Abstract
Breast cancer is one of the most common cancers in women and is associated with a high mortality rate. The majority of deaths resulting from breast cancer are attributable to metastatic growth; in addition, chemoresistance is a major concern in the treatment of patients with breast cancer. However, limited drugs are available for the treatment of metastatic breast cancer. In this study, the chemoadjuvant effects of a methanolic extract from the leaves of Pseudolysimachion rotundum var. subintegrum (NC13) and an active component isolated from the plant, verminoside (Vms), were evaluated. Furthermore, their potent anti-metastatic activities were validated in vitro and in vivo in animal models. The anti-metastatic and chemosensitizing activities of NC13 and Vms on cisplatin treatment were found to be partly mediated by suppression of the epithelial–mesenchymal transition of cancer cells. Collectively, our results implied that NC13 and its bioactive component Vms could be developed as effective chemoadjuvants in combination with conventional therapeutics.
Subject terms: Cancer, Molecular medicine
Introduction
Globally, breast cancer is one of the most common cancers and a leading cause of cancer deaths, especially in westernized countries1. In 2018, approximately 2.1 million women were diagnosed with breast cancer worldwide, accounting for 25% of cases of cancer in women2. Despite the current improvement in diagnosis and therapy, breast cancer remains a major global health burden.
There are a number of chemotherapeutic drugs approved for breast cancer, including cisplatin, docetaxel, doxorubicin, gemcitabine, paclitaxel, and 5-fluorouracil3. Various factors affect the efficacy of chemotherapy, including the delivery and penetration of drugs, target cell selectivity, and drug resistance of the tumors4. Cisplatin is a platinum-based drug used to treat breast cancer and a wide range of other cancers, including lung, ovarian, brain, and bladder cancers. It is a potent DNA chelator, forming DNA-platinum adducts that subsequently induce apoptosis in cancer cells. Although the initial response to cisplatin is outstanding, resistance to the drug eventually occurs in most patients. When drug resistance occurs during therapeutic treatment, although drug-sensitive cancer cells are initially eliminated, as selected subpopulations become no longer responsive to prolonged treatment, the cancer may reoccur5. Several researchers have attempted to overcome cisplatin drug resistance through combination therapies6. For example, synergistic effects have been demonstrated with cisplatin combination therapy with other drugs, such as paclitaxel, 5-fluorouracil, doxorubicin, and gemcitabine7,8. Besides chemoresistance, drug toxicity is a major problem in current cancer therapy. Most patients with cancer who are administered chemotherapeutic agents experience harmful side effects9. For example, adverse effects due to cisplatin treatment include neurotoxicity, nephrotoxicity, and cardiotoxicity10. These side effects may make it difficult for patients to continue chemotherapy and can compromise their quality of life11. Therefore, the development of new adjuvants used for combination therapy is crucial to improve the efficacy and safety of conventional chemotherapy.
Over the past few decades, natural compounds have been shown to exert chemotherapeutic and chemopreventive effects in several cancers; some of these natural compound-derived chemotherapeutic drugs have been approved for treatment, such as paclitaxel (Taxol), first extracted from the bark of the western yew tree (Taxus brevifolia)12, and homoharringtonine, originally extracted from Cephalotaxus harringtonia13. Furthermore, a number of natural compound-based chemotherapeutic drugs are currently in clinical trials; examples include plinabulin, plitidepsin (phase III clinical trials), AGS-16C3F, polatuzumab vedotin, and PM184 (phase II clinical trials)14. In addition, some natural compounds are considered to be potential candidate adjuvants for combination chemotherapy. For example, the combination of cisplatin with wogonin or triptolide was shown to have synergetic effects in treating several cancers15. Verminoside (Vms) is isolated from a methanolic extract of the leaves of Pseudolysimachion rotundum var. subintegrum (NC13), which is a known Asian traditional medicine used for the treatment of inflammatory diseases such as bronchitis and asthma16. Recent studies have shown that NC13 exerts anti-inflammatory effects in various diseases, especially in chronic obstructive pulmonary disease17. In this study, we evaluated the efficacy of NC13 and Vms as chemoadjuvants for cisplatin and further validated the anti-cancer effects of these combination therapeutics in vitro and in vivo in mouse models of breast cancer.
