Abstract
Phosphatidylinositol-4,5-bisphosphate (PI-4,5-P2) is critical for synaptic vesicle docking and fusion and generation of the second messengers, diacylglycerol and inositol-1,4,5-trisphosphate. PI-4,5-P2 can be generated by two families of kinases: type 1 phosphatidylinositol-4-phosphate 5-kinases, encoded by PIP5K1A, PIP5K1B and PIP5K1C, and type 2 phosphatidylinositol-5- phosphate 4-kinases, encoded by PIP4K2A, PIP4K2B, and PIP4K2C. While the roles of the type 1 enzymes in brain function have been extensively studied, the roles of the type 2 enzymes are poorly understood. Using selective antibodies validated by genetic deletion of pip4k2a or pip4k2b in mouse brain, we characterized the location of the enzymes, PI5P4Kα and PI5P4Kß, encoded by these genes. In mice, we demonstrate that PI5P4Kα is expressed in adulthood, whereas PI5P4Kß is expressed early in development. PI5P4Kα localizes to white matter tracts, especially the corpus callosum, and at a low level in neurons, while PI5P4Kß is expressed in neuronal populations, especially hippocampus and cortex. Dual labeling studies demonstrate that PI5P4Kα co-localizes with the oligodendrocyte marker, Olig2, whereas PI5P4Kß co-localizes with the neuronal marker, NeuN. Ultrastructural analysis demonstrates that both kinases are contained in axon terminals and dendritic spines adjacent to the synaptic membrane, which support a potential role in synaptic transmission. Immunoperoxidase analysis of macaque and human brain tissue demonstrate a conserved pattern for PI5P4Kα and PI5P4Kß. These results highlight the diverse cell-autonomous expression of PI5P4Kα and PI5P4Kß and support further exploration into their role in synaptic function in the brain.
Keywords: Phosphoinositide, phosphatidylinositol-5-phosphate 4-kinase, PIP4K, brain, neuron, oligodendrocyte, RRID:AB_1127270, RRID:AB_353929, RRID:AB_2164572, RRID:AB_2223210, RRID:AB_10622025, RRID:AB_561049, RRID:AB_10711040, RRID:AB_2269374, RRID:AB_2300649, RRID:AB_1904103, RRID:AB_2096811
GRAPHICAL ABSTRACT

Introduction
The phosphoinositide kinases, along with their corresponding phosphatases and phospholipases, regulate key functions of the phosphoinositide family of lipids. These functions include proliferation and migration, metabolic adaptation to growth acceleration, and survival in the setting of genotoxic stress (Toker, 2002). The type 2 phosphatidylinositol-5-phosphate 4-kinases (PI5P4Ks), composed of a family of 3 isoforms (α, β and γ), catalyze the formation of phosphatidylinositol 4,5-bisphosphate (PI-4,5-P2) by phosphorylating phosphatidylinositol 5-phosphate (PI-5-P) at the 4 position of the inositol ring. These kinases play a role in a variety of disease states, including cancer, insulin signaling, and oxidative stress (Carricaburu et al., 2003; Jones et al., 2006; Keune et al., 2012; Emerling et al., 2013; Jones et al., 2013; Jude et al., 2015; Sharma et al., 2019; Wang et al., 2019).
The PI5P4K family of lipid kinases is currently thought to contribute predominantly to intracellular PI-4,5-P2 formation to regulate dynamics of intracellular signaling pathways. Several studies have now demonstrated that the PI5P4Ks mediate autophagy, particularly during periods of nutrient stress, by promoting autophagosome-lysosome fusion (Emerling et al., 2013; Mackey et al., 2014; Lundquist et al., 2018). These kinases also regulate cellular growth pathways, including the mammalian target of rapamycin (mTOR) and phosphatidylinositol 3-kinase (PI3K) pathways (Carricaburu et al., 2003; Gupta et al., 2013). Furthermore, they regulate early endosomal homeostasis during clathrin-mediated endocytosis, a key step in synaptic vesicle trafficking (Kamalesh et al., 2017). Of particular interest, these enzymes also play a major role in suppressing the activity of the type 1 phosphatidylinositol-4-phosphate 5-kinases through direct binding to these enzymes (Wang et al., 2019). Although the expression and function of these kinases have been explored in oncogenic signaling pathways and in settings of nutrient stress, few studies have examined their role in the brain.
PI-4,5-P2 is a critical mediator of synaptic function in the brain through its role in synaptic vesicle docking and fusion, leading to successful synaptic vesicle release (Honigmann et al., 2013). Dysfunction in PI-4,5-P2 production is associated with enhanced synaptic depression, a reduced readily releasable pool of vesicles, delayed endocytosis, and reduced recycling (Di Paolo et al., 2004). In the brain, most PI-4,5-P2 is created through phosphorylation of PI-4-P by the type 1 PIP kinase, PI4P5Kγ (Volpicelli-Daley et al., 2010). However, this work did not investigate the expression or localization of the PI5P4Ks in the brain.
Prior studies have demonstrated that the PI5P4Ks are highly expressed in the mouse brain as compared to other organs. PI5P4Kβ localizes to the ventricular zone of the developing mouse brain, which may suggest its involvement in the stem cell niche in normal brain (Akiba et al., 2002). PI5P4Kγ, like PI4P5Kγ, is expressed in neurons and localizes to intracellular vesicles (Clarke et al., 2009). PI5P4Kα is expressed in glial cells and causes dysregulation of the glutamate transporter, excitatory amino acid transporter 3 (EAAT3) (Fedorenko et al., 2009), expressed in neurons, glial cells, and glioma. Dysregulation of the EAAT3 system has been associated with numerous neurodegenerative diseases, epilepsy, and schizophrenia (Danbolt, 2001), with large genome-wide association studies linking PIP4K2A polymorphisms to increased inherited risk of schizophrenia (Schwab et al., 2006; He et al., 2007; Rethelyi et al., 2010; Thiselton et al., 2010). While germline deletion of individual pip4k2a, pip4k2b or pip4k2c genes results in viable mice with relatively subtle phenotypes, germline deletion of both pip4k2a and pip4k2b results in neonatal lethality and deletion of pip4k2b and pip4k2c results in early embryonic lethality (Emerling et al., 2013). These results indicate that these genes have some essential but redundant functions. Though the distribution and localization of PI5P4Kγ has been well characterized in the brain, there have not been any studies examining the specific localization of PI5P4Kα and PI5P4Kβ in the brain and whether this localization is conserved in mice and primates.
Given that the PI5P4Ks are highly expressed in the brain but without known expression patterns of cell type specificity, we first examined the regional localization of PI5P4Kα and PI5P4Kβ in the mouse brain, using immunoperoxidase light and electron microscopy and Western blot. We then studied the localization of the PI5P4Ks in macaque and human brain tissue. This is the first comprehensive analysis of the expression and localization of PI5P4Kα and PI5P4Kβ in the brain. The differential localization of these kinases within the brain may shed light on the cell-autonomous function of these kinases in the normal brain and in brain disease states.
Materials and Methods
Animal and human studies
A total of 9 neonatal and 63 adult mice were used in this study. Both sexes were used. pip4k2a−/− and pip4k2b−/− mice were bred on a C57BL/6 background as previously described (Lamia et al., 2004; Emerling et al., 2013). Mice were provided ad libitum food (5LA1 diet) and water in a room with a 12:12 light/dark cycle (lights on at 0600). One adult male macaque was used in this study and was maintained as previously described (Schmid et al., 2014). All mouse and macaque studies were approved by the Institutional Animal Care and Use Committee and Weill Cornell Medicine and were compliant with the 2011 8th Edition of the NIH guidelines for the Care and Use of Laboratory Animals. De-identified normal post-mortem brain tissue from a male patient was obtained from the Department of Pathology at New York Presbyterian Hospital. All human studies were approved by the Institutional Review Board at Weill Cornell Medicine.
Antibodies
The following commercial antibodies, which have been extensively validated elsewhere, were used: PI5P4Kα (1:500, Santa Cruz sc-100406), PI5P4Kα (1:500, Abgent AW5494), PI5P4Kβ (1:500, CST 9694S), β-actin (1:5000, Abcam ab6276), and GAPDH (1:500, CST). Antibody information is displayed in Table 1.
Table 1.
Antibody Characteristics
| Antibody Name |
Immunogen | Host | Antibody Type |
Dilution | Manufacturer | Catalog #, RRID |
|---|---|---|---|---|---|---|
| PI5P4Kα | Human recombinant PI5P4Kα | Mouse | Monoclonal | 1:500 | Santa-Cruz | sc-100406, 1127270 |
| PI5P4Kα | Synthetic peptide of C-terminal region of PI5P4Kα | Rabbit | Polyclonal | 1:500 for western blot, 1:1000 for IHC | Abgent | AW5494, 353929 |
| PI5P4Kβ | Synthetic PI5P4Kβ | Rabbit | Polyclonal | 1:500 | Cell Signaling | 9694S, 2164572 |
| β-actin | Synthetic peptide corresponding to amino acid residues 1-14 that were slightly modified (DDDIAALVIDNGS GK) conjugated to KLH | Mouse | Monoclonal | 1:5000 | Abcam | ab6276, 2223210 |
| GAPDH | Synthetic peptide corresponding to residues near C-terminus of human GAPDH | Rabbit | Monoclonal | 1:500 | Cell Signaling | 5174s, 10622025 |
| GFAP | Native GFAP purified from pig spinal cord | Mouse | Monoclonal | 1:1000 | Cell Signaling | 3670T, 561049 |
| NeuN | Recombinant fragment of human NeuN corresponding to amino acids 1-100 (N-terminal) | Mouse | Monoclonal | 1:1000 | Cell Signaling | ab104224, 10711040 |
| Olig2 | Recombinant human Olig2 | Mouse | Monoclonal | 1:1000 | Sigma | 387M-15, 2814812 |
PI5P4Kα and PI5P4Kβ:
Specificity of PI5P4Kα antibody from Abgent and Santa-Cruz and PI5P4Kβ from Cell Signaling was tested by western blot in lysates of wild-type or pip4k2a−/− or pip4k2b−/− brains. Specificity of PI5P4Kα from Abgent and PI5P4Kβ antibody from Cell Signaling was tested by immunoperoxidase and immunofluorescence in brains of wild-type tissue as compared to brains of pip4k2a and pip4k2b knock-out mice, respectively.
