Abstract
Sjögren’s Syndrome (SjS) is a chronic, systemic autoimmune disease causing xerostomia, xerophthalmia, and systemic symptoms. The principal pathological finding in SjS is the accumulation of lymphocytes in exocrine glandular tissue and elsewhere, leading to secretory dysfunction and other abnormalities. A rational therapeutic approach might be to interfere with lymphocyte migration to the periphery from central lymphoid tissues. We thus examined in an animal model of SjS the effects of Fingolimod (FTY720, Gilenya™), which interferes with migration of lymphocytes to peripheral sites. Fingolimod induces sequestration of lymphocytes in lymphoid organs by altering lymphocyte expression of sphingosine-1-phosphate receptors. In the C57Bl/6.NOD.Aec1Aec2 (AEC) model of SjS, Fingolimod reduced circulating T and B cell numbers. Treatment of AEC mice with Fingolimod increased salivary output and decreased the size of salivary gland infiltrates. Oral Fingolimod thus merits further consideration in the management of SjS in humans.
1. Introduction:
Sjögren’s Syndrome (SjS) is a chronic autoimmune disease affecting the exocrine glands, causing xerostomia and xerophthalmia. Pathologically, the lesions are persistent and progressive focal mononuclear cell infiltrates within the glands. In addition to localized glandular dysfunction and immune cell invasion, certain systemic features also mark the disease, including circulating autoantibodies against ribonucleoprotein particles Ro (Sjögren’s-syndrome-related antigen A – SS-A) and La (SS-B), fatigue with accompanying joint and muscular pain, and neurological complaints. Non-Hodgkin lymphoma also occurs at an increased rate in patients with SjS [1,2].
The most severe manifestations of SjS in humans are extraglandular, and are the result of ectopic lymphocyte infiltration of tissues. The immune cells infiltrating exocrine glands and extraglandular sites are predominantly B- and T lymphocytes, but there are also smaller numbers of macrophages, NK cells, and dendritic cells [3,4]. These infiltrating cells may interfere with glandular function through destruction of glands, secretion of inflammatory cytokines, and local production of autoantibodies. Infiltrating cells may form germinal centers [5,6], which can be indicative of future lymphoma development [7,8,9]. Despite increased understanding of the pathogenesis of SjS, at present no cure exists and no therapy prevents disease progression.
As cell infiltration plays a role in the pathogenesis of SjS [3.4], targeting this step may prove successful for altering disease progression. Fingolimod (FTY720) was discovered during a chemical derivation program of myriocin, an atypical amino acid isolated from the thermophilic fungi Isaria Sinclarii. Initial studies revealed structural similarities between sphingosine and fingolimod and demonstrated phosphorylation of fingolimod by sphingosine-kinase 2 [9–11]. Phosphorylated fingolimod binds to lymphocyte S1P receptors (1,3,4,5) and leads to their internalization and degradation. Signaling of lymphocyte S-1-P G-protein coupled receptors through S-1-P on lymphocytes leads to their egress from secondary lymphoid organs. In the absence of such receptors, lymphocytes are retained in secondary lymphoid organs [11]. Presumably by preventing migration of immunoreactive lymphocytes to target organs, fingolimod has shown to be effective at ameliorating disease in mouse models of multiple sclerosis [12–14], transplantation [15–23], and uveitis [24–26]. In 2010, the U.S. Food and Drug Administration approved fingolimod for patients with relapsing and remitting multiple sclerosis [10].
The objective of the present study was to determine the efficacy of fingolimod as a potential therapy for Sjögren’s syndrome. We studied the response of the C57Bl/6J.NOD-AEC1.AEC2 (AEC) spontaneous mouse model of SjS to oral fingolimod. AEC mice were derived from a C57Bl/6 background, onto which chromosomal intervals IDD5 from chromosome 1 and IDD3 from chromosome 3 of the non-obese diabetic (NOD) mouse strain were bred [27]. The genes within these intervals led to development of autoimmune exocrinopathy but not diabetes [28], providing a model of primary SJS in the absence of other autoimmune diseases. Fingolimod reduced circulating lymphocytes in aged mice with disease. Treatment reduced salivary lymphocytic infiltrates. Oral fingolimod also improved salivary output. These findings should encourage trials of fingolimod and similar agents in treating SjS.
2. Material and methods
2.1. Mice
C57BL/6.NOD-Aec1Aec2 (AEC) mice were obtained from the University of Florida, Gainesville. AEC mice were housed in the Lewis Katz School of Medicine (LKSOM) animal facility under specific pathogen-free conditions with a light-dark cycle of 12 hours. Animals had access to food and water ad libitum. All procedures and housing were performed in accordance with a protocol approved by the Temple University Institutional Animal Care and Use Committee following the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Experiments began when male and female mice were between 24–32 weeks old and continued for 6 weeks.
2.2. Reagents
Fingolimod was obtained from Novartis Pharmaceuticals (Basel, Switzerland). 300mg fingolimod was reconstituted in 30mL sterile/LPS free saline (Baxter Sodium Chloride Irrigation 0.9%). The solution was passed through a 0.22uM syringe filter and 1mL aliquots were stored at −20 degrees C. Stock solutions were diluted weekly to 0.03 mg/mL for administration (100 microlitres per 10g mouse body weight for a final dose of 0.3mg/kg). Pilocarpine hydrochloride was obtained from MP Biomedicals (Solon, Ohio) and isoproterenol hydrochloride from Spectrum Chemical Manufacturing Corporation (New Brunswick, NJ). O.C.T. compound was purchased from Tissue-Tek (Torrance, CA). Antibodies used were from BD Bioscience (San Jose, California) (anti-CD19-PE), eBioscience (San Diego, California) (anti-CD3-PerCP-Cy5.5), and Life Technologies (Grand Island, NY) (anti-TCRα/β-AlexaFluor-488, anti-rat-Cy3).