Methods
Plant material and isolation of bioactive components
The plant samples were obtained from the Korea Research Institute of Bioscience and Biotechnology (KRIBB). The voucher specimen (KRIB 0020697) was deposited in the KRIBB, Cheong-ju si, Chungcheongbuk-do, Korea. The isolation of Vms from NC13 was described previously18. In summary, the dried stems and leaves of NC13 (2.0 kg) were extracted three times with methanol (MeOH) at room temperature to obtain 198.7 g of solid extract. The MeOH extract (1.0 g) was subjected to preparative reverse phase chromatography (GRACE C18, 10 µm, Grace Davison Discovery Sciences, Hesperia, CA, USA) and was eluted isocratically using 25% MeOH in a H2O solution. The fractions (Frs. 1–6) were collected and concentrated in a rotary evaporator under reduced pressure. The Fr. 4 was chromatographed using a middle performance liquid chromatography column with RPC-18 (Zeoprep C18, 10 μm, 20 × 250 mm, Zeochem, Louisville, USA) and eluted using a gradient mixture of CH3OH-H2O (20% → 100%) to yield five subfractions (F4a–4e). The fraction F4b was separated by semipreparative high-performance liquid chromatography (HPLC) (Atlantis T3, 5 μm, 19 × 250 mm, Waters, Milford, USA, 18% CH3CN in H2O) to afford isovanillyl catalpol (NC106, 10.7 mg). The F4c was separated by semipreparative HPLC (Synergy Polar-RP 4 μm, 21.2 × 250 mm, Phenomenex, Torrance, CA, USA, 22% CH3CN in H2O) to afford Vms (23.5 mg) and picroside II (NC105, 12.4 mg). The F4d was separated by Sephadex LH-20 (Pharmacia Biotech AB, Uppsala, Sweden, 90% CH3OH in H2O) to afford 6-O-veratroyl catalpol (NC107, 6.1 mg).
Materials
Cisplatin (P4394) was purchased from Sigma (USA). BCA protein assay kit (23225) was purchased from Thermo-Scientific (USA). Nitrocellulose membranes (1060003) were purchased from GE (USA). E-cadherin (#3195), Snail (#3879), Slug (#9585), Vimentin (#5741), p-ERK (#9106), ERK (#4695), and pan-keratin (#4545) antibodies were purchased from Cell signaling technology (USA). β-Actin (Sc-47778) antibody was purchased from Santa Cruz (USA). Ki-67 antibody (ab15580) was purchased from Abcam (USA). IR800 dye-conjugated rabbit (#926-32213) and mouse (#926-32212) secondary antibodies were purchased from Li-COR Biosciences (USA). Secondary antibody labeled with Cy3 (#711-165-152) was purchased from Jackson ImmunoResearch (USA) and Biotin-rabbit IgG (656140) from Invitrogen, and streptavidin-HRP (P0397) from DAKO (USA).
Cell culture
MCF7 and MDA-MB-231 breast cancer cells were acquired from the American Type Culture Collection (ATCC, VA, USA). MCF7 and MDA-MB-231 cells were cultured in DMEM supplemented with 1% penicillin/streptomycin and 10% fetal bovine serum, all of which were obtained from Hyclone (MA, USA), and maintained in a 5% CO2 incubator at 37 °C.
Cell viability assay
Cell viability was measured by performing MTT (3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide) assays (Invitrogen, CA, USA). Cells (8 × 103 cells/well) were seeded in 96-well plate. On day after, either NC13 or Vms were treated for 44 h, and then the MTT reagent was added for another 4 h. After removing culture media, formazan was dissolved in 100 μL of DMSO, and absorbance was measured at 590 nm using a microplate reader (Infinite M1000, Tecan).
Wound healing assay
MDA-MB-231 breast cancer cells were seeded in 96-well ImageLock plates (Essen BioScience, MI, USA) and incubated at 95–100% confluency. Wound-Maker (Essen BioScience) was used to make a scratch in each well, and the cells were washed two times. After cells were treated with compounds such as cisplatin, Rosi, NC13 or Vms in serum free media, cellular migration images were taken every 2 h by using the IncuCyte Live-Cell Imaging System (Essen BioScience).
