Abstract
Purpose:
Young adults are disproportionately affected by the current opioid crisis. Although medications for opioid use disorder (MOUD) are broadly effective, with reductions in morbidity and mortality, the particular effectiveness of MOUD among young adults is less well understood.
Methods:
This secondary analysis compared young adults (ages 18–25) to older adults (26+) in a large comparative effectiveness trial (“XBOT”) that randomized subjects to extended-release naltrexone (XR-NTX) or sublingual buprenorphine-naloxone for six months. Opioid relapse was defined by opioid use over 4 consecutive weeks or 7 consecutive days, using urine testing and self-report.
Results:
Among subjects in the intent to treat (ITT) sample (n=570, all randomized participants), a main effect of age group was found, with higher relapse rates relapse among young adults (70.3%) compared to older adults (58.2%), with an odds ratio (OR) of 1.72, (95% CI=[1.08, 2.70]) p=0.02. In the per-protocol sample (n=474, only participants who started medication), relapse rates were higher among young adults (66.3%) compared to older adults (50.8%), OR=1.91 (95% CI=[1.19, 3.06]). Among the ITT sample, survival analysis revealed a significant time-by-age group interaction (p=.01) with more relapse over time in young adults. No significant interactions between age and medication group were detected.
Conclusions:
Young adults have increased rates of relapse compared to older adults, perhaps because of vulnerabilities that increase their risk for treatment dropout and medication non-adherence, regardless of medication assigment. These results suggest that specialized, developmentally-informed interventions may be needed to improve retention and successful treatment of OUD among young adults.
Keywords: Opioid use disorder, Young adults, Buprenorphine, Naltrexone, Addiction treatment
Introduction
Young adults in the United States are disproportionately affected by the current opioid crisis, with 1.1% (392,000) diagnosed with opioid use disorder (OUD) in 2016 and the highest per capita rates of misuse of both prescription opioids and heroin [1]. Further, approximately two-thirds of all overdose deaths among young adults in recent years involved opioids. With the recent availability of high purity heroin and especially of very high potency, illicitly manufactured fentanyl analogs, the rates of increases in overdose deaths have outstripped the rates of increase in use and OUD [2].Assessment of effective treatments for OUD in this vulnerable population is a top public health priority.
Medications for opioid use disorder (MOUD) include an opioid agonist (methadone), partial agonist (buprenorphine), and antagonist (naltrexone). These pharmacologically distinct approaches have demonstrated effectiveness and are the standard of care for adults with OUD. Although these treatments are well established in older adults, there is less available data on MOUD in youth [3].The existing small body of research on MOUD in young adults provides evidence of efficacy, and MOUD is endorsed by the American Academy of Pediatrics as the recommended standard for adolescents and young adults [4][5][6] [Robinson, PEDIATRICS Volume 145, 2020].
A recent review of buprenorphine treatment in adolescents and young adults concluded that buprenorphine should be considered a first line treatment in youth as it is for older adults [7] as it consistently produces improved outcomes compared to treatment without MOUD. Data on extended release naltrexone (XR-NTX) in young adults is even sparser than for buprenorphine; although, observational studies provide support [8] and suggest treatment effects comparable to buprenorphine [9].
Despite the growing body of evidence that MOUD is effective for youth, including a large-scale study of claims data demonstrating that it considerably improves treatment retention for youth [10], youth tend to have poorer engagement in and response to MOUD. For example, in a naturalistic study of adolescents in buprenorphine treatment, retention rates were approximately 25% at 6 months and 10% at one year [6]. Another study found that emerging adults (ages 18–25) in buprenorphine treatment were retained in treatment at a lower rate and were more likely to use opioids and drop out during treatment, compared to the older adults in the clinic [11]. Reasons for overall poorer outcomes in youth may include features of their substantial and special developmental vulnerability, including: lack of economic and social independence from their families, early onset of SUD, limited engagement in clinical care or low motivation to change [12][13][14], subjective sense of invincibility, immature executive function, high rates of psychiatric comorbidity [15], OUD and MOUD-related stigma, misinformation about OUD risk and the potential benefits of MOUD, biases against medication, difficulties with enduring medication adherence, and insurance and regulatory restrictions [16][17][18][19][20][21][22].
Despite calls for more use of and research on MOUD in the vulnerable youth population, scientists and practitioners have been slow to respond. Additionally, access to and engagement of adolescents and young adults in MOUD treatment has been alarmingly low [10][23][24]. For example, a recent retrospective cohort study of 2.4 million youth determined that only 23.5% of youth with identified OUD received medication within 3 months of diagnosis [10], and XR-NTX is especially underutilized in youth [5]. There is a dearth of research examining how age and developmental vulnerability comparatively impacts treatment matching and outcome. No study with an experimental design has compared the effectiveness of different MOUDs in youth.
The XBOT study is, to date, the largest comparative effectiveness trial of daily sublingual buprenorphine (BUP-NX) versus monthly extended-release injection naltrexone (XR-NTX) [25][26][27]. Its main findings were: more patients had success initiating buprenorphine than XR-NTX (induction failure); among the Intention to Treat (ITT) sample (all randomized participants) relapse rates were modestly lower among the buprenorphine patients because of early relapse in those that did not successfully initiate XR-NTX and among the per-protocol sample (only those who successfully initiated either medication) there was no difference in relapse rates.
The XBOT study offers the opportunity to examine outcomes of MOUD in the young adult subgroup. We therefore conducted a secondary analysis of the XBOT trial, to compare the effectiveness of MOUD treatment in the young adult subgroup versus the older adult participants, and to examine whether the two medication treatments differ in their outcomes in the younger subgroup. We hypothesized that prior findings of poorer treatment response would be confirmed, and that XR-NTX would confer an advantage because of its potential benefit for adherence.