Glial fibrillary acidic protein (GFAP):
This antibody was produced by immunizing animals with native GFAP purified from pig spinal cord. The specificity of this antibody was verified by Cell Signaling Technology by performing immunofluorescence of SNB19 cells (positive for GFAP) and HeLa cells (negative for GFAP). Cell Signaling Technology also demonstrated that this antibody detects astrocytes in rat hippocampus by immunofluorescence.
Neuronal Nuclei (NeuN):
This antibody was produced by immunizing animals with a recombinant fragment of NeuN corresponding to human NeuN aa 1-100 (N-terminal). The specificity of this antibody was verified by Abcam by demonstrating positive staining in pyramidal cells in rat hippocampus by immunofluorescence and in mouse cerebellum by immunoperoxidase staining. Oligodendrocyte transcription factor 2 (Olig2): This antibody was produced by immunizing animals with recombinant human Olig2. The specificity of this antibody was verified by immunoperoxidase staining in human astrocytoma and glioblastoma tissue (Mokhtari et al., 2005; Otero et al., 2011).
Western blot
Whole-cell lysates were prepared from whole mouse brains using CST buffer (Cell Signal Technology) containing protease inhibitor (Sigma), and were rotated at 4°C for 30 minutes before DNA was pelleted. Total protein (30 μg) was loaded on 4-12% Tris-Glycine gels (Thermo-Fisher) for SDS-PAGE. Blots were incubated with the following primary antibodies: PI5P4Kα (1:500, Santa Cruz sc-100406), PI5P4Kα (1:500, Abgent AW5494), PI5P4Kβ (1:500, CST 9694S), β-actin (1:5000, Abcam), and GAPDH (1:500, CST). Blots were then incubated with either mouse or rabbit secondary antibodies as appropriate, and developed using Dura or Femto enhanced chemiluminescence (Pierce).
Immunoperoxidase and immunofluorescence
For immunoperoxidase staining and immunofluorescence, mice were injected with sodium pentobarbital (150 mg/kg, i.p.) and perfused transcardially with 2% heparin-saline followed by 30 ml 3.75% acrolein and 2% PFA in 0.1 M phosphate buffer (PB; pH 7.4). The brains were removed from the skull, post-fixed in 1.9% acrolein and 2% PFA in PB for 30 minutes, and then placed in PB. Coronal sections through the brains were cut (40-μm thick) using a Vibratome (Leica Microsystems) and stored in cryoprotectant (30% sucrose and 30% ethylene glycol in 0.1 PB) at −20°C until use (Milner et al., 2011).
For embryonic brain analysis, cervical dislocation was performed on pregnant mothers containing fetuses at E15.5 or E18.5. Mouse fetuses were removed, and brains were extracted from the skull. Whole brains were submerged in 4% PFA in PBS at room temperature for 2 days and then for 1 day at 4°C. PFA was replaced with PBS containing sodium azide and stored at 4°C until ready to use. Post-natal mice underwent cervical dislocation, and brains were removed, submerged in 4% PFA in PBS as above, and stored in PBS containing sodium azide until ready to use. For embryonic and post-natal brains used for determining PI5P4Kα and PI5P4Kβ localization across development, whole brains were prepared in sheets embedded in matrix (NeuroScience Associates Labs, Knoxville, TN). Free-floating sheets containing brains were then used for subsequent experiments.
For immunoperoxidase and immunofluorescence, sections were rinsed in PB to remove cryoprotectant and then incubated in 1% sodium borohydride in PB for 30 minutes to remove unbound aldehydes. Sections were washed in 8–10 changes of PB until all the gaseous bubbles disappeared and then placed in 0.1 M Tris-buffered saline (TS), pH 7.6. All brain sections were then incubated in blocking buffer (5% normal donkey serum (Jackson ImmunoResearch) and 0.3% Triton-X 100 (Sigma) in PBS, pH 7.4) and avidin/biotin blocking kit (Vector Laboratories, SP-2001) at room temperature, and then incubated with primary antibodies in blocking buffer for 24 hours at room temperature followed by 48 hours at 4°C. The following primary antibodies were used: rabbit anti-PI5P4Kα (1:500, Abgent AW5494), rabbit anti-PI5P4Kβ (1:1000, CST 9694S), mouse anti-Olig2 (1:1000, Sigma 387M-15), mouse anti-GFAP (1:1000, CST 3670T), and mouse anti-NeuN (1:1000, Abcam ab104224). For immunoperoxidase staining, the secondary antibodies used were biotinylated donkey anti–rabbit IgG (1:400, Jackson ImmunoResearch, catalog 713-065-147). For immunofluorescence, the secondary antibodies used were donkey anti-rabbit AlexaFluor 488 and donkey anti-mouse AlexaFluor 568 (Thermo Fisher). Sections were coverslipped with Prolong Gold Antifade Mountant containing DAPI (Thermo Fisher).
Brain regions in embryonic, post-natal, and adult mice were identified using developmental (Paxinos, 1991; Altman and Bayer, 1995) and adult brain atlases (Hof et al., 2000). Three mice at each pre- and post-natal age were analyzed. For light microscopy, images were acquired on a Zeiss Axio Observer.Z1 inverted microscope and captured using the same exposure time/channel/experiment between different genotypes by AxioCam MRC camera. For immunofluorescence, images were acquired on a Zeiss LSM 880 Laser Scanning Confocal Microscope equipped with ZEN software (Zeiss).
Electron microscopy
Sections were processed for electron microscopy as previously described (Milner et al., 2011). Briefly, 4-8-week-old WT, pip4k2a−/− mice, and pip4k2b−/− mice were overdosed with sodium pentobarbital (150 mg/kg, i.p.) and perfused transcardially with 2% heparin-saline followed by 30 ml, 3.75% acrolein, and 2% PFA in PB. The brains were removed from the skull, post-fixed in 1.9% acrolein and 2% PFA in PB for 30 minutes, and then placed in PB. Coronal sections through the brains were cut (40-μm thick) using a Vibratome (Leica Microsystems) and stored in cryoprotectant at −20°C until use.
For PI5P4Kα and PI5P4Kβ staining, coronal sections (n = 3 animals per genotype) were rinsed in PB to remove cryoprotectant and then incubated in 1% sodium borohydride in PB for 30 minutes to remove unbound aldehydes. Sections were washed in 8–10 changes of PB until all the gaseous bubbles disappeared and then placed in 0.1 M Tris-buffered saline (TS), pH 7.6. Sections were then incubated on a shaker sequentially in 0.5% BSA in TS (30 min) and primary antibodies (rabbit anti-PI5P4Kα (1:500, Abgent) and rabbit anti-PI5P4Kβ (1:1000, CST 9594) in 0.1% BSA in TS) for 1 day at room temperature (~23°C), followed by 2 days at 4°C. For peroxidase staining, sections were incubated in 1:400 biotinylated goat anti-rabbit IgG (Jackson ImmunoResearch, catalog 705- 065-147), 30 minutes, 1:100 peroxidase-avidin complex (Vectastain Elite Kit, Vector Laboratories, catalog PK-6100) for 30 minutes, and DAB (Sigma-Aldrich) and H2O2 in TS for 3 minutes. All incubations were separated by 2-3 washes in TS.
For immunogold detection of PI5P4Kα and PI5P4Kβ, sections were rinsed in 0.01 M PBS (pH 7.4), incubated in blocking buffer (0.8% BSA, 0.2% gelatin, 0.02% BSA in 0.02 M PBS) for 30 minutes, and placed overnight in a 1:50 dilution of donkey anti–rabbit IgG with bound 10-nm colloidal gold (Electron Microscopy Sciences, catalog 25704) diluted in blocking buffer. The gold particles were fixed to the tissue in 2% glutaraldehyde in 0.01 M PBS and rinsed in PBS followed by 0.2 M sodium citrate buffer (pH 7.4). The bound silver-gold particles were enhanced using a Silver IntenSE M kit (catalog RPN491; GE Healthcare) for 7 minutes.
Sections were rinsed in 0.1 M PB and then post-fixed in 2% osmium tetroxide in PB for 1 hour, dehydrated, embedded with Epon 812 (Electron Microscopy Sciences) between 2 sheets of Aclar plastic, and left to cure at 60°C overnight. Sections were selected, mounted on EMBed chucks (Electron Microscopy Sciences) and trimmed to 1–1.5 mm–wide trapezoids. Ultrathin sections (~65 nm thick) within 0.1–0.2 μm to the tissue-plastic interface were cut on a Leica Ultracut ultratome, collected into copper mesh grids, and counterstained with uranyl acetate and Reynold’s lead citrate. Sections were viewed and photographed using a FEI Tecnai Biotwin electron microscope equipped with a digital camera (Advanced Microscopy Techniques, software version 3.2).
Ultrastructural image analysis
The data analysis procedure is similar to previously described (Eagleson et al., 2013). Profiles in the CA1 region were identified using standard morphological criteria (Peters et al., 1991). Briefly, terminal profiles contained numerous synaptic vesicles and usually ranged about 0.2 - 0.3 μm in diameter. Dendritic profiles contained regular microtubular arrays and were usually postsynaptic to axon terminal profiles. Dendritic spines were small (about 0.1–0.2 μm in diameter), abutted terminals, and sometimes emanated from dendritic shafts. Immunoperoxidase labeling for PI5P4Kα and PI5P4Kβ was distinguished as an electron-dense reaction product precipitate. For quantification, all labeled axon terminals and dendritic spines from the CA1 region of hippocampus from either PI5P4Kα or PI5P4Kβ-stained brain sections were counted from 120 μm2 area from 3 mice per condition.