Western blot analysis
Cell lysates were prepared with NETN buffer (1% NP-40, 1 mM EDTA, 20 mM Tris–Cl, pH 7.5, 100 mM NaCl, 5 mM sodium pyrophosphate, 1 mM sodium orthovanadate, and 50 mM NaF), and protein concentration was measured using the BCA protein assay kit. Approximately 40 μg of proteins were resolved using 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to nylon membranes. After 1 h blocking in 5% skim milk, the membranes were further incubated with the primary antibody overnight at 4 °C. The membranes were then washed three times with TBST buffer and incubated in IR800 dye-conjugated secondary antibodies for another hour. Immunodetection was performed using an Odyssey CLx scanner (Li-COR Biosciences).
Immunofluorescence
MDA-MB-231 and MCF7 cells were seeded on coverslips overnight. After the indicated treatment, cells were washed with phosphate buffered saline (PBS) and fixed with 4% paraformaldehyde in PBS for 30 min. After rinsing, fixed cells were blocked with NH4Cl for 5 min and permeabilized with 0.1% Triton X-100 in PBS. Coverslip samples were incubated with the primary antibody and Vimentin overnight at 4 °C, followed by the secondary antibody labeled with Cy3 for 1 h at room temperature. DAPI was co-stained. Images were acquired with an Olympus Fv-1000 confocal microscope.
Dosage information
For in vitro experiments, we tested several doses of NC13 or Vms, and thus, 5 μg/mL and 10 μM (5.244 μg/mL) were selected as the optimal concentrations for NC13 and Vms based on CompuSyn analysis, respectively (also see Supplementary Fig. S5). The in vivo dosage of NC13 and Vms was tested at 20 mg/kg based on our previous study17 of their anti-inflammatory effects. Briefly, NC13 and picroside II, one of the catalpol derivatives isolated from NC13, displayed anti-inflammatory effects at 15 or 30 mg/kg oral administration without reported toxicity, and thus, mice were fed 20 mg/kg of either NC13 or Vms mixed with a powdered chow diet during the experimental period. The doses of NC13 and Vms are equivalent to 97.29 mg/day for 60 kg human19. As 80–160 mg of an NC13 based drug (YPL) is administered to human subjects in a clinical trial in USA, our 20 mg/kg dose is considered to be physiologically relevant.
Animal study
Animals were used in accordance with protocols approved by Institutional Animal Care and Use Committee of the Ulsan National Institute of Science and Technology (UNISTIACUC-20-07). In our previous study, we developed the MMTV-FP635 mouse model for whole-body imaging of mammary tumor progression20. In this model, the infrared-fluorescence protein (FP635) is from mammary epithelial cells, specifically expressed under the control of a MMTV promoter. By crossing with MMTV-PyMT mice (MMTV-PyMT/FP635), we can monitor tumor growth and metastasis via whole-body fluorescent scanner, longitudinally without sacrificing the mice. For PyMT/FP635 mouse model, 8-week-old female mice received intraperitoneal injection of cisplatin, dissolved in PBS and sonicated for 3 min before use. For the first week, 2.5 mg/kg of cisplatin was administered and 1.5 mg/kg of cisplatin for the rest of the weeks. Mice were fed powdered chow diet mixed with either NC13 or Vms (20 mg/kg) during the experimental period. Tumor growth was monitored every week using an in vivo fluorescence imaging system (Bruker, Germany). After 10 weeks of cisplatin administration, animals were sacrificed, and lung and tumor samples were harvested for further experiments. In a tumor allograft model with BALB/C mouse, wild-type 9-week-old male mice were fed powdered chow diets mixed with either NC13 or Vms (20 mg/kg) during the experimental period. After 1 week of NC13 or Vms pre-treatment, 0.5 × 105 of 4 T-1 cells were subcutaneously injected into the mammary fat pad. When the tumors reached approximately 100 mm3, 2.5 mg/kg of cisplatin was intraperitoneally injected twice in a week. Tumor growth was measured using digital caliper twice in a week, and after 6 weeks of cisplatin administration, animals were sacrificed, and lung and tumor samples were harvested for further experiments.
Tissue staining and immunohistochemistry
Tissue samples were excised and fixed in buffered 10% formalin for 48 h. After dehydration and paraffin embedding, each tissue section was subjected to staining with hematoxylin and eosin (H&E) or was further processed for immunohistochemistry. After antigen unmasking with a citrate-based buffer and hydrogen peroxide treatment, sections were incubated with primary antibodies against Ki-67, followed by Biotin-rabbit IgG and streptavidin-HRP. Images were acquired with an Olympus FSX100 inverted microscope.
