Abstract
Group B Streptococcus (GBS) causes adverse pregnancy outcomes and neonatal disease. The recommended preventative measure is intrapartum antibiotic prophylaxis, which impacts early onset neonatal disease but fails to protect against chorioamnionitis, preterm labor, stillbirth, or late-onset disease. Novel prevention methods are therefore needed. Use of probiotics including Lactobacillus spp., has been suggested given that they are dominant members of the lower reproductive tract microbiome. Although Lactobacillus was shown to reduce recto-vaginal colonization of GBS, no studies have examined how Lactobacillus impacts GBS in the extraplacental membranes. As Lactobacillus has been detected in the placental membranes, we sought to characterize GBS-Lactobacillus interactions in vitro using a colonizing and invasive GBS strain. While live Lactobacillus did not affect growth or biofilms in GBS, co-culture with L. gasseri contributed to an over 200-fold increase in GBS association with decidualized human endometrial stromal cells for both GBS strains (p < 0.005). Increased association did not result in increased invasion (p > 0.05) or host cell death, though some GBS and Lactobacillus combinations contributed to a significant reduction in host cell death (p < 0.05). Since Lactobacillus secretes many inhibitory compounds, the effect of Lactobacillus supernatants on GBS was also examined. The supernatants inhibited GBS growth, biofilm formation and invasion of host cells, though strain dependent effects were observed. Notably, supernatant from L. reuteri 6475 broadly inhibited growth in 36 distinct GBS strains and inhibited GBS growth to an average of 46.6% of each GBS strain alone. Together, these data show that specific Lactobacillus strains and their secreted products have varying effects on GBS and its interaction with cells of the extraplacental membranes that could impact pathogenesis. Understanding these interactions could help guide new treatment options aimed at reducing GBS-associated maternal complications and disease.
Keywords: Lactobacillus, group B Streptococcus, pregnancy, placental membranes, biofilm, probiotic
1. INTRODUCTION
Group B Streptococcus (Streptococcus agalactiae, GBS) is a global human health threat, contributing to neonatal infections and deaths as well as adverse pregnancy outcomes such as chorioamnionitis (infection of the extraplacental membranes), premature birth, preterm premature rupture of membranes (PPROM), and stillbirth [1]. GBS remains the leading cause of neonatal pneumonia, sepsis and meningitis in the United States [2]. In 2018, the Centers for Disease Control and Prevention reported the incidence of early- and late-onset GBS disease to be 0.25 and 0.28 per 1,000 live births, respectively [3]. GBS contributes to premature birth and stillbirth by infecting the fetus following its ascension from the vagina [4], while early-onset neonatal disease can occur due to aspiration of GBS during childbirth following ascension [1]. Although maternal vaginal GBS colonization is typically asymptomatic, GBS chorioamnionitis has been linked to inflammation and subsequent fetal infections [5,6].
Current consensus guidelines in the United States recommend that mothers testing positive for GBS between 36 and 37 weeks gestation receive intrapartum antibiotic prophylaxis (IAP) [2]. This practice has reduced the rate of early-onset neonatal sepsis occurring within the first week of life but has not affected late onset disease [7] or other adverse pregnancy outcomes. While effective at protecting against early-onset infection, several hundred women need to be treated with IAP for each case of early-onset sepsis that is averted [8]. Additionally, antibiotic treatment is known to affect both the maternal vaginal microbiome as well as the neonatal gut microbiome, which are both important for neonatal development [9–11]. The multiple shortcomings with IAP [12] combined with growing concerns about antibiotic resistance have resulted in increased interest in alternative therapies for the prevention of GBS-associated neonatal and pregnancy complications disease. As maternal GBS colonization is the primary risk factor for neonatal infection [1], novel therapies should aim to eradicate colonization or reduce the bacterial density.
Lactobacillus, a dominant member of the vaginal and cervical microbiota [13], has been examined for use as a probiotic against a variety of bacteria including GBS. For example, human trials using orally-administered Lactobacillus alone or in combination with other probiotic species, have shown variable, but promising success in reducing recto-vaginal colonization in pregnant mothers [14–17]. Reasons for variability include differences in the length of intervention, strain characteristics and dosage [16], though less is known about how Lactobacillus affects GBS and ascending infections. Some studies, however, have found that Lactobacillus can reduce the risk of premature birth triggered by inflammation [18–20]. To our knowledge, no prior studies have been conducted to determine how Lactobacillus affects GBS ascension of the vaginal tract or GBS virulence phenotypes such as attachment to and invasion of placental cells.
Since Lactobacillus has been identified in the human extraplacental membranes using metagenomic techniques [21], we aimed to determine whether different Lactobacillus spp. can alter the ability of GBS to associate with and invade this important barrier to the fetus. To further characterize the interaction, we examined changes in GBS growth and impact on host cell death. These in vitro studies reveal potential mechanisms used by Lactobacillus to affect colonization and premature birth, allowing for better understanding of its use as a probiotic as well as its limitations.
2. MATERIALS AND METHODS
2.1. Bacterial strains and growth conditions
GBS strains were selected based on multilocus sequence type (ST) designation, capsular serotype, and source. Lactobacillus and GBS interactions were performed using two serotype III GBS strains belonging to ST-17, which has been most associated with severe disease in neonates [22,23]. One strain, GB00112 (GB112), was isolated using a vaginal-rectal swab from a colonized mother after childbirth [24] and the second, GB00411 (GB411), from the blood of a septic newborn [25]. GBS strains were cultured in Todd-Hewitt broth (THB) or half-concentrated THB with agar (THA) at 37°C with 5% CO2.
Lactobacillus strains were selected to represent species that have been found in both the vaginal tract and extraplacental membranes via metagenomics [21] as well as the gastrointestinal tract. These species included L. reuteri, L. gasseri, and L. crispatus (Table 1). L. reuteri 6475 was isolated from breast milk, L. gasseri 33323 from the vagina [26], and L. crispatus 19390 from stool. L. reuteri 17938 is the daughter strain of L. reuteri ATCC 55730, which was isolated from breast milk and was previously shown to harbor potentially transferable resistance traits for tetracycline and lincomycin [27]. This daughter strain, however, no longer carries this plasmid. All strains were cultured in de Man, Rogosa and Sharpe (MRS, Difco 288130) broth or agar at 37°C with 5% CO2. Plating of lactobacilli and GBS co-cultures, however, was performed on Tryptic Soy Broth (TSB) supplemented with 5% Sheep Blood (Northeast Lab Services) at 37°C and 5% CO2. Lactobacillus spp. colonies were given 48 hours to grow.
Table 1.
Source of four Lactobacillus strains examined in this study.
In addition to live Lactobacillus cultures, supernatants were also isolated to evaluate the impact of Lactobacillus-associated secreted factors on GBS. Each Lactobacillus strain was loop-inoculated into 10 mL of MRS broth with or without 5 mM glycerol in a 15 mL conical tube and incubated at 37°C for 18–20 hours with the cap slightly loosened. After incubation, cultures were vortexed, centrifuged to pellet the bacteria, and filter sterilized with a 0.22 μm filter. Supernatants were used immediately. The effect of the L. reuteri supernatants on GBS growth was evaluated using a diverse set of 36 GBS strains (Table S1), while supernatant pH and hydrogen peroxide concentrations were measured in biological triplicate. The pH levels were measured using a pH reader (SevenEast pH, Mettler-Toledo GmbH). Hydrogen peroxide concentrations (μM) were determined using 50μl of supernatant in a Fluorometric Hydrogen Peroxide Assay Kit as described by the manufacturer (Sigma Aldrich; Lot#187037).
2.2. Cell Culture
Telomerase-immortalized human endometrial stromal cells (T-HESC; ATCC CRL-4003) [28] were cultured in DMEM/Nutrient Mixture Ham’s F-12 with L-glutamine (Sigma) supplemented with 1% ITS+ universal cell culture supplement premix (BD Biosciences), 1.5 g/liter sodium bicarbonate, 2% penicillin / streptomycin, and 10% charcoal-treated fetal bovine serum (HyClone), which is referred to as HESC medium herein. For all cell experiments, the T-HESC line was decidualized (dT-HESCs) by incubating with 0.5 mM 8-Bromo-cyclic AMP (cAMP) (Sigma) for three to six days as described [29]. These cells are also referred to as decidual cells. Assays were only performed when cells reached a 100% confluent monolayer so that only decidual cell surfaces were available for bacterial interactions.
