Abstract
Background
Previous studies have reported associations between nonalcoholic fatty liver disease, periodontitis, and obesity. Serum immunoglobulin G (IgG) antibody titer against Porphyromonas gingivalis, a major pathogen of periodontitis, is an established indicator of periodontal infection. However, the relationship between the antibody titer and liver enzyme levels has not been clarified yet. A study in the elderly was needed to evaluate the effect of long-term persistent bacterial infection on liver function. The objective of this study was to investigate the association between liver function and infection by P. gingivalis, and the effect of obesity on the association.
Methods
A cross-sectional study was conducted in adult outpatients visiting Sado General Hospital, in Niigata Prefecture, Japan, from 2008 to 2010. The final participants included 192 men and 196 women (mean age 68.1 years). Multivariable logistic regression analyses were performed to assess the association between the serum IgG antibody titer and the levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), and γ-glutamine transferase (GGT) levels.
Results
In women, serum IgG antibody titers against P. gingivalis was associated with elevated ALT, but not with AST or GGT, independent of covariates (p = 0.015). No significant association was found between the antibody titer and the elevated liver enzymes in men. The effect of obesity on the relationship between antibody titer and liver enzyme levels was not statistically significant.
Conclusions
A cross-sectional analysis of adult outpatients suggested an association between P. gingivalis infection and ALT levels in women. The effect of obesity on this association was not statistically significant.
Keywords: Clinical research, Dentistry, Hepatobiliary system, Internal medicine, Liver enzymes, Periodontitis, Obesity, Porphyromonas gingivalis, Antibody
Clinical Research; Dentistry; Hepatobiliary System; Internal Medicine; Liver enzymes; periodontitis; obesity; Porphyromonas gingivalis; Antibody
1. Introduction
Periodontitis is a common chronic inflammatory disease in which periodontal tissue is destroyed by infection with bacteria (Page, 1998). Porphyromonas gingivalis is an anaerobic gram-negative bacterium known as the main pathogen of periodontitis (Scannapieco et al., 2003; Beck et al., 1996; Offenbacher et al., 1999). Increased levels of immunoglobulin G (IgG) antibodies specific to the bacteria are observed in the sera of patients with periodontitis (Murayama et al., 1988; Kojima et al., 1997). Periodontal treatment decreases the number of bacteria in periodontal pockets and reduces the serum IgG antibody titer against P. gingivalis (Horibe et al., 1995). The application of the serum IgG antibody titer against P. gingivalis as a screening test for periodontitis has been studied (Kudo et al., 2012; Furuichi et al., 2003; Sakai et al., 2001).
Many reports have shown the relationship between periodontal diseases and systemic diseases (Albandar et al., 2018). Periodontitis forms superficial ulcers in the gingival sulcus and exposes its capillaries to the biofilm (D'Aiuto et al., 2004), subsequently, periodontopathic bacteria and inflammatory cytokines enter the systemic circulation and are transmitted to distant tissues (Tomás et al., 2012). A significant association has been found between periodontitis and nonalcoholic fatty liver disease (NAFLD) (Loos et al., 2000; Alazawi et al., 2017; Iwasaki et al., 2018). Meanwhile, periodontal treatment improves aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels in sera from patients with NAFLD (Yoneda et al., 2012). In a mouse model of NAFLD, P. gingivalis infection induced fatty liver and elevated blood ALT levels (Yoneda et al., 2012). In another study, probing pocket depth, a clinical parameter of periodontitis, was associated with serum γ-glutamine transferase (GGT) independent of alcohol consumption in Japanese men (Morita et al., 2014). The antibody levels against some types of P. gingivalis fimbrial antigens were elevated in sera of patients with liver fibrosis and NAFLD (Yoneda et al., 2012; Nakahara et al., 2018). However, the relationship between liver enzyme levels and serum IgG antibody titer against P. gingivalis has not been yet clear. A study including an elderly population was needed to evaluate the association between liver function and long-term persistent infection by P. gingivalis.
Obesity is the most common risk factor for chronic liver diseases (Harris et al., 2009). The prevalence of NAFLD increases among people with obesity (James and Day, 1998; Angulo, 2002; Marchesini et al., 2003; Banderas et al., 2012). Elevated serum aminotransferase activity is observed in obesity (James and Day, 1998; Angulo, 2002). Many studies have demonstrated an association between periodontitis and metabolic syndrome or obesity (Morita et al., 2010; Nibali et al., 2013; Watanabe and Cho, 2014; Lamster and Pagan, 2017; Sakurai et al., 2019). Furthermore, obesity has been suggested to influence the relationship between periodontitis and liver function (Saito and Shimazaki, 2007; Pischon et al., 2007). However, no previous study has elucidated the effect of obesity on the relationship between liver enzyme levels and serum IgG antibody titer against P. gingivalis.
Therefore, we evaluated the association between liver enzyme levels and serum IgG antibody titer against P. gingivalis along with the effect of obesity in a cross-sectional study with outpatients including the elderly.
2. Methods
2.1. Participants
The project in Sado for Total Health (PROST) began in June 2008, as an ongoing hospital-based cohort study with outpatients of Sado General Hospital in Sado Island, Niigata, Japan, in conjunction with the Center for Inter-Organ Communication Research, Niigata University Graduate School of Medical and Dental Sciences. The aging rate of individuals aged ≥65 years in Sado City is over 40%, which is equivalent to that of Japan estimated approximately 20 years later (Niigata Prefecture, 2017). The project aims to study age-related diseases from the viewpoint of association of organs in the super-aging society.
In the present study, all 733 participants registered for PROST from 2008 to 2010 (34–89 years of age in 2008), and underwent general physical examinations, blood tests, and health-related interviews. Data regarding history of illness were collected from the clinical records. Exclusion criteria included the following: edentulous (n = 61), hemodialysis (n = 7), history of steroid or immunosuppressive therapy (n = 14), hepatitis A or B, and hepatic cirrhosis (n = 12), and missing data (n = 251). The final sample consisted of 388 participants (192 men and 196 women, 34–89 years of age) (Figure 1).
Figure 1.
Flow diagram of the present study.
