fmicb.2016.00546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang B.Y., Geng Y.H., Li Z.K., Hu H.J., Li Y.G. Production of astaxanthin from Haematococcus in open pond by two-stage growth one-step process. Aquaculture. 2009;295:275–281. doi: 10.1016/j.aquaculture.2009.06.043. [DOI] [Google Scholar]
- 26.Harker M., Tsavalos A.J., Young A.J. Factors responsible for astaxanthin formation in the Chlorophyte Haematococcus pluvialis. Bioresour. Technol. 1996;55:207–214. doi: 10.1016/0960-8524(95)00002-X. [DOI] [Google Scholar]
- 27.Boussiba S., Vonshak A. Astaxanthin accumulation in the green alga Haematococcus pluvialis1. Plant Cell Physiol. 1991;32:1077–1082. doi: 10.1093/oxfordjournals.pcp.a078171. [DOI] [Google Scholar]
- 28.Al-Qasmi M., Raut N., Talebi S., Al-Rajhi S., Al-Barwani T. World Congr. Eng. 2012. A review of effect of light on microalgae growth. [Google Scholar]
- 29.Fan L., Vonshak A., Zarka A., Boussiba S. Does astaxanthin protect haematococcus against light damage? Zeitschrift Für Naturforsch. C. 1998;53:93–100. doi: 10.1515/znc-1998-1-217. [DOI] [PubMed] [Google Scholar]
- 30.Doria E., E.Temporiti M.E., Damiani M.C., Popo-Vich C.A., Leonardi P.I., Nielsen E. Influence of light stress on the accumulation of xanthophylls and lipids in Haematococcus Pluvialis CCALA 1081 grown under autotrophic or mixotrophic conditions. J. Mar. Biol. Aquac. 2018;4:30–35. doi: 10.15436/2381-0750.18.1799. [DOI] [Google Scholar]
- 31.Domínguez A., Pereira S., Otero A. Does Haematococcus pluvialis need to sleep? Algal Res. 2019;44 doi: 10.1016/j.algal.2019.101722. [DOI] [Google Scholar]
- 32.Scharff C., Domurath N., Wensch-Dorendorf M., Schröder F.-G. Cultivation of microalgae in closed systems under artificial lighting. Acta Hortic. 2017:1157–1164. doi: 10.17660/ActaHortic.2017.1170.149. [DOI] [Google Scholar]
- 33.Blanken W., Cuaresma M., Wijffels R.H., Janssen M. Cultivation of microalgae on artificial light comes at a cost. Algal Res. 2013;2:333–340. doi: 10.1016/j.algal.2013.09.004. [DOI] [Google Scholar]
- 34.Han D., Wang J., Sommerfeld M., Hu Q. Susceptibility and protective mechanisms of motile and non motile cells of Haematococcus pluvialis (chlorophyceae) to photooxidative stress. J. Phycol. 2012;48:693–705. doi: 10.1111/j.1529-8817.2012.01147.x. [DOI] [PubMed] [Google Scholar]
- 35.Li Y., Sommerfeld M., Chen F., Hu Q. Consumption of oxygen by astaxanthin biosynthesis: a protective mechanism against oxidative stress in Haematococcus pluvialis (chlorophyceae) J. Plant Physiol. 2008;165:1783–1797. doi: 10.1016/j.jplph.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 36.Goksan T., Ak I., Kilic C. Growth characteristics of the alga Haematococcus pluvialis flotow as affected by nitrogen source, vitamin, light and aeration. Turk. J. Fish. Aquat. Sci. 2011;11:31–36. doi: 10.4194/1303-2712-v11_3_06. [DOI] [Google Scholar]
- 37.Bold H.C. The morphology of chlamydomonas chlamydogama, sp. nov. Bull. Torrey Bot. Club. 1949;76:101. doi: 10.2307/2482218. [DOI] [Google Scholar]
- 38.Bischoff H.W., Bold H.C. Phycologic, University of Texas; Austin, Texas: 1963. Some Soil Algae from Enchanted Rock and Related Algal Species. [Google Scholar]
- 39.Sun H., Kong Q., Geng Z., Duan L., Yang M., Guan B. Enhancement of cell biomass and cell activity of astaxanthin-rich Haematococcus pluvialis. Bioresour. Technol. 2015;186:67–73. doi: 10.1016/j.biortech.2015.02.101. [DOI] [PubMed] [Google Scholar]