2.3. Bacterial growth curves
To examine the effect of live Lactobacillus and its supernatants on GBS, growth curves were generated by serial plating or using a plate reader (Beckman Coulter, Inc). For the Lactobacillus and GBS co-culture experiments, overnight cultures were washed once with PBS and diluted to an equivalent optical density (OD)600 of 0.1 in HESC infection media. Each culture was inoculated 1:10 to have a starting culture with a 1:1 ratio of each bacteria. Samples were collected hourly for six hours and differentially plated on Tryptic Soy Agar (TSA) supplemented with 5% Sheep Blood. To examine the effect of supernatants, 100 μL of 0.1 OD 600 culture in HESC infection media was added to a 96-well plate with 25 μL of supernatant or additional infection media. Time points were collected every 15 minutes for eight hours by plate reader (BioTek Cytation 3 Imager). The Area Under the Curve (AUC) was calculated using GraphPad Prism 6. Significant differences were determined by comparing the AUC of three biological replicates by unpaired ANOVA.
2.4. Biofilm assays
Biofilm production was quantified as described in our prior study [30]. Briefly, overnight cultures of GBS and Lactobacillus were diluted to an equivalent OD600 of 0.1 and resuspended in Tryptic Soy Broth supplemented with 1% dextrose (TSBd); 50μl of each culture was added to a 96-well plate for co-culture biofilms. Mono-culture wells contained 50 μl of culture and 50 μl of media. Lactobacillus supernatant was added to GBS in monoculture at the beginning of the incubation period at 10% v/v of the total volume of the well. Plates were incubated for 48 hours at 37°C and 5% CO2. After incubation, wells were washed twice and 100 μl of crystal violet was added. After a ten-minute incubation, crystal violet was removed, and the wells were washed four times with 150 μl of PBS. Remaining crystal violet was solubilized with 100% ethanol; 50 μl was taken from each well, and absorbance at OD595 was determined using a plate reader (Beckman Coulter, Inc). The total absorbance was calculated by subtracting the average of the media controls and multiplying by four. Significance was determined by unpaired ANOVA of at least three biological replicates of technical quadruplicates.
2.5. Association with and invasion of decidual cells
Monolayers of dT-HESCs were washed thrice with phosphate buffered saline (PBS) before infection with bacterial cultures. Overnight bacterial cultures of GBS and Lactobacillus were washed once with PBS and re-suspended in HESC infection media. dT-HESC cells were infected at a multiplicity of infection (MOI) of ten bacterial (GBS) cells per host cell (MOI = 10) for both monoculture and co-culture wells to assure the same number of GBS cells were available to affect the decidual cells.
Co-culture wells had an additional MOI of 10 of each Lactobacillus strain. For the experiments with Lactobacillus supernatants, 10% v/v of supernatant was added to each well at the beginning of the two-hour incubation. An equivalent volume of HESC infection media was added to each control well to control for total volume between wells. Following a two-hour incubation at 37°C in atmospheric conditions, samples were taken from each well to quantify the final colony forming units (CFU) of GBS.
To calculate the number of associated cells (attached and invaded), wells were washed three times to remove unattached cells and host cells were disrupted using Triton-X as described [31]. Wells were scraped and thoroughly re-suspended before plating for CFU. To enumerate intracellular bacteria, extracellular bacteria were killed with 100 μg/ml of gentamicin (Gibco) and 5 μg/ml of penicillin G (Sigma) for one hour prior to the Triton-X treatment and enumeration steps described above. The percent of associated cells was determined by dividing the associated cells by the final CFU of each well. The invasion frequencies were calculated by dividing each well by the average of the three technical replicates of the final CFU. All presented data represent the average of three biological replicates of three technical replicates.
2.6. Cytotoxicity assays
Monolayers of dT-HESCs were cultured in 24-well plates. Cells were infected as described above and/or treated with 10% v/v of Lactobacillus supernatant. After incubation, cells were washed twice with PBS and treated with 4 μM ethidium homodimer 1 (Molecular Probes) suspended in PBS as described previously [32]. Plates were incubated at room temperature for 30 minutes without light, and fluorescence was measured at 528-nm excitation and 617-nm emission using a plate reader. The total number of cells in each well was calculated by adding 0.1% (wt/v) Saponin (Sigma) and incubating for at least 20 minutes before repeating the fluorescence reading. The percent permeability (cell death) was calculated by dividing the initial reading by the second and multiplying by 100. Significance was determined by unpaired ANOVA of at least three biological replicates of technical triplicates.
3. RESULTS
3.1. Impact of live Lactobacillus strains on growth of GBS
To evaluate growth of each GBS strain in the T-HESC media used for each experiment, growth curves were performed with GBS in monoculture and co-culture with Lactobacillus. GBS growth was measured for the two GBS strains, GB00112 (GB112) and GB00411 (GB411), grown alone and in co-culture with the four Lactobacillus strains (Figure S1A). To determine if there was a difference in growth, the AUC was calculated and compared across strains (Figure S1B). Co-culturing GBS with any of the four Lactobacillus strains, however, did not significantly affect the growth of GBS (p > 0.05). Because colonies of Lactobacillus were not detectable in co-culture after the first time point due to the higher concentration of GBS, we were unable to calculate Lactobacillus growth in co-culture.
3.2. Effect of live Lactobacillus on GBS biofilm formation