The protocol of the present study was in accordance with the Helsinki Declaration of 2002 and approved by the Medical Ethics Committee at Niigata University (Approval number 511). All participants provided written informed consent. In case it was difficult for them to sign by themselves, their deputies signed. This was a human observational study, and the report was prepared in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
2.2. Blood test data
The levels of AST, ALT, and GGT were measured from the blood tests that should be performed for all patients undergoing treatment. In addition, serum high-sensitivity CRP (hsCRP), serum cytokines, interleukin-1β (IL-1β), interleukin-6 (IL-6), and TNF-α levels were measured using a portion of the collected blood for this project. HsCRP values were measured with a latex particle enhanced immunoassay (NA Latex CRP Kit, Dade Behring, Tokyo, Japan). Serum cytokine levels were measured using the Bio-Plex200 Suspension Array System (Bio-Rad Laboratories, Hercules, CA, USA) by Filgen Inc. (Nagoya, Japan).
The standard values for each serum liver enzyme level were based on the Japanese Committee for Clinical Laboratory Standard Guidelines. Elevated liver enzyme levels were defined as >30 U/L for AST for both men and women, >23 U/L in women and >42 U/L in men for ALT, and >32 U/L in women and >64 U/L in men for GGT.
2.3. Measurement of serum IgG antibody titers against P. gingivalis
Sera were obtained from participants with written informed consent and stored at -30 °C. Serum IgG titers to P. gingivalis 381 sonicated preparation were determined by PCL Japan, Inc. (Tokyo, Japan) with the enzyme-linked immunosorbent assay (ELISA) using the same method as in a previous study (Kudo et al., 2012). The analyses were calibrated using pooled sera from 10 healthy volunteers without periodontitis (aged 20–29 years). The mean ± 2SD (ELISA unit) of the controls was defined as the value 1 for the standard.
2.4. Covariates
Alcohol consumption, smoking habits, age, blood pressure, diabetes, and body mass index (BMI) were considered as covariates, based on previous studies reporting the risk factors for elevated serum liver enzyme levels. Alcohol consumption and smoking habits were surveyed using a self-administered questionnaire. The responses to the alcohol drinking habits questionnaire were as follows: (1) “I drink more than 1 day a week”, (2) “I was a drinker before”, (3) “I'm a social drinker” and (4) “I do not drink alcohol at all”. This study categorized social drinkers and past drinkers into the “No drinking habits” and “Drinking habits” groups, respectively. Similarly, the responses for smoking habits were as follows: (1) “I have a smoking habit,” (2) “I had a smoking habit before,” and (3) “I do not smoke at all.” Past smokers were categorized into the “Have smoking habits” group. Age, blood pressure, diabetes, and BMI were recorded during the PROST registration. Blood pressure was measured twice. The definition of hypertension was the average blood pressure ≥140 and/or ≥90 mmHg, and/or the use of antihypertensive medication. Diabetes was defined as the person diagnosed with it. Height and weight were measured for all participants. Obesity was defined as a body mass index (BMI) ≥ 25 kg/m2 for the Asian criteria (WHO Expert Consultation, 2004).
2.5. Statistical analyses
In a previous report (Kudo et al., 2012), six cut-off values for serum IgG antibody titers to P. gingivalis (1.00, 1.68, 3.36, 5.04, 6.72, and 8.40) were used to assess the association with periodontitis. In this study, serum IgG antibody titers against P. gingivalis were categorized using two types of cut-off points: the tertile and the six cut-off values mentioned above.
The characteristics were compared between the participants with tertile categories of serum IgG antibody titer against P. gingivalis determined by Mann-Whitney U tests or Fisher's exact tests. Univariate association of elevated serum liver enzyme levels with serum IgG antibody titer against P. gingivalis or other explanatory variables were evaluated using the Mann-Whitney U test, chi-square test, or Fisher's exact test. Multivariable logistic regression was used to analyze the association between elevated serum liver enzyme levels as outcomes and serum IgG antibody titer against P. gingivalis as an explanatory variable, adjusted for the covariates mentioned above.
To evaluate the effect of obesity on this association, the adjusted odds ratios of the six cut-off values of the antibody titer were calculated from multivariable logistic regression analyses according to the presence or absence of obesity. Statistical analyses and calculations were performed using the software SPSS version 19 for Windows (IBM Corp., Armonk, New York, USA). The alpha level was 0.05.
3. Results
3.1. Comparisons of selected characteristics by the tertile categories of serum IgG antibody titers against P. gingivalis
The participants were divided into men and women, and further divided into three groups based on the tertile categories of the serum IgG antibody titer against P. gingivalis. Age, number of teeth, number of people with hypertension, obesity, diabetes, elevated AST, elevated ALT, elevated GGT, smoking habits, and drinking habits are shown in Table 1. In women, the number of smokers in the third tertile group of antibody titers was significantly higher than that in the first and second tertile groups (p = 0.037). There was no significant difference in other factors between the tertiles of antibody titers in both men and women (Table 1).
Table 1.
Comparisons of selected characteristics by the tertile categories of serum IgG antibody titer against P. gingivalis.
| Tertile categories of serum IgG antibody titer against P. gingivalis |
||||||
|---|---|---|---|---|---|---|
| Men (n = 192) |
Women (n = 196) |
|||||
| 1st tertile | 2nd tertile | 3rd tertile | 1st tertile | 2nd tertile | 3rd tertile | |
| Antibody titer | -1.2–4.1 | 4.2–15.2 | 15.3–148.8 | -1.3–3.9 | 3.9–9.3 | 9.5–109.7 |
| Age (years) | 70 (61, 74) | 67 (63, 73) | 67 (63, 73) | 71 (62, 75) | 67 (62, 76) | 66 (61, 74) |
| Number of teeth | 25 (14, 27) | 22 (16, 26) | 22 (16, 26) | 22 (15, 27) | 21 (12, 25) | 22 (12, 27) |
| Hypertension (yes/no) | 45/19 | 38/26 | 43/21 | 44/22 | 45/20 | 39/26 |
| Obesity (yes/no) | 31/33 | 20/44 | 21/43 | 29/37 | 23/42 | 27/38 |
| Diabetes (yes/no) | 36/28 | 25/39 | 32/32 | 22/45 | 22/42 | 17/48 |
| AST (IU/l) | 22 (18, 25) | 24 (18, 30) | 24 (18, 30) | 21 (15, 27) | 22 (20, 26) | 24 (21, 27) |
| ALT (IU/l) | 21 (16, 26) | 20 (16, 26) | 20 (16, 26) | 18 (14, 23) | 18 (15, 23) | 21 (16, 25) |
| GGT (IU/l) | 30 (24, 52) | 29 (21,44) | 29 (28, 44) | 17 (13, 25) | 18 (15, 29) | 21 (16, 25) |
| Smoking (yes/no) | 44/20 | 42/22 | 43/21 | 2/64 | 1/64 | 7/58∗ |
| Drinking (yes/no) | 43/21 | 49/15 | 37/27 | 6/60 | 6/59 | 4/61 |
Abbreviations: AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma-glutamyltransferase; Values represent median (25th percentile, 75th percentile) for continuous variables and number of participants for categorical variables. Mann-Whitney U tests or Chi-square tests (Fisher's exact tests for expected value < 10) were performed. ∗p < 0.05.