2.3. Animal Experiments
2.3.1. Administration of fingolimod
Based on doses used by previous investigators (ref 13 and others), we compared 0.3 mg/kg fingolimod orally (the most commonly published dose) and 1 mg/kg doses of fingolimod to normal mice (n=3) and measured blood lymphocyte counts at 18 hrs and at 4 and 7 days. At both doses, there was nearly identical depletion (60%) at 18 hrs and 4 days, with return to normal by 7 days. We thus chose to use the lower dose. We administered 0.3mg/kg of fingolimod diluted in saline to mice three times per week via oral gavage. Control animals received a comparable volume (based on their weight) of saline via oral gavage. Animals were monitored to ensure fingolimod or saline were administered safely and directly without fluid accumulation in lungs.
2.3.2. Saliva Collection
For saliva collections, mice were first weighed then injected intraperitoneally (i.p) with 100uL stimulant solution of isoproterenol (1mg/ mL) and pilocarpine (2mg/mL) dissolved in phosphate buffered saline (PBS). After 1 minute, saliva was collected by hand via a micropipetter for 10 minutes. Saliva was stored in 1.5mL Eppendorf tubes on ice. Samples were spun at 10.0 g to remove particulates and before the volume of saliva measured. Saliva was stored at −80 degrees C. Saliva measurements were normalized to the weights of the animals.
2.4. Flow Cytometry
Blood was collected from animals during ongoing experiments via tail vein or terminally via cardiac puncture. 40–50 microliters of blood was collected in 1.5mL Eppendorf tubes containing 100 microliters 1.5mM EDTA in PBS. Red blood cells were lysed with 1mL ACK lysis buffer for 1–2 minutes on ice then washed and re-suspended with fluorescence-activated cell sorting (FACS) staining buffer. Cells were pelleted and counted. Cells were stained with CD19-PE (1:100) and CD3-PerCP-Cy5.5 (1:100). Samples were washed, fixed in 4% paraformaldehyde solution and analyzed using a Becton-Dickinson FACSCanto.
2.5. Histology
2.5.1. Hematoxylin & Eosin (H&E) Staining
At termination of experiments, submandibular glands were excised and adjoining cervical lymph nodes removed. One gland was preserved in phosphate buffered formalin (PBF) solution for subsequent H&E staining. The other gland was snap frozen in O.C.T. medium for subsequent cryostat sectioning and immunofluorescence staining (see below). PBF preserved glands were processed at the histology core of LKSOM. Glands were embedded in paraffin, sectioned at 4uM, and H&E stained. The histological score of the infiltrates was graded from 1 to 4 as has been done in previous studies [4].
2.5.2. Immunofluorescence Staining