Methods
Brief Characteristics of Parent Study
The methods and design [25][26][27] of the parent multi-site trial are presented elsewhere. For the parent study, participants (ages 18 and over) seeking acute care for OUD were recruited during an index residential treatment episode from the routine patient flow at eight different specialty SUD treatment sites. Subjects were randomized in a 1:1 allocation ratio to either daily sublingual BUP-NX or monthly injectable XR-NTX, having agreed that they would accept either as a randomized assignment. Patients were inducted onto the assigned medication through the study, and then continued through the study in outpatient medication treatment for 24 weeks. During the study intervention, assigned medications were provided for up to 24 weeks. For the purposes of the parent study, those subjects that did not start assigned medication, discontinued assigned medication, or met relapse criteria were considered to have discontinued study treatment. Patients were followed weekly during study treatment for 24 weeks, and then again at 28 and 36 weeks post end of treatment. All sites obtained local Institutional Review Board approval and all participants provided written informed consent.
Present study and sample
This secondary analysis included all randomized participants from the intent-to-treat sample (N=570). Participants were divided into two groups based on age: young adults (ages 18–25) vs. older adults: ages 26 and up). This age cut-off was used based on common definitions in the existing literature [28]. A number of patients (96/570, 16.8%) failed to initiate the medication to which they were assigned, largely due to failure to complete detoxification required to begin naltrexone. Thus, we also examine the “per protocol” sample of patients (n=474) who successfully initiated, i.e. received at least one dose of assigned study medication.
Outcome Measures
The first outcome, induction status, was defined as failing to initiate the study medication (yes/no) the participant was randomized to receive. A participant failed to initiate if they never received a single dose of the medication. The second outcome, relapse (yes/no), was defined as relapsing at any point after day 20 post-randomization over 24-week follow-up, indicating either a return to regular opioid use or dropout from treatment. Relapse was operationalized as 4 or more consecutive weeks of any non-study opioid use (by urine toxicology, or self-report, or failure to provide a urine sample); or 7 or more consecutive days of self-reported non-study opioid use. Self-reported substance use was collected with the Time Line Follow Back [29]. Urine toxicology was done on weekly urine samples that were tested for opioids (buprenorphine, methadone, morphine [heroin, codeine, morphine], oxycodone). The third outcome, time to relapse (in days), which was the primary outcome of the parent trial, was defined as the time from randomization to the start of relapse. Participants who did not relapse were censored at the end of the 24-weeks.
Statistical Analyses
Among the ITT sample, baseline differences in demographic, clinical and substance use measures between age groups were assessed using t-tests for continuous measures and chi-square tests for categorical measures. In order to assess whether age moderated the effect of treatment on failure to induct onto study medication, and/or the effect of treatment on relapse, a logistic regression model estimating the probability of each of these outcomes was fit including the effects of age (younger vs. older), treatment (XR-NTX vs. BUP-NX), and their interaction. If the interaction was not significant, it was omitted from the model and only main effects were assessed.
Further, to assess whether the effects of treatment and age remained constant over follow-up time, Cox proportional hazard models were fit including treatment-by-time and age-by-time interactions. Both the logistic models on relapse and Cox-proportional hazard model for time to relapse were fit using the ITT sample (n=570), and then the per protocol sample (n=474). The per protocol sample was defined as all participants who were randomized and were inducted onto study medication.
All models controlled for site as a random effect and were fit using SAS version 9.4. All statistical tests were two-sided with a significance level of 5%.
Results
Baseline characteristics
Table 1 shows baseline characteristics by age group. Overall, most participants in the parent study (n=570) were white men, aged 25–45 years (mean= 34 yrs), had a primary heroin use disorder, were using by injection, were single, unemployed, and Medicaid-insured. There were 111 (19.5%) young adults ages 18–25 vs. 459 (80.5%) older adults (ages >25). Compared to the older adults, young adults were more likely to be female (40.5% vs. 27.0%) and less likely to have ever been married (9.0% vs. 39.4%). Other baseline differences compared to the older adults include an earlier age of onset of opioid use among young adults (17.1 years vs. 22.3 years); higher rates of past 30 days cannabis use among young adults (60.4% vs. 40.7%); and somewhat higher rates of injection use among young adults, though not significantly different (70.3% vs. 61.4%, p=.08). No significant differences were found between young adults and older adults on baseline depression symptom severity, prevalence of psychiatric disorders, withdrawal symptoms, or severity of OUD.
Table 1.
