Abstract
We wanted to evaluate the prognostic value of common histopathological variables in a large cohort of patients with cancer in the mobile tongue as such information can be important for treatment stratification of the individual patient, especially for patients with low-stage disease. In addition, we wanted to investigate whether an alternative scoring model with fewer options would compromise the prognostic value. One hundred fifty patients with oral tongue squamous cell carcinomas that were treated in curative intent and with available HE-stained tumor sections were included. We reclassified all tumors and performed univariate and multivariate survival analyses of histopathological and clinical variables. For the complete cohort, lymph node status, grade of differentiation, perineural infiltration, and lymphocytic infiltration were independent prognosticators. In the low-stage disease group, independent prognostic factors were tumor size, grade of differentiation, and lymphocytic infiltrate. For patients with low-stage disease, a histo-score combining the scores for tumor differentiation and lymphocytic infiltrate identified a group of patients with particularly low survival, as patients with moderately or poorly differentiated tumors and little lymphocytic infiltrate had a less favorable 5-year survival outcome than patients in the high-stage disease group. This study shows that a histo-score combining tumor differentiation and lymphocytic infiltration should be given special consideration in treatment planning. Our results also illustrate that many variables can be scored with fewer options than previously suggested to increase their reproducibility, and still maintain their prognostic value.
Electronic supplementary material
The online version of this article (10.1007/s00428-020-02875-9) contains supplementary material, which is available to authorized users.
Keywords: Oral squamous cell carcinoma, Low-stage, Differentiation, Lymphocyte infiltrate, Histo-score, Prognostic factors
Introduction
About half of all malignant tumors in the oral cavity arise in the mobile, anterior two-thirds of the tongue, and more than 90% of them are squamous cell carcinomas (SCC) [1]. The aggressiveness of oral cavity tongue (OT) SCC varies markedly, even for small tumors without lymph node metastases [2]. The search for morphological tumor traits that reliably predict the prognosis for the individual patient has been going on for decades [3–5]. Such prognostic markers could help clinicians select the optimal treatment for individual patients that could increase the chances of being cured of the disease, and at the same time minimize the side effects from overtreatment. The TNM system classifies tumors based on their size and depth of invasion (T), neck node involvement (N), and distant metastasis (M). Along with the International Union against cancer (UICC) staging, these factors are today the best survival prognosticators for cancers in the oral cavity [6]. On the group level, patients with low-stage disease (stages I–II; T1–2, N0M0) have an estimated higher survival rate compared with patients with high-stage disease (stages III–IV) [7, 8]. However, there is a need to find markers that can differentiate between aggressive and more indolent tumors for individual patients within the same stage.
Various aspects of a tumor’s morphology and growth pattern can be evaluated on hematoxylin and eosin (HE)-stained tumor sections. Several of these characteristics have been proposed as prognostic markers in oral cancer [4, 9, 10]. However, despite some reports of prognostic usefulness, none of these markers has been implemented in clinical practice, mostly due to lack of coherence between studies. There are several putative explanations for the lack of consistency between prognostic studies. Many are based on small patient cohorts and do not control for parameters known to affect prognosis, such as intraoral location and stage [11–14]. This biases the actual prognostic value of the markers in question. Furthermore, the evaluation of histopathological criteria is subjective, and different pathologists may interpret the same criterion differently [15, 16]. In a recent study, we found poor inter- and intra-observer agreement when evaluating a selection of proposed histopathological prognostic markers in oral SCC, even though the observers had mutual training sessions and were experienced pathologists [17]. Improved agreement was obtained by reducing the number of scoring alternatives for each parameter. This suggests that fewer options for each parameter might increase the robustness of histopathological prognostic markers, provided that the reduction of scoring alternatives does not compromise the prognostic value. In the current study, we evaluated the prognostic value of a number of proposed histopathological variables as they were originally proposed, as well as with a reduced number of scoring alternatives, in a large, homogenous cohort of OTSCC. Our results show that some histopathological markers, individually and in combination, can add significant prognostic information for OTSCC. Our study further highlights the importance of controlling for known risk factors such as tumor size and lymph node metastasis when evaluating putative prognostic markers.
Materials and methods
Cohort of patients
The Norwegian Oral Cancer (NOROC) study is a retrospective study that includes patients diagnosed with oral cavity SCC in Norway from January 1, 2005, through December 31, 2009.The NOROC study includes patients with strict oral cavity SCC [8]. In the present study, the relevant ICD-10 codes were C02, which refer to cancers in the mobile tongue. Of the original NOROC cohort, 273 patients (45%) had OTSCC. From them, we included only the primary, treatment-naïve patients who were treated in curative intent and from whom we had HE-stained sections from biopsies or resections available, altogether 150 patients.