Data and statistical analysis
All the data were analyzed by using GraphPad Prism software (Version 7, USA) and presented as mean ± standard error of the mean. Statistical significance was evaluated by one-way analysis of variance followed by post-hoc Tukey’s tests for multiple comparison. P-values less than 0.05 were considered statistically significant difference.
Results
Isolation of Vms as one of the major bioactive components of NC13
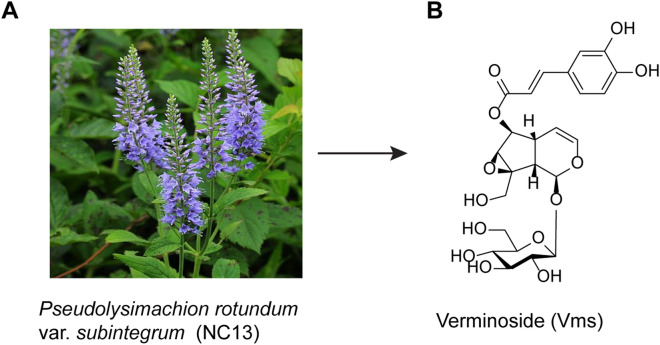
Vms is one of the major bioactive components of NC13 (voucher specimen: KRIB 0020697), and its chemical structure is displayed in Fig. 1 and Supplementary Fig. S1–4, along with a brief description of the isolation process.
Figure 1.
Chemical structure of verminoside (Vms) isolated from p. rotundum var. subintegrum (NC13). Also see Supplementary Fig. S5–4.
Combination therapy with either NC13 or Vms augments chemosensitivity to cisplatin
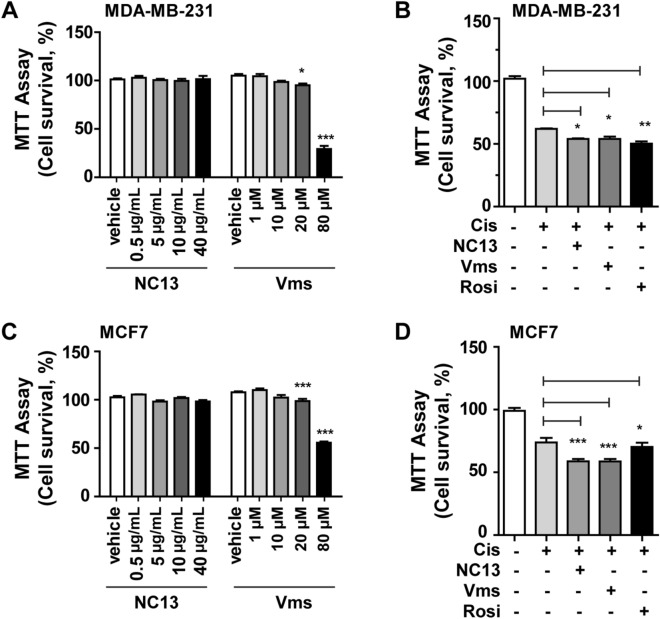
To evaluate the efficacy of NC13 and Vms as chemoadjuvants in cisplatin treatment, we first found the optimal concentrations at which there were no cellular toxicity effects. NC13 showed no cytotoxic effects on cellular proliferation below a concentration of 40 μg/mL, whereas Vms showed cytotoxic effects at a concentration of over 10 μM (= 5.244 μg/mL) (Fig. 2A,C). Therefore, we determined 5 μg/mL and 10 μM as the optimal concentrations for NC13 and Vms, respectively. The viability of breast cancer cell lines, such as MDA-MB-231 and MCF7, were determined at 48 h after NC13 or Vms treatment. Because most of the NC13 and Vms-induced synergistic effects on cisplatin monotherapy were found to be exerted at 48 h after drug treatment based on the CompuSyn analysis (Supplementary Fig. S5), we used these selected dosages and time for the in vitro experiments.
Figure 2.
Chemoadjuvant effect of NC13 or Vms on the cellular survival of MDA-MB-231 and MCF7 cells. (A,C) MDA-MB-231 and MCF7 cells were treated with increasing concentrations of either NC13 or Vms for 48 h. (B,D) The cells were treated with either NC13 (5 µg/mL) or Vms (10 µM) in the presence of cisplatin. After 2 days, the MTT assay was performed to assess cellular viability. Rosiglitazone (Rosi, 20 μM) was used as a positive control. All data are presented as mean ± SEM. Statistical significance was evaluated by one-way ANOVA, followed by post hoc tukey’s multiple comparison test. *P < 0.05, **P < 0.01.