5uM frozen sections of O.C.T.-preserved glands were made by hand using a cryostat. Frozen sections were allowed to dry for 2 hours at room temperature before storage at −20°C. For staining, slides were brought to room temperature and a liquid blocker PAP pen (Daido Sangyo, Tokyo, Japan) was used to make a hydrophobic barrier around sections. Slides were rehydrated in 1X Tris buffered saline (TBS) for 20 minutes at room temperature with shaking, then blocked with 1X TBS containing 5% bovine serum albumin (BSA) and 0.1% Tween-20 for 20 minutes at room temperature with shaking. Sections were incubated with hamster-anti-mouse TCR-AlexaFluor-488 (1:100) and rat-anti-mouse CD19-Biotin (1:200) in 1X TBS containing 5% BSA and 0.1% Tween-20 for 1 hour at room temperature in a humidified chamber. Slides were washed with 1X TBS containing 5% BSA and 0.1% Tween-20 for 5 minutes then incubated with anti-rat Cy3 antibody (1:1000) in 1X TBS containing 5% BSA and 0.1% Tween-20 for 1 hour at room temperature in a humidified chamber. Slides were washed for 10 minutes with 1X TBS containing 5% BSA and 0.1% Tween-20 three times. Slides were mounted and cover-slipped with FluoroGel anti-fade mounting reagent (Electron Microscopy Sciences). They were viewed with an Olympus fluorescence microscope.
2.6. Statistical Analysis
All results are expressed as mean ± SEM. Data were analyzed using Graphpad Prism 5 software. Using Student’s t test for independent samples, statistical significance was designated with p values <0.05 (*p<0.05, **p<0.01, *** p<0.001).
3. Results:
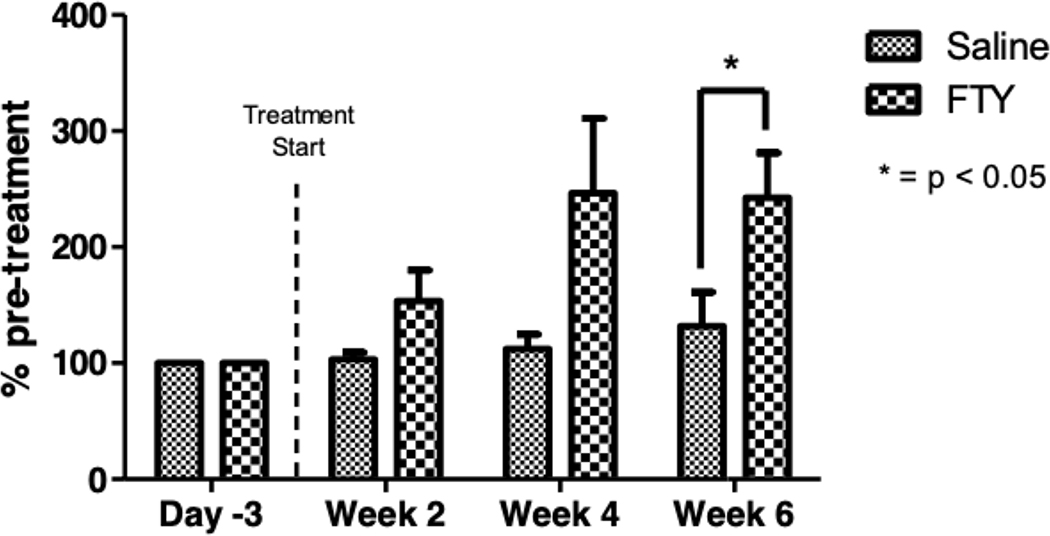
3.1. Circulating lymphocyte counts are markedly reduced in fingolimod-treated C57Bl/6J.NOD-AEC1.AEC2 mice
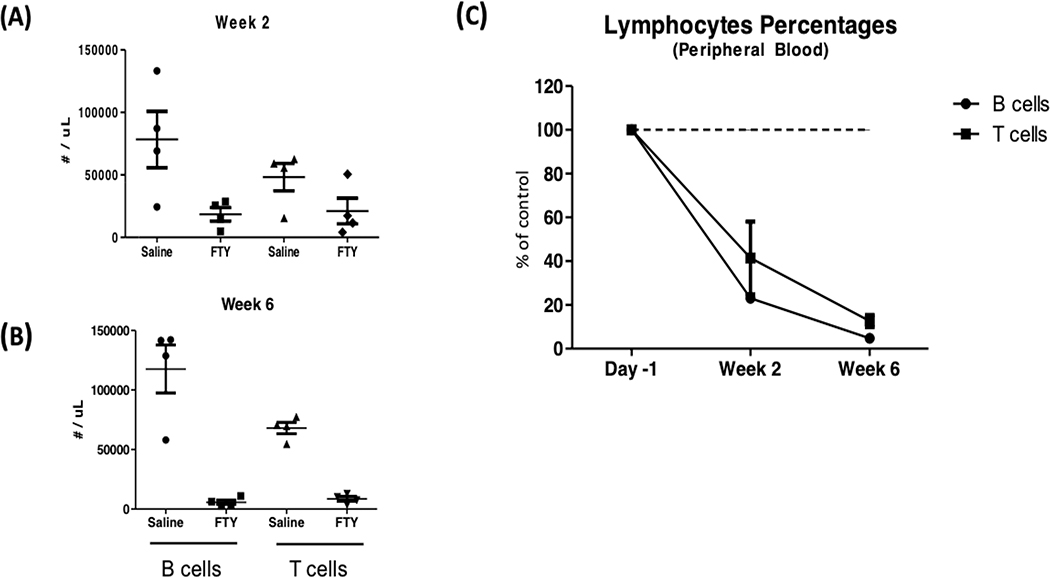
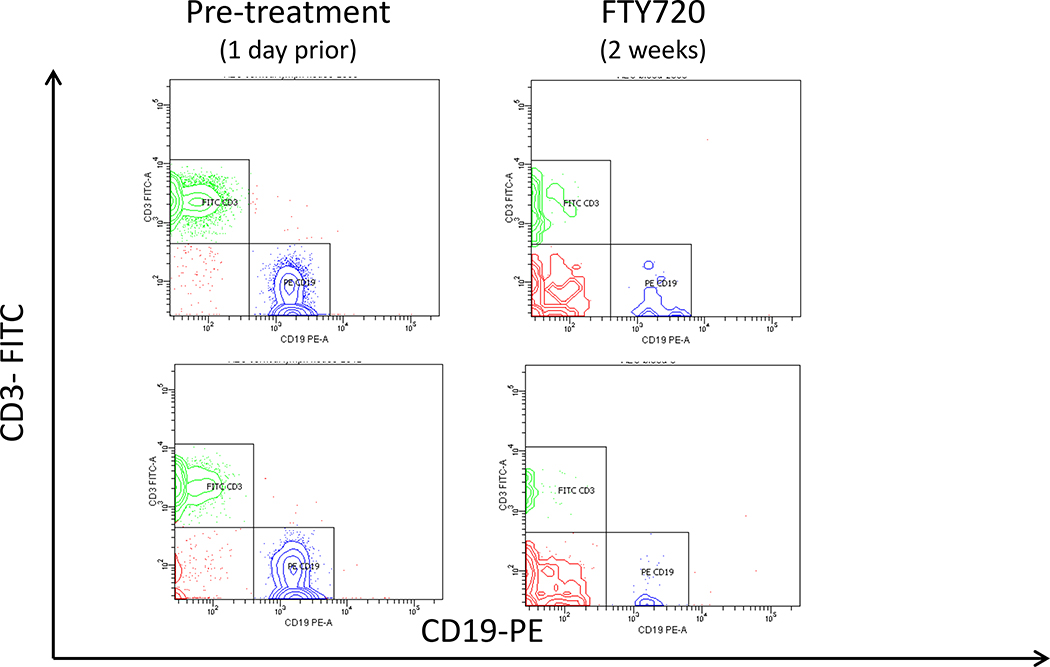
Previous studies have shown that treatment with fingolimod sequesters lymphocytes in secondary lymphoid organs, thereby reducing the numbers of lymphocytes in circulation [29,30]. To determine if fingolimod similarly reduced circulating lymphocytes in SjS mice, we treated AEC mice with 0.3mg/kg fingolimod via oral gavage three times per week for 8 weeks. Representative flow cytometry data are shown in Figure 1a, where profound depletion of both T and B lymphocytes was seen after treatment. We observed that 2 weeks of treatment reduced circulating B cell numbers by 77% and circulating T cell numbers by 41%, compared to animals treated with vehicle (saline) only (Figure 1b). Continued treatment with fingolimod further reduced lymphocytes counts and by week 6 of treatment, B cells decreased by 95% and T cells by 88% compared to vehicle treated animals.
Figure 1a.
Reduced circulating lymphocytes in C57BL/6J.NOD-AEC1.AEC2 mice after fingolimod administration. Shown are flow cytometry data from representative 30 week old Aec females before and after a two week treatment course with fingolimod. Note the marked decrease in both T cells (CD3+) and B cells (CD19+)
Figure 1b.
Panel A shows the number of T lymphocytes (CD3+) and panel B the number of B lymphocytes (CD19+) at baseline and two and six weeks after oral therapy with fingolimod. In Panel C, data are expressed as percentage of pre-treatment levels. Control mice received saline. Groups of four female mice, aged 12 weeks. The experiment was repeated twice with similar results, with a total of nine treated and nine control mice in these depletion experiments.