- 40.Li Y., Miao F., Geng Y., Lu D., Zhang C., Zeng M. Accurate quantification of astaxanthin from Haematococcus crude extract spectrophotometrically. Chin. J. Oceanol. Limnol. 2012;30:627–637. doi: 10.1007/s00343-012-1217-5. [DOI] [Google Scholar]
- 41.Sumanta N., Imranul Haque C., Nishika J., Suprakash R. Spectrophotometric analysis of chlorophylls and carotenoids from commonly grown fern species by using various extracting solvents. Res. J. Chem. Sci. 2014;4:63–69. doi: 10.1055/s-0033-1340072. [DOI] [Google Scholar]
- 42.Martínez M.E., Jiménez J.M., El Yousfi F. Influence of phosphorus concentration and temperature on growth and phosphorus uptake by the microalga Scenedesmus obliquus. Bioresour. Technol. 1999;67:233–240. doi: 10.1016/S0960-8524(98)00120-5. [DOI] [Google Scholar]
- 43.Ding D., Chen S., Peng S., Jiang C., Zheng L., Li J. Strategies of phosphorus utilization in an astaxanthin-producing green alga Haematococcus pluvialis, a comparison with a bloom-forming cyanobacterium Microcystis wesenbergii. Aquat. Ecol. 2019;53:679–688. doi: 10.1007/s10452-019-09718-z. [DOI] [Google Scholar]
- 44.Belotti G., de Caprariis B., De Filippis P., Scarsella M., Verdone N. Effect of Chlorella vulgaris growing conditions on bio-oil production via fast pyrolysis. Biomass Bioenergy. 2014;61:187–195. doi: 10.1016/j.biombioe.2013.12.011. [DOI] [Google Scholar]
- 45.Mühlroth A., Winge P., El Assimi A., Jouhet J., Maréchal E., Hohmann-Marriott M.F., Vadstein O., Bones A.M. Mechanisms of phosphorus acquisition and lipid class remodeling under P limitation in a marine microalga. Plant Physiol. 2017;175:1543–1559. doi: 10.1104/pp.17.00621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma R., Li Y., Lu Y. Sequencing and characterization of novel PII signaling protein gene in microalga Haematococcus pluvialis. Mar. Drugs. 2017;15:304. doi: 10.3390/md15100304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun X.-M., Ren L.-J., Zhao Q.-Y., Ji X.-J., Huang H. Microalgae for the production of lipid and carotenoids: a review with focus on stress regulation and adaptation. Biotechnol. Biofuels. 2018;11:272. doi: 10.1186/s13068-018-1275-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Faraloni C., Torzillo G. Carotenoids. InTech; 2017. Synthesis of antioxidant carotenoids in microalgae in response to physiological stress. [DOI] [Google Scholar]
- 49.Kang C.D., Han S.J., Choi S.P., Sim S.J. Fed-batch culture of astaxanthin-rich Haematococcus pluvialis by exponential nutrient feeding and stepwise light supplementation. Bioprocess Biosyst. Eng. 2010;33:133–139. doi: 10.1007/s00449-009-0362-5. [DOI] [PubMed] [Google Scholar]
- 50.Abd Elfata R., Wagih Abou G. Influence of various concentrations of phosphorus on the antibacterial, antioxidant and bioactive components of green microalgae Scenedesmus obliquus. Int. J. Pharmacol. 2017;14:99–107. doi: 10.3923/ijp.2018.99.107. [DOI] [Google Scholar]
