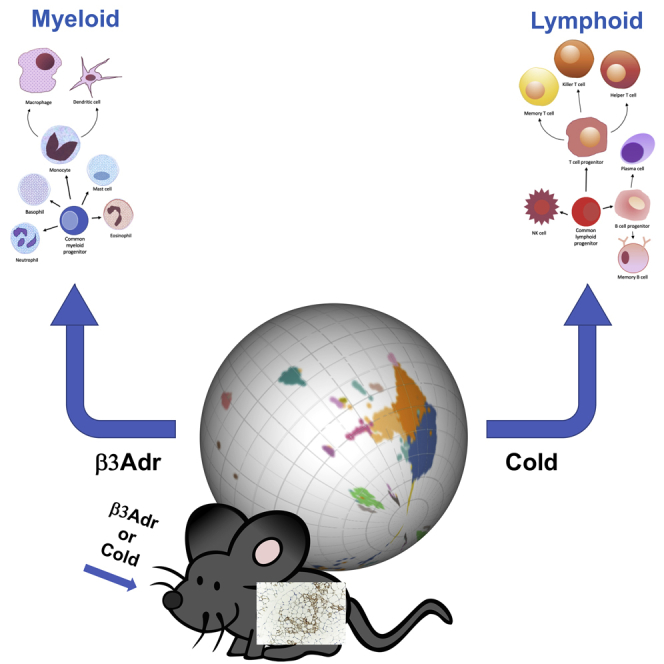
Summary
White adipose tissue (WAT) is a dynamic tissue, which responds to environmental stimuli and dietary cues by changing its morphology and metabolic capacity. The ability of WAT to undergo a beige remodeling has become an appealing strategy to combat obesity and its comorbidities. Here, by using single-cell RNA sequencing, we provide a comprehensive atlas of the cellular dynamics during beige remodeling. We reveal drastic changes both in the overall cellular composition and transcriptional states of individual cell subtypes between Adrb3- and cold-induced beiging. Moreover, we demonstrate that cold induces a myeloid to lymphoid shift of the immune compartment compared to Adrb3 activation. Further analysis showed that, Adrb3 stimulation leads to activation of the interferon/Stat1 pathways favoring infiltration of myeloid immune cells, while repression of this pathway by cold promotes lymphoid immune cell recruitment. These findings highlight that pharmacological mimetics may not provide the same beneficial effects as physiological stimuli.
Subject Areas: Biological Sciences, Endocrinology, Omics, Transcriptomics
Graphical Abstract
Highlights
-
•
ScSeq reveals an extensive remodeling during browning of white adipose tissue
-
•
Cold and β3Adr agonist treatment results in distinct brown/beige remodeling
-
•
Cold induces a myeloid to lymphoid shift of the immune compartment
-
•
β3Adr agonism leads to an interferon/Stat response and infiltration of myeloid cells
Biological Sciences; Endocrinology; Omics; Transcriptomics
Introduction
Adipose tissue is a central metabolic organ for whole-body energy homeostasis. An imbalance between energy intake and energy expenditure increases adiposity and can lead to severe metabolic disease (Sun et al., 2011). White adipose tissue (WAT) plays a key role as a reservoir for triglyceride storage, whereas brown adipose tissue (BAT) dissipates energy as heat through mitochondrial uncoupling. Under appropriate stimulation, such as cold exposure or beta-3-adrenergic receptor (ADRB3) stimulation, WAT can adopt a thermogenic phenotype, sustained by emergence of uncoupling protein 1 (UCP1) expressing cells (Kajimura et al., 2015). These cells, called beige or brite fat cells, share the same energy-burning capacity as BAT through substrate oxidation but present a distinct molecular and developmental origin. Increasing whole-body thermogenic capacity by activating BAT and promoting beige cells emergence may represent a promising strategy to counteract the development of obesity and diabetes (Cannon and Nedergaard, 2004; Bartelt et al., 2011). Indeed, activation of thermogenesis plays a critical role in promoting a shift in energy expenditure in obese and type 2 diabetes (T2D) individuals through a potent glucose and lipid clearance to fuel thermogenesis. Essentially, all studies to date show that beiging of WAT prevents high-fat, diet-induced insulin resistance and weight gain, resulting in positive metabolic indicators, such as insulin sensitivity and euglycemia (Berbée et al., 2015). Additionally, adult human BAT has been identified with more of a brite/beige character than classic rodent brown fat (Sharp et al., 2012; Cypess et al., 2009). Therefore, a better understanding of mechanisms controlling beige adipogenesis could lead to the development of new therapies for metabolic diseases.
WAT is a complex organ consisting of a mixture of mature adipocytes and stromal vascular cells (SVCs). SVCs, comprising 80% of WAT cells, are a dynamic and complex assortment of resident immune cells, vascular cells, mesenchymal stem cells (MSCs), and pre-adipocytes that can change with development and WAT remodeling (Eto et al., 2009). Furthermore, these changes in cell population can play an important role in the capacity of the tissue to respond to the metabolic needs of the body (Kahn et al., 2019; Choe et al., 2016). While a lot of effort has been devoted to defining the cellular plasticity during obesity, little is known about the landscape of these changes during early beige adipogenesis. Although some studies have attempted to define beige adipogenesis, the focus has been on characterizing progenitor cell origin and fate decisions using either mouse lineage tracing models or cell sorting (Rajbhandari et al., 2019; Vishvanath et al., 2016; Sanchez-Gurmaches and Guertin, 2014). Moreover, previous investigations have used an ADRB3 activator (CL316,243) as the tool to model WAT response to cold (Lee et al., 2017; Burl et al., 2018; Rajbhandari et al., 2019). Indeed, ADRB3 activation in vivo by CL 316,243 (CL) provides a means to rapidly induce WAT beiging; however, whether CL induces the same adipose tissue remodeling as cold exposure remains to be determined. Herein, we provide a comprehensive atlas of WAT SVC cellular subtypes and address the change in complexity of the tissue during early response to cold and CL using single-cell RNA sequencing (scRNAseq) technology. Our results identify critical cell subpopulations and their dynamic changes that occur following cold or CL treatment. In combination with flow cytometry, we demonstrate that immune cells with a myeloid origin expand in response to CL treatment while cold exposure leads to expansion of lymphoid cells, mainly B cells, CD4, and CD8 T cells. Mechanistically, this immune shift is likely controlled by activation of the interferon pathway and Stat-1 phosphorylation.