Baseline demographic, clinical, and substance use measures by age group for the intent-to-treat sample (n=570)
| Youth ≤25 (n=111) | Adults >25 (n=459) | Difference btw Groups | |||
|---|---|---|---|---|---|
| Measure | N | % or M (SD) | N | % or M (SD) | p-value |
| Gender | 0.0051 | ||||
| Male | 66 | 59.5% | 335 | 73.0% | |
| Female | 45 | 40.5% | 124 | 27.0% | |
| Hispanic Ethnicity (% Hispanic) | 20 | 18.0% | 79 | 17.2% | 0.8404 |
| Marital Status | <.0001 | ||||
| Have been married | 10 | 9.0% | 181 | 39.4% | |
| Never Married | 101 | 91.0% | 275 | 59.9% | |
| Unknown | 0 | 0.0% | 3 | 0.7% | |
| Employment (% Not Employed) | 71 | 64.0% | 289 | 63.0% | 0.8445 |
| IV Use (% yes) | 78 | 70.3% | 282 | 61.4% | 0.0834 |
| Primary Opioid | 0.4009 | ||||
| Buprenorphine | 2 | 1.8% | 6 | 1.3% | |
| Opioid analgesics | 14 | 12.7% | 76 | 16.6% | |
| Methadone | 0 | 0.0% | 7 | 1.5% | |
| Heroin | 94 | 85.5% | 369 | 80.6% | |
| Primary Opioid Cost ($/day) | 110 | 92.4 (66.6) | 458 | 93.8 (77.7) | 0.8535 |
| Age at onset of opioid use | 111 | 17.1 (2.7) | 459 | 22.3 (7.4) | <.0001 |
| Duration of Opioid Use (years) | 111 | 6.0 (2.6) | 459 | 14.1 (9.3) | <.0001 |
| First Treatment Episode (% yes) | 43 | 38.7% | 166 | 36.2% | 0.6137 |
| Stimulant use (30d prior to adm) (% yes) | 62 | 55.9% | 234 | 51.0% | 0.3562 |
| Sedative use (30d prior to adm) (% yes) | 34 | 30.6% | 130 | 28.3% | 0.6298 |
| Heavy alcohol use (30d prior to adm) (% yes) | 25 | 22.5% | 122 | 26.6% | 0.3806 |
| Cannabis use (30d prior to adm) (% yes) | 67 | 60.4% | 187 | 40.7% | 0.0002 |
| HAM-D Score (range: 0–52) | 111 | 8.3 (6.2) | 458 | 9.1 (6.6) | 0.2726 |
| Any Psych Disorders (% yes) | 79 | 71.2% | 302 | 65.8% | 0.2803 |
| High | 44 | 39.6% | 183 | 39.9% | |
Induction onto MOUD
Age did not significantly moderate the effect of treatment on failure to induct onto study medication (age-by-treatment interaction: F(1,559)=0.63, p=.427). This suggests there that are no significantly different effects of treatment between the two age groups on failure to initiate medication. Similar to findings from the parent study, a main effect of treatment on medication induction was found such that participants randomized to XR-NTX had significantly higher odds of induction failure compared to those randomized to BUP-NX (OR=6.51, 95% CI=[3.69, 11.48], p<.001). But there were no significantly different effects of treatment between the two age groups on failure to initiate medication. The rates of failure to initiate assigned XR-NTX were 18.37% for young adults vs. 29.91% for older adults, while the rates of failure to initiate BUP-NX were 6.45% for young adults vs. 5.78% for older adults. The main effect of age group on initiation was not significant (p=.230).
Relapse (ITT sample)
The unadjusted relapse rates are presented in Table 2. For the ITT sample, the unadjusted 24-week relapse rate in the young adult group was 70.3% overall (compared to 58.8% for older adults), 72.6% for those assigned to BUP-NX (compared to 52.4% for older adults) and 67.3% for those assigned to XR-NTX (compared to 65.0% for older adults). However, the age by treatment interaction was not significant (F(1,559)=2.29, p=.131). Similar to the parent study ITT analysis, the odds of relapse were significantly greater among those assigned to XR-NTX, compared to those assigned to BUP-NX (OR=1.48, 95% CI=[1.05, 2.09], p=.026). Additionally, there was a significant main effect of age such that the odds of relapse were higher among young adults compared to older adults (OR=1.71, 95% CI=[1.08, 2.72], p=.022).
Table 2.
Unadjusted 24-week relapse rates of the overall sample and by randomized treatment assignment for the intent to treat and per protocol sample
| Overall | Naltrexone | Buprenorphine | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | N | % Not Relapsed | N | % Relapsed | N | % Not Relapsed | N | % Relapsed | N | % Not Relapsed | N | % Relapsed |
| ITT | (N=570) | (N=283) | (N=287) | |||||||||
| ≤25 | 33 | 29.7 | 78 | 70.3 | 16 | 32.7 | 33 | 67.3 | 17 | 27.4 | 45 | 72.6 |
| >25 | 189 | 41.2 | 270 | 58.8 | 82 | 35.0 | 152 | 65.0 | 107 | 47.6 | 118 | 52.4 |
| Per protocol | (N=474) | (N=204) | (N=270) | |||||||||
| ≤25 | 33 | 33.7 | 65 | 66.3 | 16 | 40.0 | 24 | 60.0 | 17 | 29.3 | 41 | 70.7 |
| >25 | 185 | 49.2 | 191 | 50.8 | 82 | 50.0 | 82 | 50.0 | 103 | 48.6 | 109 | 51.4 |
Relapse (per protocol sample)
For the per protocol sample, the unadjusted relapse rate in the young adult group was 66.3% overall (compared to 50.8% for older adults), 70.7% for those assigned to BUP-NX (compared to 51.4% for older adults) and 60.0% for those assigned to XR-NTX (compared to 50.0% for older adults). The age group by treatment interaction was not significant (F(1,463)=0.67, p=.414). As in the parent study, the odds of relapse were not significantly different among those randomized and inducted onto XR-NTX, compared to those randomized and inducted onto BUP-NX (p=.508). There was a main effect of age, such that the odds of relapse were significantly higher among young adults who initiated medication compared to older adults who initiated medication (OR=1.91, 95% CI=[1.19, 3.06], p=.008).
Time to Relapse
Among the ITT sample, Figure 1A shows the relapse-free survival curves by age group, and Figure 2A shows the model estimated hazard ratios of age over time. In the ITT analysis, the constancy of the relative hazard assumption was violated for both treatment and age, as evidenced by a significant treatment-by-time interaction (p=.005) and a significant age-by-time interaction (p=.012). The risk of relapse was significantly lower in the BUP-NX group than the XR-NTX group earlier in the study period but by week 8 this difference was no longer significant. For age groups, the risk of relapse did not differ significantly by age group at the start of the study period, but by week 8 the risk of relapse is significantly and progressively higher in the young adult group compared to older adults through week 24.
Figure 1.