As biofilms are thought to be important for colonization and persistence [33], we sought to determine if co-culturing GBS with live Lactobacillus would affect the ability of GBS to form biofilms. Biofilm production at 48 hours was compared between each GBS strain in monoculture and to each GBS strain in co-culture with all four Lactobacillus strains. The colonizing GB112 strain formed a biofilm of 0.86 in monoculture, while co-culturing with all four Lactobacillus strains resulted in an increase in biofilm production in this strain (Figure S2A). Specifically, co-culturing GB112 with L. reuteri 6475, L. gasseri 33323, L. reuteri 17938, and L. crispatus 19390 increased biofilm production to absorbance values of 1.36, 1.37, 1.49, and 0.97, respectively. This increase in biofilm production, however, was not statistically significant (p > 0.05). While the invasive GB411 strain formed a stronger biofilm than GB112 with an absorbance value of 1.22, the difference between the two strains was not significant (p > 0.05). Similarly, no difference was observed in GB411 biofilm production following co-culture with L. reuteri 6475, L. gasseri 33323, L. reuteri 17938, and L. crispatus 19390, which had absorbance values of 1.44, 1.50, 1.80 and 1.25, respectively (p > 0.05) (Figure S2B). No differences were observed between GB112 and GB411 and co-cultured with each Lactobacillus strain.
3.3. Lactobacillus variably affects GBS association with decidual cells
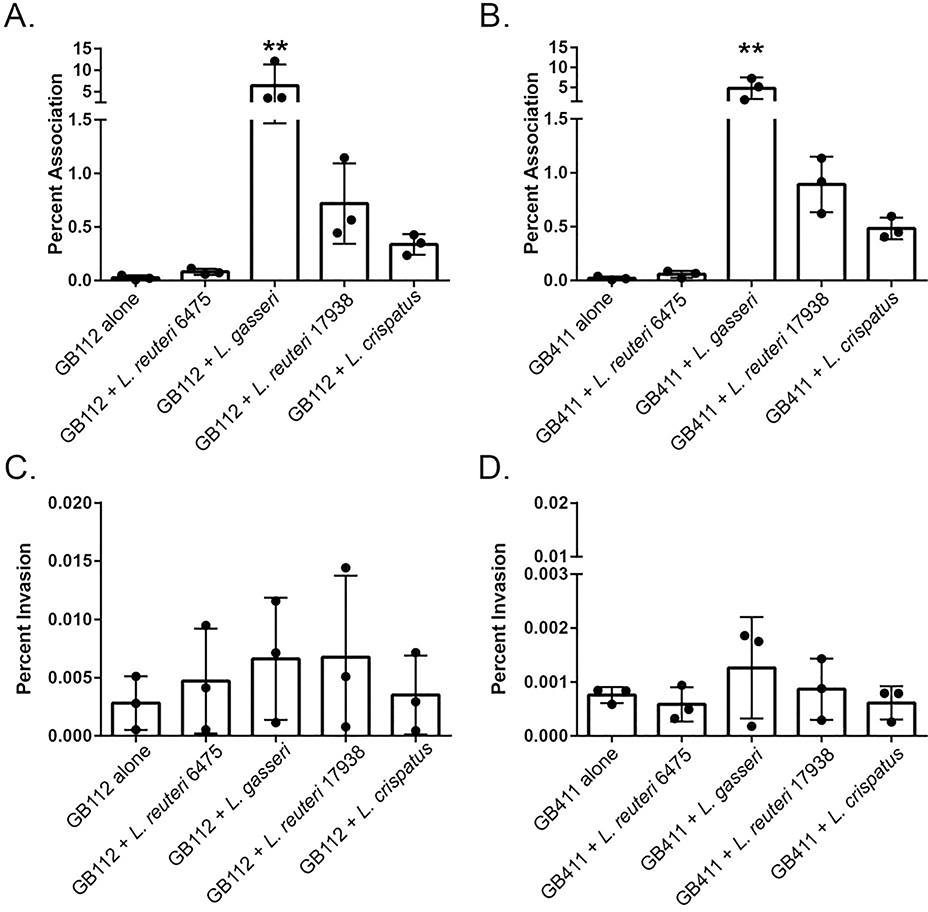
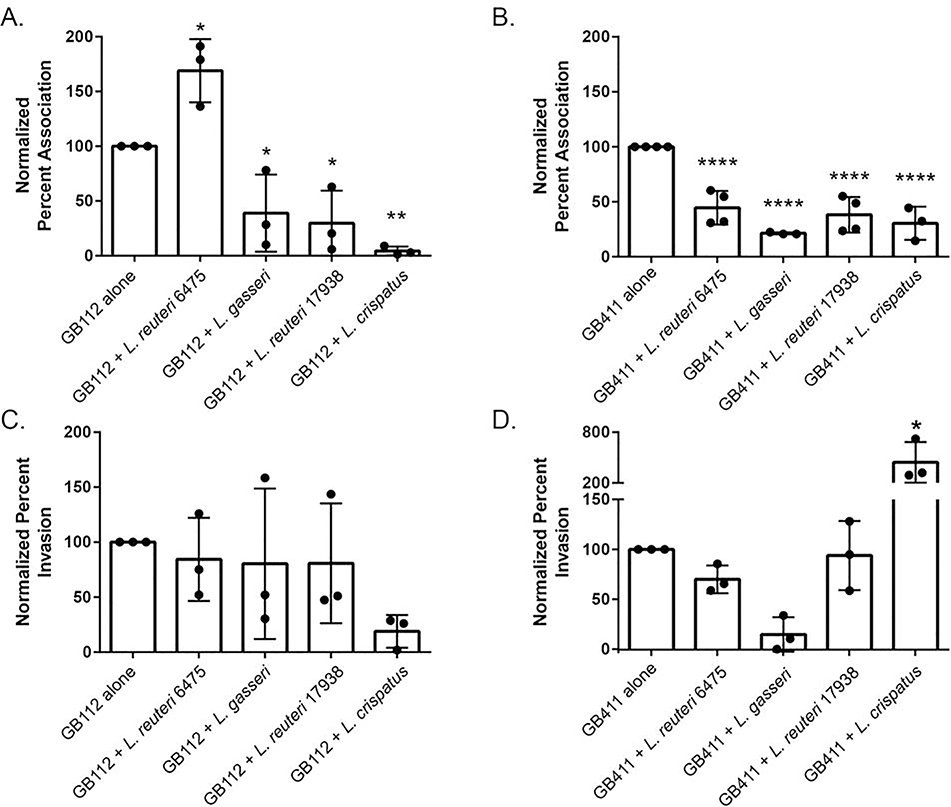
As we have demonstrated previously, dT-HESCs serve as a model of GBS attachment to and invasion of the extraplacental membranes [30–32]. We have also previously established that Lactobacillus is capable of associating with this cell line [34]. Hence, the goal of this experiment was to determine whether live Lactobacillus affects the ability of GBS to interact with this important fetal barrier. While no difference was observed in the level of association with dT-HESCs between the colonizing and invasive GBS strains of GBS (p > 0.05), differences were observed in response to co-culture with Lactobacillus. Co-culture with L. gasseri, for example, contributed to a significant 224-fold increase in association of the colonizing GB00112 strain compared to the monoculture (p < 0.005; Figure 1A). A similar effect was observed for the invasive GB00411 strain as association to dT-HESCs increased 233-fold in co-culture with L. gasseri relative to the invasive strain alone (p < 0.05; Figure 1B). Association to dT-HESCs following co-culture with L. gasseri 33323 was significantly greater for both GBS strains when compared to all other Lactobacillus and GBS combinations. No significant differences in association levels were observed between the GBS strains in monoculture compared to co-culture with both L. reuteri strains and L. crispatus (p > 0.05). Both GBS strains also demonstrated similar responses to the addition of Lactobacillus, with no observed statistical differences between each co-culture condition.
Figure 1. Live Lactobacillus variably impacts GBS association with decidual cells.
dT-HESCs were infected with a colonizing (GB112) or invasive GBS strain (GB411) at a MOI of 10 for two hours with or without an equivalent amount of each Lactobacillus strain. The percent association for A) GB112 and B) GB411 as well as the percent invasion for C) GB112 and D) GB411 were calculated relative to the total number of bacteria in the well. Experiments were completed in biological triplicates of technical triplicates. Error bars represent standard deviation between biological trials. Significance was determined by an unpaired ANOVA. p < 0.005 **
GBS invasion of the dT-HESCs was also examined. No significant difference in percent invasion was observed between the colonizing and invasive GBS strains in monoculture (p > 0.05). The percentages, however, were slightly higher for the colonizing GB112 strain. Although the addition of each Lactobacillus strain did not affect the ability of either GBS strain to invade the dT-HESCs (p > 0.05; Figures 1C and 1D), calculating the percent of associated cells that invaded resulted in a reduction for all cells when co-cultured with Lactobacillus. An average of 11.05% of the colonizing GBS cells invaded, while 5.75%, 0.10%, 0.94% and 1.04% of GBS invaded while in co-culture with L. reuteri 6475, L. gasseri, L. reuteri 17938 and L. crispatus, respectively. A similar reduction was observed with the invasive strain of GBS, with 3.68% of GBS invading in monoculture compared to 1.00%, 0.03%, 0.10% and 0.13% when co-cultured with each Lactobacillus strain, respectively. Because these experiments were conducted separately, however, we cannot determine if these differences were statistically significant as they cannot be paired in a way to allow for biological replicates.
3.4. Variation in the impact of Lactobacillus strains on GBS-induced host cell death
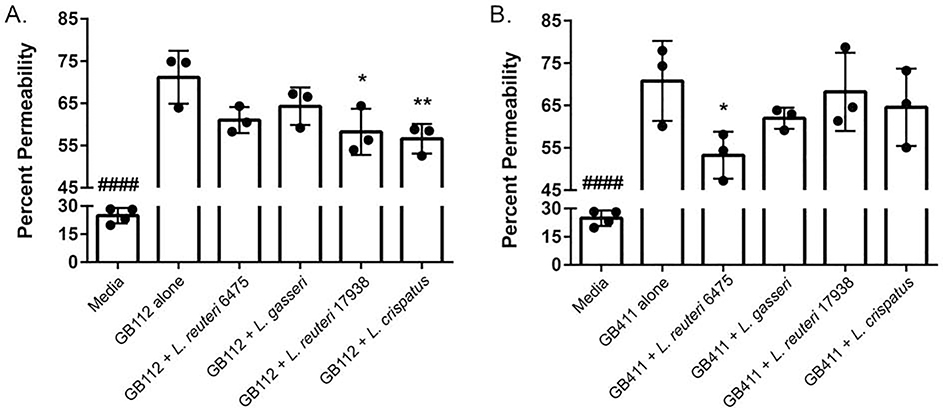
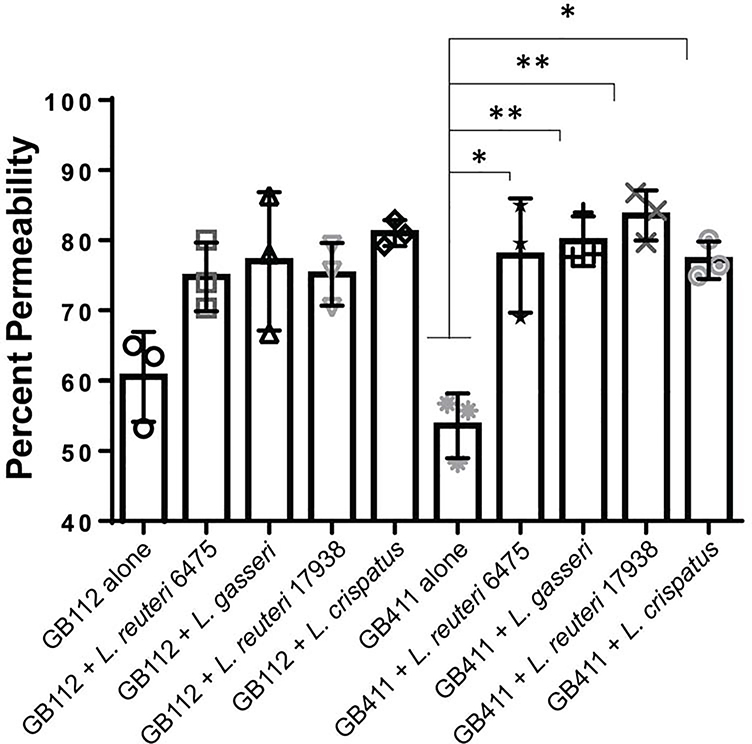
GBS is known to lyse host cells and this hemolysis could influence the ability of GBS to cross barriers like the extraplacental membranes [5]. To determine if Lactobacillus can prevent GBS-mediated cell lysis, we performed host cell permeability assays. Our prior study demonstrated that the same live Lactobacillus strains do not induce dT-HESC death [34]; therefore, the impact of GBS co-cultured with each Lactobacillus strain was evaluated. When evaluated in monoculture, both the colonizing (Figure 2A) and invasive (Figure 2B) GBS strains significantly damaged the host cells. The colonizing GB112 strain resulted in 71.16% (p < 0.00005) cell death in the four-hour period compared to the mock infection (24.85%), while the invasive GB411 strain caused 70.78% cell death (p < 0.00005). No significant difference in cell damage was observed between the colonizing and invasive strains (p > 0.05). When GBS was co-cultured with Lactobacillus, however, certain strain combinations contributed to a significant reduction in cell death. These effects were dependent on the GBS strain. For the colonizing GB112 strain, L. reuteri 17938 and L. crispatus 19390 reduced host cell death from 71.16% to 58.21% (p < 0.05) and 56.59% (p < 0.005), respectively (Figure 2A). Conversely, in the invasive GB411 strain, only L. reuteri 6475 significantly reduced host cell death from 70.78% to 53.25% (p < 0.05; Figure 2B)
Figure 2. Co-culturing GBS with Lactobacillus variably affects host cell death.