Results
Cold and β3Adr Agonist Treatment Lead to Distinct Beige Remodeling
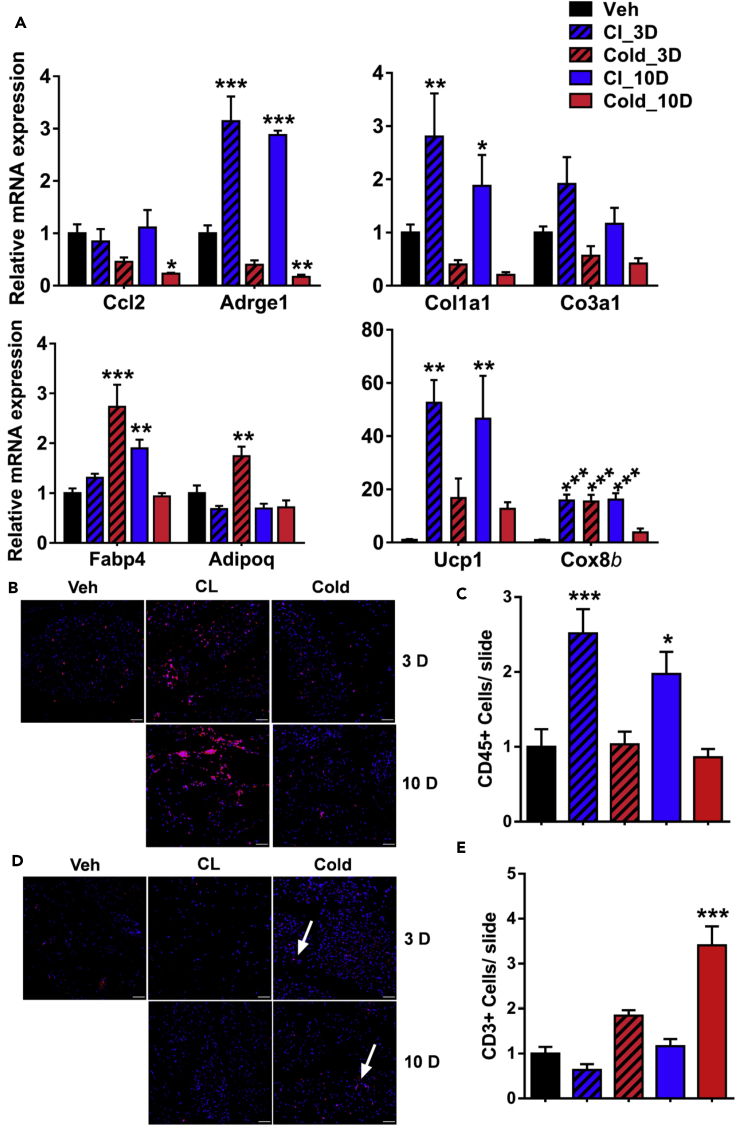
Histological analysis of C57BL/6J mice treated with Adrb3 agonist (CL316,243; CL) or cold for either 3 days or 10 days led to a comparable level of beiging within the subcutaneous inguinal WAT (iWAT) (Figure S1A). However, gene expression analysis of immune markers within the SVC fraction showed a decrease of Ccl2; the monocytes/macrophages chemoattract cytokine in cold conditions while no changes were observed in response to CL at both 3 and 10 days (Figure 1A). In agreement with these results, we found that Adrge1, a macrophage marker, was increased in CL-treated mice while decreased by cold exposure at both monitored time points (Figure 1A). Moreover, extracellular matrix (ECM) markers such as Col1a1 and Col3a1 were increased in response to CL and decreased following cold exposure. Interestingly, adipogenesis markers Fabp4 and adiponectin were only increased by cold exposure at 3 days (Figure 2A), and thermogenic markers were more responsive to CL treatment than cold. To gain more insight into the differences between cold- and Adrb3-induced immune response, we stained for CD45, a common immune cell marker (Figure 1B). We found that CL increases immune cell infiltration at both 3 and 10 days, whereas no changes were observed with cold exposure (Figures 1B and 1C). Surprisingly, we found that more T cells were observed following cold exposure than CL treatment at both monitored times (Figures 1D and 1E). Although both CL and cold induce beiging, our data suggest that the mechanisms leading to the beige phenotype have an immune component.
Figure 1.
CL and Cold Treatment Lead to Distinct Beige Remodeling
All experiments were carried out following 3 days of in vivo CL316,234 or cold exposure.
(A) Real-time PCR analysis of relevant immune (top left), ECM (top right), adipogenesis (bottom left), and thermogenesis markers (bottom right) in the SVF from iWAT.
(B) Representative image of CD45 immunofluorescent staining.
(C) Quantification of CD45 + cells (n = 5).
(D) Representative image of CD3 immunofluorescent staining.
(E) Quantification of CD453+ cells (n = 5).