Extracting clinical and histopathological data
Experienced head and neck surgeons retrieved clinical parameters from the electronic health records as previously described [8]. Of the 150 patients that underwent surgery, 72 patients had neck surgery, and for them, the N-status was based on histopathological evaluation. For the patients who did not have neck surgery, the N-status was based on clinical/radiological evaluation.
Senior pathologists re-evaluated the histopathological characteristics of the tumors, including WHO degree of differentiation, keratinization, nuclear polymorphism, perineural infiltration, lymphocyte infiltrate at the interface between tumor and surrounding stroma, and worst pattern of invasion [3, 4]. For several of these, a fairly elaborate grading system was originally suggested. In this study, we have also applied alternative versions, as described in our previous paper [17] and summarized in Table 1. The pathologists were blinded for the patients’ clinical information and outcome.
Table 1.
Variables with original and alternative grading
| Tumor characteristics | Original variables | Alternative 1 | Alternative 2 | |||
|---|---|---|---|---|---|---|
| 1.0 | Differentiation whole tumor | Well | 1.1 | Low-grade | ||
| Moderate | ||||||
| Poorly | High-grade | |||||
| 2.0 | Differentiation worst pattern | Well | 2.1 | Low-grade | ||
| Moderate | ||||||
| Poorly | High-grade | |||||
| 3.0 | Keratinization whole tumor | High | 3.1 | Low-grade | ||
| Moderate | ||||||
| Minimal | High-grade | |||||
| None | ||||||
| 4.0 | Keratinization tumor front* | High | 4.1 | Low-grade | ||
| Moderate | ||||||
| Minimal | High-grade | |||||
| None | ||||||
| 5.0 | Polymorphism whole tumor | Little/none | 5.1 | Low-grade | ||
| Moderate | ||||||
| Abundant | High-grade | |||||
| Extreme | ||||||
| 6.0 | Polymorphism tumor front* | Little/none | 6.1 | Low-grade | ||
| Moderate | ||||||
| Abundant | High-grade | |||||
| Extreme | ||||||
| 7.0 | Perineural infiltration | None | 7.1 | No | ||
| Invasive front | Yes | |||||
| Tumor center | ||||||
| 8.0 | Lymphocyte infiltrate | Marked | 8.1 | Marked | 8.2 | Abundant |
| Moderate | Not marked | |||||
| Little/none | Little | |||||
| 9.0 | Worst pattern of invasion (WPO)** | Type 1 | 9.1 | Low-grade | 9.2 | Low-grade |
| Type 2 | ||||||
| Type 3 | High-grade | |||||
| Type 4 | High-grade | |||||
| Type 5 | ||||||
We calculated survival from the date of diagnosis until the date of death or last day of follow-up, which was June 1, 2015. At that time, all patients were followed up for a minimum of 5 years or until death. Cause of death was acquired from the Norwegian Cause of Death Registry.
The study was approved by the Northern Norwegian Regional Committee for Medical Research Ethics (Protocol numbers REK Nord; 2013/1786 and 2015/1381). Patients still alive were informed about the project and had the opportunity to opt-out.
Statistical analysis
Descriptive analyses and univariate survival analyses using log-rank (Mantel-Cox) giving Kaplan-Meier survival curves were performed. Variables significant in univariate calculations were tested for collinearity before entering them into multivariable equations. Multivariate survival analyses were performed using Cox regression model. Associations were investigated using chi-square. Receiver operating characteristic curve was applied to evaluate cut-off values in binary classifications. All statistical analyses were performed using SPSS version 26. All survival analyses were significant at 0.05 level.
Results
One hundred and fifty patients with OTSCC were eligible for histopathological reclassification and included in the study. Of the tumor material available, 127 were resection specimens, 18 biopsies, and 5 unknown. Seventy-seven patients had low-stage disease (stages I and II according to TNM 8th edition), 63 had high-stage (stages III and IV) [6], and for 10 cases the information for stage was missing.
Supplementary Table 1 presents the scores for each variable for the whole cohort and after separation into low-stage and high-stage disease. Table 2 presents gender, age, TNM-status and stage, as well as calculation of 5-year disease-specific survival (DSS).
Table 2.