dT-HESCs were infected with the A) colonizing GBS strain, GB112, and B) invasive GBS strain, GB411, at a MOI of 10 with or without the equivalent amount of Lactobacillus. Following a four-hour incubation, cell permeability was detected using an ethidium homodimer, and percent permeability, or percent of cells that were permeable, was calculated by lysing the remaining cells in each well. Graphed data represents three biological replicates, and the error bars represent the standard deviation of the data. Significance was determined using an unpaired ANOVA between monoculture and co-culture. P < 0.05 *; p < 0.005 **; p < 0.00005 ####All other conditions were significantly higher than the media control.
3.5. Lactobacillus supernatants inhibit GBS growth
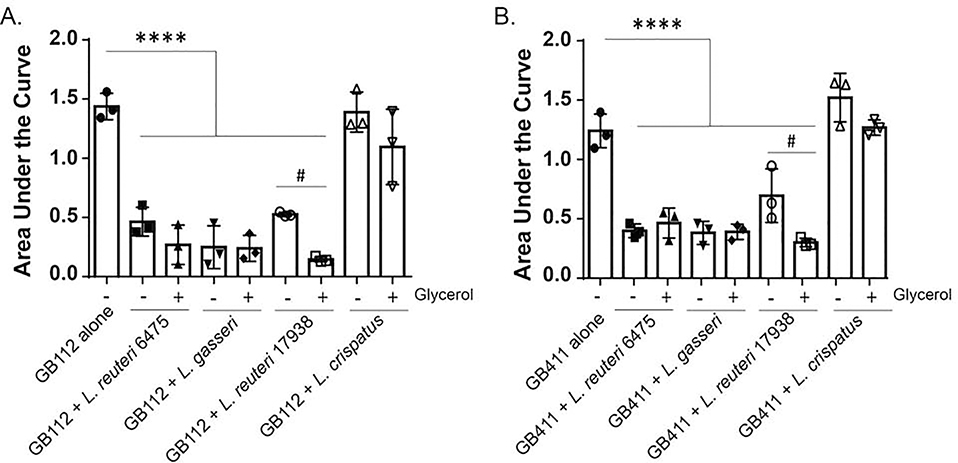
Because Lactobacillus was unable to grow in the HESC infection media, we hypothesized that it may not be producing the secondary metabolites that could inhibit GBS. To evaluate secreted metabolites or other inhibitory compounds, we grew Lactobacillus overnight and collected the supernatant. Supernatants were added at 10% v/v of supernatant to GBS, and differences in growth were assessed by calculating the AUC. Unlike the live Lactobacillus co-culture experiments, the supernatants from L. reuteri 6475, L. gasseri 33323 and L. reuteri 17938 reduced the AUC of the colonizing GB112 strain from 1.44 to 0.46 (p < 0.00005), 0.25 (p < 0.00005) and 0.53 (p < 0.00005), respectively (Figure 3A). Similarly, the same strains reduced the AUC of the invasive strain from 1.21 to 0.39 (p < 0.00005), 0.37 (p < 0.00005), and 0.68 (p < 0.0005), respectively (Figure 3B). The supernatant of L. crispatus 19390 did not affect GBS growth (p > 0.05; 1.44 to 1.39 & 1.21 to 1.50) and no statistical difference was observed between the colonizing and invasive GBS strains.
Figure 3. Effect of Lactobacillus supernatants on GBS growth with and without glycerol.
Growth of the A) colonizing GB112 strain and B) invasive GB411 strain was monitored for eight hours at OD595 when grown alone or with Lactobacillus supernatants (25% v/v) in the presence (+) and absence (−) of glycerol. The Area Under the Curve (AUC) is presented. Error bars represent the standard deviation between three biological trials. # An unpaired ANOVA was used to test differences between each supernatant condition and GBS alone. p < 0.00005 **** A Student’s t-test was used to evaluate differences based on the addition of glycerol. p < 0.05
Because L. reuteri has been shown to use glycerol to synthesize reuterin, a compound that has broad spectrum antimicrobial activities [35], we also assessed growth effects on GBS when glycerol was added. Intriguingly, the addition of glycerol increased the inhibitory effect of L. reuteri 17938 (p < 0.05). For the colonizing strain, GB112, the addition of glycerol further reduced the AUC to 0.15 following co-culture with L. reuteri 17938 (p < 0.05; Figure 3A), while it further reduced the AUC of the invasive GB411 strain to 0.29 (p < 0.005; Figure 3B). The addition of glycerol did not significantly reduce the AUC for either GBS strain upon co-culture with L. reuteri 6475, L. gasseri 33323, or L. crispatus 19390 (p > 0.05).
3.6. Lactobacillus supernatants prevent GBS biofilm formation
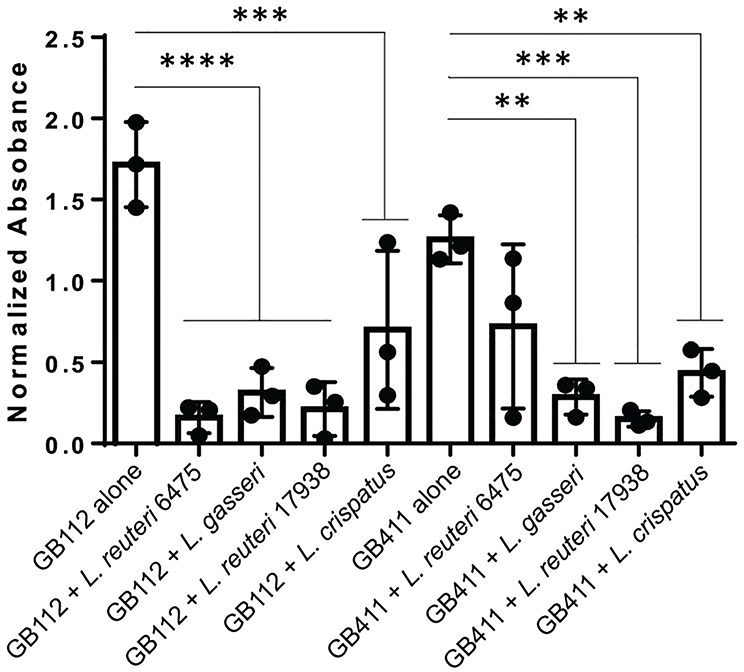
Since we found that supernatants affected GBS growth, we hypothesized that they may also impact GBS biofilm formation. Given that only one of the two L. reuteri strains was affected by glycerol addition, we only examined the effect of supernatant without glycerol. Significant differences in GBS biofilm formation were observed for all supernatants compared to the control except for the invasive GB411 strain co-cultured with L. reuteri 6475 supernatant (Figure 4). Supernatants of L. reuteri 6475, L. gasseri, L. reuteri 17938, and L. crispatus decreased biofilm formation in the colonizing strain from 1.72 to 0.16 (p < 0.00005), 0.31 (p < 0.00005), 0.21 (p < 0.00005) and 0.70 (p < 0.0005), respectively. The same supernatants also decreased biofilm production in the invasive strain from 1.26 to 0.72 (p > 0.05), 0.29 (p < 0.005), 0.15 (p < 0.0005) and 0.43 (p < 0.005). No significant differences were found between how a given supernatant affected the colonizing versus invasive strains (p > 0.05).
Figure 4. Lactobacillus supernatants reduce GBS biofilms.