Data are presented as mean ± SEM. p values. n = 5–6 animals in each group; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
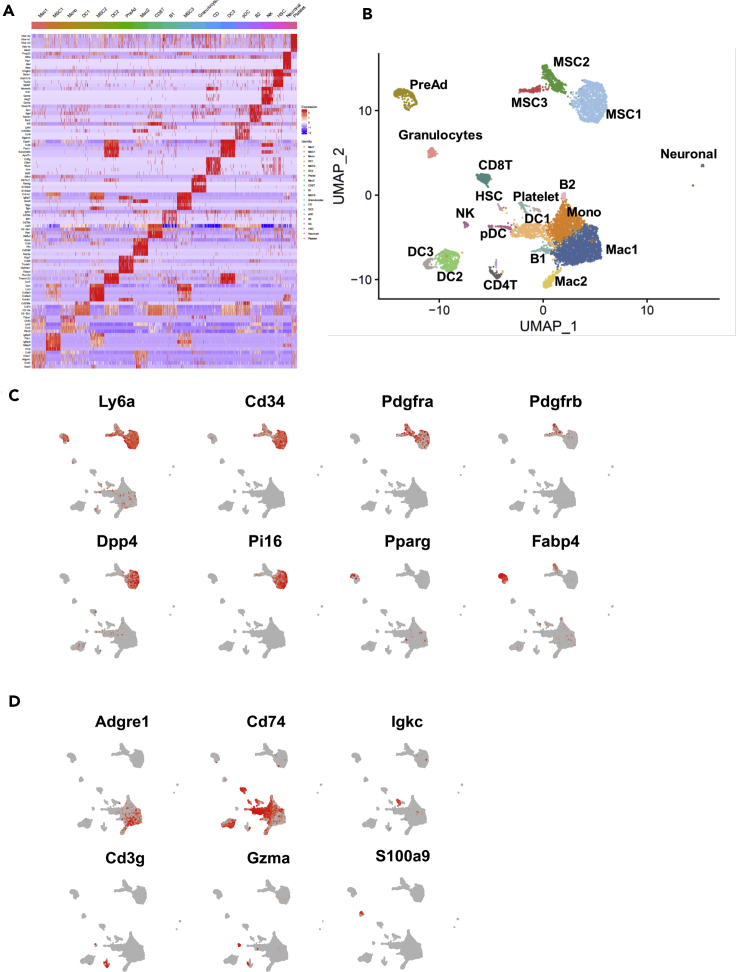
Figure 2.
Single Cell Sequencing Reveals Cellular Heterogeneity of the SVF from iWAT
(A) Gene expression heatmap showing the top 5 most differentially expressed (DE) genes (ordered by) used for biological identification of each cluster compared to all other clusters. Genes are represented in rows and ordered by decreasing p value and cell clusters in columns.
(B–D) (B) UMAP plots of iWAT SVF populations from combined data set of control, CL-, and cold-treated mice for 3 days representation, which identified 20 clusters from 6022 cells. UMAP expression plots of representative DE genes displaying expression of known as in (C) adipocyte progenitors' cell markers and in (D) immune cell markers overlaid in red across all populations.
Scale bars represent z-test-normalized gene expression in (A) and gene counts in (C and D). MSCs, mesenchymal stem cells; PreAd, committed pre-adipocytes; Mac1, M1 macrophages; Mac2, M2 macrophages; Mono, monocytes; DC, dendritic cells; pDC, plasmacytoid dendritic cell; CD4T, CD4+ T cells; CD8T, CD8+ T cells; B, B cells; NKs, natural killer cells; HSCs, myeloid progenitor cells.
Single Cell Sequencing Reveals an Extensive Remodeling of Stroma Vascular Compartment during Browning/Beiging of WAT
To understand the full extent of the divergent cellular response to CL and cold, we performed single cell RNA sequencing (scRNA-seq) analysis of the SVC fraction isolated from iWAT of control, CL-, or cold-treated mice for 3 days to capture early changes. Data from all three treatments were pooled together to identify subpopulations within the captured cells. Unsupervised clustering using gene markers singled out 20 distinct cell clusters (Figure 2A). We assigned putative biological identities to each cluster by manual annotation using established gene expression patterns, as well as by interrogating a gene expression atlas (Su et al., 2004; Ravasi et al., 2010). The annotation resulted in several groups of cells including pre-adipocytes, mesenchymal stem cells, immune cells, and neuronal cells (Figure 2B). Previous reports identified two to three cellular subpopulations with an adipogenic potential that were defined as mesenchymal progenitor cells (MSCs) (Merrick et al., 2019; Hepler et al., 2018; Burl et al., 2018). However, these data were either generated from Pdrgfrβ+ sorted cells or assuming that canonical MSC markers Cd34, Pdgfrα, Lys6a (Sca1) are co-expressed within the same cell. Because CD34 and Sca1 are the major markers of stemness for most of mice progenitors, we plotted them across our single-cell data to discriminate between possible MSCs and other cells subtype. We found that four clusters which we named MSC1, MSC2, MSC3, and pre-adipocytes express both markers (Figures 2B and 2C). Pdgfrα was expressed in most of the cells in the three MSC subpopulations and was highly expressed in MSC2 cluster. Dpp4 and Pi16, markers previously proposed as interstitial progenitors, were exclusively expressed in MSC1. Fabp4 was expressed in both MSC2 and pre-adipocytes, and Ppary was exclusively expressed by the pre-adipocyte cluster suggesting that the MSC2 state may precede the pre-adipocytes state (Figure 2C). Genes encoding ECM components were expressed at different levels within the four clusters, with MSC2 cluster expressing the largest number of ECM-related genes (Figure 2A and Table S1). Interestingly, collagen-type expression was found to be different between populations. Indeed, Col14a was identified as a marker for MSC1 cluster, Col15a as a marker for MSC2 cluster, while both marked MSC3 cluster (Table S1).