3.2. Univariate association of elevated serum liver enzymes with serum IgG antibody against P. gingivalis or other explanatory variables
In men, the number of people with hypertension was significantly higher in the elevated AST group than in the normal group, and the number of people with drinking habits was significantly higher in the group with elevated GGT than in the normal group (p = 0.009 and p = 0.011, respectively) (Table 2).
Table 2.
Univariate association of elevated serum liver enzyme levels with serum IgG antibody titers against P. gingivalis or other explanatory variables.
| Participants | Variables | AST |
ALT |
GGT |
|||
|---|---|---|---|---|---|---|---|
| Normal level | Elevated level | Normal level | Elevated level | Normal level | Elevated level | ||
| Men | Number of participants | 158 | 34 | 180 | 12 | 161 | 31 |
| Antibody titer | 8.5 (3.0, 16.9) | 13.03 (4.9, 28.9) | 9.1 (3.3, 17.3) | 12.8 (2.5, 35.8) | 9.2 (3.2, 17.9) | 11.9 (3.1, 16.3) | |
| (1st/2nd/3rd tertile) | 56/53/49 | 8/11/15 | 60/61/59 | 4/3/5 | 51/55/55 | 13/9/9 | |
| Age (yrs.) | 68 (62, 74) | 72 (64, 75) | 69 (63, 74) | 71 (62, 77) | 69 (63, 75) | 65 (60, 73) | |
| Number of teeth | 23 (13, 27) | 24 (15, 26) | 24 (14, 27) | 24 (9, 27) | 23 (14, 26) | 26 (10, 28) | |
| Hypertension (yes/no) | 97/61 | 29/5∗ | 117/63 | 9/3 | 101/60 | 25/6 | |
| Obesity (yes/no) | 59/99 | 13/21 | 65/115 | 7/5 | 56/105 | 16/15 | |
| Diabetes (yes/no) | 78/80 | 15/19 | 86/94 | 7/5 | 79/82 | 14/17 | |
| Smoking habit (yes/no) | 110/48 | 19/15 | 121/59 | 8/4 | 105/56 | 24/7 | |
| Drinking habit (yes/no) | 108/50 | 21/13 | 122/58 | 7/5 | 102/59 | 27/4∗ | |
| Women | Number of participants | 172 | 24 | 142 | 54 | 163 | 33 |
| Antibody titer | 5.7 (2.7, 12.5) | 6.6 (2.7, 11.7) | 5.4 (2.5, 10.8) | 8.8 (2.8, 16.1) | 5.7 (2.6, 12.5) | 7.4 (2.3, 12.0) | |
| (1st/2nd/3rd tertile) | 58/57/57 | 8/8/8 | 51/51/40 | 15/14/25∗ | 56/54/53 | 10/11/12 | |
| Age (yrs) | 69 (62, 75) | 65 (61, 72) | 71 (62, 76) | 65 (60, 69)∗ | 69 (62, 75) | 67 (60, 71) | |
| Number of teeth | 21 (11, 27) | 23 (15, 27) | 20 (10, 25) | 25 (19, 27)∗ | 21 (11, 26) | 23 (17, 27) | |
| Hypertension (yes/no) | 110/62 | 18/6 | 92/50 | 36/18 | 107/56 | 21/12 | |
| Obesity (yes/no) | 69/103 | 10/14 | 51/91 | 28/26 | 62/101 | 17/16 | |
| Diabetes (yes/no) | 55/117 | 6/18 | 40/102 | 21/33 | 50/113 | 11/22 | |
| Smoking habit (yes/no) | 10/162 | 0/24 | 7/135 | 3/51 | 9/154 | 1/32 | |
| Drinking habit (yes/no) | 13/159 | 3/21 | 12/130 | 4/50 | 14/149 | 2/31 | |
Abbreviations: AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma-glutamyltransferase. Values represent median (25th percentile, 75th percentile) for continuous variables and number of participants for categorical variables. Elevated AST level was defined as >30 IU/l. Elevated ALT levels were defined as >42 IU/l for men and >23 IU/l for women. Elevated GGT levels were defined as >64 IU/l for men and >32 IU/l for women. Mann-Whitney U tests for continuous variables, Fisher's exact tests for 2 × 2 contingency tables, and chi-square tests for 2 × 3 contingency tables were performed. ∗p < 0.05.
On the other hand, women participants in the elevated ALT level group had a significantly lower age, a significantly higher number of teeth, and a significantly higher number of participants in the third tertile of the antibody than in the normal group (p = 0.001, p = 0.002, and p = 0.045, respectively) (Table 2).
3.3. Adjusted odds ratios of serum IgG antibody titers against P. gingivalis for elevated liver enzymes
Multiple logistic regression analyses were conducted to evaluate whether each tertile category of the serum IgG antibody titer against P. gingivalis was associated with elevated liver enzyme levels (Tables 3, 4, and 5). In women, a significant association was observed between the third tertile of the antibody titer against P. gingivalis and elevated ALT (p = 0.015, OR = 2.80, 95%CI = 1.22–6.44).
Table 3.
Logistic regression analyses for elevated AST.