GBS was grown for 48 hours with or without 10% v/v Lactobacillus supernatants to measure biofilm production. Normalized absorbance was calculated by taking OD595, subtracting the media control and multiplying by four. Error bars represent the standard deviation between biological trials. Statistical differences between GBS alone and with supernatant added were calculated in PRISM 6 with unpaired ANOVA. P < 0.005 ** p < 0.0005 *** p < 0.00005 ****
3.7. Impact of Lactobacillus supernatants on GBS interactions with dT-HESCs
To further assess the effects of Lactobacillus supernatants, we characterized how the addition of each supernatant would affect GBS association with and invasion of dT-HESCs. As this experimental design accounts for growth within the time span of the experiment by presenting the data as a percent of the CFU at the end of the incubation period, differences are independent of the growth changes observed. Because lactobacilli are lactic acid producing bacteria, we were concerned that the supernatants could damage the host cells, which are sensitive to pH changes. However, we observed no significant effect on host cell permeability for all supernatants except for those recovered from L. gasseri (p < 0.05; Figure S3). Supernatants from L. gasseri significantly increased host cell permeability from 27.27% to 59.84% in three hours (p < 0.05); therefore, results using this supernatant in combination with dT-HESCs must be interpreted carefully as changes may be due to supernatant-induced host cell permeability.
Generally, the addition of all Lactobacillus supernatants reduced association of GBS with dT-HESCs except for L. reuteri. When Lactobacillus was grown without glycerol, the supernatant of L. reuteri 6475 increased association of the colonizing strain to 169.90% of the strain alone. Conversely, L. gasseri 33323, L. reuteri 17938, and L. crispatus 19390 reduced association of the colonizing GB112 strain to 35.85% (p > 0.05), 29.74% (p < 0.05) and 4.41% (p < 0.005), respectively (Figure 5A). The addition of glycerol during Lactobacillus growth also resulted in reduced association for the colonizing GB112 strain, but this decrease was not statistically significant. By contrast, supernatants from L. reuteri 6475, L. gasseri 33323, L. reuteri 17938, and L. crispatus 19390 grown with glycerol reduced association to 41.85% (p > 0.05), 14.16% (p < 0.005), 72.27% (p > 0.05) and 50.00% (p > 0.05) of the invasive strain without supernatant added.
Figure 5. Effect of Lactobacillus supernatant on GBS association with and invasion of dT-HESCs.
The colonizing GB112 and invasive GB411 strains were added to dT-HESCs at a MOI of 10 for two hours with 10% v/v of supernatant from each Lactobacillus strain; GBS without Lactobacillus supernatant was included for comparison. The percent of associated GBS was calculated for A) GB112 and B) GB411 as was the percent of invaded bacteria for C) GB112 and D) GB411. Percentages were calculated relative to the total number of bacteria in the well and were examined as a percentage of each GBS strain alone to account for variation between trials. Experiments were completed in at least biological triplicates of technical triplicates. Error bars represent standard deviation between biological trials. Significance between GBS alone and with supernatant was determined by an unpaired ANOVA. p < 0.05 *
Association of the invasive GB411 strain was also reduced by Lactobacillus supernatants (Figure 5B). Supernatants from L. reuteri 6475, L. gasseri 33323, L. reuteri 17938, and L. crispatus 19390 grown in the absence of glycerol reduced association of the invasive GB411 strain to 44.50% (p < 0.00005), 21.36% (p < 0.00005), 38.17% (p < 0.00005) and 30.49% (p < 0.00005). Similarly, supernatants made with glycerol also significantly reduced association. Association of the invasive strain was reduced to 21.95% (p < 0.00005), 34.38% (p < 0.00005), 27.36% (p < 0.00005) and 23.99% (p < 0.00005) by L. reuteri 6475, L. gasseri 33323, L. reuteri 17938, and L. crispatus 19390, respectively. No significant differences were observed between the colonizing and invasive strains based on interactions with a given supernatant except for L. reuteri 6475 grown without glycerol. This strain contributed to increased association of the colonizing GB112 strain but reduced association of the invasive GB411 strain (p < 0.00005).
Because invasion of the extraplacental membranes is an important step in in utero infection for GBS, we also sought to assess the effect of these Lactobacillus supernatants on intracellular invasion. As with the association experiments, supernatant was added at 10% v/v, and the percent invasion was normalized to the invasion of the GBS strain alone to account for variation between replicates. Controlling for variation among biological replicates, however, did not account for all the variation observed in these experiments. Indeed, the level of invasion observed for the colonizing GB112 strain was particularly variable for all supernatants except L. crispatus, (Figure 5C). Given this high degree of variability, no significant differences were observed for invasion by GB112 following incubation with each Lactobacillus supernatant (p > 0.05). For the invasive GB411 strain, the most variable invasion percentages were observed with the L. reuteri 17938 supernatant. Only the addition of L. crispatus supernatant contributed to a significant increase in GB411 invasion of dT-HESCs (Figure 5D).
3.8. Impact of Lactobacillus supernatants on host cell death differs by GBS strain
As maintenance of the extraplacental membranes is important for a successful pregnancy, we also examined if the Lactobacillus supernatants altered the amount of host cell damage induced by GBS. To this end, we examined host cell death in the presence of both GBS and Lactobacillus supernatants five hours post-infection. Indeed, we observed an increase in host cell death that was dependent on the GBS strain. The colonizing GB112 strain of GBS caused 60.5% cell death alone, but with the addition of supernatants from L. reuteri 6475, L. gasseri, L. reuteri 17938, and L. crispatus, cell death increased to 74.8%, 77.0%, 75.1% and 81.0% (Figure 6). These increases were not statistically significant (p > 0.05). By contrast, the invasive GB411 strain had increased host cell death in the presence of Lactobacillus supernatants from all four strains. Percent host cell death increased from 53.6% alone to 77.8% (p < 0.05), 79.9% (p < 0.005), 83.6% (p < 0.005) and 77.1% (p < 0.05) with L. reuteri 6475, L. gasseri, L. reuteri 17938, and L. crispatus, respectively. No significant differences were observed for host cell death with the colonizing strain versus the invasive strain using the same supernatant.
Figure 6. Lactobacillus supernatants increase GBS-induced host cell death.
dT-HESCs were infected with GB112 and GB411 (MOI = 10) with 10% v/v Lactobacillus supernatant for four hours. Cell permeability was detected using an ethidium homodimer, and percent permeability was calculated by lysing the remaining cell in each well. Graphed data are three biological replicates with error bars to represent the standard deviation. An unpaired ANOVA tested for differences between GBS alone and with supernatant. p < 0.05 *; p < 0.005 **
3.9. Supernatant from Lactobacillus reuteri 6475 broadly inhibits GBS strains
To determine if growth inhibition of GBS was specific to the two strains, GB411 and GB112, a larger sampling of 36 strains representing diverse sequence types (STs), serotypes and sources (Table S1) was examined. Only the impact of L. reuteri 6475 supernatant was evaluated. Among all 36 strains tested, GBS growth was broadly inhibited, averaging 46.6% of each GBS strain alone and ranging from 39.2% to 54.7% (Figure S4). No significant differences, however, were observed across strains based on the level of growth inhibition or after stratifying by strain characteristics, namely ST, capsule type and source.
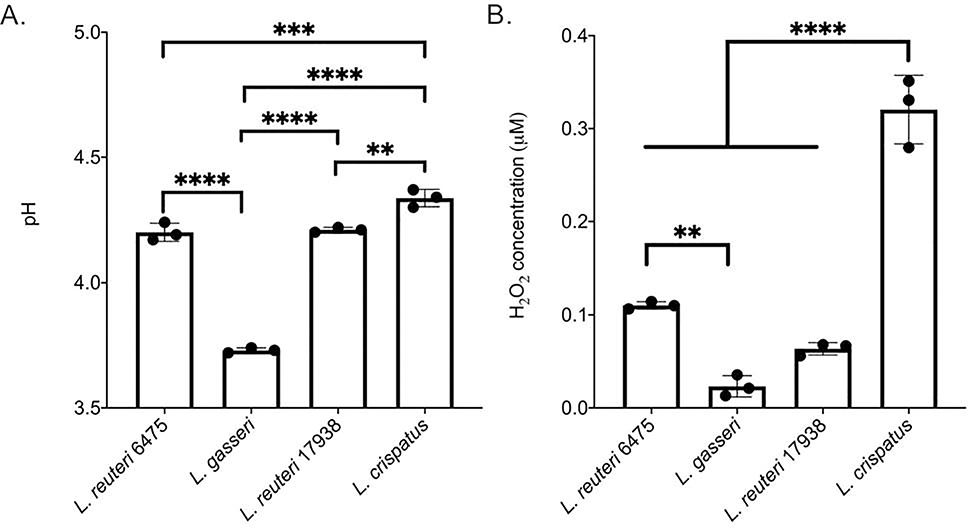
3.10. Supernatants have variable H2O2 and pH levels
To better understand the effects of Lactobacillus supernatants, we examined two properties of the supernatants that have been previously shown to affect other species: pH and H2O2 concentration. The pH was similarly acidic for all supernatants, but L. gasseri was significantly lower than all other strains at 3.73 (Figure 7A; p < 0.0001). pH of L. reuteri 6475, L. reuteri 17938 and L. crispatus supernatants were 4.20, 4.21 and 4.34, respectively. There were also significant differences between L. crispatus and both of the L. reuteri strains (p < 0.001, ANOVA). Hydrogen peroxide concentrations were also different across the Lactobacillus strains examined. L. crispatus supernatants, for instance, had a significantly greater concentration of hydrogen peroxide (0.32 μM) compared to all other strains (Figure 7B; p < 0.0001, ANOVA). L. reuteri 6475 had a greater concentration (0.11 μM) than L. reuteri 17938 (0.06 units per ul, p < 0.001, ANOVA) and L. gasseri (0.02 μM), but the difference was not significant for L. gasseri. Similarly, no significant difference was observed between L. gasseri and the L. reuteri 17938 strain (p > 0.05 ANOVA).