Our merged data from the 3 conditions allowed for the identification of 13 distinct immune clusters (Figure 2B and Table S1). We used unsupervised annotation and cell-type-specific markers to interpret and identify the resulting 13 immune clusters based on literature searches and the Immunological Genome Project database (Figure 2D) (Jojic et al., 2013; Yoshida et al., 2019). We identified 3 clusters that express macrophage markers such as Adgre1 (Figure 2D). Proinflammatory cytokines such Ccl2, Ccl6, Ccl9, Ccl12, Cxcl2 were highly expressed in M1 macrophage cluster (Mac1); Mrc1, Cd 209, Lyve1, Cd36, and Mmp9 expressing macrophages were annotated as M2 macrophages (Mac2), and a mixed monocyte/macrophage cluster was marked by the expression of Ccr2, Lyz2, Cy6c2, Ms4a4c, Tyrobp, and Cd52 (Figure 2A and Table S1). Four clusters were annotated as dendritic cells (DC1, DC2, DC3, and pDC) expressing the common DC cell markers such as CD74 and two B-cell clusters expressing genes such as Igkc (Figures 2A and 2D, Table S1). We also identified Cd4 T cells (CD4T), Cd8 T cells (CD8T), natural killer cells (NKs), and a mixed population of granulocytes (Figures 2A, 2B and 2D, Table S1). In conclusion, our scRNAseq revealed twenty subpopulations of cells with distinct markers, although the functions of these subsets remain to be elucidated. These findings provide a point of reference for examining occurrences in each cell subset during beiging of adipose tissue.
Cold and b3Adr Agonist Differentially Alter the Adipose Resident Immune Compartment
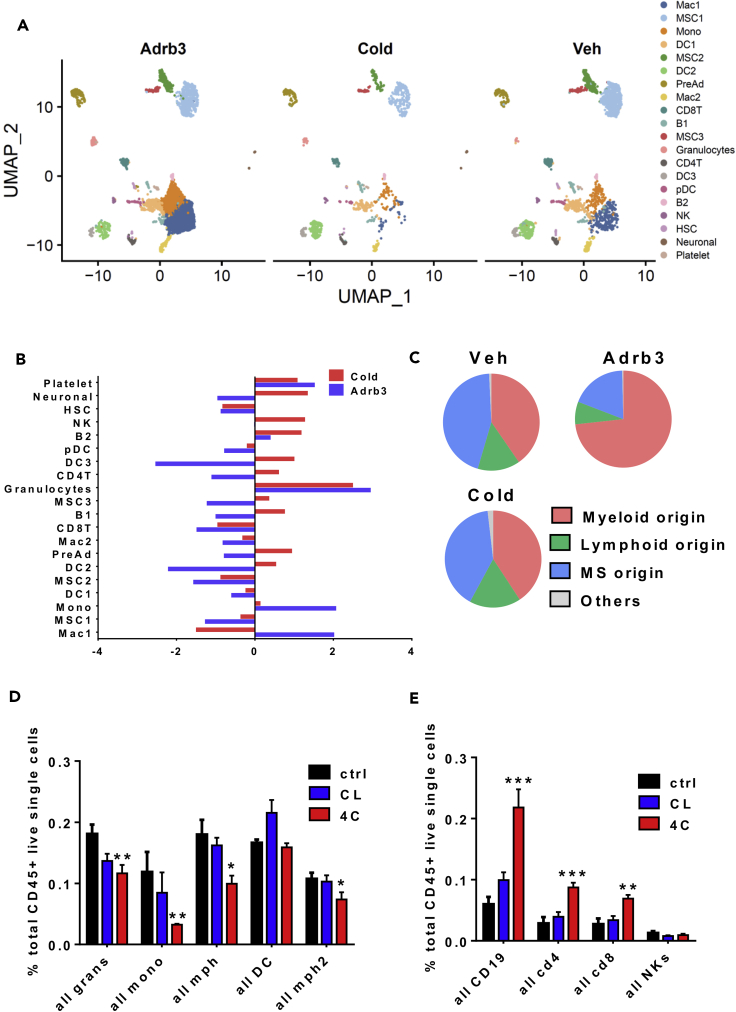
To commence such an examination, we performed a side-by-side comparison of the control, CL-, and cold-treated data sets. While the data showed that the twenty clusters are represented within the three conditions, our analysis revealed drastic changes both in the overall cellular composition and transcriptional states of individual cell subtypes (Figure 3A and 3B). Initial analysis of Uniform Manifold Approximation and Projection (UMAP) maps of the data showed that the pre-adipocyte population increased in CL condition compared to cold and control treatment (Figure 3A). However, normalization of the data to total number of cells sequenced per condition revealed that only cold increased the pre-adipocyte population (Figures 3B and S2A). MSC3 cluster showed the same pattern while both MSC1 and MSC2 clusters were reduced by both cold and CL. However, the reduction was more prominent with CL than cold (Figure 3B). Normalized macrophages and monocyte populations were increased by CL and reduced by cold. In contrast, CD4T cells, NKs, and all B cells were increased by cold and reduced by CL treatment (Figure 3B). These results suggest a dissimilar immune cell response to CL and cold. Interestingly, the cell populations that are increased with CL are mostly from a myeloid origin, whereas cold promotes an increase of immune cells of lymphoid origin (Figure 3C). We verified the results obtained by scRNAseq using flow cytometry of the iWAT stromal vascular fraction (SVF) from mice treated with a vehicle, CL, or cold for 3 days. We used a panel of antibodies that allowed detailed assessment of multiple immune subsets previously identified by the scRNAseq including B cells, CD4+ and CD8+ T cells, NK cells, DC cells, granulocytes, M1 and M2 macrophages, and monocytes (Figures S3B and S3C). In concordance with previous results, immune cells with a myeloid origin were reduced by cold compared to vehicle- or CL-treated mice (Figure 3D). More importantly, cold induced lymphoid origin immune cells including B cells (CD19+), CD4, and CD8 T cells compared to the other treatments. All together, these data suggest that cold- and CL-induced beiging involves a different immune remodeling leading to the same level of UCP1+ adipocytes (Figure S1A). Indeed, activation of Adrb3 induces a specific activation of cells with a myeloid origin such as macrophages while cold leads to increased recruitment of immune cells with a lymphoid origin, suggesting that CL treatment is not able to activate the full immune system to mimic the cold.