To investigate whether either NC13 or Vms had chemoadjuvant effects in cisplatin monotherapy, MDA-MB-231 and MCF7 cells were treated with NC13 or Vms in the presence of cisplatin, and cell survival was determined using the MTT assay. Optimal dosages of cisplatin were also determined by CompuSyn analysis, considering IC50 values of cisplatin for each breast cancer cell line (Supplementary Fig. S5). In the MDA-MB-231 cells, treatment with NC13 or Vms in combination with cisplatin significantly suppressed cell survival compared with the effect of cisplatin alone (Fig. 2B). Similarly, for the MCF7 cells, the combination treatment significantly augmented the efficacy of cisplatin for the suppression of cancer cell survival (Fig. 2D). Rosiglitazone (rosi), used as a positive control drug, has synergistic effects in cisplatin combination therapy; nevertheless, it has no cytotoxicity by Rosi alone likely with NC13 or Vms21–23. Notably, the efficacy of the combination of cisplatin with either NC13 or Vms was comparable or superior to that of the treatment with rosi. These results suggested that combination treatment of cisplatin with either NC13 or Vms sensitized breast cancer cells to the anti-cancer effects of cisplatin on cancer cell survival in vitro.
NC13 or Vms attenuates the epithelial–mesenchymal transition (EMT) in breast cancer cells in vitro
It is well known that the EMT, in which cancer cells lose their epithelial properties and adopt mesenchymal characteristics, leads to reorganization of the actin cytoskeleton, decreased cell-to-cell contact, and loss of cell polarity24. As a result, invasive and metastatic activities increase in cancer cells. Beyond the cellular invasion and metastasis, multiple studies have suggested that sensitivity to chemotherapeutic drugs is closely associated with the EMT of cancer cells25. EMT contributes to chemoresistance in several ways, including EMT-related microRNA and cytokine production24, and EMT-driven cancer cell stemness is one of the most well-known mechanisms.
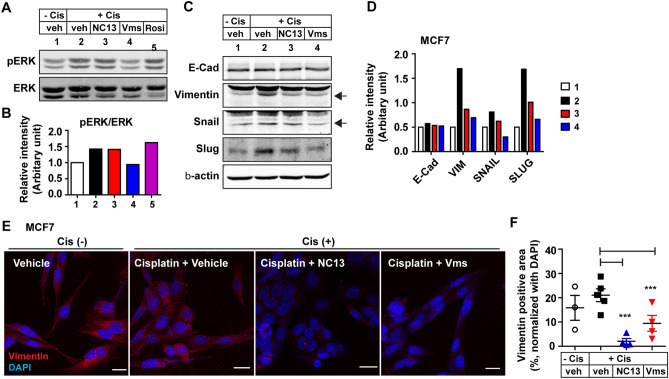
Therefore, we examined the effect of NC13 and Vms on the EMT process in breast cancer cells. As functional indices of EMT, we determined the cell migration ability of the MDA-MB-231 cells following treatment with either NC13 or Vms in the presence or absence of cisplatin. Both NC13- and Vms-treated cells displayed reduced migration ability compared with that of the control cells in the presence or absence of cisplatin, as determined by the cell migration assay in vitro (Fig. 3A,B). The extracellular signal-regulated kinase (ERK) pathways are well known to regulate various biological processes including proliferation, cell migration, and invasion in response to environmental stimuli such as chemotherapy26,27. Treatment of the MDA-MB-231 cells with cisplatin elevated the activity of the ERK signaling pathways compared to that in the vehicle group without cisplatin. However, the combination of cisplatin with NC13 and Vms showed no additional (or synergistic) effect on the cisplatin-induced ERK activation (Fig. 3C,D). This showed that NC13 or Vms has a limited effect on cisplatin-mediated ERK activation in the MDA-MB-231 cells. To further explore whether the EMT processes were altered by the adjuvant therapy, the levels of EMT markers such as vimentin, snail, slug, N-cadherin, and E-cadherin were analyzed. Overall, cisplatin treatment increased the levels of mesenchymal cell markers such as N-cadherin, vimentin, snail, and slug compared to that in control groups (no cisplatin groups) in the MDA-MB-231 cells (Fig. 3E,F). Furthermore, the levels of these mesenchymal cell markers significantly decreased by the combination of cisplatin with NC13 and Vms, comparable to the levels after treatment with Rosi in combination with cisplatin, used as a positive control (Fig. 3E,F). The level of E-cadherin, an epithelial cell marker, was not affected by the combination of cisplatin with NC13 and Vms compared with that in the control groups (no cisplatin groups) (Fig. 3E,F). These results indicated that combination of both NC13 and Vms with cisplatin may contribute to altering the levels of mesenchymal marker proteins rather than activating upstream signaling pathways such as the ERK. This was further validated with immunofluorescence staining of vimentin in the MDA-MB-231 cells, which strongly suggested that combined treatment with either NC13 or Vms significantly attenuated the cisplatin-associated increase of the EMT process (Fig. 3G,H).