| Participants | Variables | Crude OR (95%CI) | Adjusted OR (95%CI) | |
|---|---|---|---|---|
| Men | Serum IgG antibody titers against P. gingivalis | The 1st tertile | Reference | Reference |
| The 2nd tertile | 1.45 (0.54–3.89) | 1.63 (0.57–4.63) | ||
| The 3rd tertile | 2.14 (0.84–5.49) | 2.29 (0.86–6.10) | ||
| Age | 1.04 (0.99–1.09) | 1.04 (0.98–1.09) | ||
| Number of teeth | 1.00 (0.96–1.05) | 1.02 (0.97–1.08) | ||
| Hypertension | 3.65 (1.34–9.93)∗ | 3.43 (1.21–9.75)∗ | ||
| Obesity | 1.04 (0.48–2.23) | 1.08 (0.47–2.46) | ||
| Diabetes | 0.81 (0.38–1.71) | 0.96 (0.43–2.15) | ||
| Smoking habit | 0.55 (0.26–1.18) | 0.73 (0.32–1.66) | ||
| Drinking habit | 0.75 (0.35–1.61) | 0.85 (0.36–1.99) | ||
| Women | Serum IgG antibody titers against P. gingivalis | The 1st tertile | Reference | Reference |
| The 2nd tertile | 1.02 (0.36–2.90) | 1.11 (0.38–3.23) | ||
| The 3rd tertile | 1.02 (0.36–2.90) | 1.07 (0.37–3.11) | ||
| Age | 0.98 (0.93–1.02) | 0.98 (0.93–1.03) | ||
| Number of teeth | 1.03 (0.98–1.09) | 1.03 (0.97–1.09) | ||
| Hypertension | 1.69 (0.64–4.48) | 2.09 (0.74–5.88) | ||
| Obesity | 1.07 (0.45–2.54) | 0.88 (0.36–2.19) | ||
| Diabetes | 0.71 (0.27–1.89) | 0.68 (0.25–1.84) | ||
| Drinking habit | 1.75 (0.46–6.64) | 1.64 (0.41–6.54) |
Smoking habit as a variable was excluded from the analysis in women because of small number of smokers. AST, aspartate aminotransferase. Elevated AST level was defined as >30 IU/l. OR, odds ratio; 95% CI, confidence interval; ∗p < 0.05.
Table 4.
Logistic regression analyses for elevated ALT.
| Participants | Variables | Crude OR (95%CI) | Adjusted OR (95%CI) | |
|---|---|---|---|---|
| Men | Serum IgG antibody titers against P. gingivalis | The 1st tertile | Reference | Reference |
| The 2nd tertile | 0.74 (0.16–3.44) | 0.99 (0.20–4.97) | ||
| The 3rd tertile | 1.27 (0.33–4.97) | 1.52 (0.37–6.18) | ||
| Age | 1.03 (0.96–1.11) | 1.04 (0.96–1.13) | ||
| Number of teeth | 1.00 (0.93–1.07) | 1.01 (0.93–1.09) | ||
| Hypertension | 1.62 (0.42–6.18) | 1.43 (0.35–5.88) | ||
| Obesity | 2.48 (0.76–8.12) | 2.74 (0.79–9.51) | ||
| Diabetes | 1.53 (0.47–5.00) | 1.64 (0.47–5.75) | ||
| Smoking habit | 0.98 (0.28–3.37) | 1.23 (0.32–4.67) | ||
| Drinking habit | 0.67 (0.20–2.19) | 0.75 (0.21–2.71) | ||
| Women | Serum IgG antibody titers against P. gingivalis | The 1st tertile | Reference | Reference |
| The 2nd tertile | 0.93 (0.41–2.13) | 1.31 (0.55–3.15) | ||
| The 3rd tertile | 2.13 (0.99–4.55) | 2.80 (1.22–6.44)∗ | ||
| Age | 0.95 (0.91–0.98)∗ | 0.97 (0.93–1.01) | ||
| Number of teeth | 1.07 (1.03–1.12)∗ | 1.07 (1.02–1.12)∗ | ||
| Hypertension | 1.09 (0.56–2.11) | 1.39 (0.66–2.94) | ||
| Obesity | 1.92 (1.02–3.62)∗ | 1.64 (0.81–3.30) | ||
| Diabetes | 1.62 (0.84–3.13) | 1.71 (0.84–3.48) | ||
| Drinking habit | 0.87 (0.27–2.81) | 0.77 (0.20–2.95) |
Smoking habit as a variable was excluded from the analysis in women because of small number of smokers. ALT, alanine aminotransferase. Elevated ALT levels were defined as >42 IU/l for men, and >23 IU/l for women. OR, odds ratio; 95% CI, confidence interval; ∗p < 0.05.
Table 5.
Logistic regression analyses for elevated GGT.
| Participants | Variables | Crude OR (95%CI) | Adjusted OR (95%CI) | |
|---|---|---|---|---|
| Men | Serum IgG antibody titers against P. gingivalis | The 1st tertile | Reference | Reference |
| The 2nd tertile | 0.64 (0.25–1.63) | 0.68 (0.25–1.88) | ||
| The 3rd tertile | 0.64 (0.25–1.63) | 0.70 (0.26–1.89) | ||
| Age | 0.96 (0.92–1.00) | 0.96 (0.91–1.01) | ||
| Number of teeth | 1.02 (0.97–1.06) | 1.00 (0.95–1.05) | ||
| Hypertension | 2.48 (0.96–6.38) | 2.98 (1.06–8.41)∗ | ||
| Obesity | 2.00 (0.92–4.34) | 1.59 (0.68–3.68) | ||
| Diabetes | 0.86 (0.40–1.85) | 0.97 (0.41–2.28) | ||
| Smoking habit | 1.83 (0.74–4.51) | 1.83 (0.70–4.83) | ||
| Drinking habit | 3.90 (1.30–11.71)∗ | 3.77 (1.20–11.84)∗ | ||
| Women | Serum IgG antibody titers against P. gingivalis | The 1st tertile | Reference | Reference |
| The 2nd tertile | 1.14 (0.45–2.90) | 1.65 (0.63–4.34) | ||
| The 3rd tertile | 1.27 (0.51–3.18) | 1.50 (0.57–3.92) | ||
| Age | 0.97 (0.93–1.01) | 0.98 (0.94–1.03) | ||
| Number of teeth | 1.04 (0.99–1.09) | 1.03 (0.98–1.08) | ||
| Hypertension | 0.92 (0.42–2.00) | 0.96 (0.41–2.22) | ||
| Obesity | 1.73 (0.82–3.67) | 1.67 (0.75–3.71) | ||
| Diabetes | 1.13 (0.51–2.51) | 1.08 (0.48–2.44) | ||
| Drinking habit | 0.69 (0.15–3.18) | 0.61 (0.13–2.95) |
Smoking habit as a variable was excluded from the analysis in women because of small number of smokers. GGT, gamma-glutamyltransferase. Elevated GGT levels were defined as >64 IU/l for men and >32 IU/l for women. OR, odds ratio; 95% CI, confidence interval; ∗p < 0.05.