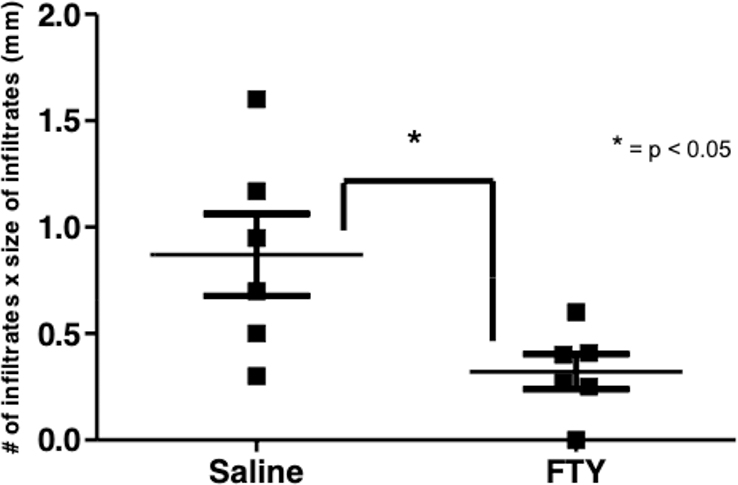
3.2. Changes in salivary gland infiltrates with fingolimod treatment.
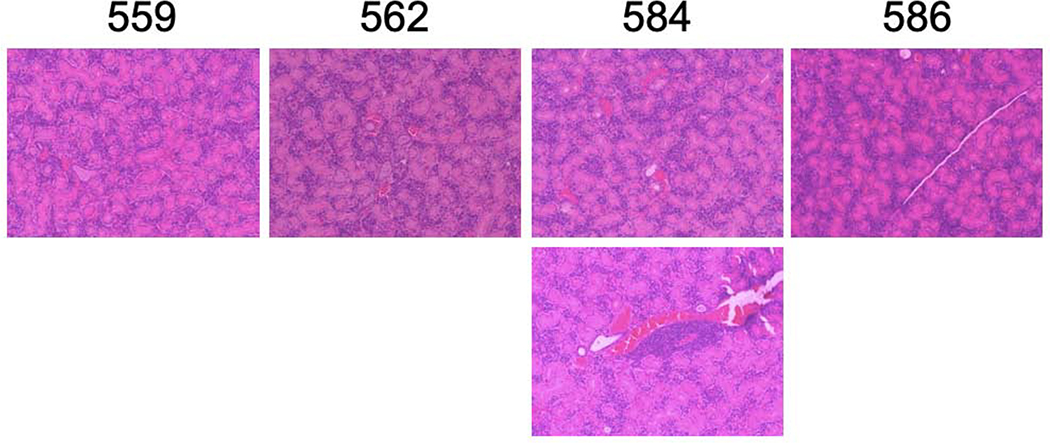
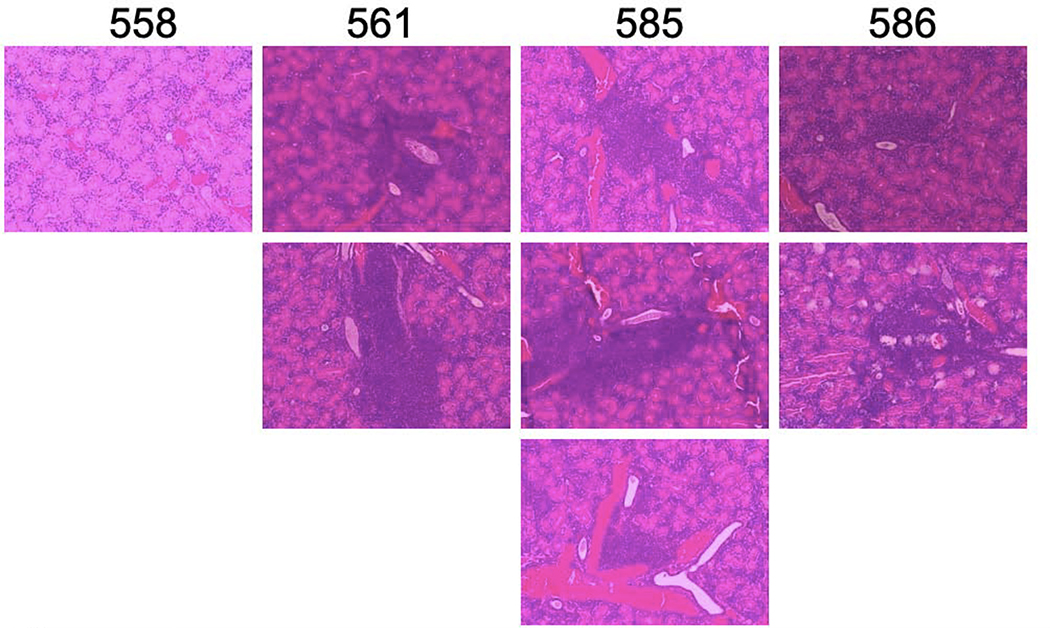
Because we saw a sustained decrease in peripheral blood lymphocyte numbers in AEC mice with repeated administration of fingolimod, we wanted to determine if lymphocytic infiltrates within salivary glands were also altered with fingolimod treatment. We treated four AEC mice with fingolimod and four with saline. At the end of 6 weeks of treatment, salivary glands of mice were paraffin embedded, sectioned, and stained with hematoxylin and eosin for histological scoring. We examined salivary gland sections in a blinded fashion. Both number and size of infiltrates were determined and recorded. Representative H and E sections from this experiment are shown in Figures 2a and 2b. Fingolimod treated mice had much smaller salivary lymphocytic infiltrates.
Figure 2.
a and b. Salivary gland infiltrates are smaller in fingolimod treated AEC mice.H&E staining was performed on paraffin-embedded sections from individual mice. Images are representative. Figure 2a shows salivary glands from fingolimod treated mice, Figure 2b glands from saline treated mice Magnification 100X.