Clinicopathological characteristics related to 5-year disease specific survival (DSS). Number and percent of patients in each group, and in addition percentage of patients with 5-year DSS
| Variable | n | % | DSS % | p *(DSS) | |
|---|---|---|---|---|---|
| Gender | Male | 92 | 61.3 | 70.7 | 0.702 |
| Female | 58 | 38.7 | 69.0 | ||
| Age (year) group | ≤ 50 | 29 | 19.3 | 79.3 | 0.015 |
| 51–60 | 29 | 19.3 | 65.5 | ||
| 61–70 | 44 | 29.3 | 70.5 | ||
| 71–80 | 31 | 20.7 | 67.7 | ||
| ≥ 81 | 17 | 11.3 | 64.7 | ||
| pT 8th Edition | pT1 | 48 | 35.6 | 87.5 | 0.006 |
| pT2 | 53 | 39.3 | 64.2 | ||
| pT3 | 34 | 25.2 | 64.7 | ||
| N** | N0 | 108 | 72.0 | 82.4 | 0.001 |
| N+ | 40 | 26.7 | 37.5 | ||
| Nx/Unknown | 2 | 1.3 | |||
| Stage | Low stage | 77 | 55.0 | 82.8 | < 0.001 |
| High stage | 63 | 42.0 | 44.6 | ||
| Nx/Unknown | 10 | 6.7 |
*Significant at 0.05 level
**Combination of cN and pN. If neck dissection was performed the result on pN was superior to cN
Survival
The 5-year DSS was 64.8% for the whole group and 82.8% and 44.6% for the low- and high-stage group, respectively.
Univariate analyses
In Table 3, 5-year DSS from univariate analyses are listed for each variable, both with original and alternative versions of grading. For the whole cohort, the following variables were significantly associated with 5-year DSS: degree of differentiation (1.0 and 1.1), keratinization of the whole tumor (3.0 and 3.1), keratinization at tumor front (4.0 and 4.1), perineural infiltration (7.0 and 7.1), lymphocytic infiltrate (8.0, 8.1, and 8.2), and worst pattern of infiltration (9.2).
Table 3.
Variables (both original and alternative grading) and 5-year disease-specific survival in univariate calculations for the whole cohort, and for low-stage disease and high-stage disease separately. The percentage of patients surviving according to different grading is specified under DSS%
| Whole cohort (n = 150) | Low-stage (n = 78) | High-stage (n = 63) | |||||
|---|---|---|---|---|---|---|---|
| Variables | All | All | All | ||||
| DSS % | p (n) | DSS% | p (n) | DSS% | p (n) | ||
| 1.0 | Differentiation, whole tumor | 92.3/64.0/16.7 | < 0.001 (127)* | 94.7/84.6/40.0 | 0.002 (63)* | 75.0/47.7/0 | 0.055 (55) |
| 1.1 | 70.4/16.7 | < 0.001* | 87.7/40.0 | 0.001* | 51.1/0 | 0.025 * | |
| 2.0 | Differentiation, worst pattern | 100/72.0/56.7 | 0.074 (124) | 100/88.9/76.9 | 0.248 (63) | 100/50.0/40.0 | 0.646 (54) |
| 2.1 | 75.4/56.7 | 0.053 | 90.9/76.7 | 0.121 | 52.6/40.0 | 0.676 | |
| 3.0 | Keratinization whole tumor | 85.0/68.0/53.6/47.6 | 0.038 (124)* | 82.4/91.3/83.3/66.7 | 0.402 (61) | 83.3/46.2/30.8/22.2 | 0.137 (54) |
| 3.1 | 73.3/51.0 | 0.010 * | 87.5/76.2 | 0.238 | 53.1/27.3 | 0.078 | |
| 4.0 | Keratinization, tumor front | /100/70.3/59.7 | 0.047 (120)* | ÷/100/89.5/75.8 | 0.133 (62) | ÷/100/47.1/44.1 | 0.665 (52) |
| 4.1 | 100/63.3 | 0.025* | 100/80.8 | 0.146 | 100/45.1 | 0.374 | |
| 5.0 | Nuclear polymorphism | 84.0/64.4/60.0/52.0 | 0.147(125) | 100/85.7/84.6/50.0 | 0.004 (62)* | 33.3/42.1/40.0/50.0 | 0.767 (54) |
| 5.1 | 71.4/56.4 | 0.097 | 92.3/69.6 | 0.018* | 40.0/44.8 | 0.673 | |
| 6.0 | Nuclear polymorphism, tumor front | 66.7/75.0/68.8/57.5 | 0.507 (120) | 100/94.7/64.2/64.7 | 0.054 (63) | 20.0/46.7/40.0/54.5 | 0.363 (52) |
| 6.1 | 72.9/62.5 | 0.302 | 96.2/75.0 | 0.029* | 40.0/50.0 | 0.343 | |
| 7.0 | Perineural infiltration | 70.0/52.9/28.6 | 0.003 (114)* | 84.0/71.4/50.0 | 0.250 (59) | 52.8/33.3/20.0 | 0.046 (50)* |
| 7.1 | 70.0/45.8 | 0.020* | 84.0/66.7 | 0.208 | 52.8/28.6 | 0.098 | |