Figure 3.
Chemoadjuvant effect of NC13 or Vms on the EMT in MDA-MB-231 breast cancer cells. (A) MDA-MB-231 cells were scratched, and representative images were taken after 24 h of either NC13 (5 μg/ml) or Vms (5 μg/ml, 9.53 μM) treatment in the presence or absence of cisplatin (20 μM). Rosi (10 μM) is used as a positive control. (B) The wound density was quantified in the indicated treatment. (C) Western blotting analysis was performed to determine ERK phosphorylation in indicated groups, and (D) their band intensity was quantified. (E) The protein levels of EMT markers, including E-Cadherin, N-Cadherin, Vimentin, and Slug were determined, and (F) their band intensity was quantified. (G) Immunofluorescence staining of vimentin and (H) the signal intensity was quantified for the indicated treatments (scale bar = 20 μm). All data are presented as mean ± SEM. Statistical significance was evaluated by one-way ANOVA, followed by post hoc tukey’s multiple comparison test. *P < 0.05, ***P < 0.001 vs. vehicle (− cis); #P < 0.05, ###P < 0.001 vs. vehicle (+ cis). Uncropped blots are shown in the Supplementary information.
Consistently, the ERK signaling pathways were not altered or even slightly reduced in the NC13 and Vms groups, respectively, compared to the case in the vehicle group in the presence of cisplatin (Fig. 4A,B). In addition, the protein levels of EMT markers, particularly vimentin and snail, significantly decreased by NC13 and Vms treatment in combination with cisplatin compared with those in the cisplatin control group (Fig. 4C,D). Immunostaining of vimentin in the MCF7 cells clearly indicated that the EMT was suppressed by NC13 and Vms in the cisplatin treatment group compared with that in the vehicle group (Fig. 4E,F). Collectively, these data suggested that the combination treatment of cisplatin with NC13 or Vms enhanced the suppression of the EMT phenotype in both MDA-MB-231 and MCF7 cells, partly through suppression of the EMT process.
Figure 4.
Chemoadjuvant effect of NC13 or Vms in cisplatin treatment on the suppression of the EMT in MCF7 breast cancer cells. (A) Western blotting analysis was performed to determine ERK phosphorylation and (B) the band intensity was quantified. (C) The protein levels of EMT markers, including E-Cadherin, Vimentin, Snail, and Slug were determined by western blots, and (D) the band intensity was quantified. (E) Immunofluorescence staining of vimentin and (F) the signal intensity was quantified for the indicated treatments (scale bar = 20 μm). All data are presented as mean ± SEM. Statistical significance was evaluated by one-way ANOVA, followed by post hoc tukey’s multiple comparison test. ***P < 0.001. Uncropped blots are shown in the Supplementary information.