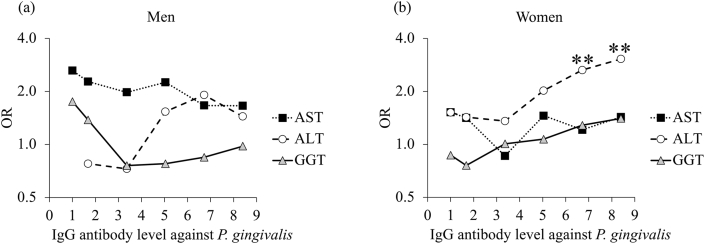
Figure 2 shows the results of multiple logistic regression analyses using the six cut-off values (1.00, 1.68, 3.36, 5.04, 6.72, and 8.40) of serum IgG antibody titer against P. gingivalis. In women, a significant association was obtained between the serum IgG antibody titer against P. gingivalis, and ALT levels at the cut-off values of 6.72 and 8.40 (p = 0.005 and p = 0.001, respectively).
Figure 2.
Adjusted odds ratios of serum IgG antibody titers against P. gingivalis for elevated liver enzyme levels. Multiple logistic regression analyses were performed in (a) men and (b) women. The odds ratio of the antibody titer over each cut-off value was calculated by adjusting for age, number of teeth, hypertension, obesity, drinking habit, and smoking habit (except in women due to the small number of smokers). ∗p < 0.05, ∗∗p < 0.01.
3.4. Adjusted odds ratios of serum IgG antibody titer against P. gingivalis for elevated liver enzymes with or without obesity
Figure 3 shows adjusted odds ratios from multiple logistic regression analyses to evaluate the effect of obesity on the association between serum IgG antibody titers to P. gingivalis and elevated liver enzymes. For men with an antibody titer over 3.36, the odds ratios were consistently higher with obesity than without obesity, although none of the odds ratios was statistically significant. In women, the IgG antibody titer against P. gingivalis higher than the cut-off titers 6.72 and 8.40 showed significant associations with the elevated ALT regardless of the presence or absence of obesity.
Figure 3.
Adjusted odds ratios of serum IgG antibody titers against P. gingivalis for elevated liver enzyme levels with or without obesity. The odds ratios for (a) elevated AST in men, (b) elevated ALT in men, (c) elevated GGT in men, (d) elevated AST in women, (e) elevated ALT in women, and (f) elevated GGT in women. Multiple logistic regression analyses were performed. The odds ratio of the antibody titer over each cut-off value was calculated in participants with or without obesity adjusted for age, number of teeth, hypertension, drinking habit, and smoking habit (except in women due to the small number of smokers). Obesity was defined as a BMI ≥25 kg/m2 ∗p < 0.05.
3.5. Correlation analyses between serum hsCRP, cytokine levels, and IgG antibody titer against P. gingivalis
In both men and women, regardless of the presence or absence of obesity, no significant correlation was observed between serum IgG antibody titer against P. gingivalis and hsCRP, IL-1β, IL-6, or TNF-α levels (p > 0.05) (Table 6).
Table 6.
Correlation coefficient between serum IgG antibody titer against P. gingivalis, cytokines and hsCRP.
| Serum IgG antibody titers to P. gingivalis |
||||
|---|---|---|---|---|
| Men |
Women |
|||
| β | p-value | β | p-value | |
| IL-1β | 0.01 | 0.95 | -0.02 | 0.82 |
| IL-6 | 0.10 | 0.18 | -0.09 | 0.23 |
| TNF-α | -0.03 | 0.70 | -0.07 | 0.36 |
| hsCRP | 0.11 | 0.15 | 0.01 | 0.86 |
Adjusted for age, body mass index. IL-1β, interleukin-1β; IL-6, interleukin-6; TNF-α, Tumor Necrosis Factor-α; hsCRP, high-sensitivity C-reactive protein.
4. Discussion
This is the first report to investigate the relationship between liver function and infection with periodontopathic bacteria in adults including the elderly. Since sufficient time and skill are required to perform an accurate periodontal examination, serum IgG antibody titer against P. gingivalis is useful to determine the relationship between periodontitis and systemic diseases in a large population (Dye et al., 2009; Eke, 2013). We found an association between elevated ALT and serum IgG antibody titers against P. gingivalis in women. A statistically significant association was observed only with the 3rd tertile category, or at a cut-off value > 6.72 of the antibody titers. According to a previous report (Kudo et al., 2012), the recommended cut-off value of serum IgG antibody titer against P. gingivalis was 1.68 for screening for the presence of periodontitis. A titer greater than 6.72 corresponded to more severe periodontitis (Kudo et al., 2012). On the other hand, ALT is one of the important indicators of declined liver function and is increased as a result of fatty liver, independent of an alcoholic or nonalcoholic association (Rakha et al., 2010). Although both AST and ALT are deviation enzymes, ALT is mainly localized in the liver, unlike AST, which is distributed not only in the liver but also in the myocardium, skeletal muscle, and kidneys. Therefore, the results of this study suggest an association between declined liver function and the presence of severe infection by P. gingivalis in women.
According to a report (Saito et al., 2006) in healthy Japanese women (20–59 years old), significant associations between periodontitis and serum AST, ALT, and GGT levels were found by multivariable linear regression analyses. Another previous study in Japan (Morita et al., 2014), in which most of the participants were men aged 39–64 years old, reported a significant association between elevated GGT and periodontitis independent of drinking habit. In the present study, only ALT levels in women were significantly associated with serum IgG antibody titers against P. gingivalis. The difference in age range may be a cause of the inconsistencies between the results from the previous reports and the present study. Indeed, 85% of the participants were 60 years or older in our study. Additionally, the participants in our study were outpatients of a hospital, whereas participants in previous studies were recruited during medical check-ups.
We hypothesized bidirectional relationships between elevated liver enzyme levels, serum IgG antibody titers against P. gingivalis, and obesity. Previous studies have suggested that periodontitis affects liver function by hepatic dyslipidemia, and obesity has been reported to be a risk factor for periodontitis (Saito and Shimazaki, 2007). However, we did not find a significant difference in the IgG antibody titer against P. gingivalis between obesity and non-obesity. Adjusted odds ratios of the antibody titer for liver enzymes were higher in obese men than in non-obese men, but the difference was not statistically significant (Figure 3). Similarly, obese and non-obese women tended to have different odds ratios, but they were significant only for ALT and for cut-off levels of antibody titers >6.72 (Figure 3). Therefore, we are unable to conclude whether obesity modifies the relationship between liver enzymes and serum IgG antibody titers against P. gingivalis based on our results. Further investigations with a larger number of participants, including data on indicators for dyslipidemia, may lead to a greater understanding in this field in the future.