| 8.0 | Lymphocytic infiltration | 87.5/67.2/50.0 | 0.008 (123)* | 100/87.5/56.3 | 0.003 (63)* | 62.5/40.0/47.6 | 0.711 (54) |
| 8.1 | 87.5/60.0 | 0.021* | 100/77.1 | 0.050* | 62.5/43.5 | 0.409 | |
| 8.2 | 72.9/50.0 | 0.007* | 91.5/56.3 | 0.001* | 45.5/47.6 | 0.821 | |
| 9.0 | WPOI | 100/70.6/82.8/58.9/56.3 | 0.190 (120) | 100/80.0/100/77.8/66.7 | 0.224 (62) | ÷/50.0/50.0/42.3/50.0 | 0.997 (52) |
| 9.1 | 73.7/65.3 | 0.461 | 83.3/84.0 | 0.999 | 50,0/45.7 | 0.908 | |
| 9.2 | 79.2/58.3 | 0.022* | 93.1/75.8 | 0.061 | 50.0/44.4 | 0.902 | |
*Significant at 0.05 level
For patients with low-stage disease, differentiation of the whole tumor (1.0 and 1.1), nuclear polymorphism whole tumor (5.0 and 5.1), nuclear polymorphism at tumor front (6.1), and lymphocytic infiltrate (8.0, 8.1, and 8.2) were significantly associated with DSS. For patients with high-stage disease, differentiation of whole tumor (1.1) and perineural infiltration (7.0) were the only significant prognosticators of DSS.
Separate calculations were also performed for resection specimens only (biopsies excluded) with results similar to those for all tumors (resections and biopsies), and it is presented in Supplementary Table 2.
Multivariate analyses
We performed multivariate analysis of the histopathological variables that were significant in univariate calculations, with separate analyses for original and alternative grading of the variables. Additionally, T and lymph node status was included in the equation for the whole cohort, and T for the low-stage disease group. For the whole cohort, this included differentiation, keratinization, perineural infiltration, and lymphocytic infiltration for both original and alternative scoring gradings, and WPOI was additionally included in the alternative version. Keratinization of the whole tumor and keratinization of the tumor front were collinear, and only keratinization of the whole tumor (3.0/3.1) was included in multivariate analyses. For patients with low-stage disease differentiation, nuclear polymorphism and lymphocytic infiltration were included. Nuclear polymorphism of the whole tumor and in the tumor front were collinear, and only polymorphism of whole tumor (5.0/5.1) was included in the multivariate analyses. Independent prognosticators for the complete patient cohort were lymph node status (N, p < 0.001), differentiation of whole tumor (1.1, p = 0.022), perineural infiltration (7.0, p = 0.025), and lymphocytic infiltration (8.2, p = 0.048). In the low-stage group, T (p = 0.003), differentiation of whole tumor (1.1, p = 0.022), and lymphocytic infiltrate (8.2, p = 0.003) were all independent variables.
Combined histo-score
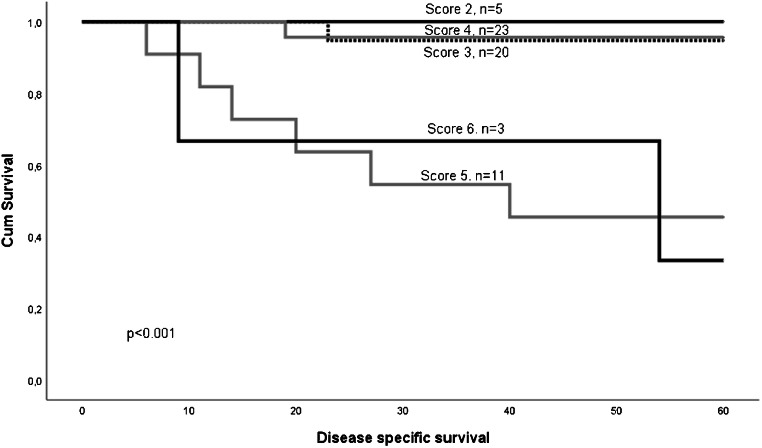
For the low-stage group, we created a combined score, called histo-score, based on tumor differentiation and lymphocytic infiltrate (Fig. 1). The histo-score was calculated by summarizing the individual score of differentiation and lymphocyte infiltration (Supplementary Table 3). Using the original grading, the lowest score was 2 and the highest was 6. There was a highly significant difference in survival between the groups (p < 001), Fig. 2. Of the 48 patients with scores 2, 3, or 4, only two patients died of the disease within 5 years (DSS = 95.8%). Of the 14 patients with a score of 5 or 6, eight patients died within 5 years (DSS 42.9%). The area under the ROC curve was estimated to be 0.748. The combined histo-score based on the alternative grading differentiation 1.1 and lymphocytic infiltration 8.2 showed the same significant prognostic power (p < 0.001).