- 51.Beck W.S., Hall E.K. Confounding factors in algal phosphorus limitation experiments. PLoS One. 2018;13 doi: 10.1371/journal.pone.0205684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu Y., Ibrahim I.M., Harvey P.J. The influence of photoperiod and light intensity on the growth and photosynthesis of Dunaliella salina (chlorophyta) CCAP 19/30. Plant Physiol. Biochem. 2016;106:305–315. doi: 10.1016/j.plaphy.2016.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baroli I., Melis A. Photoinhibitory damage is modulated by the rate of photosynthesis and by the photosystem II light-harvesting chlorophyll antenna size. Planta. 1998;205:288–296. doi: 10.1007/s004250050323. [DOI] [PubMed] [Google Scholar]
- 54.Lamers P.P., Martens D.E., Wijffels H. Phototrophic pigment production with microalgae: biological constraints and opportunities. J. Phycol. 2014;50:229–242. doi: 10.1111/jpy.12173. [DOI] [PubMed] [Google Scholar]
- 55.Li F., Cai M., Lin M., Huang X., Wang J. Differences between motile and nonmotile cells of Haematococcus pluvialis in the production of astaxanthin at different light intensities. Mar. Drugs. 2019;17:1–14. doi: 10.3390/md17010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Powell N., Shilton A., Chisti Y., Pratt S. Towards a luxury uptake process via microalgae – defining the polyphosphate dynamics. Water Res. 2009;43:4207–4213. doi: 10.1016/j.watres.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 57.Brown N., Shilton A. Luxury uptake of phosphorus by microalgae in waste stabilisation ponds: current understanding and future direction. Rev. Environ. Sci. Biotechnol. 2014;13:321–328. doi: 10.1007/s11157-014-9337-3. [DOI] [Google Scholar]
- 58.Arnon D.I. The light reactions of photosynthesis. Proc. Natl. Acad. Sci. 1971;68:2883–2892. doi: 10.1073/pnas.68.11.2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fork D.C., Herbert S.K. Electron transport and photophosphorylation by Photosystem I in vivo in plants and cyanobacteria. Photosynth. Res. 1993;36:149–168. doi: 10.1007/BF00033035. [DOI] [PubMed] [Google Scholar]
- 60.Gimmler H. Correlations between photophosphorylation and light-induced conformational changes of chloroplasts in whole cells of the halophilic green alga Dunaliella parva. Zeitschrift Für Pflanzenphysiologie. 1973;68:289–307. doi: 10.1016/S0044-328X(73)80014-5. [DOI] [Google Scholar]
- 61.Yahia E.M., Carrillo-López A., Barrera G.M., Suzán-Azpiri H., Bolaños M.Q. Postharvest Physiol. Biochem. Fruits Veg. Elsevier; 2019. Photosynthesis; pp. 47–72. [DOI] [Google Scholar]
- 62.Kobayashi M., Kurimura Y., Sakamoto Y., Tsuji Y. Selective extraction of astaxanthin and chlorophyll from the green alga Haematococcus pluvialis. Biotechnol. Tech. 1997;11:657–660. doi: 10.1023/a:1018455209445. [DOI] [Google Scholar]
- 63.Lee E., Ahn H., Choe E. Effects of light and lipids on chlorophyll degradation. Food Sci. Biotechnol. 2014;23:1061–1065. doi: 10.1007/s10068-014-0145-x. [DOI] [Google Scholar]
- 64.Wahidin S., Idris A., Shaleh S.R.M. The influence of light intensity and photoperiod on the growth and lipid content of microalgae Nannochloropsis sp. Bioresour. Technol. 2013;129:7–11. doi: 10.1016/j.biortech.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 65.Amini Khoeyi Z., Seyfabadi J., Ramezanpour Z. Effect of light intensity and photoperiod on biomass and fatty acid composition of the microalgae, Chlorella vulgaris. Aquac. Int. 2012;20:41–49. doi: 10.1007/s10499-011-9440-1. [DOI] [Google Scholar]
- 66.Wong Y.K., Ho Y.H., Ho K.C., Lai Y.T., Tsang P.M., Chow K.P., Yau Y.H., Choi M.C., Ho C.R.S. Effects of light intensity, illumination cycles on microalgae Haematococcus Pluvialis for production of astaxanthin. J. Mar. Biol. Aquac. 2016;2:1–6. doi: 10.15436/2381-0750.16.1083. [DOI] [Google Scholar]