Figure 7. pH levels and hydrogen peroxide concentration vary among Lactobacillus supernatants.
Lactobacillus supernatants were collected from overnight cultures of each strain and evaluated for A) pH and B) hydrogen peroxide (μM) levels. Significance was measured using a one-way ANOVA after confirmation of a normal distribution by the Shapiro-Wilk test. ** p < 0.005; *** p< 0.001; **** p < 0.0001
4. DISCUSSION
Efforts to reduce the burden of perinatal GBS infections through antibiotic treatment have not been fully successful [2,12]. While reducing rates of early-onset neonatal sepsis, there has been no effect on late-onset disease [7] or other GBS-related adverse pregnancy outcomes. Further, the use of antibiotics is not without potential negative effects, including those on maternal and neonatal microbiomes [9–11], increased risk of E. coli-associated disease, and antibiotic resistance [36,37]. For this reason, alternative therapies, including probiotics, have been suggested. Herein, we sought to characterize the effects of different Lactobacillus strains and their secreted factors on GBS and its interactions with cells (dT-HESCs) that represent those found in the extraplacental membranes. To determine if GBS-lactobacilli interactions vary across GBS strains, we first compared interactions among the invasive GB411 and colonizing GB112 strains, which both belong to the ST-17 lineage that has been shown to disproportionately affect neonates [22,23].
Surprisingly, we found that live Lactobacillus did not affect growth or biofilm production for either GBS strain. These results, however, are likely due to the inability of Lactobacillus to grow in both the infection media and the biofilm media, and hence, these findings may not accurately represent the impact on GBS. Future work should therefore evaluate these interactions with different media, particularly given the inhibitory effects observed when some Lactobacillus supernatants were examined. It would also be interesting, for example, to determine the relative composition of Lactobacillus and GBS within each biofilm as previous work showed inhibition of biofilm formation in Pseudomonas fluorescens and Bacillus cereus by Lactobacillus spp. [38]. Given the phenotypic variation observed among the four Lactobacillus strains evaluated herein, it is likely that distinct Lactobacillus strains respond differently to different pathogens and environmental signals. Indeed, the prior study of bacterial biofilms utilized a strain of Lactobacillus plantarum [38], which not evaluated in our study.
Nonetheless, it is notable that certain Lactobacillus strains could alter GBS interactions with dT-HESCs. While we have shown that each Lactobacillus strain was capable of attaching to dT-HESCs [34], most of the strains evaluated did not increase the ability of GBS to associate with host cells. The exception was L. gasseri as co-culture significantly increased association of both the colonizing and invasive GBS strains, suggesting that these species interact in a way that promotes GBS attachment. Prior studies have shown that this strain of Lactobacillus can aggregate with other pathogens [39] and GBS [40], which could enhance colonization of both species if the aggregate is secured to the host cells. It is important to note that increased association may not necessarily be a negative outcome as association did not lead to an increase in invasion or death of dT-HESCs. Indeed, these findings are in line with those from a prior study that showed co-aggregation of GBS and Lactobacillus spp. was inhibitory on GBS viability [40]. Future studies involving the use of advanced microscopy tools could enhance understanding of co-aggregation, particularly to dT-HESCs and other cells in vitro.
Because of the concerns regarding poor growth of Lactobacillus in the media used for the experiments, we were unable to evaluate the impact of specific secreted factors that may be produced by each Lactobacillus strain. The role of factors such as bacteriocins, which have been isolated from L. acidophilus [41] and other lactic acid bacteria [42], should be evaluated in future studies. Instead, we evaluated the effect of Lactobacillus supernatants representing a compilation of secreted factors, on GBS growth and biofilm production. Since biofilms are a congregation of cells surrounded by a polysaccharide matrix [33], we hypothesize that the observed reduction in biofilm production may be due to the limited growth of GBS. Indeed, previous work has demonstrated the importance of environmental conditions for GBS biofilm production [43], suggesting this may be the primary reason for reduced levels. It is interesting to note, however, that though supernatant from L. crispatus did not significantly inhibit growth, it did significantly inhibit biofilm formation (p < 0.005). It is therefore possible that another mechanism of biofilm reduction exists in some strains. Nonetheless, this growth deficiency may explain the effects on biofilm production but it does not explain the effects on GBS association with dT-HESCs, which was also reduced by L. crispatus and other Lactobacillus supernatants. Because the experimental design of the association assays accounts for growth by testing the CFUs at the end of the incubation period, these reductions are due to another factor. Further characterization of the supernatants is critical to identify those factors that are have contributed to reductions in GBS association levels. Indeed, we hypothesized that reuterin, a compound with broad spectrum antimicrobial activities produced by L. reuteri [35], may be one of these factors. Because L. reuteri uses glycerol to synthesize reuterin, we evaluated whether adding glycerol would result in increased GBS inhibition, particularly for the two L. reuteri strains. An increase in inhibition was only observed by L. reuteri 17938, suggesting that it may be capable of producing reuterin unlike the other L. reuteri strain.
The different Lactobacillus supernatants also variably impacted GBS invasion. Although the host cell death assay time points are three hours later than those for the invasion assays, these results could suggest that the host cells are becoming more permeable within the time frame of the invasion assay. If this cell death is increased or decreased during the first two hours of the experiment, then it may explain the high variability between biological replicates; the assay requires the host cell to be non-permeable to protect the invaded GBS from antibiotic treatment. It is also notable that host cell death was increased with the combination of GBS plus the supernatants since the supernatants alone did not affect host cell death with the exception of L. gasseri. This finding suggests that GBS can alter its interactions with host cells in response to the supernatant, which was more pronounced in the invasive GBS strain compared to the colonizing strain. This variation further suggests that there may be differences in how these two strains respond to microbial cues, which is consistent with prior studies. For example, key stressors like antibiotics and reactive oxygen species, have been shown to alter GBS interactions with host cells including increasing macrophage uptake [44]. Additional experiments are needed to determine how GBS alters its transcription or protein profile to better understand these changes. An increase in GBS virulence in response to Lactobacillus supernatants is of particular importance because the extraplacental membranes are critical for maintaining a health pregnancy.
The examination of pH level and hydrogen peroxide concentrations in each Lactobacillus supernatant identified more interesting differences between the Lactobacillus strains. The lower pH of the L. gasseri supernatant may have contributed to its effect on dT-HESC viability; however, we did not see a significant difference in the relative reduction in GBS growth between the strains that could be explained solely by this difference in pH. This result differs from those findings from a prior study that showed a pH-dependent effect of the supernatants [40]. Specifically, those supernatants with a pH below 4.0 could completely kill GBS, while those with levels between 4.0 and 4.5 simply reduced growth. Despite observing a L. gasseri supernatant pH level of 3.73 in our study, we did not observe GBS killing, but rather, a reduction in GBS was detected. This difference could also be explained by differences in GBS strains. Similarly, differences in hydrogen peroxide concentrations were observed in the Lactobacillus supernatants. Hydrogen peroxide is a reactive oxygen species that can lead to peroxidation of membrane lipids and increased membrane permeability of bacteria such as Salmonella enterica, Escherichia coli, Staphylococcus aureus and Streptococcus faecalis [45]. Although the L. crispatus supernatant had the highest concentration of hydrogen peroxide, we did not observe a corresponding difference in GBS growth inhibition, biofilm production, or association and invasion of dT-HESCs. Indeed, L. crispatus did not reduce GBS growth and was comparable to the other Lactobacillus strains for inhibition of GBS biofilms, association and invasion. Because GBS is resistant to hydrogen peroxide [46,47], these data suggest that hydrogen peroxide concentration is not likely contributing to the GBS inhibition observed.
Since we observed effects on growth and association with dT-HESCs, future work should focus on examining the different components of the supernatants to determine which components are responsible for each phenotype. The supernatants could be fractionated and examined individually for the effects observed on association and growth. This fractionation could also allow for separation of positive effects of the supernatant, such as inhibition of GBS growth, from negative effects including increased GBS-induced host cell death. Identification of growth inhibitory compounds would be of particular interest due to its broad impact on GBS growth across a diverse set of strains.