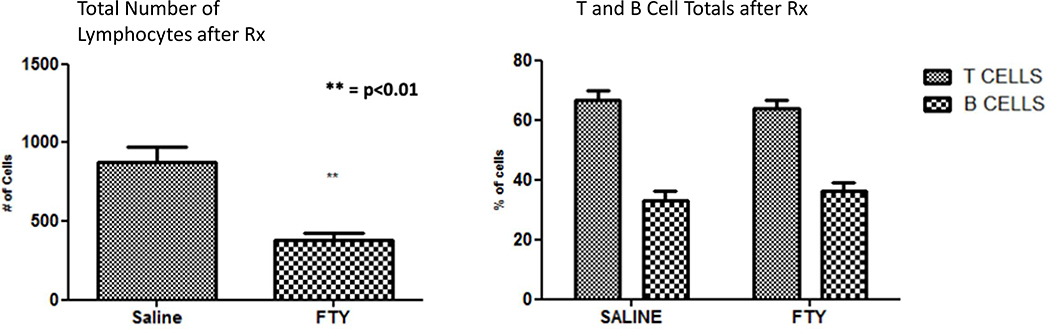
We repeated this experiment, this time with with six fingolimod treated and six control mice. Histology was similar to what is shown in Figure 2. The histological focus scores of fingolimod treated animals were significantly lower than that of vehicle treated animals (Figure 3 a and b). We used immunofluorescence to determine the composition of lymphocytes infiltrating the salivary glands of treated vs. control mice. Numbers of infiltrating lymphocytes were markedly reduced in the fingolimod group (Figure 4). The degree of T and B cell depletion was similar (Figure 5), as the ratio of T to B lymphocytes was similar after fingolimod treatment (Figure 6). Tissue immunofluorescence studies showed clustering of T and B lymphocytes, and depletion of both T and B cells.
Figure 3.
Salivary focus scores from fingolimod treated vs. untreated mice. Two cohorts of six mice were studied in this experiment.
Figure 4.
The numbers of both B and T cells within the salivary gland infiltrates decreased in the fingolimod treated group to approximately the same degree. The left panel depicts reduction in total numbers of cells from infiltrates, while the right panel shows reduced T and B cells in infiltrates from fingolimod treated mice.
Figure 5.
Representative immunofluorescence staining of B and T cells (red and green respectively) in infiltrates from control and fingolimod treated mice. There was a global reduction in both T and B cells.
Figure 6.
Stimulated salivary flow rates in a cohort of fingolimod treated and control AEC mice. There was a significant increased flow of saliva in treated mice at 6 weeks. Mice in this experiment were 30–32 weeks old.
3.3. Fingolimod treatment enhances salivary flow in C57Bl/6J.NOD-AEC1.AEC2 mice
In SjS, glandular dysfunction accompanies aberrant infiltration of lymphocytes and foci formation within exocrine glands; therefore, we hypothesized that because fingolimod alters lymphocyte trafficking and reduced infiltration of salivary glands, it may also alter disease progression. To determine if fingolimod treatment could alter the course of disease, we treated aged male and female AEC mice with 0.3mg/kg fingolimod three times per week for 6 weeks. Stimulated salivary flow measurements were made once every 2 weeks for 6 weeks. We saw a significant increase in saliva production in fingolimod treated animals after 6 weeks of treatment, compared to vehicle treated animals (Figure 6).
4. Discussion:
The complex nature of autoimmune disease pathogenesis makes designing targeted therapies to halt or reverse disease progression a difficult process. Biological therapies targeting key players of the immune system, such as cytokines and lymphocytes, have been tested in the autoimmune diseases systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA). Some therapies efficacious in RA or SLE have been tried as treatment for SjS. These include the anti-TNF therapies infliximab [33] (chimeric monoclonal antibody) and enteracept [34] (human fusion protein) as well as rituximab [35] (anti-CD20). In clinical trials, however, only rituximab shows promise thus far, and has mostly been studied in patients with late stage disease [35]. Currently approved therapies for early stage SJS target only symptoms of disease (e.g. artificial tears, oral gels/sprays/lozenges, muscarinic agonists pilocarpine and cevimiline) [36]. There is a need for newer, better-targeted therapies that help alleviate symptoms as well as target systemic manifestations of SjS and halt disease progression. To further this goal, we examined the efficacy of fingolimod on a mouse model (AEC) of SjS.
Our initial studies showed that treatment of AEC mice with fingolimod induced a significant and sustained reduction in circulating lymphocyte numbers, comparable to that seen in other mouse strains. Both B and T cells play a role in the pathogenesis of SjS at the systemic and glandular level. When lymphocytes infiltrate the exocrine, most likely from the periphery, they disrupt the architecture of the gland. Additionally, both B and T cells can cause specific damage to the gland. B cells secrete autoantibodies targeting both ubiquitous autoantigens (e.g. SS-A/Ro, SS-B/La, α-fodrin) and exocrine gland specific antigens (e.g. muscarinic M3 receptor). Certain autoantibodies may form immune complexes with apoptotic cells, which may thereby intensify the proinflammatory environment within exocrine glands [37]. M3R autoantibodies, which bind to the M3 muscarinic receptor, can interfere with normal parasympathic nervous system signaling and diminish exocrine gland function [38–40]. T cells are also thought to play many roles in disease progression as a result of their ability to activate B cells, to induce apoptosis, and to secrete damaging pro-inflammatory cytokines [41,42].
Because both B and T lymphocytes contribute to glandular disease, we hypothesized that inhibiting the circulation of lymphocytes may inhibit further infiltration into glands, thereby ameliorating disease symptoms. First, we confirmed that 0.3mg/kg fingolimod induces peripheral lymphopenia in AEC mice consistent with results seen in mouse models of EAE [43–45], uveitis [46–48], and transplantation [49–53]. Upon histological examination of salivary glands from AEC mice, we determined that fingolimod treatment significantly reduced the size of foci within salivary glands of mature female mice compared to vehicle treated control animals (figure 2); these data parallel the decrease in size of exocrine gland infiltrates seen in rituximab treated patients [54]. Together, these findings suggest that peripheral lymphocytes continuously enter glands and may serve as a source for continuous immune cell influx into the glands as disease progresses.