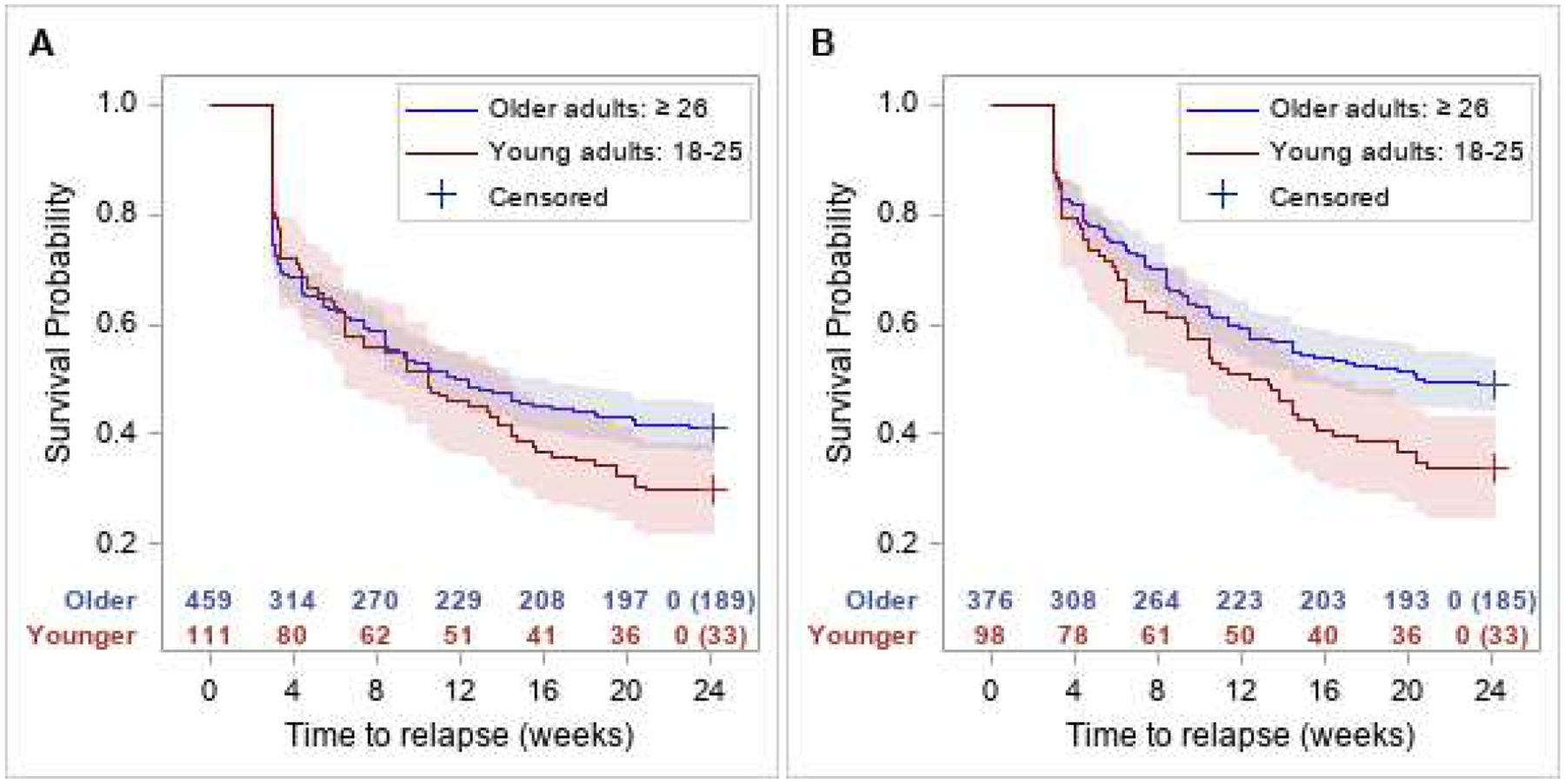
Relapse-free survival curves with 95% confidence intervals by age group among the ITT sample (A: left panel) and among the per protocol sample (B: right panel). Corresponding number of subjects at risk are presented along x-axis along with number censored at week 24.
Figure 2.
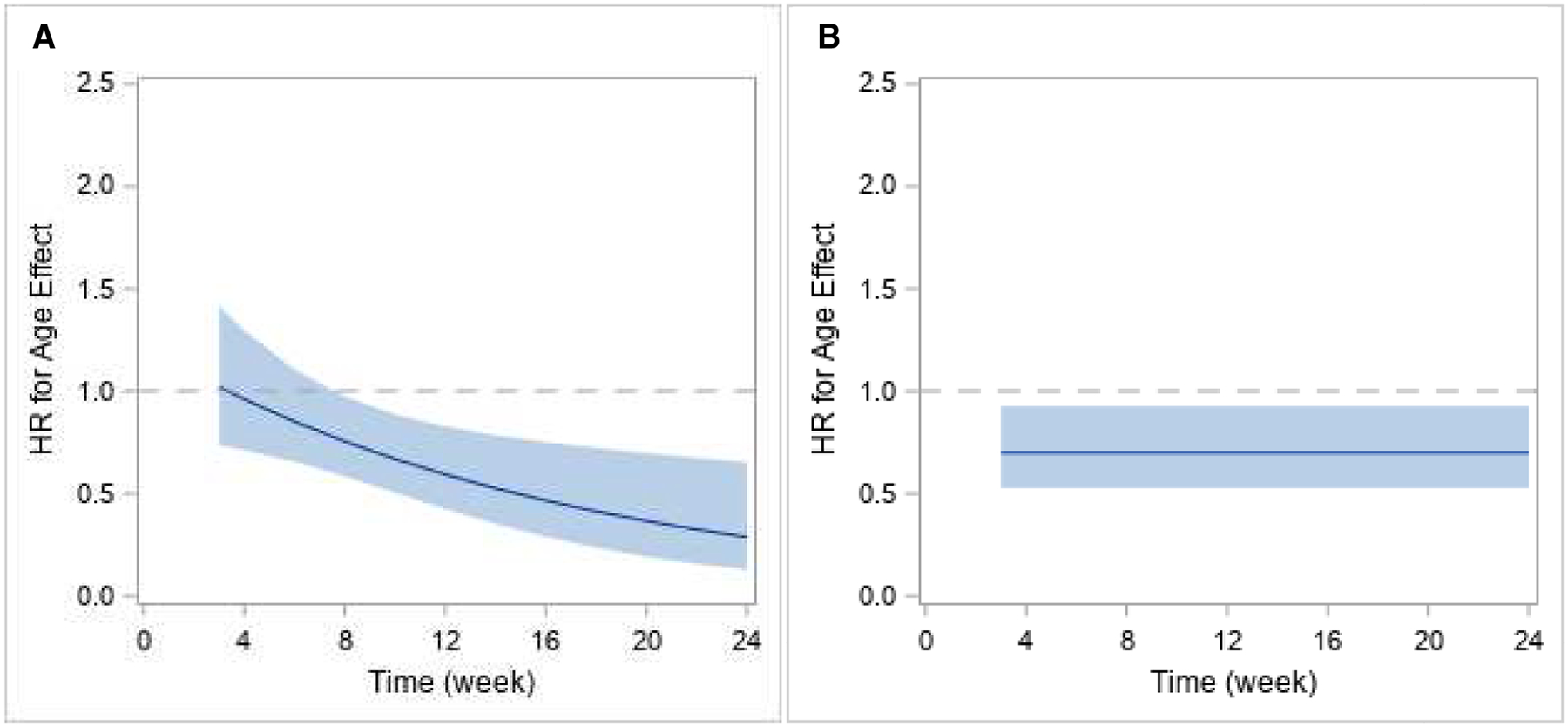
Model estimated hazard-ratio along with corresponding 95% confidence interval over time for effect of age group on risk of relapse among the ITT sample (A: left panel) and the per protocol sample (B: right panel). (HR > 1 favors young adults, HR< 1, favors older adults.)