- 67.Imamoglu E., Sukan F.V., Dalayd M.C. Effect of different culture media and light intensities on growth of Haematococcus pluvialis. Int. J. Nat. Eng. Sci. 2007;1:5–9. [Google Scholar]
- 68.Tao W., Wenhui G., Jian L., Bo Z., Guanghua P., Daling Z., Guangce W. Effect of photoperiod on microalgae Haematococcus pluvialis. Chin. Bull. Bot. 2013;48:168–173. doi: 10.3724/SP.J.1259.2013.00168. [DOI] [Google Scholar]
- 69.Grant C., Louda J. Microalgal pigment ratios in relation to light intensity: implications for chemotaxonomy. Aquat. Biol. 2010;11:127–138. doi: 10.3354/ab00298. [DOI] [Google Scholar]
- 70.He Q., Yang H., Wu L., Hu C. Effect of light intensity on physiological changes, carbon allocation and neutral lipid accumulation in oleaginous microalgae. Bioresour. Technol. 2015;191:219–228. doi: 10.1016/j.biortech.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 71.Ferreira V.S., Pinto R.F., Sant’Anna C. Low light intensity and nitrogen starvation modulate the chlorophyll content of Scenedesmus dimorphus. J. Appl. Microbiol. 2016;120:661–670. doi: 10.1111/jam.13007. [DOI] [PubMed] [Google Scholar]
- 72.George B., Pancha I., Desai C., Chokshi K., Paliwal C., Ghosh T., Mishra S. Effects of different media composition, light intensity and photoperiod on morphology and physiology of freshwater microalgae Ankistrodesmus falcatus – a potential strain for bio-fuel production. Bioresour. Technol. 2014;171:367–374. doi: 10.1016/j.biortech.2014.08.086. [DOI] [PubMed] [Google Scholar]
- 73.Hagen C., Grünewald K., Schmidt S., Müller J. Accumulation of secondary carotenoids in flagellates of Haematococcus pluvialis (Chlorophyta) is accompanied by an increase in per unit chlorophyll productivity of photosynthesis. Eur. J. Phycol. 2000;35:75–82. doi: 10.1080/09670260010001735651. [DOI] [Google Scholar]
- 74.Aflalo C., Meshulam Y., Zarka A., Boussiba S. On the relative efficiency of two- vs. one-stage production of astaxanthin by the green alga Haematococcus pluvialis. Biotechnol. Bioeng. 2007;98:300–305. doi: 10.1002/bit.21391. [DOI] [PubMed] [Google Scholar]
- 75.Olaizola M. Commercial production of astaxanthin from Haematococcus pluvialis using 25,000-liter outdoor photobioreactors. J. Appl. Phycol. 2000;12:499–506. [Google Scholar]
- 76.Li F., Cai M., Lin M., Huang X., Wang J., Zheng X., Wu S., An Y. Accumulation of astaxanthin was improved by the nonmotile cells of Haematococcus pluvialis. Biomed. Res. Int. 2019;2019:1–7. doi: 10.1155/2019/8101762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lorenz R.T., Cysewski G.R. Commercial potential for Haematococcus microalgae as a natural source of astaxanthin. Trends Biotechnol. 2000;18:160–167. doi: 10.1016/S0167-7799(00)01433-5. [DOI] [PubMed] [Google Scholar]
- 78.D’Alessandro E.B., Antoniosi Filho N.R. Concepts and studies on lipid and pigments of microalgae: a review. Renew. Sustain. Energy Rev. 2016;58:832–841. doi: 10.1016/j.rser.2015.12.162. [DOI] [Google Scholar]
- 79.da C. Coêlho A.A., Barros M.U.G., Bezerra J.H.C., da Silva W.A., Moreira R.L., Farias W.R.L.F. Growth of the microalgae Tetraselmis tetrathele and nitrate depletion in culture medium Guillard f/2 and Conway. Acta Sci. Biol. Sci. 2013;35:163–168. doi: 10.4025/actascibiolsci.v35i2.13971. [DOI] [Google Scholar]