Primate tissue and immunoperoxidase labeling
A male Macaque was deeply anesthetized with ketamine 15 mg/kg IM potentiated by xylazine 2 mg/kg IM (Rompun, Haver) and transcardially perfused with 4% PFA in PB. Coronal sections (40 μm thick) were cut on a vibratome and stored in cryoprotectant at −20°C until use. Sections were rinsed in PB to remove cryoprotectant and then incubated in 1% sodium borohydride in PB for 30 minutes to remove unbound aldehydes. Sections were washed in 8–10 changes of PB until all the gaseous bubbles disappeared and then placed in 0.1 M Tris-buffered saline (TS), pH 7.6. All brain sections were then incubated in blocking buffer (5% normal donkey serum (Jackson ImmunoResearch) and 0.3% Triton-X 100 (Sigma) in PBS, pH 7.4) and avidin/biotin blocking kit (Vector Laboratories, SP-2001) at room temperature, and then incubated with primary antibodies in blocking buffer for 24 hours at room temperature followed by 48 hours at 4°C. Immunoperoxidase staining was performed using the primary antibodies, PI5P4Kα antibody (1:1000, Abgent AW5494) and PI5P4Kβ antibody (1:500, CST 9694S), followed by secondary antibody incubation with biotinylated donkey anti-rabbit secondary antibodies and then DAB incubation. Sections were mounted on gelatin-coated slides, dehydrated, and coverslipped with Permount (Fisher). Slide images were captured with an Observer.Z1 (Carl Zeiss), and digital images were acquired with an AxioCam MRc camera and AxioVision 4.8.
Human tissue samples and immunoperoxidase labeling
Post-mortem human brain tissue from the temporal lobe (containing the hippocampus) was post-fixed in acrolein in PBS and sectioned (40 μm thick) on a vibratome and stored in cryoprotectant at −20°C until use. Sections were rinsed in PB to remove cryoprotectant and then incubated in 1% sodium borohydride in PB for 30 minutes to remove unbound aldehydes. Sections were washed in 8–10 changes of PB until all the gaseous bubbles disappeared and then placed in 0.1 M Tris-buffered saline (TS), pH 7.6. All brain sections were then incubated in blocking buffer (5% normal donkey serum (Jackson ImmunoResearch) and 0.3% Triton-X 100 (Sigma) in PBS, pH 7.4) and avidin/biotin blocking kit (Vector Laboratories, SP-2001) at room temperature, and then incubated with primary antibodies in blocking buffer for 24 hours at room temperature followed by 48 hours at 4°C. Immunoperoxidase labeling was performed using the primary antibodies, PI5P4Kα antibody (1:1000, Abgent AW5494) and PI5P4Kβ antibody (1:500, CST 9694S), followed by secondary antibody incubation with biotinylated donkey anti-rabbit secondary antibodies and then DAB incubation. Sections were mounted on gelatin-coated slides, dehydrated, and coverslipped with Permount (Fisher). Slide images were captured with an Observer.Z1 (Carl Zeiss), and digital images were acquired with an AxioCam MRc camera and AxioVision 4.8.
Statistics
All of the data were analyzed with the program GraphPad Prism version 7.0 (GraphPad Software). A two-tailed student’s t-test was used to analyze significance between groups in electron microscopy experiments.
Results
Antibody Characterization
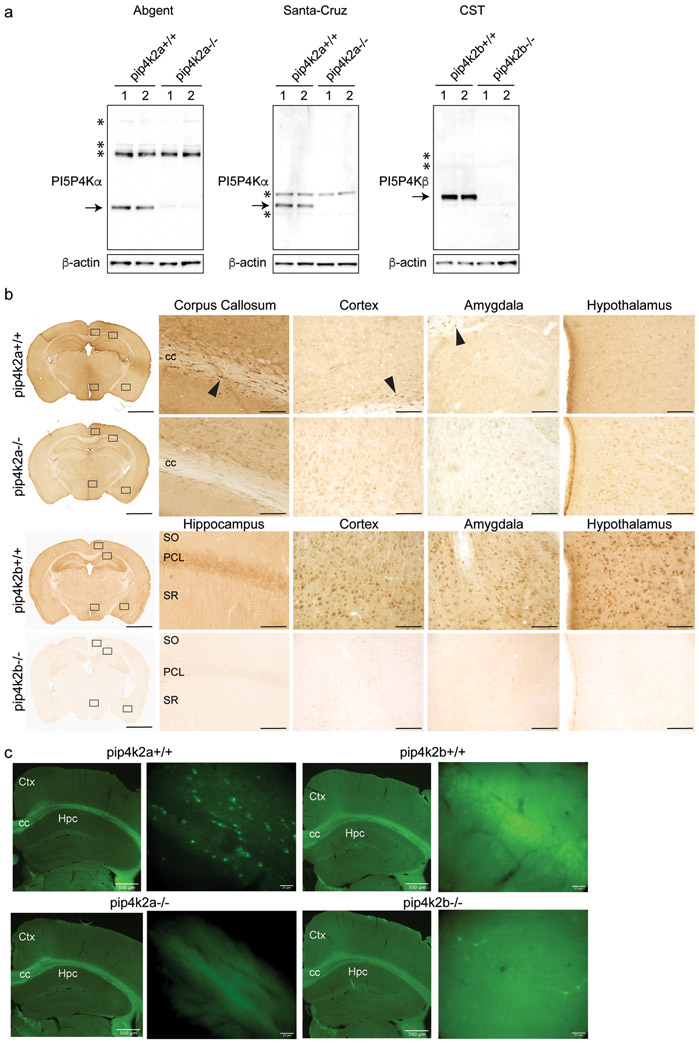
Previous studies have localized PI5P4Kγ protein and PIP4K mRNA in mouse brain (Clarke et al., 2009), but no studies have examined localization of PI5P4Kα and PI5P4Kβ protein in the brain of any species. We tested the specificity of PI5P4Kα and PI5P4Kβ antibody in wild-type and pip4k2a−/− and pip4k2b−/− knock-out mice by Western blot and immunoperoxidase staining using two commercially available antibodies (Fig. 1a). We also demonstrate specificity of PI5P4Kα and PI5P4Kβ antibodies in wild-type and respective knockout mouse brain tissue by immunoperoxidase (Fig. 1b) and immunofluorescence (Fig. 1c). PI5P4Kα immunolabeling was found throughout the corpus callosum and was present in scattered cells in cortex, basolateral amygdala, and hypothalamus in wild-type, but not knockout, sections (Fig. 1b, c). Likewise, PI5P4Kβ immunolabeling was found in the hippocampal pyramidal cell layer, cortex, basolateral amygdala, and hypothalamus in wild-type, but not knockout mice (Fig. 1b, c).
Figure 1.
Confirmation of antibody specificity for PI5P4Kα and PI5P4Kβ in mouse brain
a. Western blot analysis of whole brain lysates in wild-type and pip4k2a knockout mouse brain shows specificity of 2 antibodies (Abgent and Santa-Cruz) for PI5P4Kα, and specificity of Cell Signal antibody (CST9594S) for PI5P4Kβ in wild-type compared to knockout brains. Arrows, specific bands that are not present in knockout brain lysates; *, non-specific bands.
b. Immunoperoxidase staining for PI5P4Kα (top two rows) (Abgent) and PI5P4Kβ (bottom two rows) in wild-type and knockout brains shows positive signal in wild-type brains and lack of staining in pip4k2a and pip4k2b knockout brains. Corpus callosum, cortex, basolateral amygdala, and hypothalamus are shown for PI5P4Kα staining, and hippocampus, cortex, basolateral amygdala, and hypothalamus are shown for PI5P4Kβ staining. Scale bars for immunoperoxidase images are 2 mm for low-magnification images on left and 125 μm for high-magnification images on right.
c. Immunofluorescence for PI5P4Kα (left) (Abgent) and PI5P4Kβ (right) in wild-type and knockout brains shows positive PI5P4Kα signal in corpus callosum and positive PI5P4Kβ signal in hippocampus in wild-type brains and lack of staining in pip4k2a and pip4k2b knockout brains. Scale bar length is indicated for each bar.
CC, corpus callosum; PCL, pyramidal cell layer; SO, stratum oriens; SR, stratum radiatum; Hpc, hippocampus; Ctx, cortex
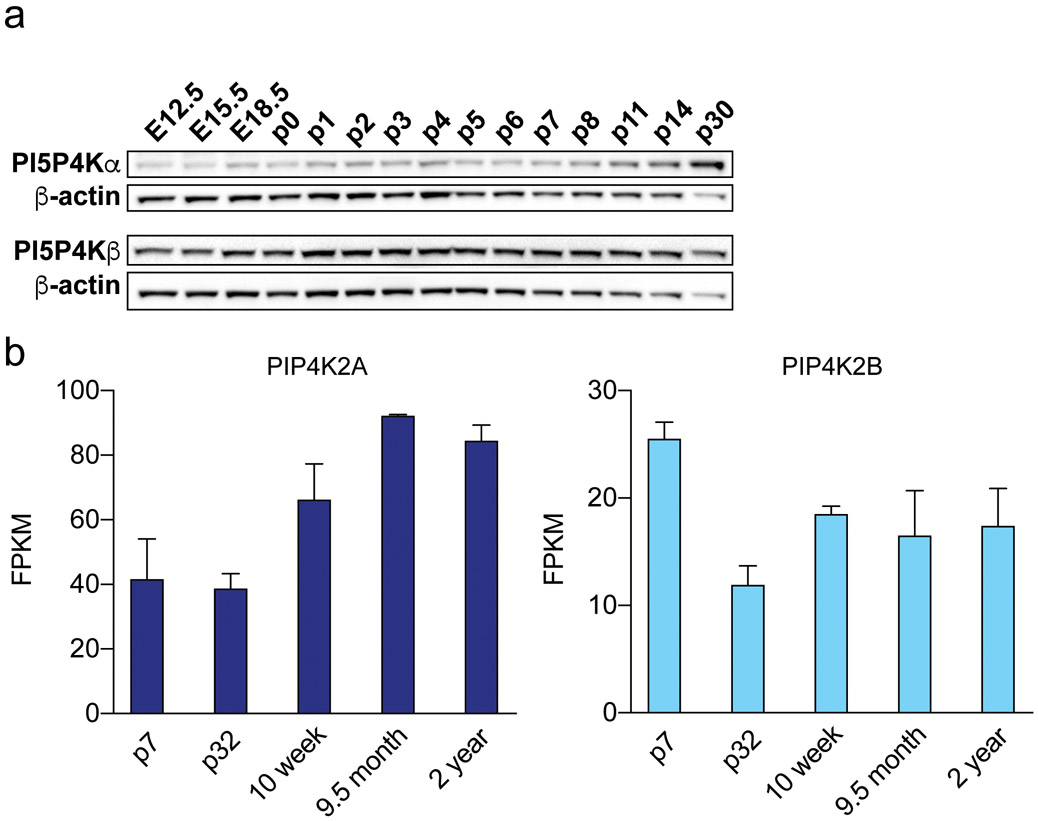
PI5P4Kα and PI5P4Kß are differentially expressed in mouse brain during development
To characterize the expression of PI5P4Kα and PI5P4Kβ in mouse brain across development, we performed Western blot of whole brain lysates from mouse brains at 3 embryonic time-points and 12 post-natal time-points. We found that expression of PI5P4Kα increased in post-natal stages, around P11, further increasing up until p30 (Fig. 2a). On the other hand, PI5P4Kβ expression remained constant from embryonic day 12.5 (E12.5) to post-natal day 30 (p30). These data correlate with findings from the BrainRNASeq database, a transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex (Zhang et al., 2014). This database shows that PIP4K2A expression increases from early post-natal development at p7 up until 9.5 months and 2 years of age (Fig. 2B) and that PIP4K2B expression is highest at early post-natal development at p7 and decreases thereafter (Fig. 2b).