Fig 3A shows the relapse survival curves for the ITT sample among the young adults by treatment, with no significant interaction, that is, no significant difference from the sample as a whole (which showed an advantage in the ITT sample for BUP-NX).
Figure 3.
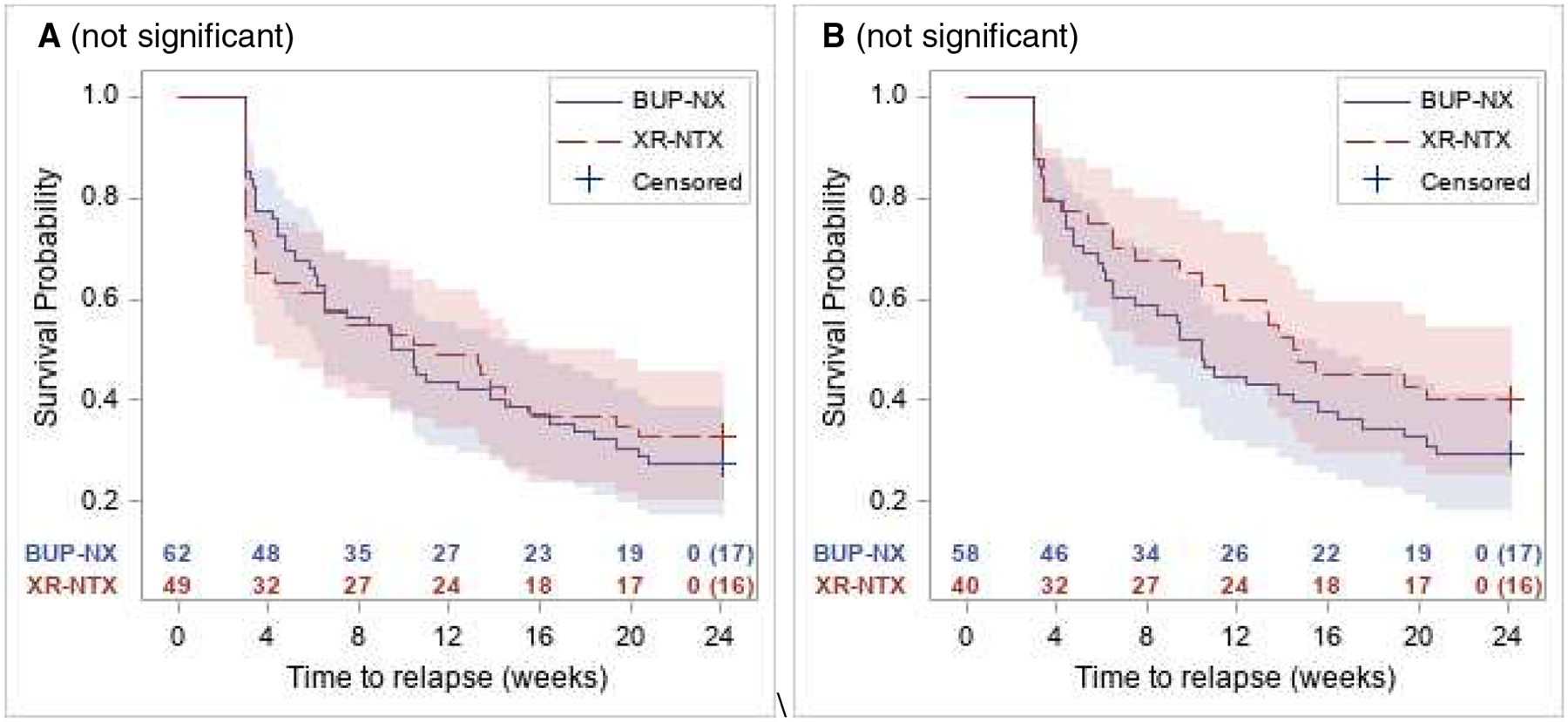
Relapse-free survival curves with 95% confidence intervals in young adults by treatment among the ITT sample (A: left panel) and among the per protocol sample (B: right panel). Corresponding number of subjects at risk are presented along x-axis along with number censored at week 24.