We expected that cytokines and hsCRP produced in periodontitis would be a link between serum IgG antibody titer against P. gingivalis and elevated liver enzyme levels. Higher hsCRP and IL-6 levels have been observed in sera from patients with periodontitis compared to healthy controls, and the concentrations were significantly decreased after periodontal treatment (Nakajima et al., 2010; Yamazaki et al., 2005). In an obese rat model, experimental periodontitis increased gene expression levels of TNF-α and CRP in liver tissue, and IL-6 and CRP in adipose tissue (Endo et al., 2010). However, in our study, serum IgG antibody titers against P. gingivalis were not correlated with serum hsCRP, IL-1β, IL-6, or TNF-α levels, regardless of the presence or absence of obesity. Therefore, it is unlikely that increased proinflammatory mediators in periodontitis elevated ALT levels in women in this study. Several biological mechanisms have been proposed to explain the association between periodontitis and systemic diseases. Oral administration of P. gingivalis in mice has been suggested to induce dysbiosis in the gastrointestinal microbiota, exacerbate intestinal permeability, and disseminate enterobacteria in the liver (Arimatsu et al., 2014; Nakajima et al., 2015). Further study is needed to determine the mechanism that explains the observed association between serum IgG antibody titers against P. gingivalis and elevated ALT levels in this study.
Alcohol consumption is a well-known risk factor for reduced liver function. GGT levels are elevated in alcoholic fatty liver and are affected by even small amounts of alcohol (Wang and Yue, 2011). In contrast, a study reported no differences in ALT levels between NAFLD and alcoholic fatty liver (Rakha et al., 2010). In this study, the proportion of participants with a drinking habit was 67% of men and 8% of women. Although a univariate association between elevated GGT levels and drinking habit was observed in men, it was nonexistent after the adjustment for covariates.
The mean age of women in the present study was 68.5 ± 9.4, and most of them were postmenopausal women. It has been suggested that estrogen deficiency is associated with periodontitis in postmenopausal women (Inagaki et al., 2001; Yoshihara et al., 2004; Iwasaki et al., 2013). In addition, the effects of sex hormones on NASH and NAFLD have been reported (Xin et al., 2015; Kamada et al., 2011). It might be speculated that estrogen deficiency affected the results in this study.
The limitations of the present study were the small number of participants and the absence of periodontal clinical parameters. In addition, the cross-sectional design of the study does not show causative relationships. The mechanism of association between the serum IgG antibody titer against P. gingivalis and the liver enzyme level is unknown. Further large-scale studies and additional research will be conducted in the PROST cohort.
In conclusion, a cross-sectional analysis of adult outpatients including the elderly showed an association between the serum IgG antibody titer against P. gingivalis and ALT levels in women.
Declarations
Author contribution statement
N. Sugita and A. Yoshihara: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
T. Kobayashi, H. Yoshie, K. Tabeta, K. Nakamura, O. Onodera, N. Endo and I. Narita: Conceived and designed the experiments; Wrote the paper.
K. Takamisawa: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
S. Komatsu: Performed the experiments; Wrote the paper.
M. Wakasugi, A. Yokoseki, T. Momotsu and K. Sato: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by a Grant-in-Aid for the Project in Sado for Total Health (PROST) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, Japan.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We would like to thank Editage (www.editage.jp) for English language editing.
References
- Alazawi W., Bernabe E., Tai D., Janicki T., Kemos P., Samsuddin S., Syn W.K., Gillam D., Turner W. Periodontitis is associated with significant hepatic fibrosis in patients with non-alcoholic fatty liver disease. PLoS One. 2017 Dec 8;12(12) doi: 10.1371/journal.pone.0185902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albandar J.M., Susin C., Hughes F.J. Manifestations of systemic diseases and conditions that affect the periodontal attachmentapparatus: case definitions and diagnostic considerations. J. Periodontol. 2018 Jun;89(Suppl 1):S183–S203. doi: 10.1002/JPER.16-0480. [DOI] [PubMed] [Google Scholar]
- Angulo P. Nonalcoholic fatty liver disease. N. Engl. J. Med. 2002 Apr 18;346(16):1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- Arimatsu K., Yamada H., Miyazawa H., Minagawa T., Nakajima M., Ryder M.I., Gotoh K., Motooka D., Nakamura S., Iida T., Yamazaki K. Oral pathobiont induces systemic inflammation and metabolic changes associated with alteration of gut microbiota. Sci. Rep. 2014 May 6;4:4828. doi: 10.1038/srep04828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banderas D.Z., Escobedo J., Gonzalez E., Liceaga M.G., Ramírez J.C., Castro M.G. γ-Glutamyl transferase: a marker of nonalcoholic fatty liver disease in patients with the metabolic syndrome. Eur. J. Gastroenterol. Hepatol. 2012;24(7):805–810. doi: 10.1097/MEG.0b013e328354044a. [DOI] [PubMed] [Google Scholar]
- Beck J., Garcia R., Heiss G., Vokonas P.S., Offenbacher S. Periodontal disease and cardiovascular disease. J. Periodontol. 1996 Oct;67(10 Suppl):1123–1137. doi: 10.1902/jop.1996.67.10s.1123. [DOI] [PubMed] [Google Scholar]
- D'Aiuto F., Parkar M., Andreaou G., Brett P.M., Ready D., Tonetti M.S. Periodontitis and atherogenesis: causal association or simple coincidence? J. Clin. Periodontol. 2004 May;31:402–411. doi: 10.1111/j.1600-051X.2004.00580.x. [DOI] [PubMed] [Google Scholar]