Figure 2.
Developmental expression of PI5P4Kα and PI5P4Kβ in the mouse brain
a. Western blot analysis of PI5P4Kα (Abgent) and PI5P4Kβ in whole brain lysates at the indicated developmental timepoints.
b. RNAseq analysis of PIP4K2A and PIP4K2B expression from the BrainRNAseq database at the indicated developmental timepoints.
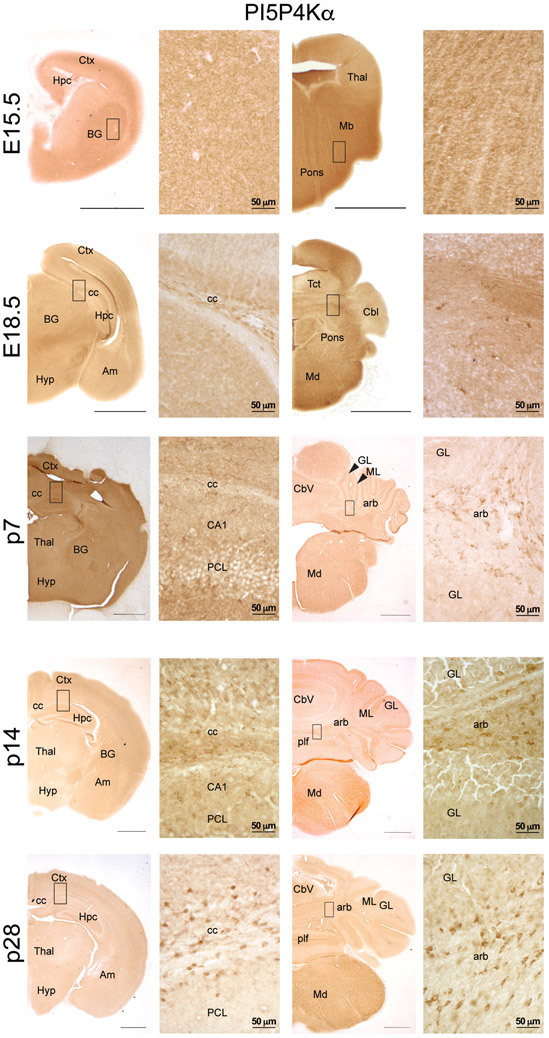
By immunoperoxidase, we evaluated localization of PI5P4Kα and PI5P4Kβ across development. We found that PI5P4Kα-immunolabeling was contained in white matter, particularly in corpus callosum, beginning around p14 (Fig. 3). We also found PI5P4Kα–labeled cells in white matter tracks in the cerebellum, including the arbor vitae, beginning at E18.5 and becoming more prominent by p28 (Fig. 3). PI5P4Kα expression was absent in the molecular and granular layers of the cerebellum at all time-points.
Figure 3.
Immunoperoxidase staining for PI5P4Kα (Abgent) at the indicated developmental timepoints. Scale bars for low-magnification images = 150 μm, scale bars for high-magnification images are indicated for each bar; Ctx, cortex; Hpc, hippocampus; BG, basal ganglia; Thal, thalamus; Mb, midbrain; Hyp, hypothalamus; Am, amygdala; Tct, tectum; Cbl, cerebellum; Md, medulla; CC, corpus callosum; PCL, pyramidal cell layer; Cbv, cerebellar vermis; GL, granule cell layer of the cerebellum; ML, molecular cell layer of the cerebellum; plf, posterolateral fissure; arb, arbor vitae; arrowheads identify relevant named structures
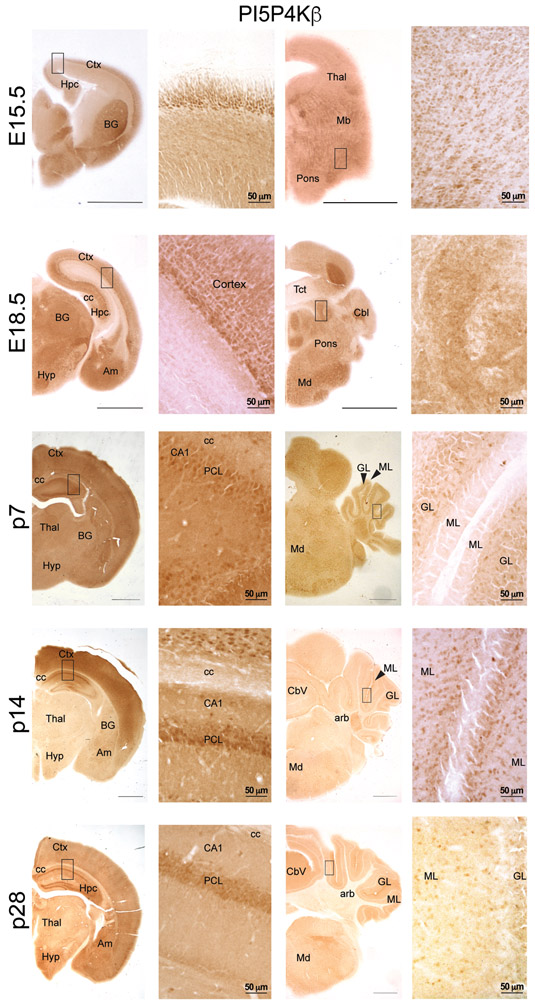
PI5P4Kβ-immunolabeling was evident early in development at E15.5, especially in cortex, basal ganglia, and hippocampus. In the E15.5 cortex, labeled cells were densest in the cortical plate. However, by E18.5, PI5P4Kβ-positive cells were primarily found in the ventricular zone. This pattern of labeling was similar at the remaining developmental time points. At E15.5, PI5P4Kβ-labeled cells were found scattered throughout the basal ganglia with a greater concentration in the region of the lateral migratory steam. The distribution of PI5P4Kβ-labeled cells in the basal ganglia was similar at post-natal stages of development (not shown). The E15.5 hippocampus contained PI5P4Kβ-labeled cells, but the laminae were indistinct at this time point. By E18.5, cells in the pyramidal cell layer, especially CA1, were prominently labeled with PI5P4Kβ. By p7, PI5P4Kβ-immunoreactivity was distinguishable within pyramidal cells and interneurons as well as granule cells (Fig. 4). In the cerebellum, PI5P4Kβ expression was evident in the granular layer beginning at p7 and in the molecular layer beginning at p14. PI5P4Kβ expression was absent in the arbor vitae of the cerebellum at all time-points. These findings demonstrate that PI5P4Kα and PI5P4Kβ exhibit not only differential levels of expression during development but also regional diversity throughout the mouse brain.
Figure 4.
Immunoperoxidase staining for pi5p4kβ at the indicated developmental timepoints. Scale bars for low-magnification images = 150 μm, scale bars for high-magnification images are indicated for each bar; Ctx, cortex; Hpc, hippocampus; BG, basal ganglia; Thal, thalamus; Mb, midbrain; Hyp, hypothalamus; Am, amygdala; Tct, tectum; Cbl, cerebellum; Md, medulla; CC, corpus callosum; PCL, pyramidal cell layer; Cbv, cerebellar vermis; GL, granule cell layer of the cerebellum; ML, molecular cell layer of the cerebellum; plf, posterolateral fissure; arb, arbor vitae; arrowheads identify relevant named structures
PI5P4Kα and PI5P4Kβ are differentially expressed in the adult mouse brain
To further differentiate the regional levels of PI5P4Kα and PI5P4Kβ expression in the mouse brain, we determined the localization of PI5P4Kα and PI5P4Kβ in 9 different brain regions by dissection of each region prior to preparing whole cell lysates and subsequent Western blot. We found that PI5P4Kα and PI5P4Kβ are expressed in each region of the brain and spinal cord that we assayed (Fig. 5 a,b). However, we found that both PI5P4Kα and PI5P4Kβ expression are highest in cortex and lowest in spinal cord, with PI5P4Kβ also having relatively high levels of expression in olfactory bulb, caudate putamen, thalamus, hippocampus, and midbrain.
Figure 5.
Differential expression of PI5P4Kα and PI5P4Kβ in the mouse brain by western blot analysis
a. Western blot of lysates obtained from the indicated brain regions. Abgent antibody was used to detect PI5P4Kα.
b. Quantification of western blot band intensities from A (n=3 mice per group).