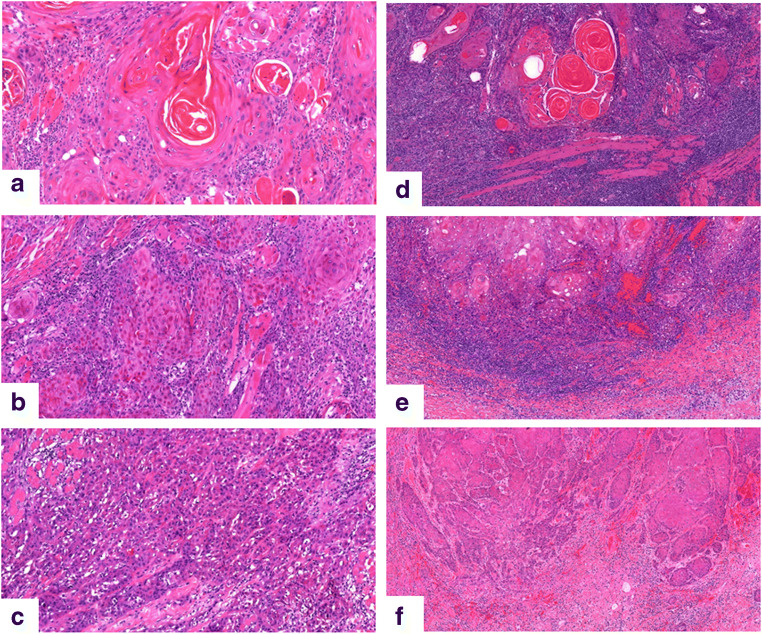
Fig. 1.
Tumor differentiation and lymphocyte infiltration. Well, moderate, and poorly differentiated tumor in a–c. Marked, moderate, and little lymphocyte infiltration in d–f
Fig. 2.
Kaplan-Meier curve showing the results after combining the variables of differentiation (1.0) and lymphocytic infiltrate (8.0). Scoring alternatives are shown in Table 3. Patients with low score (2–4) had a statistically better survival than those with high score (5–6). Figure 2 Kaplan-Meier curve showing survival of patients with low-stage disease stratified according to the histo-score
There were no common denominators for the patients with low-stage disease and low versus high histo-score who died with respect to age, gender, T-stage, keratinization, or worst pattern of infiltration. Additionally, we explored whether there was a difference between different treatment options (with or without neck dissection, with or without postsurgical radiotherapy), but we could not find any associations.
Discussion
Reliable, prognostic markers that can supplement tumor staging are lacking for oral cavity cancer. As tumors of the same stage can have different degrees of aggressiveness, there is a need to find additional markers to assist the treatment planning and to predict the outcome of individual patients. Oral cancer is most prevalent in developing countries [18]. Thus, markers that do not require expensive equipment or reagents, such as histopathological traits that can be assessed on HE-stained sections, are especially valuable. In the present study, we have evaluated the prognostic power of a number of histopathological variables suggested for oral cancer, where results from previous studies are contradictory [19, 20]. We have tested them in a large, homogenous cohort of patients with OTSCC, in which clinical and histopathological parameters are well controlled. Our hypothesis was that the lack of consistency of prognostic value in previous studies can be partly explained by small cohorts of patients and the inclusion of tumors from various intraoral locations. Furthermore, scoring of histopathological parameters is subjective, as reflected by the poor inter- and intra-rater agreement [17]. Several of the histological variables have been proposed with three to six options for scoring, sometimes with subtle differences between each alternative. Grouping categories and thereby reducing the number of scoring alternatives can make the scoring easier and more reproducible [17]. Therefore, we also tested the prognostic power of the variables as they were originally proposed, as well as with broader and fewer categories.
As expected, the well-established prognostic markers’ tumor size and lymph node metastases were independent predictors of survival. Additionally, we found that tumor differentiation was an independent prognosticator of survival both for the whole cohort and for patients with low-stage disease. WHO lists differentiation as a prognostic marker for oral cavity cancer, and the degree of differentiation is usually described in pathology reports [21]. However, due to many studies reporting low prognostic value of differentiation for oral SCC, clinicians rarely give it much emphasis during treatment planning [22, 23]. Our results indicate that this marker has significant prognostic power.