While we noted the absence of lymphocytic infiltrates in some fingolimod treated mice, others had residual, smaller infiltrates even after 6 weeks of treatment. We determined that those residual small infiltrates had fewer cells than vehicle treated animals, but these smaller infiltrates had the same percentage of B and T cells as control animals. These remaining infiltrating lymphocytes may be of an effector memory phenotype, as effector memory cells have lost CCR7 expression and thus are not subject to fingolimod mediated retention. Additionally, previous studies in our lab have shown that some of the infiltrating T cells in salivary glands of AEC mice are of the effector memory phenotype [55]. We have yet to determine if the residual small infiltrates seen in fingolimod treated animals are specifically of a memory phenotype.
We also saw an increase in saliva production by fingolimod treated female and male AEC mice concurrent with reduced infiltrate size, most notably after 4 to 6 weeks of fingolimod treatment. This seemed to correlate with the increased degree of lymphocyte disappearance from the peripheral blood with the passage of time. We were surprised to note that vehicle treated animals did not continue to lose salivary function as they aged. This could be an unintended consequence of frequent gavaging of the animals and potential stimulation of the vagus nerve or the excess fluid from frequent oral gavaging in addition to normal fluids consumed ad libitum.
Further investigation into the mechanism of action of fingolimod in AEC mice may help to elucidate the pathogenesis of SjS disease. It would be of interest in future studies to compare the amount of cell death occurring in the salivary glands of untreated, vehicle treated and fingolimod treated animals. It would also be of interest to quantify immunoglobulin deposition within the glands, and see if it is altered by fingolimod treatment, since we saw nearly no B cells in the infiltrates after fingolimod administration.
We did not look at serum immunoglobulins, autoantibodies, or indices of inflammation in this study. AEC mice have modest increases in IgM, but in our hands do not develop anti-Ro and anti-La autoantibodies, nor do they develop anti-chromatin autoantibodies [56]. This probably reflects the slow pace of development of disease in this model.
The details of the infiltrative process in the AEC model need further investigation. It is possible that lymphocytes accumulate from inward migration, from local replication, or from failure to die; however, our studies support the hypothesis that lymphocytes accumulate from inward migration. Not only may fingolimod provide a therapeutic option for SJS patients but also fingolimod can be utilized to study disease progression and pathogenesis in this mouse model.
In conclusion, these findings encourage further exploration of fingolimod and related drugs in the treatment of Sjögren’s syndrome.
Highlights.
Fingolimod (FTY720), a sphingosine-1-P antagonist, has been used to block trafficking of lymphocytes to the periphery
Sjogren’s syndrome is characterized by lymphocytes infiltrating exocrine glands
We found that treatment of a Sjogren’s syndrome mouse model (AEC1/AEC2) with fingolimod reduced salivary gland infiltrates
Concomitantly, fingolimod treated mice showed improvement of stimulated saliva production
Fingolimod (a licensed drug) has the potential to improve salivary function in Sjogren’s syndrome and deserves further investigation
Acknowledgment:
We thank Dr. Marc Monestier for helpful comments
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Kassan SS and Moutsopoulos HM. Clinical manifestations and early diagnosis of Sjogren’s syndrome. Arch Int Med 2004;164:1275–84. [DOI] [PubMed] [Google Scholar]
- [2].Zintzaras E, Voulgarelis M, Moutsopoulos HM. The risk of lymphoma development in autoimmune diseases: a meta-analysis. Arch Int Med 2005;165:2337–44 [DOI] [PubMed] [Google Scholar]
- [3].Manoussakis MN, Boiu S, Korkolopoulou P, et al. Rates of infiltration by macrophages and dendritic cells and expression of interleukin-18 and interleukin-12 in the chronic inflammatory lesions of Sjögren’s syndrome: Correlation with certain features of immune hyperactivity and factors associated with high risk of lymphoma development. Arthritis Rheum. 2007;56:3977–88. [DOI] [PubMed] [Google Scholar]
- [4].Adamson TC 3rd, Fox RI, Frisman DM, Howell FV. Immunohistologic analysis of lymphoid infiltrates in primary Sjögren’s syndrome using monoclonal antibodies. J Immunol. 1983;130:203–08. [PubMed] [Google Scholar]
- [5].Risselada AP, Looije MF, Kruize AA, Bijlsma JW, van Roon JA. The role of ectopic germinal centers in the immunopathology of primary Sjögren’s syndrome: A systematic review. Semin Arthritis Rheum. 2012. [DOI] [PubMed] [Google Scholar]
- [6].Salomonsson S, Jonsson MV, Skarstein K, et al. Cellular basis of ectopic germinal center formation and autoantibody production in the target organ of patients with sjogren’s syndrome. Arthritis Rheum. 2003;48:3187–3201. [DOI] [PubMed] [Google Scholar]
- [7].Jonsson MV, Theander E, Jonsson R. Predictors for the development of non-Hodgkin lymphoma in primary Sjögren’s syndrome. Presse Med. 2012;41(9 Pt 2):e511–6. [DOI] [PubMed] [Google Scholar]