- 80.Moreno Osorio J.H., Pinto G., Pollio A., Frunzo L., Lens P.N.L., Esposito G. Start-up of a nutrient removal system using Scenedesmus vacuolatus and Chlorella vulgaris biofilms. Bioresour. Bioprocess. 2019;6:27. doi: 10.1186/s40643-019-0259-3. [DOI] [Google Scholar]
- 81.Xin L., Hong-ying H., Ke G., Ying-xue S. Effects of different nitrogen and phosphorus concentrations on the growth, nutrient uptake, and lipid accumulation of a freshwater microalga Scenedesmus sp. Bioresour. Technol. 2010;101:5494–5500. doi: 10.1016/j.biortech.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 82.Katsuda T., Shiraishi H., Ishizu N., Ranjbar R., Katoh S. Effect of light intensity and frequency of flashing light from blue light emitting diodes on astaxanthin production by Haematococcus pluvialis. J. Biosci. Bioeng. 2008;105:216–220. doi: 10.1263/jbb.105.216. [DOI] [PubMed] [Google Scholar]
- 83.Goksan T., Ak I. Vegetative growth of the green alga Haematococcus pluvialis cultivated in different light-path lengths. Asian J. Plant Sci. 2006;5:455–460. doi: 10.3923/ajps.2006.455.460. [DOI] [Google Scholar]
- 84.Zhang W., Zhou X., Zhang Y., Cheng P., Ma R., Cheng W., Chu H. Enhancing astaxanthin accumulation in Haematococcus pluvialis by coupled light intensity and nitrogen starvation in column photobioreactors. J. Microbiol. Biotechnol. 2018;28:2019–2028. doi: 10.4014/jmb.1807.07008. [DOI] [PubMed] [Google Scholar]
- 85.Kobayashi M., Kakizono T., Nagai S. Enhanced carotenoid biosynthesis by oxidative stress in acetate-induced cyst cells of a green unicellular alga, Haematococcus pluvialis. Appl. Environ. Microbiol. 1993;59:867–873. doi: 10.1128/aem.59.3.867-873.1993. http://www.ncbi.nlm.nih.gov/pubmed/16348895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Krinsky N.I. The biological properties of carotenoids. Pure Appl. Chem. 1994;66:1003–1010. [Google Scholar]
- 87.Krinsky N.I. Carotenoids. Birkhauser Verlag Basel; Stuttgart: 1971. Function. [Google Scholar]
- 88.Krinsky N.I. Carotenoid protection against oxidation. Pure Appl. Chem. 1979;51:649–660. [Google Scholar]
- 89.Ben-Amotz A., Shaish A., Avron M. Mode of action of the massively accumulated β-carotene of Dunaliella bardawil in protecting the alga against damage by excess irradiation. Plant Physiol. 1989;91:1040–1043. doi: 10.1104/pp.91.3.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fábregas J., Otero A., Maseda A., Domínguez A. Two-stage cultures for the production of astaxanthin from Haematococcus pluvialis. J. Biotechnol. 2001;89:65–71. doi: 10.1016/S0168-1656(01)00289-9. [DOI] [PubMed] [Google Scholar]
- 91.Wayama M., Ota S., Matsuura H., Nango N., Hirata A., Kawano S. Three-dimensional ultrastructural study of oil and astaxanthin accumulation during encystment in the green alga Haematococcus pluvialis. PLoS One. 2013;8 doi: 10.1371/journal.pone.0053618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pérez-López P., González-García S., Jeffryes C., Agathos S.N., McHugh E., Walsh D., Murray P., Moane S., Feijoo G., Moreira M.T. Life cycle assessment of the production of the red antioxidant carotenoid astaxanthin by microalgae: from lab to pilot scale. J. Clean. Prod. 2014;64:332–344. doi: 10.1016/j.jclepro.2013.07.011. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.