A revised grading for lymphocytic infiltration where the categories for marked and moderate infiltration were combined was also an independent prognostic marker for low-stage disease and for the whole patient cohort. Grouping categories generates larger groups for statistical analyses, and this can affect the significance level. However, the cutoff for dichotomizing the original three-tier variable was important. The variable only had independent prognostic power when separating the tumors with low lymphocytic infiltration from those with moderate and abundant infiltration. This suggests that lymphocyte infiltration is tightly related to the biology of the tumor and that the tumors with little infiltration may take a more aggressive course. This is in line with previous studies showing that a rich lymphocyte infiltration is associated with favorable prognosis [24–26].
By incorporating the degree of differentiation and lymphocytic infiltration in a combined histo-score, we were able to define a subgroup of patients with low-stage disease that had a much lower survival rate than the rest of the low-stage disease patients. Interestingly, the survival in this subgroup was even less favorable than patients with high-stage disease (42.9 versus 46.6%). This indicates that patients with poorly differentiated tumors with a weak lymphocytic response should be regarded as high-risk patients who need special attention, even if the tumors are small and without lymph node metastases.
Perineural infiltration was a significant prognostic marker for the whole cohort and for patients with high-stage disease, but not for low-stage disease. One could assume that nerve bundles are more abundant in deeper parts of the oral mucosa, and tumors probably need to invade deeper than in T1 and T2 tumors for this to be a relevant marker. This illustrates the importance of evaluating prognostic markers in homogenous groups of tumors and controlling for known risk factors.
We found that alternative grading (fewer options) of histopathological variables only altered their prognostic value only to a minor extent. A previous study comparing inter- and intra-rater agreement showed significantly better agreement when using an alternative grading with fewer options, compared to the more elaborate original grading [17]. This supports the use of variables with fewer options as they improve the reproducibility of the scoring without reducing the prognostic power of the variables. A simplification of scoring models has been introduced for many cancers. The reproducibility for uterine endometrial endometrioid carcinoma was found to be higher with a binary tumor grading system [27]. In the latest WHO-classification of tumors in the GI tract, the adenocarcinomas are stratified into a two-tiered grading system, low-grade and high-grade, where grading is based on the least differentiated component [28].
The present study is retrospective, and this approach gives a larger risk of variation in how clinical variables are reported in the electronic health records compared with prospective studies. When subgrouping, some groups became small, which increases the risk of underpowered statistical analyses and thereby underestimating the prognostic power of some variables. Our cohort included some tumors from which we had only biopsy samples for histopathological evaluation, which makes evaluation less certain. Therefore, we performed separate statistical analyses excluding the grading of biopsies (supplementary tables), but this did not alter the results significantly.
Conclusion
Our study on a large, homogenous tumor cohort of OTSCC shows that a histo-score combining tumor differentiation and lymphocytic infiltration identified a subgroup among the low-stage disease patients that had lower DSS than the average patients with high-stage disease. This subgroup should be given special consideration in treatment planning. Our results also illustrate that many variables can be scored with fewer options than previously suggested to increase their reproducibility, and still maintain their prognostic value.
Electronic supplementary material
(SAV 12 kb)
(DOCX 23 kb)
(DOCX 19 kb)
(DOCX 17 kb)
Acknowledgments
We thank Peter Jebsen for valuable input in the discussion of the evaluation of variables. The clinicians Olav Jetlund, Gunnhild Karevold, Ása Karlsdóttir, and Ellen Jaatun have contributed to the discussion and collection of clinical data. We thank Helse Nord (Nonprofit organization) for financial support.
Authors’ contributions
Inger-Heidi Bjerkli has been essential for building the database, conducting analyses, and writing the paper. Elin Hadler-Olsen and Lars Uhlin-Hansen have designed the research study and contributed in scoring histopathological variables, critically evaluating results, and writing the paper. Elisabeth Sivy Nginamau, Helene Laurvik, Tine M. Søland, and Daniela Elena Costea have performed scoring of histopathological variables and contributed to the scientific content of the paper. Sonja E. Steigen has designed the research study and contributed in scoring histopathological variables, conducting analyses, critically evaluating results, and writing the paper. All authors have read and commented on the final draft of the paper.
Funding Information
Open Access funding provided by UiT The Arctic University of Norway.
Data availability
Statistics file with generated data is provided.
Compliance with ethical standards
Conflict of interest
Elisabeth Sivy Nginamau has declared that she has been a consultant for MSD Norway.