Combination of NC13 or Vms with cisplatin attenuates metastatic growth in MMTV-PyMT mammary tumor mice in vivo
As we have isolated several major components from NC13 plant extracts including Vms, picroside II (NC105), isovanilly catalpol (NC106), and 6-O-veratroyl catalpol (NC107) (Supplementary Fig. S1–4), these components were also tested for their chemoadjuvant effects on cisplatin. MDA-MB-231 and MCF7 breast cancer cells were treated with NC13 or its major components in combination with cisplatin, and subsequently, the cancer cell survival rate was determined (Fig. 5A,B). Similar to NC13 and Vms, treatment of NC105, NC106, and NC107 in combination with cisplatin significantly suppressed cell survival compared to that of cisplatin alone (Fig. 5A,B). Therefore, we pursued analyzing the chemoadjuvant effects of NC13 and its major components such as Vms, NC105, and NC107 in in vivo settings. NC106 was not included in this setting due to its mild adjuvant effects on the in vivo pilot study. To evaluate the chemoadjuvant effect of NC13 or its major components in combination with cisplatin in vivo animal model, the mouse mammary tumor virus-polyoma middle tumor-antigen (MMTV-PyMT) mice crossed with MMTV-infrared fluorescent protein (FP635) mice were utilized, which allow the visualization of tumor growth via fluorescent images longitudinally22. In combination with NC13 or its major components including Vms, NC105, and NC107, cisplatin treatment did not result in significant differences in body weight change, an index of drug toxicity (Fig. 5C). We further confirmed that cisplatin combination treatment with NC13 nor Vms did not induce adverse cytotoxic side effects in an in vivo experimental setting as determined by hepatotoxicity such as ALT, AST activities and histological assessment of liver tissue sections (Supplementary Fig. S6). Moreover, the chemoadjuvant activity of NC13 and its major components on the primary tumor growth was limited as compared to that in cisplatin alone (Fig. 5D,E). Next, we subsequently analyzed pulmonary metastasis as determined by in vivo fluorescent imaging (Fig. 5F). In combination with NC13 or Vms, cisplatin treatment significantly reduced metastatic growth compared with cisplatin alone or in combination with either NC105 or NC107 treatment (Fig. 5F,G), suggesting that the chemoadjuvant activity of NC13 in suppression of metastatic growth is mostly mediated by Vms, rather than NC105 or NC107, in the MMTV-PyMT settings. Accordingly, H&E staining and immunohistochemical staining for ki-67 indicated that the combination treatment of cisplatin with NC13 or Vms significantly inhibited pulmonary metastasis in a MMTV-PyMT mammary tumor mouse model (Fig. 5H–J).
Figure 5.
Anti-metastatic effects of NC13 in combination with cisplatin are mostly mediated by Vms in the MMTV-PyMT breast cancer mouse model. (A,B) The breast cancer cells were treated with either NC13 or its isolated components including Vms (5 µg/mL), NC105—Picroside II (5 µg/mL), NC106—Isovanilly catalpol (5 µg/mL), and NC107—6-o-veratroyl catalpol (5 µg/mL) in the presence of cisplatin (10 μM). After 2-days, an MTT assay was performed to assess cellular viability. Rosi (10 μM) was used as a positive control. (C–J) MMTV-PyMT mice were injected with Cisplatin and fed a powdered diet mixed with either NC13 or its isolated bioactive components including Vms, NC105 and NC107 during the period of tumor progression. (C) Body weight was measured. (D) Fluorescent images of tumor burden in whole body were presented and (E) the tumor burden in each group was quantified. (F) Representative fluorescent images of lung metastatic burden are shown and (G) quantification of metastatic burden. (H) H&E staining and (I) immunohistochemistry of ki-67 were performed on lung tissues and (J) their areas were quantified (scale bar = 20 μm). All data are presented as the mean ± SEM. Statistical significance was evaluated by one-way ANOVA, followed by post hoc tukey’s multiple comparison test. *P < 0.05, **P < 0.01, ***P < 0.001.
Combination of NC13 or Vms with cisplatin attenuates metastatic growth of 4T-1 breast cancer cells in vivo
We further confirmed the chemoadjuvant activity of NC13 or Vms in the suppression of tumor metastasis by using a 4T-1 tumor allograft model. The 4T-1 mammary carcinoma cell line was originally developed by Miller and his coworkers; it is derived from the mammary tumors of BALB/C mice. As this cancer cell is transplantable to mammary glands, highly aggressive, and able to spontaneously metastasize to other organs, the 4T-1 tumor implantation model is suitable for a human breast cancer study.