Figure 3.
CL and Cold Differently Alter the Adipose Resident Immune Compartment
(A) Side-by-side UMAP plots of iWAT SVF populations from control, CL-, or cold-treated mice for 3 days.
(B) Bar chart showing population fold changes in relative abundance of each cluster induced by 3 days of CL or cold treatment compared to vehicle-treated mice.
(C) Proportions of cell populations grouped by the cellular origin.
(D) Quantification of frequency of selected myeloid cell subsets in the iWAT depot after 3 days of CL or cold treatment compared to vehicle-treated mice (n = 6) performed by flow cytometry.
(E) Quantification of frequency of selected lymphoid cell subsets in the iWAT depot after 3 days of CL or cold treatment compared to vehicle-treated mice (n = 6) performed by flow cytometry.
Data are presented as mean ± SEM. p values. n = 5–6 animals in each group; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
b3Adr Agonist-Induced Browning/Beiging Lead to an Interferon/Stat1 Response
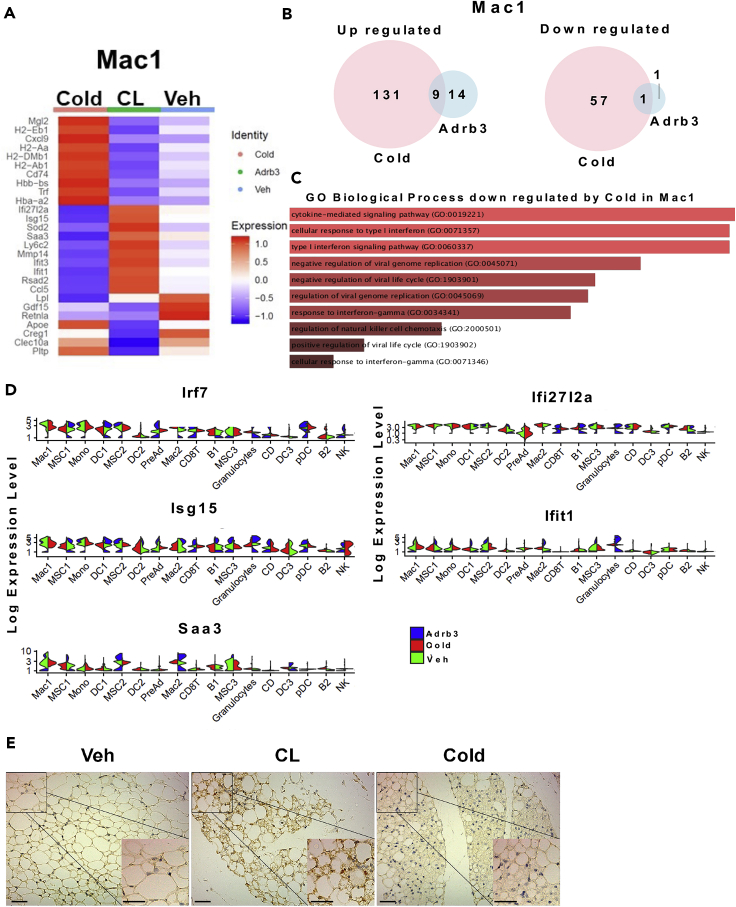
To gain more insight into the mechanisms controlling the shift from myeloid to lymphoid immune cell recruitments upon cold exposure compared to CL, we performed differential gene expression analyses between the same clusters using the treatments as a variable. The results showed that both immune cells with a myeloid origin or a lymphoid origin activate a different set of genes in response to either CL or cold (Figures S4A and S4B). Because the activation of the monocytes and macrophages precedes the activation of lymphoid cells such as T cells and B cells, we focused on macrophages. Differential analysis revealed that cold induced the upregulation of 140 genes while CL upregulated 23 genes, only 9 of those were overlapping with cold. We also identified 57 genes downregulated by cold while CL only decreased 2 genes (Figure 4A and 4B). Gene ontology analysis of genes downregulated by cold showed an enrichment of genes associated with biological processes including cytokine-mediated signaling, cellular response to type 1 interferon, and type 1 interferon signaling pathway (Figure 4D). We next examined the expression of genes induced by interferons such as Irf7, Isg15, Ifit1, and Saa3 across all the identified clusters. Surprisingly, we found that interferon target genes are induced in most immune population regardless of their origin. To further confirm these results, we stained tissue from vehicle-, CL-, or cold-treated mice with antibody against pStat1, an interferon-induced signaling component. Our data showed that CL treatment induced a considerable phosphorylation of Stat1 in SVF cells. Collectively, these results strongly suggest that repression of the interferon/pStat1 pathway controls the shift from myeloid to lymphoid immune cell recruitment during cold exposure compared to CL.
Figure 4.
CL-Induced Beiging Leads to an Interferon/Stat1 Response
(A) Heatmap of differentially expressed genes in Mac1 cluster of iWAT from mice after 3 days of CL or cold treatment.
(B) Venn diagram of up (left) and down (right) regulated genes within Mac1 cluster after 3 days of CL or cold treatment.
(C) GO-driven pathway analysis of DE downregulated gene by cold in Mac1.
(D) Violin plots for the expression of interferon response genes (expression in each cell is shown along with the probability density of gene expression).
(E) Representative images of immunohistochemistry of P-Stat1 (Tyr701) in iWAT section from mice treated for 3 days with vehicle, CL, or cold.