Ethics approval
The study was approved by the Northern Norwegian Regional Committee for Medical Research Ethics (Protocol numbers REK Nord; 2013/1786 and 2015/1381).
Code availability
Not applicable
Consent to participate/publication
Not applicablle
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Weatherspoon DJ, Chattopadhyay A, Boroumand S, Garcia I. Oral cavity and oropharyngeal cancer incidence trends and disparities in the United States: 2000-2010. Cancer Epidemiol. 2015;39(4):497–504. doi: 10.1016/j.canep.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rusthoven K, Ballonoff A, Raben D, Chen C. Poor prognosis in patients with stage I and II oral tongue squamous cell carcinoma. Cancer. 2008;112(2):345–351. doi: 10.1002/cncr.23183. [DOI] [PubMed] [Google Scholar]
- 3.Bryne M, Boysen M, Alfsen CG, Abeler VM, Sudbo J, Nesland JM, et al. The invasive front of carcinomas. The most important area for tumour prognosis? Anticancer Res. 1998;18(6B):4757–4764. [PubMed] [Google Scholar]
- 4.Brandwein-Gensler M, Teixeira MS, Lewis CM, Lee B, Rolnitzky L, Hille JJ, Genden E, Urken ML, Wang BY. Oral squamous cell carcinoma: histologic risk assessment, but not margin status, is strongly predictive of local disease-free and overall survival. Am J Surg Pathol. 2005;29(2):167–178. doi: 10.1097/01.pas.0000149687.90710.21. [DOI] [PubMed] [Google Scholar]
- 5.Brandwein-Gensler M, Smith RV, Wang B, Penner C, Theilken A, Broughel D, Schiff B, Owen RP, Smith J, Sarta C, Hebert T, Nason R, Ramer M, DeLacure M, Hirsch D, Myssiorek D, Heller K, Prystowsky M, Schlecht NF, Negassa A. Validation of the histologic risk model in a new cohort of patients with head and neck squamous cell carcinoma. Am J Surg Pathol. 2010;34(5):676–688. doi: 10.1097/PAS.0b013e3181d95c37. [DOI] [PubMed] [Google Scholar]
- 6.The TNM (2016) In: Brierley JD, Gospodarowicz MK, Wittekind C (eds) Classification of malignant tumours 8th edition. Wiley
- 7.Listl S, Jansen L, Stenzinger A, Freier K, Emrich K, Holleczek B, et al. Survival of patients with oral cavity cancer in Germany. PLoS One. 2013;8(1):e53415. doi: 10.1371/journal.pone.0053415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bjerkli IH, Jetlund O, Karevold G, Karlsdottir A, Jaatun E, Uhlin-Hansen L, et al. Characteristics and prognosis of primary treatment-naive oral cavity squamous cell carcinoma in Norway, a descriptive retrospective study. PLoS One. 2020;15(1):e0227738. doi: 10.1371/journal.pone.0227738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manjula M, Angadi PV, Priya NK, Hallikerimath S, Kale AD. Assessment of morphological parameters associated with neural invasion in oral squamous cell carcinoma. J Oral Maxillofac Pathol. 2019;23(1):157. doi: 10.4103/jomfp.JOMFP_178_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang WC, Chang CF, Li YH, Yang CY, Su RY, Lin CK, Chen YW. A histopathological evaluation and potential prognostic implications of oral squamous cell carcinoma with adverse features. Oral Oncol. 2019;95:65–73. doi: 10.1016/j.oraloncology.2019.06.012. [DOI] [PubMed] [Google Scholar]
- 11.Jerjes W, Upile T, Petrie A, Riskalla A, Hamdoon Z, Vourvachis M, et al. Clinicopathological parameters, recurrence, locoregional and distant metastasis in 115 T1-T2 oral squamous cell carcinoma patients. Head Neck Oncol. 2010;2:9. doi: 10.1186/1758-3284-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, Bai S, Carroll W, Dayan D, Dort JC, Heller K, et al. Validation of the risk model: high-risk classification and tumor pattern of invasion predict outcome for patients with low-stage oral cavity squamous cell carcinoma. Head Neck Pathol. 2013;7(3):211–223. doi: 10.1007/s12105-012-0412-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimizu S, Miyazaki A, Sonoda T, Koike K, Ogi K, Kobayashi JI, et al. Tumor budding is an independent prognostic marker in early stage oral squamous cell carcinoma: With special reference to the mode of invasion and worst pattern of invasion. PLoS One. 2018;13(4):e0195451. doi: 10.1371/journal.pone.0195451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palazon-Bru A, Mares-Garcia E, Lopez-Bru D, Mares-Arambul E, Gil-Guillen VF, Carbonell-Torregrosa MA. A systematic review of predictive models for recurrence and mortality in patients with tongue cancer. Eur J Cancer Care (Engl) 2019;28(6):e13157. doi: 10.1111/ecc.13157. [DOI] [PubMed] [Google Scholar]