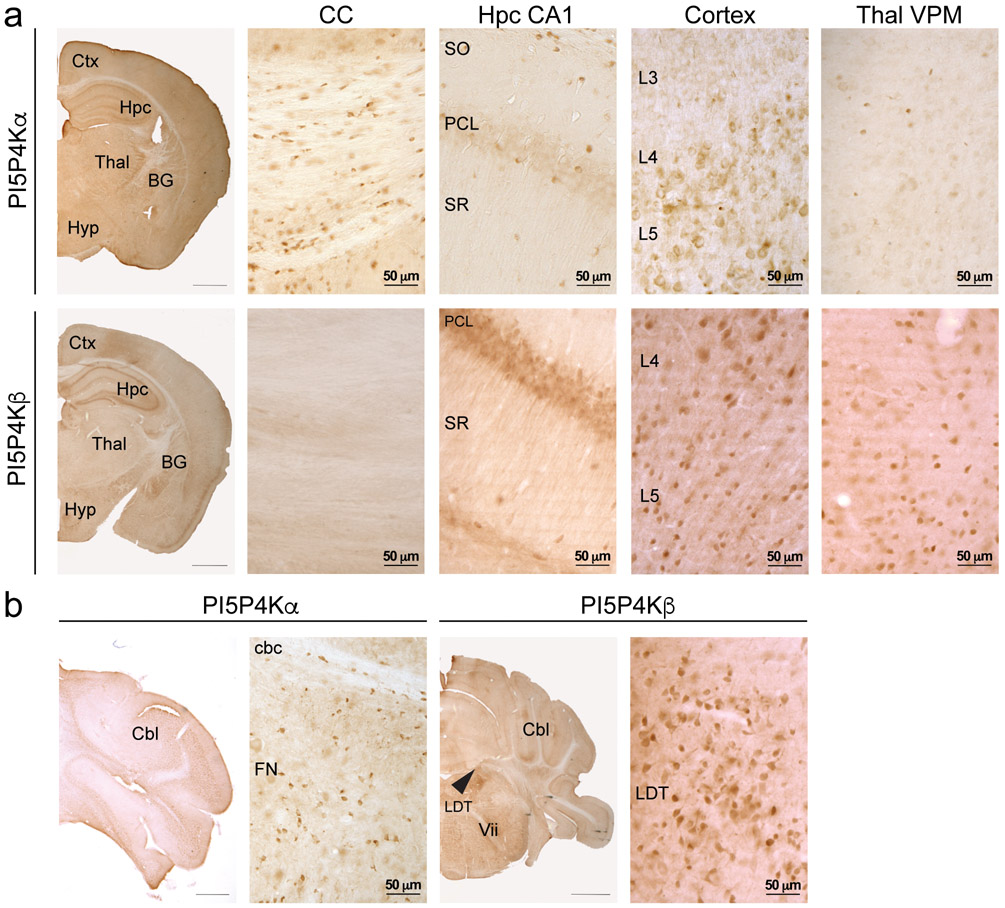
We next surveyed the expression of PI5P4Kα and PI5P4Kβ in the adult mouse brain through immunoperoxidase staining. PI5P4Kα expression is highest in the corpus callosum, with a scattered pattern of expression in populations of cells within layer 5 of cortex, the CA1 region of hippocampus, the ventral posteromedial nucleus of the thalamus, and the cerebellar commissure and funicular nucleus of the cerebellum (Fig. 6a and b). Conversely, strong PI5P4Kβ expression was found throughout the brain in the hippocampal pyramidal cell layer, layers 4 and 5 of cortex, ventral posteromedial nucleus of the thalamus, and laterodorsal tegmental nucleus (Fig. 6a and b). Expression of PI5P4Kβ was absent within the corpus callosum.
Figure 6.
Immunoperoxidase distribution of PI5P4Kα and PI5P4Kβ in the mouse brain
a. At the level of rostral hippocampus (top set of panels), PI5P4Kα (Abgent) is expressed in oligodendrocytes throughout the corpus callosum, in neurons and oligodendrocytes in cortex and in oligodendrocytes in the midbrain. PI5P4Kα is also expressed at low levels in neurons in hippocampus. In the cerebellum (bottom set of panels), PI5P4Kα is expressed in oligodendrocytes and some neurons.
b. At the level of rostral hippocampus (top set of panels), PI5P4Kβ is expressed in neurons in the hippocampal pyramidal cell layer, in cortical neurons, and in neurons in the midbrain. PI5P4Kβ expression is absent in the corpus callosum. In the bottom set of panels, PI5P4Kβ is expressed in neurons in the laterodorsal tegmental nucleus (arrowhead).
Ctx, cortex; Hpc, hippocampus; BG, basal ganglia; Thal, thalamus; Hyp, hypothalamus; Cbl, cerebellum; CC, corpus callosum; PCL, pyramidal cell layer; SO, stratum oriens; SR, stratum radiatum; L3, cortical layer 3; L4, cortical layer 4, L5, cortical layer 5; cbc, cerebellar commissure; FN, funicular nucleus; LDT, laterodorsal tegmental nucleus; Vii, 7th cranial nerve. Scale bars for low-magnification images = 1 mm, scale bars for high-magnification images are indicated for each bar.
PI5P4Kα and PI5P4Kβ are expressed in different cell types in mouse brain, and this differential expression is also observed in macaque and human brain
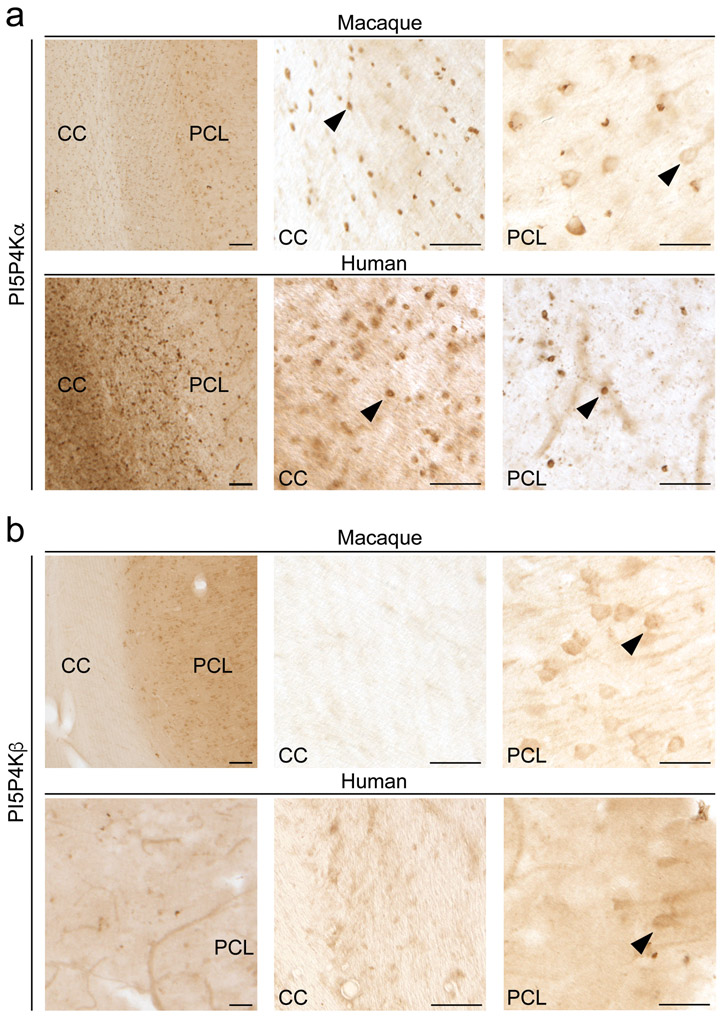
To determine the cellular identity of PI5P4Kα and PI5P4Kβ-expressing cells in the mouse brain, we performed immunofluorescent analysis with cell type-specific markers. Using double labeling immunofluorescence, we found that PI5P4Kα expression is restricted mainly to oligodendrocytes and some neuronal populations, whereas PI5P4Kβ expression is restricted to neurons. PI5P4Kα co-localized with the oligodendrocyte marker, Olig2, in corpus callosum but did not co-localize with the astrocyte marker, GFAP (Fig. 7a-c). PI5P4Kα was not detectable at the immunofluorescent level in neurons within the hippocampal pyramidal cell layer. On the other hand, diffuse PI5P4Kβ immunoreactivity was visualized in NeuN-labeled neurons throughout the brain, but did not co-localize with Olig2 or GFAP (Fig. 7d-f). We performed immunoperoxidase studies in macaque and human brains to confirm our findings in the mouse brain. We found PI5P4Kα expression in oligodendrocytes in macaque and human corpus callosum and also in neurons in macaque and human hippocampus, and we found PI5P4Kβ expression in neurons in macaque and human hippocampus (Fig. 8a and b). These findings indicate conservation of cell type specificity for PI5P4Kα and PI5P4Kβ among mouse, macaque, and human species, with more robust neuronal PI5P4Kα expression in macaque and human species.
Figure 7.
Immunofluorescent characterization of cell type expression of PI5P4Kα and PI5P4Kβ in the mouse brain
PI5P4Kα (Abgent) does not localize with GFAP in the hippocampal pyramidal cell layer (a) but localizes with Olig2 in corpus callosum (b). PI5P4Kα expression is not evident at the immunofluorescent level in neurons within the hippocampal pyramidal cell layer (c). PI5P4Kβ localizes with NeuN (f) but not Olig2 (d) or GFAP (e) in the hippocampal pyramidal cell layer. Arrowheads identify positive staining for PI5P4Kα and PI5P4Kβ.
CC, corpus callosum; PCL, pyramidal cell layer; SO, stratum oriens; SR, stratum radiatum Scale bars = 30 μm.
Figure 8.
Immunoperoxidase distribution of PI5P4Kα and PI5P4Kβ in the macaque and human brain
a. PI5P4Kα (Abgent) is expressed in oligodendrocytes (arrowheads) in corpus callosum and neurons in macaque hippocampus. PI5P4Kα is expressed in oligodendrocytes in human corpus callosum and hippocampus.
b. PI5P4Kβ is expressed in neurons (arrowheads) in hippocampus in macaque and human brain.
Arrowheads identify positive staining for PI5P4Kα and PI5P4Kβ. CC, corpus callosum; PCL, pyramidal cell layer.
Scale bars on left panel = 150 μm; scale bars on right 2 panels = 50 μm.