Among the per protocol sample, Figure 1B shows the relapse-free survival curves by age group, and Figure 2B shows the model estimated hazard ratios of treatment and age over time. For the per protocol sample, the proportional hazards assumption was not violated (treatment-by-time interaction: p=.776; age-by-time interaction: p=.119), with the HR estimates for treatment and for age remaining constant over time. That is, there was no variation over time in the relative hazards of relapse related to either treatment group or age group. There was no significant difference in the risk of relapse between treatment groups (p=.488), but there was a significant difference in the risk of relapse between age groups. Younger adults had a higher risk of relapse over time compared to older adults (HR=1.43, p=.013).
Fig 3B shows the relapse survival curves among the young adults in the per-protocol sample by treatment, with no significant interaction, that is, no significant difference from the sample as a whole (which showed no significant difference in the per-protocol sample between BUP-NX and XR-NTX).
Discussion
This study examined the association of age group with MOUD treatment outcomes in a secondary analysis comparing young adult participants (ages 18–25; n=111) vs. older adults (ages 26 and up; n=459) from the XBOT comparative effectiveness trial of XR-NTX vs. BUP-NX [25]. Three outcomes were examined – rates of successful induction onto medication, rates of relapse at 24 weeks, and relapse-free survival over time. Age group did not have a significant impact on rates of medication induction success/failure. Age group did have a significant impact on treatment effectiveness, for both treatment effectiveness outcomes examined (rates of relapse at 24 weeks, and relapse-free survival over time), and for both analysis samples examined – (the ITT group and the per protocol group). Young adults had significantly worse outcomes in all four of these treatment effectiveness analyses (24-week relapse ITT, 24-week per protocol, relapse-free survival ITT, relapse-free survival per protocol).
Our results are consistent with previous findings that younger age generally confers worse treatment prognosis in OUD treatment [6][11]. Poorer treatment response in young adults presumably reflects various features of the well-known developmental vulnerability of young adults. As there is no evidence of any lesser direct biological efficacy of MOUD based on age, differential response is more likely to involve aspects of medication adherence, motivation for change, treatment engagement and retention, and possibly co-morbidities. Little, if any, research has directly addressed these factors in youth OUD treatment response. Immature executive function would be an intuitive candidate for exploration, though these issues of potential mechanism are beyond the scope of the present study.
There was no significant interaction with age group on success/failure to initiate medications. The finding of a significant “induction hurdle” for naltrexone compared to buprenorphine occurred in both age groups and was consistent with the parent study. As in the parent study, the difference between rates of relapse events in the ITT and per-protocol samples was largely accounted for by the occurrence of early relapse among XR-NTX induction failures. Just as for older adults, the impediments to naltrexone induction in young adults leave considerable room for improvement, highlighting the importance of the body of work that seeks to identify strategies for reducing barriers to naltrexone initiation. One such strategy might be the facilitation of longer lengths of stay in residential treatment to allow more sufficient time for an opioid free washout period without anxieties over the risk of precipitated withdrawal, perhaps particularly relevant for young adults who may be especially distress intolerant. Another strategy is the use of accelerated induction protocols [30][31][32]. This might have particular relevance to youth because of impatience and impulsiveness that can be aspects of developmental vulnerability.
While younger age was associated with worse outcomes for both medications, there was no significant impact of age on the comparative effectiveness of the two medications. While the model did not show a significant moderation by age group, the unadjusted raw numbers were in the direction of greater relapse rates for BUP-NX than for XR-NTX. In the ITT analysis, 24 week young adult relapse rates were 72.6% for those assigned to BUP-NX and 67.3% for those assigned to XR-NTX (a minor difference but in the opposite direction as the older adults and the parent study), and in the per protocol analysis 24 week young adult relapse rates were 70.7% for those assigned to BUP-NX and 60.0% for those assigned to XR-NTX. It is possible that a sample with larger numbers of young adults could help with further exploration of the question of differential medication response. One might speculate that a long acting formulation medication like XR-NTX would be particularly useful for youth, given difficulties with adherence, although there was no advantage shown in this study. The same speculation might also pertain to newly developed extended release buprenorphine formulations [33][34].
Strengths of this study include: largest young adult sample to date in a study examining role of age in MOUD outcomes, largest young adult sample and first study with experimental design to examine treatment outcomes in young adults with more than one type of MOUD, and first study to examine role of age on initiation of either XR-NTX or BUP-NX. Limitations include: relatively small young adult sample size compared to older adult sample, limiting power to test interactions, secondary analysis with post-hoc hypotheses, lack of exploration of potential co-factors that may have served as mechanisms, moderators, or mediators of the effect of age group (such as cognitive function and others).
Future research should focus on development of models of care that attempt to overcome the treatment outcome gap in the younger age group, targeting barriers to treatment engagement and retention, and especially medication adherence. Additional investigation should also include larger young adult sample sizes, exploration of possible mediators such as age of onset, medication adherence, executive function measures, motivation measures, psychiatric comorbidities, non-opioid substance use, and others. Such explorations may be partially informed by the differential baseline characteristics of the young adults in this sample, that is, greater proportion female, younger age of onset, greater rates of cannabis use, and lower rates of ever having been married (and likely more dependent on family of origin). Based on the finding that the ITT sample survival curves of the two age groups start to diverge progressively at 8 weeks, it may be fruitful to explore within-treatment phenomena at or preceding that time point.
Despite having lower overall effectiveness compared to older adults, this analysis also serves to highlight that young adults do respond positively to both of these medications, reinforcing the emerging body of work and consensus that MOUD should be incorporated into the standard of care as first line. While many of the young adult participants did relapse, many did not--29.7% in the ITT sample and 33.7% in the per protocol sample. And while those rates are 11.5% and 15.5% below the unadjusted numbers for the older adults respectively, they still represent a vast improvement over treatment without MOUD. Both buprenorphine and XR-naltrexone are available in SUD specialty and, increasingly, in primary care settings. The effectiveness gap conferred by age further reminds us of the particular vulnerabilities and special needs of youth, and the imperative to develop and implement developmentally-informed strategies to improve engagement, retention, and medication adherence in youth with OUD. Such strategies, for further future exploration, could include: family involvement, home delivery of medications [35], electronic reminders and other messaging, age-specific engagement approaches, young adult specialty programs [9], lower barrier delivery models [36], phased treatment with higher intensity early on, and others.