CONCLUSIONS
While current literature examines the effect of Lactobacillus on vaginal-rectal colonization, we sought to determine if distinct lactobacilli could also impact GBS phenotypes that are important for ascending infections, including growth, biofilm production, association with and invasion of decidual cells, and host cell death. Because the decidua is the outermost cell layer of the extraplacental membranes, it represents a potential early contact point for bacteria, including GBS, which ascend from the cervicovaginal region. Hence, it is biologically plausible that lactobacilli and GBS could be physically close to each other in the lower reproductive tract and near the decidua of the extraplacental membranes. While we show that live Lactobacillus had a minimal impact on GBS, some Lactobacillus supernatants variably affected growth, biofilm production, association with and death of dT-HESCs. Some of these effects, namely increased association and induction of host cell death, are concerning when considering the importance of the extraplacental membranes in maintaining a healthy pregnancy. Therefore, these findings further demonstrate the importance of a thorough examination of the effects of any potential alternative therapies.
Supplementary Material
Highlights.
Several Lactobacillus spp. had variable effects on group B Streptococcus (GBS) association with decidualized human endometrial stromal cells but no effect on cell invasion or cell death
Different Lactobacillus supernatants containing secreted inhibitory compounds were capable of inhibiting GBS growth, biofilm formation and host cell invasion
Supernatant from one L. reuteri strain demonstrated a broad level of growth inhibition in 36 distinct GBS strains of varying serotypes and multilocus sequence types
Collectively, these data indicate that specific Lactobacillus strains and their secreted products have varying effects on GBS that could impact pathogenesis or protection from invasive disease
Acknowledgments
We thank Dr. H. Dele Davies of the University of Nebraska Medical Center for use of the GBS strains and Drs. Cindy Arvidson and Poorna Visvanathan at Michigan State University (MSU) for kindly providing the Lactobacillus strains. We also thank Drs. Neal Hammer and Cindy Arvidson for their scientific discussions and editing of prior versions of this manuscript.
Funding: Support for this work was provided by the National Institutes of Health (grant numbers AI134036, HD090061 to DMA, JAD and SDM) as well as the MSU Foundation (to SDM). Student support was provided to MS through the Bertina Wentworth Scholar Award from the MSU Department of Microbiology and Molecular Genetics. The funders played no role in any aspect of this study or manuscript.
Footnotes
Declarations of interest: none
Conflict of interests
The authors declare that no commercial or financial conflicts of interest exist.
Data statement: All data are available within the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Verani JR, McGee L, Schrag SJ, Centers for Disease Control and Prevention, Prevention of perinatal group B streptococcal disease--revised guidelines from CDC, 2010., MMWR. (2010). 59 (RR10): 1–32. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5910a1.htm [PubMed] [Google Scholar]
- [2].American College of Obstetricians and Gynecologists, Prevention of Group B streptococcal early-onset disease in newborns: ACOG Committee Opinion, Number 797, Obstet. Gynecol. 135 (2020) e51–e72. 10.1097/AOG.0000000000003668. [DOI] [PubMed] [Google Scholar]
- [3].Center for Disease Control and Prevention, Active Bacterial Core Surveillance (ABCs) Report Emerging Infections Program Network group B Streptococcus, 2018 (2020). https://www.cdc.gov/abcs/reports-findings/survreports/gbs18.pdf.
- [4].Romero R, Gomez-Lopez N, Winters AD, Jung E, Shaman M, Bieda J, Panaitescu B, Pacora P, Erez O, Greenberg JM, Ahmad MM, Hsu CD, Theis KR, Evidence that intra-amniotic infections are often the result of an ascending invasion - A molecular microbiological study, J. Perinat. Med. (2019). 10.1515/jpm-2019-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Whidbey C, Harrell MI, Burnside K, Ngo L, Becraft AK, Iyer LM, Aravind L, Hitti J, Adams Waldorf KM, Rajagopal L, A hemolytic pigment of Group B Streptococcus allows bacterial penetration of human placenta, J. Exp. Med. 210 (2013) 1265–1281. 10.1084/jem.20122753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Vornhagen J, Quach P, Boldenow E, Merillat S, Whidbey C, Ngo LY, Adams Waldorf KM, Rajagopal L, Bacterial hyaluronidase promotes ascending GBS infection and preterm birth, mBio. 7 (2016) 1–10. 10.1128/mBio.00781-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Schrag SJ, Verani JR, Intrapartum antibiotic prophylaxis for the prevention of perinatal group B streptococcal disease: Experience in the United States and implications for a potential group B streptococcal vaccine., Vaccine. (2012) 1–7. 10.1016/j.vaccine.2012.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bevan D, White A, Marshall J, Peckham C, Modelling the effect of the introduction of antenatal screening for group B Streptococcus (GBS) carriage in the UK, BMJ Open. 9 (2019). 10.1136/bmjopen-2018-024324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Aloisio I, Mazzola G, Corvaglia LT, Tonti G, Faldella G, Biavati B, Di Gioia D, Influence of intrapartum antibiotic prophylaxis against group B Streptococcus on the early newborn gut composition and evaluation of the anti-Streptococcus activity of Bifidobacterium strains, Appl. Microbiol. Biotechnol. 98 (2014) 6051–6060. 10.1007/s00253-014-5712-9. [DOI] [PubMed] [Google Scholar]
- [10].Roesch LFW, Silveira RC, Corso AL, Dobbler PT, Mai V, Rojas BS, Laureano ÁM, Procianoy RS, Diversity and composition of vaginal microbiota of pregnant women at risk for transmitting Group B Streptococcus treated with intrapartum penicillin, PLoS One. 12 (2017). 10.1371/journal.pone.0169916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Stearns JC, Simioni J, Gunn E, McDonald H, Holloway AC, Thabane L, Mousseau A, Schertzer JD, Ratcliffe EM, Rossi L, Surette MG, Morrison KM, Hutton EK, Intrapartum antibiotics for GBS prophylaxis alter colonization patterns in the early infant gut microbiome of low risk infants, Sci. Rep. 7 (2017). 10.1038/s41598-017-16606-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Aronoff DM, Blaser MJ, Disturbing the neonatal microbiome is a small price to pay for preventing early-onset neonatal group B streptococcus disease: AGAINST: Against relying on antibiotics to prevent early-onset neonatal group B Streptococcus disease, BJOG An Int. J. Obstet. Gynaecol. 127 (2020) 229 10.1111/1471-0528.15988. [DOI] [PubMed] [Google Scholar]
- [13].Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SSK, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, Brotman RM, Davis CC, Ault K, Peralta L, Forney LJ, Vaginal microbiome of reproductive-age women, Proc. Natl. Acad. Sci. U. S. A. 108 (2011) 4680–4687. 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hanson L, Vandevusse L, Duster M, Warrack S, Safdar N, Feasibility of oral prenatal probiotics against maternal group B Streptococcus vaginal and rectal colonization, J. Obstet. Gynecol. Neonatal Nurs. 43 (2014) 294–304. 10.1111/1552-6909.12308. [DOI] [PubMed] [Google Scholar]
- [15].Ho M, Chang YY, Chang WC, Lin HC, Wang MH, Lin WC, Chiu TH, Oral Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 to reduce Group B Streptococcus colonization in pregnant women: A randomized controlled trial, Taiwan. J. Obstet. Gynecol. 55 (2016) 515–518. 10.1016/j.tjog.2016.06.003. [DOI] [PubMed] [Google Scholar]
- [16].Olsen P, Williamson M, Traynor V, Georgiou C, The impact of oral probiotics on vaginal group B streptococcal colonisation rates in pregnant women: A pilot randomised control study, Women Birth. 31 (2018) 31–37. 10.1016/j.wombi.2017.06.012. [DOI] [PubMed] [Google Scholar]
- [17].Sharpe M, Shah V, Freire-Lizama T, Cates EC, McGrath K, David I, Cowan S, Letkeman J, Stewart-Wilson E, Effectiveness of oral intake of Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 on Group B Streptococcus colonization during pregnancy: a midwifery-led double-blind randomized controlled pilot trial, J. Matern. Neonatal Med. (2019) 1–8. 10.1080/14767058.2019.1650907. [DOI] [PubMed] [Google Scholar]