Discussion
In the present study, we reveal a complete atlas of cellular complexity of the SVF during beige remodeling. All cell populations were present within iWAT from control mice, suggesting that cells are at a paused-like state to maintain tissue homeostasis. Cold and CL treatments lead to a large modulation of the cellular composition to achieve a beige phenotype. Previous studies have identified two distinct MSC populations within the epidermal WAT (eWAT) and three MSCs within the iWAT (Merrick et al., 2019; Burl et al., 2018; Hepler et al., 2018). Our current work reveals the existence of four distinct MSC populations harboring an adipogenic potential which express classical stemness markers and can be distinguished by specific markers including different collagen subtypes. The co-existence of the four populations could explain the high adipogenic potential of the iWAT compared to the eWAT. While all MSCs exist within the control mice iWAT, cold and CL treatments lead to major changes both in the cell number and signaling pathways of individual MSC subtypes. Interestingly, pre-adipocytes and MSC3 cluster were increased in response to cold compared to CL, suggesting that cold leads to significantly more expansion of those populations. However, further studies will be needed to determine if there are any differences between cold and CL stimulation in the recruitment potential to adipocyte progenitors.
Large changes within the immune fraction composition were also observed. In agreement with previous reports from both whole tissue RNA sequencing and scRNAseq, we found that Adrb3-induced beiging leads to an increase of macrophage recruitment (Lee et al., 2016; Nguyen et al., 2011; Burl et al., 2018). At a more global scale, immune cells derived from myeloid origin were increased in response to CL compared to cold. In contrast, cold promoted the recruitment of lymphoid originated immune cells including B cells, CD4, and CD8 T cells. This suggests that a shift from myeloid to lymphoid immune cells is an important step to promote the high level of beiging attained in response to cold. Furthermore, it assumes a functional interaction of lymphoid cells with activated MCSs to induce complete beige remodeling. The differences in the origin of the immune cell populations involved in cold- versus Adrb3-induced beiging will be important to address in future studies looking into the immune implications in thermogenesis.
Our results further showed that the interferon/Stat1 signaling pathway is activated by CL suggesting an importance of these pathways in myeloid activation during CL-induced beiging. Previous work on human peripheral blood mononuclear cells showed that interferon synthesis was suppressed by catecholamines and favors a type 2 cytokine through Adrb2 stimulation (Wahle et al., 2005). Furthermore, neural inputs have been shown to increase lymphocyte numbers in vitro and in vivo (Agarwal and Marshall, 2000; Araujo et al., 2019). Other studies have documented a need to suppress Interferon/Stat1 signal transduction pathways and transcription factors downstream of cytokines to drive differentiation of Th subtypes (Naka et al., 2001; Yu et al., 2004) Interestingly, catecholamines have been shown to promote an anti-inflammatory effect by inhibiting Stat1 phosphorylation and favor Th2 cytokine type secretion such as IL13 and IL4 (Ishii et al., 2015). Our single-cell data show that Adrb3 activation alone leads to recruitment of myeloid immune cells and an increase of Stat1 phosphorylation, suggesting that CL is not sufficient to create the cytokine/cellular microenvironment for lymphoid immune recruitment to achieve a cold-like beige remodeling. These studies support a model in which catecholamines released during cold exposure lead to lymphoid immune recruitment through the suppression of the interferon response activated by Adrb3 stimulation alone. Moreover, our results suggest the involvement of different coordinated signaling pathways to induce beige remodeling during cold and open the possibility that different signaling can lead to distinct adipocytes with an equivalent beige phenotype. Those observations revealed that Adrb3-induced beiging mimics a hypermetabolic-like response found in the context of thermal injury or cachexia (Petruzzelli et al., 2014; Sidossis et al., 2015; Patsouris et al., 2015). Indeed, the hypermetabolic response is characterized by a profound increase in free fatty acids and glycerol release from fat and increase in myeloid immune cell infiltration ultimately resulting in significant elevations in resting energy expenditure (Jeschke et al., 2014),. Moreover, Adrb3 activation has been implicated in burn-associated beiging (Kulp et al., 2010; Jeschke et al., 2011). Our data suggest that, although both cold and CL lead to an initial metabolic benefit, prolonged CL exposure is potentially futile and could become devastating, specifically in an already inflamed context such as obesity. More attention should be focused on the immune microenvironments when developing new pharmacological approaches to induce thermogenesis.
In conclusion, these data provide a comprehensive atlas of the cellular dynamics during beige remodeling within WAT. We shed light on the complexity of the immune microenvironment and highlight the differences in the immune cell populations infiltrated in response of Adrb3- and cold-induced beiging. A better understanding of the signaling pathways and the cellular intra-organ communication influencing beige remodeling during Adrb3 and cold stimulation could ultimately lead to novel strategies to increase energy expenditure and protect against obesity.
Limitations of the Study
Our study reveals that cold induces a myeloid to lymphoid shift of the immune compartment compared to Adrb3 activation. We showed that Adrb3 stimulation leads to activation of the interferon/Stat1 signaling pathway suggesting an importance of these pathways in myeloid activation during CL-induced beiging. However, further investigations of myeloid cells are needed to demonstrate the exact mechanism by which interferon/Stat1 signaling is activated by Adrb3 stimulation. Myeloid cells do not express Adrb3, so it is likely that Adrb3 activation leads to secretion of cytokines by adipocytes that in turn activate the interferon/Stat1 pathway. Blocking secretion of these cytokines could be important to induce the recruitment of lymphoid cells. We also do not know the specific cytokines involved in lymphoid cell recruitment and the exact role of these lymphoid cells. We suggest that Adrb2 stimulation by catecholamines released upon cold exposure suppresses interferon/Stat1 signaling and favors the recruitment of lymphoid cells. More in vivo experiments including myeloid cell-specific knockout mice models and/or Adrb2 agonists are needed to demonstrate that activation of Adrb2 is involved in the myeloid to lymphoid shift upon cold exposure.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Stephen R Farmer, Boston University School of Medicine (sfarmer@bu.edu).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
The data sets/code generated during this study are available at GEO accession GSE159966: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE159966.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was supported by the NIH/NIDDK grants DK117161 and DK117163. N.R was supported by the American Heart Association (AHA) fellowship (17POST33660875). We thank Hu Tianmu and Yuriy Alekseyev of the BUSM Single Cell Sequencing Core for their advice and assistance. We also thank the Boston University School of Medicine (BUSM) Flow Cytometry Core Facility for support.