- [8].Theander E, Vasaitis L, Baecklund E, et al. Lymphoid organisation in labial salivary gland biopsies is a possible predictor for the development of malignant lymphoma in primary Sjögren’s syndrome. Ann Rheum Dis. 2011;70:1363–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ramos-Casals M, Brito-Zeron P, Siso-Almirall A, Bosch X, Tzioufas AG. Topical and systemic medications for the treatment of primary sjogren’s syndrome. Nat Rev Rheumatol. 2012;8:399–411. [DOI] [PubMed] [Google Scholar]
- [10].Brinkmann V, Billich A, Baumruker T, et al. fingolimod (fingolimod): Discovery and development of an oral drug to treat multiple sclerosis. Nat Rev Drug Discov. 2010;9(11):883–97. [DOI] [PubMed] [Google Scholar]
- [11].Brinkmann V, Davis MD, Heise CE, et al. The immune modulator fingolimod targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277:21453–57. [DOI] [PubMed] [Google Scholar]
- [12].Fujino M, Funeshima N, Kitazawa Y, et al. Amelioration of experimental autoimmune encephalomyelitis in lewis rats by fingolimod treatment. J Pharmacol Exp Ther. 2003;305:70–7. [DOI] [PubMed] [Google Scholar]
- [13].Kataoka H, Sugahara K, Shimano K, et al. fingolimod, sphingosine 1-phosphate receptor modulator, ameliorates experimental autoimmune encephalomyelitis by inhibition of T cell infiltration. Cell Mol Immunol. 2005;2:439–48. [PubMed] [Google Scholar]
- [14].Webb M, Tham CS, Lin FF, et al. Sphingosine 1-phosphate receptor agonists attenuate relapsing-remitting experimental autoimmune encephalitis in SJL mice. J Neuroimmunol. 2004;153:108–21. [DOI] [PubMed] [Google Scholar]
- [15].Song J, Ito T, Matsuda C, et al. Inhibition of donor-derived T cells trafficking into target organs by fingolimod during acute graft-versus-host disease in small bowel transplantation. Clin Exp Immunol. 2006;146:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sedlakova K, Muckersie E, Robertson M, Filipec M, Forrester JV. fingolimod in corneal concordant xenotransplantation. Transplantation. 2005;79:297–303. [DOI] [PubMed] [Google Scholar]
- [17].Lima RS, Nogueira-Martins MF, Silva HT Jr, Pestana JO, Bueno V. fingolimod treatment prolongs skin graft survival in a completely incompatible strain combination. Transplant Proc. 2004;36:1015–17. [DOI] [PubMed] [Google Scholar]
- [18].Hwang MW, Matsumori A, Furukawa Y, et al. fingolimod, a new immunosuppressant, promotes long-term graft survival and inhibits the progression of graft coronary artery disease in a murine model of cardiac transplantation. Circulation. 1999;100:1322–29. [DOI] [PubMed] [Google Scholar]
- [19].Yanagawa Y, Hoshino Y, Kataoka H, et al. fingolimod, a novel immunosuppressant, prolongs rat skin allograft survival by decreasing T-cell infiltration into grafts. Transplant Proc. 1999;31:1227–29. [DOI] [PubMed] [Google Scholar]
- [20].Kunikata S, Nagano T, Nishioka T, Akiyama T, Kurita T. Immunosuppressive action of fingolimod for renal allograft a rat model. Transplant Proc. 1999;31:1157–59. [DOI] [PubMed] [Google Scholar]
- [21].Budde K, Schutz M, Glander P, et al. fingolimod (fingolimod) in renal transplantation. Clin Transplant. 2006;20 Suppl 17:17–24. [DOI] [PubMed] [Google Scholar]
- [22].Brinkmann V, Pinschewer DD, Feng L, Chen S. fingolimod: Altered lymphocyte traffic results in allograft protection. Transplantation. 2001;72:764–69. [DOI] [PubMed] [Google Scholar]
- [23].Chiba K, Yanagawa Y, Masubuchi Y, et al. fingolimod, a novel immunosuppressant, induces sequestration of circulating mature lymphocytes by acceleration of lymphocyte homing in rats. I. fingolimod selectively decreases the number of circulating mature lymphocytes by acceleration of lymphocyte homing. J Immunol. 1998;160:5037–44. [PubMed] [Google Scholar]
- [24].Commodaro AG, Peron JP, Lopes CT, et al. Evaluation of experimental autoimmune uveitis in mice treated with fingolimod. Invest Ophthalmol Vis Sci. 2010;51:2568–74. [DOI] [PubMed] [Google Scholar]
- [25].Kurose S, Ikeda E, Tokiwa M, Hikita N, Mochizuki M. Effects of fingolimod, a novel immunosuppressant, on experimental autoimmune uveoretinitis in rats. Exp Eye Res. 2000;70:7–15. [DOI] [PubMed] [Google Scholar]
- [26].Copland DA, Liu J, Schewitz-Bowers LP, et al. Therapeutic dosing of fingolimod (fingolimod) prevents cell infiltration, rapidly suppresses ocular inflammation, and maintains the blood-ocular barrier. American Journal of Pathology. 2012;180:672–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Brayer J, Lowry J, Cha S, et al. Alleles from chromosomes 1 and 3 of NOD mice combine to influence Sjögren’s syndrome-like autoimmune exocrinopathy. J Rheumatol. 2000;27:1896–1904. [PubMed] [Google Scholar]
- [28].Cha S, Nagashima H, Brown VB, Peck AB, Humphreys-Beher MG. Two NOD idd-associated intervals contribute synergistically to the development of autoimmune exocrinopathy (Sjögren’s syndrome) on a healthy murine background. Arthritis Rheum. 2002;46:1390–98. [DOI] [PubMed] [Google Scholar]
- [29].Mandala S, Hajdu R, Bergstrom J, et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346–49. [DOI] [PubMed] [Google Scholar]
- [30].Pinschewer DD, Ochsenbein AF, Odermatt B, Brinkmann V, Hengartner H, Zinkernagel RM. fingolimod immunosuppression impairs effector T cell peripheral homing without affecting induction, expansion, and memory. J Immunol. 2000;164:5761–70. [DOI] [PubMed] [Google Scholar]
- [31].Suzuki S, Enosawa S, Kakefuda T, et al. A novel immunosuppressant, fingolimod, with a unique mechanism of action, induces long-term graft acceptance in rat and dog allotransplantation. Transplantation. 1996;61:200–205. [DOI] [PubMed] [Google Scholar]