- 15.Kujan O, Khattab A, Oliver RJ, Roberts SA, Thakker N, Sloan P. Why oral histopathology suffers inter-observer variability on grading oral epithelial dysplasia: an attempt to understand the sources of variation. Oral Oncol. 2007;43(3):224–231. doi: 10.1016/j.oraloncology.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 16.Gavrielides MA, Gallas BD, Lenz P, Badano A, Hewitt SM. Observer variability in the interpretation of HER2/neu immunohistochemical expression with unaided and computer-aided digital microscopy. Arch Pathol Lab Med. 2011;135(2):233–242. doi: 10.1043/1543-2165-135.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steigen SE, Soland TM, Nginamau ES, Laurvik H, Costea DE, Johannessen AC et al (2020) Grading of oral squamous cell carcinomas - intra and interrater agreeability: simpler is better? J Oral Pathol Med. 10.1111/jop.12990 [DOI] [PubMed]
- 18.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 19.Rodrigues PC, Miguel MC, Bagordakis E, Fonseca FP, de Aquino SN, Santos-Silva AR, et al. Clinicopathological prognostic factors of oral tongue squamous cell carcinoma: a retrospective study of 202 cases. Int J Oral Maxillofac Surg. 2014;43(7):795–801. doi: 10.1016/j.ijom.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 20.Sinha N, Rigby MH, McNeil ML, Taylor SM, Trites JR, Hart RD, et al. The histologic risk model is a useful and inexpensive tool to assess risk of recurrence and death in stage I or II squamous cell carcinoma of tongue and floor of mouth. Mod Pathol. 2018;31(5):772–779. doi: 10.1038/modpathol.2017.183. [DOI] [PubMed] [Google Scholar]
- 21.WHO . In: Classification of Head and Neck Tumours. 4. El-Naggar AK, JKC C, Grandis JR, Takata T, Slootweg PJ, editors. Lyon: International Agency for Research on Cancer (IARC); 2017. [Google Scholar]
- 22.Sim YC, Hwang JH, Ahn KM. Overall and disease-specific survival outcomes following primary surgery for oral squamous cell carcinoma: analysis of consecutive 67 patients. J Korean Assoc Oral Maxillofac Surg. 2019;45(2):83–90. doi: 10.5125/jkaoms.2019.45.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Speight PM, Abram TJ, Floriano PN, James R, Vick J, Thornhill MH, et al. Interobserver agreement in dysplasia grading: toward an enhanced gold standard for clinical pathology trials. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120(4):474–82 e2. doi: 10.1016/j.oooo.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mihm MC, Jr, Clemente CG, Cascinelli N. Tumor infiltrating lymphocytes in lymph node melanoma metastases: a histopathologic prognostic indicator and an expression of local immune response. Lab Invest. 1996;74(1):43–47. [PubMed] [Google Scholar]
- 25.Marrogi AJ, Munshi A, Merogi AJ, Ohadike Y, El-Habashi A, Marrogi OL, et al. Study of tumor infiltrating lymphocytes and transforming growth factor-beta as prognostic factors in breast carcinoma. Int J Cancer. 1997;74(5):492–501. doi: 10.1002/(sici)1097-0215(19971021)74:5<492::aid-ijc3>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 26.Wirsing AM, Rikardsen OG, Steigen SE, Uhlin-Hansen L, Hadler-Olsen E. Characterisation and prognostic value of tertiary lymphoid structures in oral squamous cell carcinoma. BMC Clin Pathol. 2014;14:38. doi: 10.1186/1472-6890-14-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sagae S, Saito T, Satoh M, Ikeda T, Kimura S, Mori M, Sato N, Kudo R. The reproducibility of a binary tumor grading system for uterine endometrial endometrioid carcinoma, compared with FIGO system and nuclear grading. Oncology. 2004;67(5–6):344–350. doi: 10.1159/000082917. [DOI] [PubMed] [Google Scholar]
- 28.Lokuhetty D, White V, Watanabe R, Cree I. Digestive system tumours. Lyon: International Agency for Reasearch on Cancer (IARC); 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(SAV 12 kb)
(DOCX 23 kb)
(DOCX 19 kb)
(DOCX 17 kb)
Data Availability Statement
Statistics file with generated data is provided.