Acknowledgments
This work (the original parent study) was supported by grants from the NIDA National Drug Abuse Treatment Clinical Trials Network (U10DA013046, UG1/U10DA013035, UG1/U10DA013034, U10DA013045, UG1/U10DA013720, UG1/U10DA013732, UG1/U10DA013714, UG1/U10DA015831, U10DA015833, as well as NCCIH (AT010614) to MF and KW (salary support during the secondary analysis).
Abbreviations:
- BUP-NX
Buprenorphine naloxone
- ITT
Intent to treat
- MOUD
Medications for opioid use disorder
- OUD
Opioid use disorder
- SUD
Substance use disorder
- XBOT
Comparative effectiveness trial
- XR-NTX
Extended release naltrexone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts: Dr Fishman has been a consultant for Alkermes, US World Meds and Drug Delivery LLC.
Previous presentation:
Fishman, M., Wenzel, K. R., Campbell, A., Rotrosen, J., Pavilcova, M., Nunes, E. (2020, June). Young adults have worse outcomes than older adults: Secondary analysis of the X:BOT trial of extended release naltrexone versus buprenorphine for opioid use disorder. Online oral presentation at the annual meeting for the College on Problems of Drug Dependence.
References
- [1].Substance Abuse and Mental Health Services Administration. Key Substance Use and Mental Health Indicators in the United States: Results from the 2016 National Survey on Drug Use and Health, https://www.samhsa.gov/data/report/key-substance-use-and-mental-health-indicators-united-states-results-2016-national-survey
- [2].Scholl L, Seth P, Kariisa M, et al. 2018 Drug and opioid-involved overdose deaths - United States 2013–2017. Morbidity and Mortality Weekly Report 2018;67(5152):1419–1427.doi: 10.15585/mmwr.mm675152e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Minozzi S, Amato L, Bellisario C, Davoli M. Maintenance treatments for opiate-dependent adolescents. Cochrane Database Syst Rev 2014;6. doi: 10.1002/14651858.CD007210.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Levy S. Medication-Assisted Treatment of Adolescents With Opioid Use Disorders. American Association of Pediatrics News & Journals 2016;138(3). DOI: 10.1542/peds.2016-1893 [DOI] [PubMed] [Google Scholar]
- [5].Hadland SE, Wharam JF, Schuster MA, et al. Trends in receipt of buprenorphine and naltrexone for opioid use disorder among adolescents and young adults 2001–2014. JAMA Pediatr 2017;171(8):747–755. doi: 10.1001/jamapediatrics.2017.0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Matson SC, Hobson G, Abdel-Rasoul M, Bonny AE. A retrospective study of retention of opioid-dependent adolescents and young adults in an outpatient buprenorphine/naloxone clinic. J Addict Med 2014;8(3):176–82. doi: 10.1097/ADM.0000000000000035. Robinson C, Wilson J. Management of Opioid Misuse and Opioid Use Disorders Among Youth. Pediatrics. 2020 [DOI] [PubMed] [Google Scholar]
- [7].Borodovsky JT, Levy S, Fishman M, Marsch LA. Buprenorphine Treatment for Adolescents and Young Adults With Opioid Use Disorders: A narrative review. Journal of Addiction Medicine 2018;12:170–183.doi: 10.1097/ADM.0000000000000388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fishman MJ, Winstanley EL, Curran E, et al. Treatment of opioid dependence in adolescents and young adults with extended release naltrexone: Preliminary case-series and feasibility. Addiction 2010;105(9):1669–1676.doi: 10.1111/j.1360-0443.2010.03015.x. [DOI] [PubMed] [Google Scholar]
- [9].Vo HT, Robbins E, Westwood M, et al. Relapse prevention medications in community treatment for young adults with opioid addiction. Substance Abuse 2016;37(3):392–397. DOI: 10.1080/08897077.2016.1143435 [DOI] [PubMed] [Google Scholar]
- [10].Hadland SE, Bagley SM, Rodean J, et al. Receipt of Timely Addiction Treatment and Association of Early Medication Treatment With Retention in Care Among Youths With Opioid Use Disorder. Journal of the American Medical Association Pediatrics 2018;172(11):1029–1037.doi: 10.1001/jamapediatrics.2018.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Schuman-Oliver Z, Weiss RD, Hoeppner BB, et al. Emerging Adult Age Status Predicts Poor Buprenorphine Treatment Retention. Journal of Substance Abuse Treatment 2014;47(3):202–212.doi: 10.1016/j.jsat.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wu LT, Ringwalt C. Use of substance abuse services by young uninsured American adults. Psychiatric Services 2005;56(8):946–953. DOI: 10.1176/appi.ps.56.8.946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Subramaniam GA, Ives ML, Stitzer ML, Dennis ML. The added risk of opioid problem use among treatment-seeking youth with marijuana and/or alcohol problem use. Addiction 2010;105(4):686–698.doi: 10.1111/j.1360-0443.2009.02829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gandhi DH, Jaffe JH, McNary S, et al. Short-term outcomes after brief ambulatory opioid detoxification with buprenorphine in young heroin users. Addiction 2003;98(4):453–462. DOI: 10.1046/j.1360-0443.2003.00334.x [DOI] [PubMed] [Google Scholar]
- [15].Warden D, Subramaniam GA, Carmody T, et al. Predictors of attrition with buprenorphine/naloxone treatment in opioid dependent youth. Addictive Behaviors 2012;37(9)1046–1053.doi: 10.1016/j.addbeh.2012.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Volkow ND, Jones EB, Einstein EB, Wargo EM. Prevention and Treatment of Opioid Misuse and Addiction: A Review. JAMA Psychiatry 2018; 76(2):208–2016. doi: 10.1001/jamapsychiatry.2018.3126. [DOI] [PubMed] [Google Scholar]