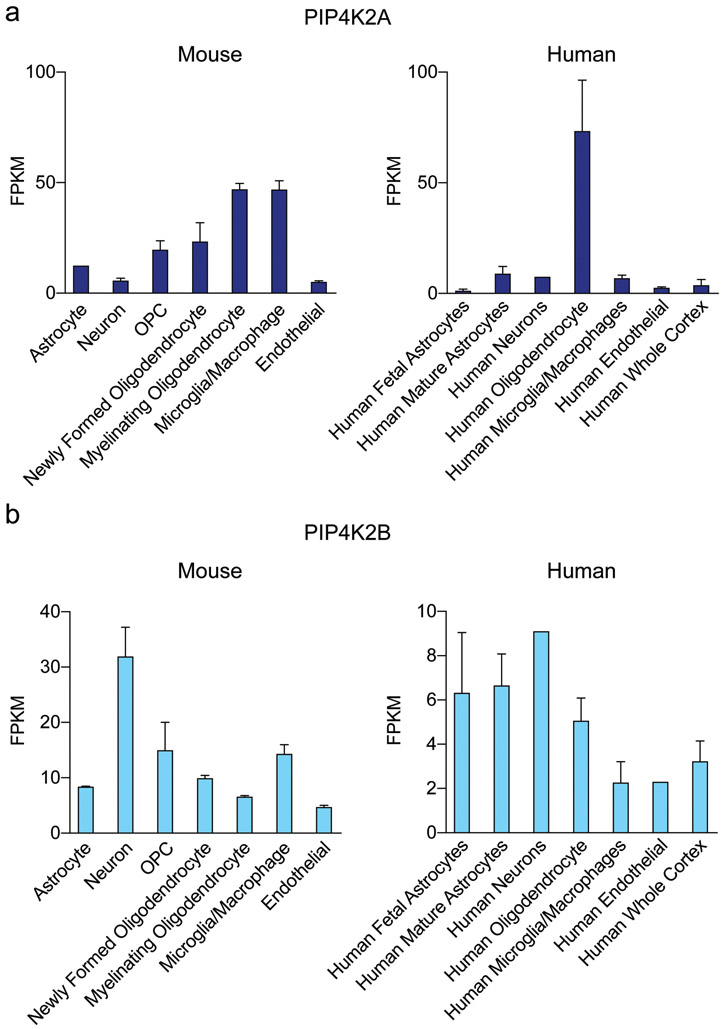
Data from the BrainRNASeq database are in agreement with our immunoperoxidase findings of PI5P4Kα and PI5P4Kβ protein levels (Zhang et al., 2014; Zhang et al., 2016). We probed this database to determine the mRNA expression levels of PIP4K2A and PIP4K2B. In the mouse brain, PIP4K2A mRNA expression is highest in oligodendrocytes but is much lower in neurons (Fig. 9a). Interestingly, PIP4K2B mRNA is also found in microglia. Similarly, in human brain, PIP4K2A mRNA expression is highest in oligodendrocytes. For PIP4K2B, mRNA expression is highest in neurons in mouse brain, whereas in human brain, astrocytes, oligodendrocytes, and neurons express nearly similar levels of PIP4K2B mRNA (Fig. 9b). The oligodendrocyte-specific localization of PIP4K2A is also supported by a recent paper demonstrating that PIP4K2A is a marker gene for oligodendrocytes (Schirmer et al., 2019).
Figure 9.
Cell type distribution of PIP4K2A and PIP4K2B throughout whole mouse brain RNAseq analysis
a. PIP4K2A is expressed mostly in oligodendrocyte lineage cells in mouse and human brain, with microglia and macrophages also displaying high levels of expression.
b. PIP4K2B is expressed mostly in neurons in mouse brain and in astrocytes and oligodendrocytes in human brain.
Ultrastructural localization of PI5P4Kα and PI5P4Kβ in the mouse brain to the synapse
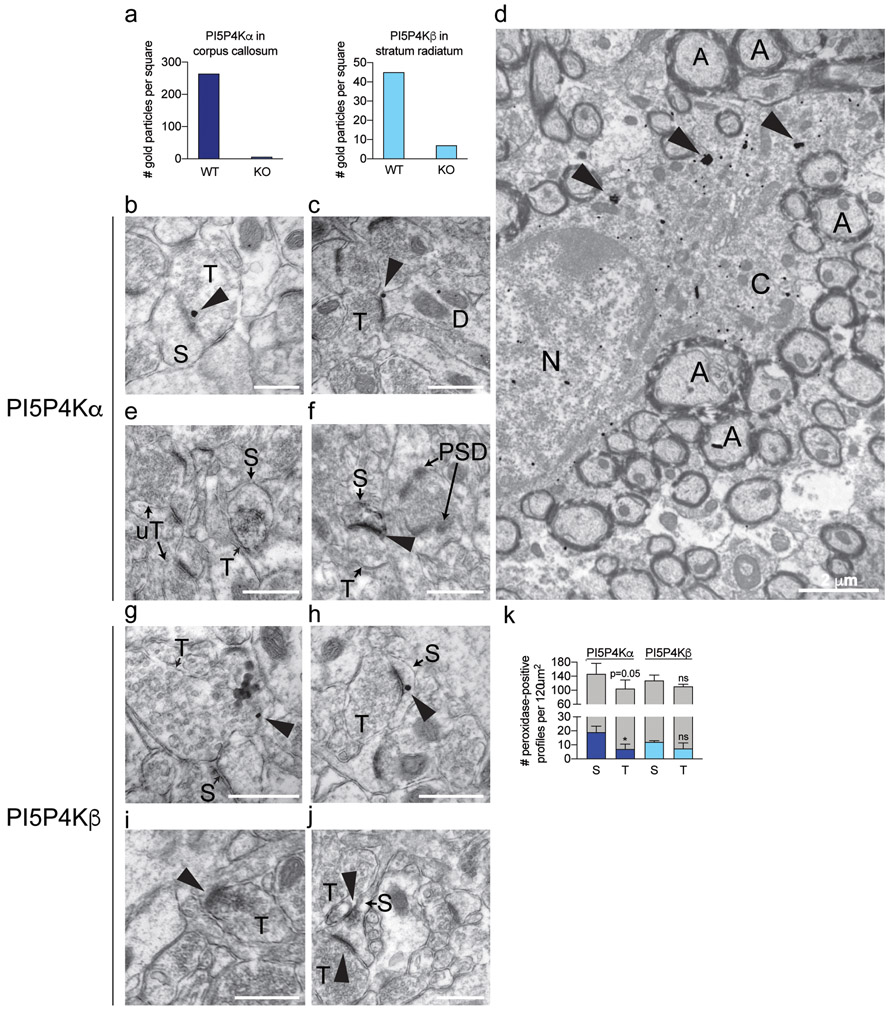
We next studied the endogenous localization of PI5P4Kα and PI5P4Kβ using peroxidase and immunogold labeling at the ultrastructural level. We confirmed specificity of our antibodies by quantifying silver-intensified gold (SIG) particles for these proteins in pip4k2a and pip4k2b wild-type and knockout brains. Indeed, we found more SIG particles in PI5P4Kα and PI5P4Kβ wild-type than corresponding knockout brains (Fig. 10a).
Figure 10.
Immuno-electron microscopy of endogenous PI5P4Kα and PI5P4Kβ expression in mouse hippocampus and corpus callosum.
a Quantification of SIG particle labeling for PI5P4Kα (Abgent) in corpus callosum and PI5P4Kβ in stratum radiatum (SR) of hippocampal CA1 from wild-type and respective knockout mice.
b-d SIG particle labeling for PI5P4Kα (arrowheads) is contained in an axon terminal, adjacent to the synapse (b), the perisynaptic zone of an asymmetric synapse in a dendritic spine in CA1 SR (c), and oligodendrocyte cytoplasm in corpus callosum (d).
e-f Immunoperoxidase labeling for PI5P4Kα (arrowheads) is contained in an axon terminal (e) and dendritic spine (f) in CA1 SR. Unlabeled terminals (uT) and post-synaptic densities (PSD) are indicated for comparison.
g-h SIG labeling for PI5P4Kβ (arrowheads) is found in an axon terminal (g) and dendritic spine (h) in CA1 SR.
i-j Immunoperoxidase labeling for PI5P4Kβ (arrowheads) is found in an axon terminal (i) and dendritic spine (j).
k Quantification of PI5P4Kα and PI5P4Kβ immunoperoxidase labeled profiles in CA1 (n=3 mice per group). Labeled profiles are indicated by dark blue (PI5P4Kα) and light blue (PI5P4Kβ) bars, and unlabeled profiles are indicated by gray bars. *, p < 0.05; ns, not significant.
Scale bars for b, c, e, f, g, h, i, j = 500 nm; scale bars for D = 2 μm.
A, axon; C, cytoplasm; S, dendritic spine; N, nucleus; T, axon terminal; uT, unlabeled axon terminal
Because we identified high levels of expression of PI5P4Kβ in the CA1 region of the hippocampus in prior analyses, we next characterized the ultrastructural labeling of the PI5P4Ks in this region. At the electron microscopy level, we were able to detect low-frequency PI5P4Kα expression that was not evident at the immunofluorescent or immunoperoxidase levels. SIG particle labeling for PI5P4Kα was localized to axon terminals, sometimes adjacent to the synaptic membrane (Fig. 10b). Similarly, we found PI5P4Kα SIG particles in dendritic spines, just adjacent to the synapse (Fig. 10c). Peroxidase labeling for PI5P4Kα was similarly localized in axon terminals and dendritic spines (Fig. 10e,f). Quantification of peroxidase-labeled profiles showed that PI5P4Kα was slightly more common in dendritic spines than axon terminals (Fig. 10k). Immunogold analysis also demonstrated ubiquitous PI5P4Kα labeling in the cytoplasm of oligodendrocytes throughout the corpus callosum (Fig. 10d), supporting our light microscopic findings of oligodendrocyte localization.
Similar to PI5P4Kα, PI5P4Kβ localized to both axon terminals and dendritic spines by immunogold (Fig. 10g-h) and peroxidase labeling (Fig. 10i-j). The overall peri-synaptic distribution for both kinases was nearly identical, with less frequent labeling in axon terminals than dendritic spines (Fig. 10k).
Discussion
In this study, we have characterized the developmental and regional localization of PI5P4Kα and PI5P4Kβ in the mouse brain. Furthermore, we have demonstrated the cell type-specific expression of these kinases in mouse, macaque, and human brain with immunoperoxidase staining and an independent RNAseq dataset. Finally, we provide the first endogenous expression of PI5P4Kα and PI5P4Kβ by electron microscopy.
Developmental expression of PI5P4Kα and PI5P4Kβ
Prior work has shown that PIP4K2A and PIP4K2B may play overlapping roles in the stress response after birth. Whereas germline deletion of either pip4k2a or pip4k2b results in normal embryonic growth and development, deletion of both genes results in early embryonic lethality, with pups dying around 12 hours after birth (Emerling et al., 2013). Data from this work showing lethality of tp53−/−; pip4k2b−/− embryos indicated that PIP4K2B may be preferentially more important than PIP4K2A shortly after birth. In support of these findings, we demonstrate through immunoperoxidase and RNAseq analysis that PI5P4Kα and PI5P4Kβ display a variable expression pattern in the mouse brain across development. PI5P4Kα is expressed later in development, whereas PI5P4Kβ is expressed at a greater level early in development and diminishes thereafter. It is possible that part of the function of PI5P4Kβ to reduce nutrient stress after birth takes place within the brain and that PI5P4Kβ is less necessary later in development.