- [18].Yeganegi M, Watson CS, Martins A, Kim SO, Reid G, Challis JRG, Bocking AD, Effect of Lactobacillus rhamnosus GR-1 supernatant and fetal sex on lipopolysaccharide-induced cytokine and prostaglandin-regulating enzymes in human placental trophoblast cells: implications for treatment of bacterial vaginosis and prevention of preterm, Am. J. Obstet. Gynecol. 200 (2009) 532.e1–532.e8. 10.1016/j.ajog.2008.12.032. [DOI] [PubMed] [Google Scholar]
- [19].Yang S, Li W, Challis JRG, Reid G, Kim SO, Bocking AD, Probiotic Lactobacillus rhamnosus GR-1 supernatant prevents lipopolysaccharide-induced preterm birth and reduces inflammation in pregnant CD-1 mice, Am. J. Obstet. Gynecol. 211 (2014) 44.e1–44.e12. 10.1016/j.ajog.2014.01.029. [DOI] [PubMed] [Google Scholar]
- [20].Li W, Yang S, Kim SO, Reid G, Challis JRG, Bocking AD, Lipopolysaccharide-induced profiles of cytokine, chemokine, and growth factors produced by human decidual cells are altered by Lactobacillus rhamnosus GR-1 supernatant, Reprod. Sci. 21 (2014) 939–947. 10.1177/1933719113519171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Prince AL, Ma J, Kannan PS, Alvarez M, Gisslen T, Harris RA, Sweeney EL, Knox CL, Lambers DS, Jobe AH, Chougnet CA, Kallapur SG, Aagaard KM, The placental membrane microbiome is altered among subjects with spontaneous preterm birth with and without chorioamnionitis, Am. J. Obstet. Gynecol. 214 (2016) 627.e1–627.e16. 10.1016/j.ajog.2016.01.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Jones N, Bohnsack JF, Takahashi S, Oliver KA, Chan MS, Kunst F, Glaser P, Rusniok C, Crook DWM, Harding RM, Bisharat N, Spratt BG, Multilocus sequence typing system for group B Streptococcus, J. Clin. Microbiol. 41 (2003) 2530–2536. 10.1128/JCM.41.6.2530-2536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Manning SD, Springman AC, Lehotzky E, Lewis MA, Whittam TS, Davies HD, Multilocus sequence types associated with neonatal group B streptococcal sepsis and meningitis in Canada, J. Clin. Microbiol. 47 (2009) 1143–1148. 10.1128/JCM.01424-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Davies HD, Adair C, McGeer A, Ma D, Robertson S, Mucenski M, Kowalsky L, Tyrell G, Baker CJ, Antibodies to capsular polysaccharides of group B Streptococcus in pregnant Canadian women: Relationship to colonization status and infection in the neonate, J. Infect. Dis. (2001). 10.1086/322029. [DOI] [PubMed] [Google Scholar]
- [25].Davies HD, Raj S, Adair C, Robinson J, McGeer A, Population-based active surveillance for neonatal group B streptococcal infections in Alberta, Canada: Implications for vaccine formulation, Pediatr. Infect. Dis. J. 20 (2001) 879–884. 10.1097/00006454-200109000-00011. [DOI] [PubMed] [Google Scholar]
- [26].Lauer E, Kandler O, Lactobacillus gasseri sp. nov., a new species of the subgenus Thermobacterium, Zentralblatt Fur Bakteriol. Angew. Und Okol. Microbiol. Abt.1 Orig.C Hyg. (1980). 10.1016/s0172-5564(80)80019-4. [DOI] [Google Scholar]
- [27].Rosander A, Connolly E, Roos S, Removal of antibiotic resistance gene-carrying plasmids from Lactobacillus reuteri ATCC 55730 and characterization of the resulting daughter strain, L. reuteri DSM 17938, Appl. Environ. Microbiol. (2008). 10.1128/AEM.00991-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Krikun G, Mor G, Alvero A, Guller S, Schatz F, Sapi E, Rahman M, Caze R, Qumsiyeh M, Lockwood CJ, A novel immortalized human endometrial stromal cell line with normal progestational response, Endocrinology. 145 (2004) 2291–2296. 10.1210/en.2003-1606. [DOI] [PubMed] [Google Scholar]
- [29].Brosens JJ, Takeda S, Acevedo CH, Lewis MP, Kirby PL, Symes EK, Krausz T, Purohit A, Gellersen B, White JO, Human endometrial fibroblasts immortalized by simian virus 40 large T antigen differentiate in response to a decidualization stimulus, Endocrinology. 137 (1996) 2225–2231. 10.1210/endo.137.6.8641169. [DOI] [PubMed] [Google Scholar]
- [30].Parker RE, Laut C, Gaddy JA, Zadoks RN, Davies HD, Manning SD, Association between genotypic diversity and biofilm production in group B Streptococcus, BMC Microbiol. 16 (2016) 86 10.1186/s12866-016-0704-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Korir ML, Knupp D, LeMerise K, Boldenow E, Loch-Caruso R, Aronoff DM, Manning SD, Association and virulence gene expression vary among serotype III group B Streptococcus isolates following exposure to decidual and lung epithelial cells, Infect. Immun. 82 (2014) 4587–4595. 10.1128/IAI.02181-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Flaherty RA, Magel M, Aronoff DM, Gaddy JA, Petroff MG, Manning SD, Modulation of death and inflammatory signaling in decidual stromal cells following exposure to group B Streptococcus, Infect. Immun. 87 (2019). 10.1128/IAI.00729-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Costerton JW, Stewart PS, Greenberg EP, Bacterial biofilms: A common cause of persistent infections, Science 284 (1999) 1318–1322. 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- [34].Shiroda M, Manning SD, Lactobacillus strains vary in their ability to interact with human endometrial stromal cells, PloS One (In Revision, 2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Spinler JK, Taweechotipatr M, Rognerud CL, Ou CN, Tumwasorn S, Versalovic J, Human-derived probiotic Lactobacillus reuteri demonstrate antimicrobial activities targeting diverse enteric bacterial pathogens, 2008. 10.1016/j.anaerobe.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, Lemons JA, Donovan EF, Stark AR, Tyson JE, Oh W, Bauer CR, Korones SB, Shankaran S, Laptook AR, Stevenson DK, Papile LA, Kenneth Poole W, Changes in pathogens causing early-onset sepsis in very-low-birth-weight infants, N. Engl. J. Med. (2002). 10.1056/NEJMoa012657. [DOI] [PubMed] [Google Scholar]
- [37].Ecker KL, Donohue PK, Kim KS, Shepard JA, Aucott SW, The impact of group B Streptococcus prophylaxis on late-onset neonatal infections, J. Perinatol. (2013). 10.1038/jp.2012.76. [DOI] [PubMed] [Google Scholar]
- [38].Jalilsood T, Baradaran A, Song AAL, Foo HL, Mustafa S, Saad WZ, Yusoff K, Rahim RA, Inhibition of pathogenic and spoilage bacteria by a novel biofilm-forming Lactobacillus isolate: A potential host for the expression of heterologous proteins, Microb. Cell Fact. 14 (2015). 10.1186/s12934-015-0283-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Spurbeck RR, Arvidson CG, Lactobacilli at the front line of defense against vaginally acquired infections, Future Microbiol. 6 (2011) 567–582. 10.2217/fmb.11.36. [DOI] [PubMed] [Google Scholar]
- [40].Marziali G, Foschi C, Parolin C, Vitali B, Marangoni A, In-vitro effect of vaginal lactobacilli against group B Streptococcus, Microb. Pathog. 136 (2019) 103692 10.1016/j.micpath.2019.103692. [DOI] [PubMed] [Google Scholar]
- [41].Gaspar C, Donders GG, Palmeira-de-Oliveira R, Queiroz JA, Tomaz C, Martinez-de-Oliveira J, Palmeira-de-Oliveira A, Bacteriocin production of the probiotic Lactobacillus acidophilus KS400, AMB Express. 8 (2018). 10.1186/s13568-018-0679-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Alvarez-Sieiro P, Montalbán-López M, Mu D, Kuipers OP, Bacteriocins of lactic acid bacteria: extending the family, Appl. Microbiol. Biotechnol. (2016). 10.1007/s00253-016-7343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Rosini R, Margarit I, Biofilm formation by Streptococcus agalactiae: Influence of environmental conditions and implicated virulence factor, Front. Cell. Infect. Microbiol. (2015). 10.3389/fcimb.2015.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Korir ML, Laut C, Rogers LM, Plemmons JA, Aronoff DM, Manning SD, Differing mechanisms of surviving phagosomal stress among group B Streptococcus strains of varying genotypes, Virulence. 8 (2017) 924–937. 10.1080/21505594.2016.1252016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Dahl TA, Midden WR, Hartman PE, Comparison of killing of gram-negative and gram-positive bacteria by pure singlet oxygen, 1989. 10.1128/jb.171.4.2188-2194.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Poyart C, Pellegrini E, Gaillot O, Boumaila C, Baptista M, Trieu-Cuot P, Contribution of Mn-cofactored superoxide dismutase (SodA) to the virulence of Streptococcus agalactiae, Infect. Immun. 69 (2001) 5098–5106. 10.1128/IAI.69.8.5098-5106.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Korir ML, Flaherty RA, Rogers LM, Gaddy JA, Aronoff DM, Manning SD, Investigation of the role that NADH peroxidase plays in oxidative stress survival in group B Streptococcus, Front. Microbiol. 9 (2018). 10.3389/fmicb.2018.02786. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.