- [17].Chang DC, Kilmas J, Wood E, Fairbairn N. Medication-assisted treatment for youth with opioid use disorder: Current dilemmas and remaining questions. The American Journal of Drug and Alcohol Abuse 2018; 44(2):143–146.doi: 10.1080/00952990.2017.1399403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Levin FR, Bisaga A, Sullivan MA, et al. A review of a national training initiative to increase provider use of MAT to address the opioid epidemic. American Journal on Addictions 2016;25(8)603–609.doi: 10.1111/ajad.12454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Liebling EJ, Yedinak JL, Green TC, et al. Access to Substance Use Treatment among Young Adults Who Use Prescription Opioids Non-Medically. Substance Abuse Treatment, Prevention, and Policy 2016;11(1):38 DOI: 10.1186/s13011-016-0082-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sharma A, Kelly SM, Mitchell SG, et al. Update on barriers to pharmacotherapy for opioid use disorders. Current psychiatry reports 2017;19(6):35.doi: 10.1007/s11920-017-0783-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Moore SK, Guarino H, Marsch LA. “This is Not Who I Want to be:” Experiences of Opioid-Dependent Youth Before, and During, Combined Buprenorphine and Behavioral Treatment. Substance Use & Misuse 2014;49(3):303–314.doi: 10.3109/10826084.2013.832328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Robinson SM, Adinoff B. The mixed message behind “Medication-Assisted Treatment” for substance use disorder. American Journal of Drug and Alcohol Abuse 2018;44(2):147–150.doi: 10.1080/00952990.2017.1362419. [DOI] [PubMed] [Google Scholar]
- [23].Alinsky RH, Zima BT, Rodean J, et al. Receipt of addiction treatment after opioid overdose among Medicaid-enrolled adolescents and young adults. JAMA Pediatrics 2020. doi: 10.1001/jamapediatrics.2019.5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bagley SM, Larochelle MR, Xuan Z, et al. Characteristics and receipt of medication treatment among young adults who experience a nonfatal opioid-related overdose. Annals of Emergency Medicine 2020;75(1):29–38.doi: 10.1016/j.annemergmed.2019.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lee JD, Nunes EV, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): A multicentre, open-label, randomised controlled trial. Lancet 2018;391(10118):309–318.doi: 10.1016/S0140-6736(17)32812-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lee JD, Nunes EV, Mpa PN, et al. NIDA Clinical Trials Network CTN-0051, Extended-Release Naltrexone vs. Buprenorphine for Opioid Treatment (X:BOT): Study design and rationale. Contemporary Clinical Trials 2016;50:253–264.doi: 10.1016/j.cct.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Nunes EV, Lee JD, Sisti D, et al. Ethical and Clinical Safety Considerations in the Design of an Effectiveness Trial: A Comparison of Buprenorphine versus Naltrexone Treatment for Opioid Dependence. Contemporary Clinical Trials 2016;51:34–43.doi: 10.1016/j.cct.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Arnett JJ. Emerging adulthood: A theory of development from the late teens through the twenties. American Psychologist 2000;55(5):469–480. [PubMed] [Google Scholar]
- [29].Sobell LC,Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption IN:Litten RZ, Allen JP, editors. Measuring alcohol consumption: Psychosocial and biochemical methods, New Jersey: Humana Press; 1992, p. 41–72. 10.1007/978-1-4612-0357-5_3 [DOI] [Google Scholar]
- [30].Sullivan M, Bisaga A, Pavlicova M, et al. Long-Acting Injectable Naltrexone Induction: A Randomized Trial of Outpatient Opioid Detoxification With Naltrexone Versus Buprenorphine. American Journal of Psychiatry 2017;174(5):459–467.doi: 10.1176/appi.ajp.2016.16050548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sibai M, Mishlen K, Nunes EV, et al. A week-long outpatient induction onto XR-naltrexone in patients with opioid use disorder. American Journal on Drug and Alcohol Abuse 2019:1–8.doi: 10.1080/00952990.2019.1700265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bisaga A, Mannelli P, Yu M, et al. Outpatient transition to extended-release injectable naltrexone for patients with opioid use disorder: A phase 3 randomized trial. Drug and Alcohol Dependence 2018;187:171–178.doi: 10.1016/j.drugalcdep.2018.02.023. [DOI] [PubMed] [Google Scholar]
- [33].Lofwall MR, Walsh SL, Nunes EV, et al. Weekly and Monthly Subcutaneous Buprenorphine Depot Formulations vs Daily Sublingual Buprenorphine With Naloxone for Treatment of Opioid Use Disorder. JAMA International Medicine 2018;178(6):764–773.doi: 10.1001/jamainternmed.2018.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Haight BR, Learned SM, Laffont CM, et al. Efficacy and safety of a monthly buprenorphine depot injection for opioid use disorder: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2019;393(10173):778–790.doi: 10.1016/S0140-6736(18)32259-1. [DOI] [PubMed] [Google Scholar]
- [35].Vo HT, Burgower R, Rozenberg I, Fishman M. Home-based delivery of XR-NTX in youth with opioid addiction. Journal of Substance Abuse Treatment 2018;85:84–89.doi: 10.1016/j.jsat.2017.08.007. [DOI] [PubMed] [Google Scholar]
- [36].Marsch LA, Moore SK, Borodovsky JT, et al. A randomized controlled trial of buprenorphine taper duration among opioid-dependent adolescents and young adults. Addiction 2016;111(8):1406–15.doi: 10.1111/add.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]