- Dye B.A., Herrera-Abreu M., Lerche-Sehm J., Vlachojannis C., Pikdoken L., Pretzl B., Schwartz A., Papapanou P.N. Serum antibodies to periodontal bacteria as diagnostic markers of periodontitis. J. Periodontol. 2009 Apr;80(4):634–647. doi: 10.1902/jop.2009.080474. [DOI] [PubMed] [Google Scholar]
- Eke P.I. Probing depth is not a reliable predictor for changes in periodontitis. J. Evid. Base Dent. Pract. 2013 Sep;13(3):107–108. doi: 10.1016/j.jebdp.2013.07.009. [DOI] [PubMed] [Google Scholar]
- Endo Y., Tomofuji T., Ekuni D., Irie K., Azuma T., Tamaki N., Yamamoto T., Morita M. Experimental periodontitis induces gene expression of proinflammatory cytokines in liver and white adipose tissues in obesity. J. Periodontol. 2010 Apr;81(4):520–526. doi: 10.1902/jop.2009.090574. [DOI] [PubMed] [Google Scholar]
- Furuichi Y., Shimotsu A., Ito H., Namariyama Y., Yotsumoto Y., Hino Y., Mishige Y., Inoue M., Izumi Y. Associations of periodontal status with general health conditions and serum antibody titers for Porphyromonas gingivalis and Actinobacillus actinomycetemcomitans. J. Periodontol. 2003 Oct;74(10):1491–1497. doi: 10.1902/jop.2003.74.10.1491. [DOI] [PubMed] [Google Scholar]
- Harris R., Card T.R., Delahooke T., Aithal G.P., Guha I.N. Obesity is the most common risk factor for chronic liver disease: results from a risk stratification pathway using transient elastography. Am. J. Gastroenterol. 2009 Nov;114(11):1744–1752. doi: 10.14309/ajg.0000000000000357. [DOI] [PubMed] [Google Scholar]
- Horibe M., Watanabe H., Ishikawa I. Effect of periodontal treatments on serum IgG antibody titers against periodontopathic bacteria. J. Clin. Periodontol. 1995 Jul;22(7):510–515. doi: 10.1111/j.1600-051x.1995.tb00798.x. [DOI] [PubMed] [Google Scholar]
- Inagaki K., Kurosu Y., Kamiya T., Kondo F., Yoshinari N., Noguchi T., Krall E.A., Garcia R.I. Low metacarpal bone density, tooth loss, and periodontal disease in Japanese women. J. Dent. Res. 2001 Sep;80(9):1818–1822. doi: 10.1177/00220345010800090901. [DOI] [PubMed] [Google Scholar]
- Iwasaki M., Taylor G.W., Nakamura K., Yoshihara A., Miyazaki H. Association between low bone mineral density and clinical attachment loss in Japanese post-menopausal females. J. Periodontol. 2013 Dec;84(12):1708–1716. doi: 10.1902/jop.2013.120613. [DOI] [PubMed] [Google Scholar]
- Iwasaki T., Hirose A., Azuma T., Ohashi T., Watanabe K., Obora A., Deguchi F., Kojima T., Isozaki A., Tomofuji T. Correlation between ultrasound-diagnosed non-alcoholic fatty liver and periodontal condition in a cross-sectional study in Japan. Sci. Rep. 2018 May 14;8(1):7496. doi: 10.1038/s41598-018-25857-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James O.F., Day C.P. Non-alcoholic steatohepatitis (NASH): a disease of emerging identity and importance. J. Hepatol. 1998 Sep;29(3):495–501. doi: 10.1016/s0168-8278(98)80073-1. [DOI] [PubMed] [Google Scholar]
- Kamada Y., Kiso S., Yoshida Y., Chatani N., Kizu T., Hamano M., Tsubakio M., Takemura T., Ezaki H., Hayashi N., Takehara T. Estrogen deficiency worsens steatohepatitis in mice fed high-fat and high-cholesterol diet. Am. J. Physiol. Gastrointest. Liver Physiol. 2011 Dec;301(6):G1031–G1043. doi: 10.1152/ajpgi.00211.2011. [DOI] [PubMed] [Google Scholar]
- Kojima T., Yano K., Ishikawa I. Relationship between serum antibody levels and subgingival colonization of Porphyromonas gingivalis in patients with various types of periodontitis. J. Periodontol. 1997 Jul;68(7):618–625. doi: 10.1902/jop.1997.68.7.618. [DOI] [PubMed] [Google Scholar]
- Kudo C., Naruishi K., Maeda H., Abiko Y., Hino T., Iwata M., Mitsuhashi C., Murakami S., Nagasawa T., Nagata T., Yoneda S., Nomura Y., Noguchi T., Numabe Y., Ogata Y., Sato T., Shimauchi H., Yamazaki K., Yoshimura A., Takashiba S. Assessment of the plasma/serum IgG test to screen for Periodontitis. J. Dent. Res. 2012 Dec;91(12):1190–1195. doi: 10.1177/0022034512461796. [DOI] [PubMed] [Google Scholar]
- Lamster I.B., Pagan M. Periodontal disease and the metabolic syndrome. Int. Dent. J. 2017 Apr;67(2):67–77. doi: 10.1111/idj.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loos B.G., Craandijk J., Hoek F.J., Wertheim-van Dillen P.M., van der Velden U. Elevation of systemic markers related to cardiovascular diseases in the peripheral blood of periodontitis patients. J. Periodontol. 2000 Oct;71(10):1528–1534. doi: 10.1902/jop.2000.71.10.1528. [DOI] [PubMed] [Google Scholar]
- Marchesini G., Bugianesi E., Forlani G., Cerrelli F., Lenzi M., Manini R., Natale S., Vanni E., Villanova N., Melchionda N., Rizzetto M. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003 Apr;37(4):917–923. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- Morita T., Yamazaki Y., Fujiharu C., Ishii T., Seto M., Nishinoue N., Sasaki Y., Kawato T., Motohashi M., Maeno M. Serum γ-glutamyltransferase level is associated with periodontal disease independent of drinking habits in Japanese adults. Med. Sci. Monit. 2014 Oct 31;20:2109–2116. doi: 10.12659/MSM.891204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita T., Yamazaki Y., Mita A., Takada K., Seto M., Nishinoue N., Sasaki Y., Motohashi M., Maeno M. A cohort study on the association between periodontal disease and the development of metabolic syndrome. J. Periodontol. 2010 Apr;81(4):512–519. doi: 10.1902/jop.2010.090594. [DOI] [PubMed] [Google Scholar]
- Murayama Y., Satoh S., Oka T., Imanishi J., Noishiki Y. Reduction of the antigenicity and immunogenicity of xenografts by a new cross-linking reagent. ASAIO (Am. Soc. Artif. Intern. Organs) Trans. 1988 Jul-Sep;34(3):546–549. [PubMed] [Google Scholar]
- Nakahara T., Hyogo H., Ono A., Nagaoki Y., Kawaoka T., Miki D., Tsuge M., Hiraga N., Hayes C.N., Hiramatsu A., Imamura M., Kawakami Y., Aikata H., Ochi H., Abe-Chayama H., Furusho H., Shintani T., Kurihara H., Miyauchi M., Takata T., Arihiro K., Chayama K. Involvement of Porphyromonas gingivalis in the progression of non-alcoholic fatty liver disease. J. Gastroenterol. 2018 Feb;53(2):269–280. doi: 10.1007/s00535-017-1368-4. [DOI] [PubMed] [Google Scholar]