Author Contributions
Conceptualization, N.R. and S.R.F.; Methodology, N.R. and S.R.F.; Investigation, N.R.; Flow cytometry analysis, N.R and A.C.B; Formal Analysis, N.R.; Mouse experiments, N.R, K.D, and B.N.C; Writing – Review & Editing, N.R. and S.R.F.
Declaration of Interests
The authors declare there are no conflicts of interest.
Published: December 18, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101765.
Supplemental Information
References
- Agarwal S.K., Marshall G.D. Beta-adrenergic modulation of human type-1/type-2 cytokine balance. J. Allergy Clin. Immunol. 2000;105:91–98. doi: 10.1016/s0091-6749(00)90183-0. [DOI] [PubMed] [Google Scholar]
- Araujo L.P., Maricato J.T., Guereschi M.G., Takenaka M.C., Nascimento V.M., de Melo F.M., Quintana F.J., Brum P.C., Basso A.S. The sympathetic nervous system mitigates CNS autoimmunity via β2-adrenergic receptor signaling in immune cells. Cell Rep. 2019;28:3120–3130.e5. doi: 10.1016/j.celrep.2019.08.042. [DOI] [PubMed] [Google Scholar]
- Bartelt A., Bruns O.T., Reimer R., Hohenberg H., Ittrich H., Peldschus K., Kaul M.G., Tromsdorf U.I., Weller H., Waurisch C. Brown adipose tissue activity controls triglyceride clearance. Nat. Med. 2011;17:200–205. doi: 10.1038/nm.2297. [DOI] [PubMed] [Google Scholar]
- Berbée J.F.P., Boon M.R., Khedoe P.P.S.J., Bartelt A., Schlein C., Worthmann A., Kooijman S., Hoeke G., Mol I.M., John C. Brown fat activation reduces hypercholesterolaemia and protects from atherosclerosis development. Nat. Commun. 2015;6:6356. doi: 10.1038/ncomms7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burl R.B., Ramseyer V.D., Rondini E.A., Pique-Regi R., Lee Y.-H., Granneman J.G. Deconstructing adipogenesis induced by β3-adrenergic receptor activation with single-cell expression profiling. Cell Metab. 2018;28:300–309.e4. doi: 10.1016/j.cmet.2018.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon B., Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol. Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- Choe S.S., Huh J.Y., Hwang I.J., Kim J.I., Kim J.B. Adipose tissue remodeling: its role in energy metabolism and metabolic disorders. Front. Endocrinol. 2016;7:30. doi: 10.3389/fendo.2016.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess A.M., Lehman S., Williams G., Tal I., Rodman D., Goldfine A.B., Kuo F.C., Palmer E.L., Tseng Y.-H., Doria A. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eto H., Suga H., Matsumoto D., Inoue K., Aoi N., Kato H., Araki J., Yoshimura K. Characterization of structure and cellular components of aspirated and excised adipose tissue. Plast. Reconstr. Surg. 2009;124:1087–1097. doi: 10.1097/PRS.0b013e3181b5a3f1. [DOI] [PubMed] [Google Scholar]
- Hepler C., Shan B., Zhang Q., Henry G.H., Shao M., Vishvanath L., Ghaben A.L., Mobley A.B., Strand D., Hon G.C. Identification of functionally distinct fibro-inflammatory and adipogenic stromal subpopulations in visceral adipose tissue of adult mice. eLife. 2018;7:e39636. doi: 10.7554/eLife.39636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii Y., Yamaizumi A., Kawakami A., Islam A., Choudhury M.E., Takahashi H., Yano H., Tanaka J. Anti-inflammatory effects of noradrenaline on LPS-treated microglial cells: suppression of NFκB nuclear translocation and subsequent STAT1 phosphorylation. Neurochem. Int. 2015;90:56–66. doi: 10.1016/j.neuint.2015.07.010. [DOI] [PubMed] [Google Scholar]
- Jeschke M.G., Gauglitz G.G., Kulp G.A., Finnerty C.C., Williams F.N., Kraft R., Suman O.E., Mlcak R.P., Herndon D.N. Long-term persistance of the pathophysiologic response to severe burn injury. PLoS One. 2011;6:e21245. doi: 10.1371/journal.pone.0021245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeschke M.G., Gauglitz G.G., Finnerty C.C., Kraft R., Mlcak R.P., Herndon D.N. Survivors versus nonsurvivors postburn: differences in inflammatory and hypermetabolic trajectories. Ann. Surg. 2014;259:814–823. doi: 10.1097/SLA.0b013e31828dfbf1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jojic V., Shay T., Sylvia K., Zuk O., Sun X., Kang J., Regev A., Koller D., Immunological Genome Project Consortium, Best A.J. Identification of transcriptional regulators in the mouse immune system. Nat. Immunol. 2013;14:633–643. doi: 10.1038/ni.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn C.R., Wang G., Lee K.Y. Altered adipose tissue and adipocyte function in the pathogenesis of metabolic syndrome. J. Clin. Invest. 2019;129:3990–4000. doi: 10.1172/JCI129187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajimura S., Spiegelman B.M., Seale P. Brown and beige fat: physiological roles beyond heat generation. Cell Metab. 2015;22:546–559. doi: 10.1016/j.cmet.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulp G.A., Herndon D.N., Lee J.O., Suman O.E., Jeschke M.G. Extent and magnitude of catecholamine surge in pediatric burned patients. Shock Augusta Ga. 2010;33:369–374. doi: 10.1097/SHK.0b013e3181b92340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.-H., Kim S.-N., Kwon H.