- [32].Suzuki S, Li XK, Shinomiya T, et al. The in vivo induction of lymphocyte apoptosis in MRL-lpr/lpr mice treated with fingolimod. Clin Exp Immunol. 1997;107:103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Mariette X, Ravaud P, Steinfeld S, Baron G, Goetz J, Hachulla E et al. Inefficacy of infliximab in primary Sjögren’s syndrome: results of the randomized, controlled Trial of Remicade in Primary Sjögren’s Syndrome (TRIPSS). Arthritis Rheum 2004;50:1270–6. [DOI] [PubMed] [Google Scholar]
- [34].Sankar V, Brennan MT, Kok MR, Leakan RA, Smith JA, Manny J et al. Etanercept in Sjögren’s syndrome: a twelve-week randomized, double-blind, placebo-controlled pilot clinical trial. Arthritis Rheum 2004;50:2240–5. [DOI] [PubMed] [Google Scholar]
- [35].O’Neill I, Scully C. Biologics in oral medicine: Sjögren syndrome. Oral Dis. 2012. [DOI] [PubMed] [Google Scholar]
- [36].Bowman S, Barone F. Biologic treatments in Sjögren’s syndrome. Presse Med. 2012;41(9 Pt 2):e495–509. [DOI] [PubMed] [Google Scholar]
- [37].Nordmark G, Alm GV, Rönnblom L. Mechanisms of Disease: primary Sjögren’s syndrome and the type I interferon system. Nature Reviews Rheumatology 2, 262–269 [DOI] [PubMed] [Google Scholar]
- [38].Bacman S, Perez Leiros C, Sterin-Borda L, Hubscher O, Arana R, Borda E. Autoantibodies against lacrimal gland M3 muscarinic acetylcholine receptors in patients with primary Sjögren’s syndrome. Invest Ophthalmol Vis Sci. 1998;39:151–56. [PubMed] [Google Scholar]
- [39].Bacman S, Sterin-Borda L, Camusso JJ, Arana R, Hubscher O, Borda E. Circulating antibodies against rat parotid gland M3 muscarinic receptors in primary Sjögren’s syndrome. Clin Exp Immunol. 1996;104:454–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Robinson CP, Brayer J, Yamachika S, et al. Transfer of human serum IgG to nonobese diabetic Ig mu null mice reveals a role for autoantibodies in the loss of secretory function of exocrine tissues in sjogren’s syndrome. Proc Natl Acad Sci U S A. 1998;95:7538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bave U, Nordmark G, Lovgren T, et al. Activation of the type I interferon system in primary Sjögren’s syndrome: A possible etiopathogenic mechanism. Arthritis Rheum. 2005;52:1185–95. [DOI] [PubMed] [Google Scholar]
- [42].Peck AB, Nguyen CQ. Transcriptome analysis of the interferon-signature defining the autoimmune process of Sjögren’s syndrome. Scand J Immunol. 2012;76:237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Fujino M, Funeshima N, Kitazawa Y, et al. Amelioration of experimental autoimmune encephalomyelitis in Lewis rats by fingolimod treatment. J Pharmacol Exp Ther. 2003;305:70–77. [DOI] [PubMed] [Google Scholar]
- [44].Kataoka H, Sugahara K, Shimano K, et al. fingolimod, sphingosine 1-phosphate receptor modulator, ameliorates experimental autoimmune encephalomyelitis by inhibition of T cell infiltration. Cell Mol Immunol. 2005;2:439–48. [PubMed] [Google Scholar]
- [45].Webb M, Tham CS, Lin FF, et al. Sphingosine 1-phosphate receptor agonists attenuate relapsing-remitting experimental autoimmune encephalitis in SJL mice. J Neuroimmunol. 2004;153:108–21. [DOI] [PubMed] [Google Scholar]
- [46].Commodaro AG, Peron JP, Lopes CT, et al. Evaluation of experimental autoimmune uveitis in mice treated with fingolimod. Invest Ophthalmol Vis Sci. 2010;51:2568–74. [DOI] [PubMed] [Google Scholar]
- [47].Kurose S, Ikeda E, Tokiwa M, Hikita N, Mochizuki M. Effects of fingolimod, a novel immunosuppressant, on experimental autoimmune uveoretinitis in rats. Exp Eye Res. 2000;70(1):7–15. [DOI] [PubMed] [Google Scholar]
- [48].Copland DA, Liu J, Schewitz-Bowers LP, et al. Therapeutic dosing of fingolimod (fingolimod) prevents cell infiltration, rapidly suppresses ocular inflammation, and maintains the blood-ocular barrier. American Journal of Pathology. 2012;180(2):672–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Yanagawa Y, Hoshino Y, Kataoka H, et al. fingolimod, a novel immunosuppressant, prolongs rat skin allograft survival by decreasing T-cell infiltration into grafts. Transplant Proc. 1999;31:1227–29. [DOI] [PubMed] [Google Scholar]
- [50].Kunikata S, Nagano T, Nishioka T, Akiyama T, Kurita T. Immunosuppressive action of fingolimod for renal allograft in a rat model. Transplant Proc. 1999;31:1157–59. [DOI] [PubMed] [Google Scholar]
- [51].Budde K, Schutz M, Glander P, et al. Fingolimod in renal transplantation. Clin Transplant. 2006;20 Suppl 17:17–24. [DOI] [PubMed] [Google Scholar]
- [52].Brinkmann V, Pinschewer DD, Feng L, Chen S. Fingolimod: Altered lymphocyte traffic results in allograft protection. Transplantation. 2001;72:764–69. [DOI] [PubMed] [Google Scholar]
- [53].Chiba K, Yanagawa Y, Masubuchi Y, et al. Fingolimod, a novel immunosuppressant, induces sequestration of circulating mature lymphocytes by acceleration of lymphocyte homing in rats. I. Fingolimod selectively decreases the number of circulating mature lymphocytes by acceleration of lymphocyte homing. J Immunol. 1998;160:5037–44. [PubMed] [Google Scholar]
- [54].Pijpe J, Meijer JM, Bootsma H, et al. Clinical and histologic evidence of salivary gland restoration supports the efficacy of rituximab treatment in Sjögren’s syndrome. Arthritis Rheum. 2009;60(11):3251–3256. [DOI] [PubMed] [Google Scholar]
- [55].Singh N, Cohen PL. The T cell in Sjögren’s syndrome: force majeure, not spectateur. 2012. J Autoimmun. 39(3):229–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Meng W, Li Y, Xue E et al. B-cell tolerance defects in the B6.Aec1/2 mouse model of Sjogren’s syndrome. J Clin Immunol 2012;22:551–564. [DOI] [PMC free article] [PubMed] [Google Scholar]