- Nakajima T., Honda T., Domon H., Okui T., Kajita K., Ito H., Takahashi N., Maekawa T., Tabeta K., Yamazaki K. Periodontitis-associated up-regulation of systemic inflammatory mediator level may increase the risk of coronary heart disease. J. Periodontal. Res. 2010 Feb;45(1):116–122. doi: 10.1111/j.1600-0765.2009.01209.x. [DOI] [PubMed] [Google Scholar]
- Nakajima M., Arimatsu K., Kato T., Matsuda Y., Minagawa T., Takahashi N., Ohno H., Yamazaki K. Oral administration of P. gingivali induces dysbiosis of gut microbiota and impaired barrier function leading to dissemination of enterobacteria to the liver. PLoS One. 2015 Jul 28;10(7) doi: 10.1371/journal.pone.0134234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibali L., Tatarakis N., Needleman I., Tu Y.K., D’Aiuto F., Rizzo M., Donos N. Clinical review: association between metabolic syndrome and periodontitis: a systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2013 Mar;98(3):913–920. doi: 10.1210/jc.2012-3552. [DOI] [PubMed] [Google Scholar]
- Niigata Prefecture . 2017. Statistics of Sado City.http://www.pref.niigata.lg.jp/sado_kenko/1204737334720.html [Google Scholar]
- Offenbacher S., Madianos P.N., Champagne C.M., Southerland J.H., Paquette D.W., Williams R.C., Slade G., Beck J.D. Periodontitis-atherosclerosis syndrome: an expanded model of pathogenesis. J. Periodontal. Res. 1999 Oct;34(7):346–352. doi: 10.1111/j.1600-0765.1999.tb02264.x. [DOI] [PubMed] [Google Scholar]
- Page R.C. The pathobiology of periodontal diseases may affect systemic diseases: inversion of a paradigm. Ann. Periodontol. 1998 Jul;3(1):108–120. doi: 10.1902/annals.1998.3.1.108. [DOI] [PubMed] [Google Scholar]
- Pischon N., Heng N., Bernimoulin J.P., Kleber B.M., Willich S.N., Pischon T. Obesity, inflammation, and periodontal disease. J. Dent. Res. 2007 May;86(5):400–409. doi: 10.1177/154405910708600503. [DOI] [PubMed] [Google Scholar]
- Rakha E.A., Adamson L., Bell E., Neal K., Ryder S.D., Kaye P.V., Aithal G.P. Portal inflammation is associated with advanced histological changes in alcoholic and non-alcoholic fatty liver disease. J. Clin. Pathol. 2010 Sep;63(9):790–795. doi: 10.1136/jcp.2010.079145. [DOI] [PubMed] [Google Scholar]
- Sakai Y., Shimauchi H., Ito H.O., Kitamura M., Okada H. Porphyromonas gingivalis-specific IgG subclass antibody levels as immunological risk indicators of periodontal bone loss. J. Clin. Periodontol. 2001 Sep;28(9):853–859. doi: 10.1034/j.1600-051x.2001.028009853.x. [DOI] [PubMed] [Google Scholar]
- Scannapieco F.A., Bush R.B., Paju S. Associations between periodontal disease and risk for atherosclerosis, cardiovascular disease, and stroke. A systematic review. Ann. Periodontol. 2003 Dec;8(1):38–53. doi: 10.1902/annals.2003.8.1.38. [DOI] [PubMed] [Google Scholar]
- Saito T., Shimazaki Y. Metabolic disorders related to obesity and periodontal disease. Periodontol. 2000. 2007;43:254–266. doi: 10.1111/j.1600-0757.2006.00186.x. [DOI] [PubMed] [Google Scholar]
- Saito T., Shimazaki Y., Koga T., Tsuzuki M., Ohshima A. Relationship between periodontitis and hepatic condition in Japanese women. J. Int. Acad. Periodontol. 2006 Jul;8(3):89–95. [PubMed] [Google Scholar]
- Sakurai S.I., Yamada S.I., Karasawa I., Sakurai A., Kurita H. A longitudinal study on the relationship between dental health and metabolic syndrome in Japan. J. Periodontol. 2019 Jul;90(7):728–746. doi: 10.1002/JPER.18-0523. [DOI] [PubMed] [Google Scholar]
- Tomás I., Diz P., Tobías A., Scully C., Donos N. Periodontal health status and bacteraemia from daily oral activities: systematic review/meta-analysis. J. Clin. Periodontol. 2012 Mar;39(3):213–228. doi: 10.1111/j.1600-051X.2011.01784.x. [DOI] [PubMed] [Google Scholar]
- Wang X.F., Yue M. Relationship between alcohol consumption and clinical manifestation of patients with fatty liver: a single-center study. Hepatobiliary Pancreat. Dis. Int. 2011 Jun;10(3):276–279. doi: 10.1016/s1499-3872(11)60046-5. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Cho Y.D. Periodontal disease and metabolic syndrome: a qualitative critical review of their association. Arch. Oral Biol. 2014 Aug;59(8):855–870. doi: 10.1016/j.archoralbio.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Expert Consultation Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004 Jan 10;363(9403):157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- Xin G., Qin S., Wang S., Wang X., Zhang Y., Wang J. Sex hormone affects the severity of non-alcoholic steatohepatitis through the MyD88-dependent IL-6 signaling pathway. Exp. Biol. Med. 2015 Oct;240(10):1279–1286. doi: 10.1177/1535370215570189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki K., Honda T., Oda T., Ueki-Maruyama K., Nakajima T., Yoshie H., Seymour G.J. Effect of periodontal treatment on the C-reactive protein and proinflammatory cytokine levels in Japanese periodontitis patients. J. Periodontal. Res. 2005 Feb;40(1):53–58. doi: 10.1111/j.1600-0765.2004.00772.x. [DOI] [PubMed] [Google Scholar]
- Yoneda M., Naka S., Nakano K., Wada K., Endo H., Mawatari H., Imajo K., Nomura R., Hokamura K., Ono M., Murata S., Tohnai I., Sumida Y., Shima T., Kuboniwa M., Umemura K., Kamisaki Y., Amano A., Okanoue T., Ooshima T., Nakajima A. Involvement of a periodontal pathogen, Porphyromonas gingivalis on the pathogenesis of non-alcoholic fatty liver disease. BMC Gastroenterol. 2012 Feb 16;12:16. doi: 10.1186/1471-230X-12-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara A., Seida Y., Hanada N., Miyazaki H. A longitudinal study of the relationship between periodontal disease and bone mineral density in community-dwelling older adults. J. Clin. Periodontol. 2004 Aug;31(8):680–684. doi: 10.1111/j.1600-051X.2004.00548.x. [DOI] [PubMed] [Google Scholar]