-J., Maddipati K.R., Granneman J.G. Adipogenic role of alternatively activated macrophages in β-adrenergic remodeling of white adipose tissue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016;310:R55–R65. doi: 10.1152/ajpregu.00355.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.-H., Kim S.-N., Kwon H.-J., Granneman J.G. Metabolic heterogeneity of activated beige/brite adipocytes in inguinal adipose tissue. Sci. Rep. 2017;7:39794. doi: 10.1038/srep39794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick D., Sakers A., Irgebay Z., Okada C., Calvert C., Morley M.P., Percec I., Seale P. Identification of a mesenchymal progenitor cell hierarchy in adipose tissue. Science. 2019;364:eaav2501. doi: 10.1126/science.aav2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka T., Tsutsui H., Fujimoto M., Kawazoe Y., Kohzaki H., Morita Y., Nakagawa R., Narazaki M., Adachi K., Yoshimoto T. SOCS-1/SSI-1-deficient NKT cells participate in severe hepatitis through dysregulated cross-talk inhibition of IFN-gamma and IL-4 signaling in vivo. Immunity. 2001;14:535–545. doi: 10.1016/s1074-7613(01)00132-7. [DOI] [PubMed] [Google Scholar]
- Nguyen K.D., Qiu Y., Cui X., Goh Y.P.S., Mwangi J., David T., Mukundan L., Brombacher F., Locksley R.M., Chawla A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–108. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsouris D., Qi P., Abdullahi A., Stanojcic M., Chen P., Parousis A., Amini-Nik S., Jeschke M.G. Burn induces browning of the subcutaneous white adipose tissue in mice and humans. Cell Rep. 2015;13:1538–1544. doi: 10.1016/j.celrep.2015.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruzzelli M., Schweiger M., Schreiber R., Campos-Olivas R., Tsoli M., Allen J., Swarbrick M., Rose-John S., Rincon M., Robertson G. A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab. 2014;20:433–447. doi: 10.1016/j.cmet.2014.06.011. [DOI] [PubMed] [Google Scholar]
- Rajbhandari P., Arneson D., Hart S.K., Ahn I.S., Diamante G., Santos L.C., Zaghari N., Feng A.-C., Thomas B.J., Vergnes L. Single cell analysis reveals immune cell-adipocyte crosstalk regulating the transcription of thermogenic adipocytes. eLife. 2019;8:e49501. doi: 10.7554/eLife.49501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravasi T., Suzuki H., Cannistraci C.V., Katayama S., Bajic V.B., Tan K., Akalin A., Schmeier S., Kanamori-Katayama M., Bertin N. An atlas of combinatorial transcriptional regulation in mouse and man. Cell. 2010;140:744–752. doi: 10.1016/j.cell.2010.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Gurmaches J., Guertin D.A. Adipocyte lineages: tracing back the origins of fat. Biochim. Biophys. Acta. 2014;1842:340–351. doi: 10.1016/j.bbadis.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp L.Z., Shinoda K., Ohno H., Scheel D.W., Tomoda E., Ruiz L., Hu H., Wang L., Pavlova Z., Gilsanz V. Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS One. 2012;7:e49452. doi: 10.1371/journal.pone.0049452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidossis L.S., Porter C., Saraf M.K., Børsheim E., Radhakrishnan R.S., Chao T., Ali A., Chondronikola M., Mlcak R., Finnerty C.C. Browning of subcutaneous white adipose tissue in humans after severe adrenergic stress. Cell Metab. 2015;22:219–227. doi: 10.1016/j.cmet.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su A.I., Wiltshire T., Batalov S., Lapp H., Ching K.A., Block D., Zhang J., Soden R., Hayakawa M., Kreiman G. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc. Natl. Acad. Sci. U S A. 2004;101:6062–6067. doi: 10.1073/pnas.0400782101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K., Kusminski C.M., Scherer P.E. Adipose tissue remodeling and obesity. J. Clin. Invest. 2011;121:2094–2101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishvanath L., MacPherson K.A., Hepler C., Wang Q.A., Shao M., Spurgin S.B., Wang M.Y., Kusminski C.M., Morley T.S., Gupta R.K. Pdgfrβ+ mural preadipocytes contribute to adipocyte hyperplasia induced by high-fat-diet feeding and prolonged cold exposure in adult mice. Cell Metab. 2016;23:350–359. doi: 10.1016/j.cmet.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahle M., Neumann R.P., Moritz F., Krause A., Buttgereit F., Baerwald C.G.O. Beta2-adrenergic receptors mediate the differential effects of catecholamines on cytokine production of PBMC. J. Interferon Cytokine Res. 2005;25:384–394. doi: 10.1089/jir.2005.25.384. [DOI] [PubMed] [Google Scholar]
- Yoshida H., Lareau C.A., Ramirez R.N., Rose S.A., Maier B., Wroblewska A., Desland F., Chudnovskiy A., Mortha A., Dominguez C. The cis-regulatory atlas of the mouse immune system. Cell. 2019;176:897–912.e20. doi: 10.1016/j.cell.2018.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C.-R., Mahdi R.M., Ebong S., Vistica B.P., Chen J., Guo Y., Gery I., Egwuagu C.E. Cell proliferation and STAT6 pathways are negatively regulated in T cells by STAT1 and suppressors of cytokine signaling. J. Immunol. 2004;173:737–746. doi: 10.4049/jimmunol.173.2.737. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets/code generated during this study are available at GEO accession GSE159966: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE159966.