Regional and cell type-specific expression of PI5P4Kα and PI5P4Kβ
Though it is known that the PI5P4Ks are highly expressed in the brain, the distribution of PI5P4Kα and PI5P4Kβ has not been previously described. We find that PI5P4Kα is contained predominantly in white matter with less frequent localization in gray matter and that PI5P4Kβ is expressed solely in gray matter. In the mouse brain, on a cellular level, PI5P4Kα is expressed most notably in oligodendrocytes, though we did observe expression in some neuronal populations. However, PI5P4Kβ is contained solely in neurons. These expression patterns are supported by studies in macaque and human brain tissue.
The restricted localization of the PI5P4Ks to distinct cell types suggests that different cellular processes may regulate their expression. One possibility is that each kinase is expressed through unique transcriptional programs in each cell type. For example, transcription factors, such as the Olig family in oligodendrocytes and the Sox family in neurons, may regulate expression of each kinase in a cell-autonomous fashion. Alternatively, PI5P4Kα and PI5P4Kβ may be regulated through post-transcriptional or post-translational mechanisms, with trafficking of each kinase to particular intracellular membranes being a highly likely mechanism of localization and potential degradation (Hinchliffe et al., 1999).
The differential localization pattern of PI5P4Kα and PI5P4Kβ highlight potential diverse functions in the brain. For example, PI5P4Kα expression in oligodendrocytes may indicate a potential role in the process of myelination. Interestingly, prior studies have implicated a polymorphism in PIP4K2A (N251S) to inherited risk for schizophrenia (Schwab et al., 2006; He et al., 2007; Rethelyi et al., 2010; Thiselton et al., 2010), with lymphocytes from schizophrenia patients expressing elevated levels of PIP4K2A (Saggers-Gray et al., 2011). Several studies have postulated white matter dysfunction in schizophrenia, arguing for a role for oligodendrocyte dysfunction in this disease (Tonnesen et al., 2018). Therefore, our findings of oligodendrocyte-specific PIP4K2A expression may connect the epidemiological studies with the white matter theory in schizophrenia.
On a molecular level, PI5P4Kα influences several neurotransmitter systems. PI5P4Kα has been shown to increase the expression of the neuronal glutamate transporter, EAAT3, while the schizophrenia-associated polymorphism, N251S, exerts a dominant-negative effect on EAAT3 expression (Fedorenko et al., 2009), resulting in reduced glutamate uptake. PI5P4Kα, but not the N251S mutant, activates neuronal M channels, which under normal physiological conditions, suppress dopaminergic transmission (Fedorenko et al., 2008). Interestingly, this mutant does not alter PI5P4Kα intrinsic kinase activity, suggesting a non-catalytic function of this polymorphism in disrupting PI5P4Kα function (Clarke and Irvine, 2013). Our data showing a peri-synaptic distribution of PI5P4Kα support these studies that demonstrate a possible role of PI5P4Kα in the molecular pathogenesis of schizophrenia.
It is also noteworthy that several genetic susceptibility loci in PI5P4Kγ have been linked to autoimmune diseases, including rheumatoid arthritis and type 1 diabetes mellitus, and that PI5P4Kγ-deficient mice exhibit a hyperimmune phenotype (Barton et al., 2008; Fung et al., 2009; Shim et al., 2016). Though there have not been as of yet any PIP4K loci linked to the development of multiple sclerosis, the predilection of PIP4K2A expression to oligodendrocytes may be relevant to demyelinating diseases. Given that both PI5P4Kα and PI5P4Kβ, but not PI5P4Kγ, exhibit kinase activity, it is possible that their differential expression in oligodendrocytes and neurons, respectively, highlights non-overlapping kinase function in these cell types. In addition, the similar pattern of expression of PI5P4Kβ and PI5P4Kγ may indicate that either PI5P4Kγ is required for appropriate localization of PI5P4Kβ or that these 2 kinases serve unique functions in neurons. For example, germline deletion of pip4k2c in mice results in a dramatic increase in S6-Kinase activity downstream of TORC1 in the mouse brain (Shim et al., 2016). Such upregulation of S6-Kinase activity may be explained by the role of PI5P4Kγ in inhibiting PI4P5K activity and downstream PI 3-kinase activity (Wang et al., 2019). Therefore, PI5P4Kα and PI5P4Kβ kinase activity could balance PI4P5Kγ inhibitory activity in a cell-autonomous manner.
Subcellular distribution of PI5P4Kα and PI5P4Kβ
The localization of PI5P4Kα and PI5P4Kβ to the perisynaptic region continues to support the role of the PI5P4Ks in intracellular PI-4,5-P2 pathways (Clarke et al., 2009). At the ultrastructural level, PI5P4Kα and PI5P4Kβ are both found in peri-synaptic regions in axon terminals and dendritic spines. Their particular localization adjacent to the synaptic membrane in both axon terminals and dendritic spines suggests that they may hold a more specific function in the brain, namely synaptic neurotransmission. Given that we did not identify their specific localization on the synaptic membrane, it is possible that these kinases serve a role in the synaptic vesicle docking or recycling process rather than vesicle fusion or release at the synapse.
Conclusions
To our knowledge, this is the first description of the endogenous ultrastructural localization of the PI5P4Ks in the brain. Their differential expression pattern in the brain highlights potentially diverse functions of these kinases, and their novel localization to the peri-synaptic region within particular axon terminals and dendritic spines lends support to the theory that these kinases may serve a supplementary role in synaptic function, whether through synaptic vesicle docking or recycling processes.
Phosphatidylinositol-4,5-bisphosphate (PI-4,5-P2) is critical for synaptic vesicle docking and fusion and is generated by either the type 1 phosphatidylinositol-4-phosphate 5-kinases (PI4P5Ks) or the type 2 phosphatidylinositol-5-phosphate 4-kinases (PI5P4Ks). In this study, we describe the neuro-anatomical localization of PI5P4Kα and PI5P4Kβ in the normal brain. PI5P4Kα is expressed in both oligodendrocytes and some neurons, while PI5P4Kβ is expressed solely in neurons. By electron microscopy, both kinases are located in a peri-synaptic distribution in axon terminals and dendritic spines, suggesting a role in neuro-transmission, possibly through synaptic vesicle docking, recycling, and/or fusion.
Acknowledgements:
Neuroanatomy EM Core; Dr. Jonathan Victor and Dr. Keith Purpura (supported by NEI R01 EY009314) for the donation of the macaque tissue.
Support: NIH grants DA08259, HL136520 (T.A.M). Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number R35CA197588. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Data Availability Statement:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Akiba Y, Suzuki R, Saito-Saino S, Owada Y, Sakagami H, Watanabe M, Kondo H. 2002. Localization of mRNAs for phosphatidylinositol phosphate kinases in the mouse brain during development. Brain Res Gene Expr Patterns 1(2):123–133. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. 1995. Atlas of prenatal rat brain development. Boca Raton, Fla: CRC Press; lxx, 589 p. p. [Google Scholar]
- Barton A, Thomson W, Ke X, Eyre S, Hinks A, Bowes J, Plant D, Gibbons LJ, Wellcome Trust Case Control C, Consortium Y, Consortium B, Wilson AG, Bax DE, Morgan AW, Emery P, Steer S, Hocking L, Reid DM, Wordsworth P, Harrison P, Worthington J. 2008. Rheumatoid arthritis susceptibility loci at chromosomes 10p15, 12q13 and 22q13. Nat Genet 40(10):1156–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carricaburu V, Lamia KA, Lo E, Favereaux L, Payrastre B, Cantley LC, Rameh LE. 2003. The phosphatidylinositol (PI)-5-phosphate 4-kinase type II enzyme controls insulin signaling by regulating PI-3,4,5-trisphosphate degradation. Proc Natl Acad Sci U S A 100(17):9867–9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke JH, Emson PC, Irvine RF. 2009. Distribution and neuronal expression of phosphatidylinositol phosphate kinase IIgamma in the mouse brain. J Comp Neurol 517(3):296–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke JH, Irvine RF. 2013. Enzyme activity of the PIP4K2A gene product polymorphism that is implicated in schizophrenia. Psychopharmacology (Berl) 230(2):329–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danbolt NC. 2001. Glutamate uptake. Prog Neurobiol 65(1):1–105. [DOI] [PubMed] [Google Scholar]
- Di Paolo G, Moskowitz HS, Gipson K, Wenk MR, Voronov S, Obayashi M, Flavell R, Fitzsimonds RM, Ryan TA, De Camilli P. 2004. Impaired PtdIns(4,5)P2 synthesis in nerve terminals produces defects in synaptic vesicle trafficking. Nature 431(7007):415–422. [DOI] [PubMed] [Google Scholar]
- Eagleson KL, Milner TA, Xie Z, Levitt P. 2013. Synaptic and extrasynaptic location of the receptor tyrosine kinase met during postnatal development in the mouse neocortex and hippocampus. J Comp Neurol 521(14):3241–3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerling BM, Hurov JB, Poulogiannis G, Tsukazawa KS, Choo-Wing R, Wulf GM, Bell EL, Shim HS, Lamia KA, Rameh LE, Bellinger G, Sasaki AT, Asara JM, Yuan X, Bullock A, Denicola GM, Song J, Brown V, Signoretti S, Cantley LC. 2013. Depletion of a putatively druggable class of phosphatidylinositol kinases inhibits growth of p53-null tumors. Cell 155(4):844–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko O, Strutz-Seebohm N, Henrion U, Ureche ON, Lang F, Seebohm G, Lang UE. 2008. A schizophrenia-linked mutation in PIP5K2A fails to activate neuronal M channels. Psychopharmacology (Berl) 199(1):47–54. [DOI] [PubMed] [Google Scholar]
- Fedorenko O, Tang C, Sopjani M, Foller M, Gehring EM, Strutz-Seebohm N, Ureche ON, Ivanova S, Semke A, Lang F, Seebohm G, Lang UE. 2009. PIP5K2A-dependent regulation of excitatory amino acid transporter EAAT3. Psychopharmacology (Berl) 206(3):429–435. [DOI] [PubMed] [Google Scholar]
- Fung EY, Smyth DJ, Howson JM, Cooper JD, Walker NM, Stevens H, Wicker LS, Todd JA. 2009. Analysis of 17 autoimmune disease-associated variants in type 1 diabetes identifies 6q23/TNFAIP3 as a susceptibility locus. Genes Immun 10(2):188–191. [DOI] [PubMed] [Google Scholar]
- Gupta A, Toscano S, Trivedi D, Jones DR, Mathre S, Clarke JH, Divecha N, Raghu P. 2013. Phosphatidylinositol 5-phosphate 4-kinase (PIP4K) regulates TOR signaling and cell growth during Drosophila development. Proc Natl Acad Sci U S A 110(15):5963–5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Li Z, Shi Y, Tang W, Huang K, Ma G, Zhou J, Meng J, Li H, Feng G, He L. 2007. The PIP5K2A gene and schizophrenia in the Chinese population--a case-control study. Schizophr Res 94(1-3):359–365. [DOI] [PubMed] [Google Scholar]
- Hinchliffe KA, Ciruela A, Letcher AJ, Divecha N, Irvine RF. 1999. Regulation of type IIalpha phosphatidylinositol phosphate kinase localisation by the protein kinase CK2. Curr Biol 9(17):983–986. [DOI] [PubMed] [Google Scholar]
- Hof P, Young W, Bloom F, Belichenko P, Celio M. 2000. Comparative Cytoarchitectonic Atlas of the C57BL/6 and 129/Sv Mouse Brains: Elsevier. [Google Scholar]
- Honigmann A, van den Bogaart G, Iraheta E, Risselada HJ, Milovanovic D, Mueller V, Mullar S, Diederichsen U, Fasshauer D, Grubmuller H, Hell SW, Eggeling C, Kuhnel K, Jahn R. 2013. Phosphatidylinositol 4,5-bisphosphate clusters act as molecular beacons for vesicle recruitment. Nat Struct Mol Biol 20(6):679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DR, Bultsma Y, Keune WJ, Halstead JR, Elouarrat D, Mohammed S, Heck AJ, D'Santos CS, Divecha N. 2006. Nuclear PtdIns5P as a transducer of stress signaling: an in vivo role for PIP4Kbeta. Mol Cell 23(5):685–695. [DOI] [PubMed] [Google Scholar]
- Jones DR, Foulger R, Keune WJ, Bultsma Y, Divecha N. 2013. PtdIns5P is an oxidative stress-induced second messenger that regulates PKB activation. FASEB J 27(4):1644–1656. [DOI] [PubMed] [Google Scholar]
- Jude JG, Spencer GJ, Huang X, Somerville TDD, Jones DR, Divecha N, Somervaille TCP. 2015. A targeted knockdown screen of genes coding for phosphoinositide modulators identifies PIP4K2A as required for acute myeloid leukemia cell proliferation and survival. Oncogene 34(10):1253–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamalesh K, Trivedi D, Toscano S, Sharma S, Kolay S, Raghu P. 2017. Phosphatidylinositol 5-phosphate 4-kinase regulates early endosomal dynamics during clathrin-mediated endocytosis. J Cell Sci 130(13):2119–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keune WJ, Jones DR, Bultsma Y, Sommer L, Zhou XZ, Lu KP, Divecha N. 2012. Regulation of phosphatidylinositol-5-phosphate signaling by Pin1 determines sensitivity to oxidative stress. Sci Signal 5(252):ra86. [DOI] [PubMed] [Google Scholar]
- Lamia KA, Peroni OD, Kim YB, Rameh LE, Kahn BB, Cantley LC. 2004. Increased insulin sensitivity and reduced adiposity in phosphatidylinositol 5-phosphate 4-kinase beta−/− mice. Mol Cell Biol 24(11):5080–5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundquist MR, Goncalves MD, Loughran RM, Possik E, Vijayaraghavan T, Yang A, Pauli C, Ravi A, Verma A, Yang Z, Johnson JL, Wong JCY, Ma Y, Hwang KS, Weinkove D, Divecha N, Asara JM, Elemento O, Rubin MA, Kimmelman AC, Pause A, Cantley LC, Emerling BM. 2018. Phosphatidylinositol-5-Phosphate 4-Kinases Regulate Cellular Lipid Metabolism By Facilitating Autophagy. Mol Cell 70(3):531–544 e539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey AM, Sarkes DA, Bettencourt I, Asara JM, Rameh LE. 2014. PIP4kgamma is a substrate for mTORC1 that maintains basal mTORC1 signaling during starvation. Sci Signal 7(350):ra104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TA, Waters EM, Robinson DC, Pierce JP. 2011. Degenerating processes identified by electron microscopic immunocytochemical methods. Methods Mol Biol 793:23–59. [DOI] [PubMed] [Google Scholar]
- Mokhtari K, Paris S, Aguirre-Cruz L, Privat N, Criniere E, Marie Y, Hauw JJ, Kujas M, Rowitch D, Hoang-Xuan K, Delattre JY, Sanson M. 2005. Olig2 expression, GFAP, p53 and 1p loss analysis contribute to glioma subclassification. Neuropathol Appl Neurobiol 31(1):62–69. [DOI] [PubMed] [Google Scholar]
- Otero JJ, Rowitch D, Vandenberg S. 2011. OLIG2 is differentially expressed in pediatric astrocytic and in ependymal neoplasms. J Neurooncol 104(2):423–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G 1991. Atlas of the developing rat brain. San Diego: Academic Press. [Google Scholar]
- Peters A, Palay SL, Webster Hd. 1991. The fine structure of the nervous system : neurons and their supporting cells. New York: Oxford University Press; xviii, 494 p. p. [Google Scholar]
- Rethelyi JM, Bakker SC, Polgar P, Czobor P, Strengman E, Pasztor PI, Kahn RS, Bitter I. 2010. Association study of NRG1, DTNBP1, RGS4, G72/G30, and PIP5K2A with schizophrenia and symptom severity in a Hungarian sample. Am J Med Genet B Neuropsychiatr Genet 153B(3):792–801. [DOI] [PubMed] [Google Scholar]
- Saggers-Gray L, Wildenauer DB, Schwab SG. 2011. Expression of PIP4K2A in lymphocyte cell lines from a sample of schizophrenia patients with previous evidence for association. Schizophr Res 130(1-3):295–296. [DOI] [PubMed] [Google Scholar]
- Schirmer L, Velmeshev D, Holmqvist S, Kaufmann M, Werneburg S, Jung D, Vistnes S, Stockley JH, Young A, Steindel M, Tung B, Goyal N, Bhaduri A, Mayer S, Engler JB, Bayraktar OA, Franklin RJM, Haeussler M, Reynolds R, Schafer DP, Friese MA, Shiow LR, Kriegstein AR, Rowitch DH. 2019. Neuronal vulnerability and multilineage diversity in multiple sclerosis. Nature 573(7772):75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid AM, Purpura KP, Victor JD. 2014. Responses to orientation discontinuities in V1 and V2: physiological dissociations and functional implications. J Neurosci 34(10):3559–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab SG, Knapp M, Sklar P, Eckstein GN, Sewekow C, Borrmann-Hassenbach M, Albus M, Becker T, Hallmayer JF, Lerer B, Maier W, Wildenauer DB. 2006. Evidence for association of DNA sequence variants in the phosphatidylinositol-4-phosphate 5-kinase IIalpha gene (PIP5K2A) with schizophrenia. Mol Psychiatry 11(9):837–846. [DOI] [PubMed] [Google Scholar]
- Sharma S, Mathre S, Ramya V, Shinde D, Raghu P. 2019. Phosphatidylinositol 5 Phosphate 4-Kinase Regulates Plasma-Membrane PIP3 Turnover and Insulin Signaling. Cell Rep 27(7):1979–1990 e1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim H, Wu C, Ramsamooj S, Bosch KN, Chen Z, Emerling BM, Yun J, Liu H, Choo-Wing R, Yang Z, Wulf GM, Kuchroo VK, Cantley LC. 2016. Deletion of the gene Pip4k2c, a novel phosphatidylinositol kinase, results in hyperactivation of the immune system. Proc Natl Acad Sci U S A 113(27):7596–7601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiselton DL, Maher BS, Webb BT, Bigdeli TB, O'Neill FA, Walsh D, Kendler KS, Riley BP. 2010. Association analysis of the PIP4K2A gene on chromosome 10p12 and schizophrenia in the Irish study of high density schizophrenia families (ISHDSF) and the Irish case-control study of schizophrenia (ICCSS). Am J Med Genet B Neuropsychiatr Genet 153B(1):323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toker A 2002. Phosphoinositides and signal transduction. Cell Mol Life Sci 59(5):761–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonnesen S, Kaufmann T, Doan NT, Alnaes D, Cordova-Palomera A, Meer DV, Rokicki J, Moberget T, Gurholt TP, Haukvik UK, Ueland T, Lagerberg TV, Agartz I, Andreassen OA, Westlye LT. 2018. White matter aberrations and age-related trajectories in patients with schizophrenia and bipolar disorder revealed by diffusion tensor imaging. Sci Rep 8(1):14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli-Daley LA, Lucast L, Gong LW, Liu L, Sasaki J, Sasaki T, Abrams CS, Kanaho Y, De Camilli P. 2010. Phosphatidylinositol-4-phosphate 5-kinases and phosphatidylinositol 4,5-bisphosphate synthesis in the brain. J Biol Chem 285(37):28708–28714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DG, Paddock MN, Lundquist MR, Sun JY, Mashadova O, Amadiume S, Bumpus TW, Hodakoski C, Hopkins BD, Fine M, Hill A, Yang TJ, Baskin JM, Dow LE, Cantley LC. 2019. PIP4Ks Suppress Insulin Signaling through a Catalytic-Independent Mechanism. Cell Rep 27(7):1991–2001 e1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O'Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, Deng S, Liddelow SA, Zhang C, Daneman R, Maniatis T, Barres BA, Wu JQ. 2014. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci 34(36):11929–11947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Sloan SA, Clarke LE, Caneda C, Plaza CA, Blumenthal PD, Vogel H, Steinberg GK, Edwards MS, Li G, Duncan JA 3rd, Cheshier SH, Shuer LM, Chang EF, Grant GA, Gephart MG, Barres BA. 2016. Purification and Characterization of Progenitor and Mature Human Astrocytes Reveals Transcriptional and Functional Differences with Mouse. Neuron 89(1):37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]