Six weeks after 4T-1 breast cancer cell implantation into the mammary glands, the mice were sacrificed, and the lung tissues were harvested. The cisplatin-treated groups showed lower body weights than the vehicle group due to the drug toxicity of cisplatin, while the combination with NC13 or Vms in cisplatin did not affect additional body weight differences compared to cisplatin alone (Fig. 6A), which was consistent with MMTV-PyMT mice. Similarly, there were no significant differences in the primary tumor growth from combination treatment with NC13 or Vms in cisplatin compared with those treated with cisplatin alone (Fig. 6B). Consistent with the results from the MMTV-PyMT models, the adjuvant chemotherapy of NC13 or Vms with cisplatin significantly suppressed the metastatic growth compared with the cisplatin control group, as determined by the numbers of metastatic nodules stained with H&E (Fig. 6C,D) and pan-keratin (Fig. 6E,F). To further assess the expression of EMT markers in tumor tissues, a western blotting analysis was performed. The mesenchymal marker vimentin was downregulated in tumors from mice treated with the combination of cisplatin with NC13 or Vms compared with mice treated with cisplatin, whereas there was no significant change in E-cadherin expression (Fig. 6G–I). Collectively, these results strongly suggested that the combination of NC13 or Vms with cisplatin inhibited the EMT in tumor cells, particularly through the downregulation of vimentin resulting in suppression of pulmonary metastasis in vivo.
Figure 6.
Chemoadjuvant effect of NC13 or Vms in cisplatin treatment on the 4T-1 breast cancer cells allograft model. (A) Body weight of the BALB/C mice administered cisplatin with either NC13 or Vms was measured compared to the vehicle group (− cisplatin). (B) Tumor volumes were measured in the indicated groups. (C) Representative H&E staining of the lung images were shown, and (D) the number of lung metastatic nodules were quantified. Arrowheads indicate tumor metastatic nodules. (E) Immunohistochemistry of pan-keratin were performed on lung tissues and (F) their area was quantified. Scale bar = 50 μm. (G) Protein levels of vimentin and E-cadherin in tumor tissue were determined by western blotting, and (H–I) quantification of band intensity from the indicated mice groups. Protein samples for the Cis + Vms group were blotted in a separated gel and analyzed with the same exposure done for the other blots including Cis + vehicle and Cis + NC13 groups. All data are presented as mean ± SEM. Statistical significance was evaluated by one-way ANOVA, followed by post hoc tukey’s multiple comparison test. *P < 0.05, **P < 0.01, ***P < 0.001. Uncropped blots are shown in the Supplementary information.
Discussion
Treatment with chemotherapeutic agents often fails in causing successful cancer remission when used in monotherapy. Hence, it is widely accepted to combine two or more chemotherapeutic drugs to achieve better outcomes, referred to as multidrug treatment. During the past few decades, studies have focused on the molecular and pharmacological factors that affect the efficacy of drug combinations, such as cellular pathways, drug interactions, optimal administration ratios, side effects, and consequently have given us a better understanding of multidrug combinations28. This progress leads us to develop combination therapeutic regimens that dramatically improve outcomes for cancer patients. For example, nowadays, a combination of cisplatin with docetaxel or gemcitabine is a first-line chemotherapeutic regimen for metastatic breast cancer patients29,30. Chemo-adjuvant therapy, on the other hand, is a combinatorial approach which employs the different forms of treatment such as chemotherapy with radiation therapy, instead of multiple anti-cancer drugs31,32. According to the National Cancer Institute (NCI) guideline, adjuvant therapy is defined as an additional cancer treatment given after the primary treatment to lower the risk that the cancer will recur. Recent advances in adjuvant therapies include combining newer agents with conventional chemotherapy. Among the various kinds of regimens, natural compounds are reported to have positive adjunctive effects in combination with chemo- or radiotherapy33. This natural compound based chemoadjuvant therapy could augment cytotoxicity to cancer cells and have additional effects on the immune response to tumors or the tumor microenvironment34.
In this study, we aimed to develop the natural compounds used for chemoadjuvants in conventional chemotherapy. We presented the first evidence of the therapeutic potential of natural products NC13 and its major component, Vms as chemoadjuvants in cisplatin monotherapy, and validated their anti-metastatic activity in vitro and in vivo animal models. The screening criteria of these compounds were based on their cytotoxic effects on breast cancer cells; thus, they have no cytotoxic effects by themselves, but they convey a synergistic (or additive) cytotoxic effect on cisplatin monotherapy. Based on this screening criteria, NC13 was firstly identified and subsequently Vms was further isolated as the major components derived from it. In the in vitro settings, NC13 and Vms exerted synergistic effects on cisplatin-induced suppression of cell growth and cell migration. Interestingly, these chemoadjuvants combined with cisplatin showed only a mild effect on primary tumor growth but they significantly suppressed metastatic growth in tumor-bearing mice including MMTV-PyMT and 4T-1 allograft models in vivo. We confirmed the potent anti-metastatic effects of NC13 combination in cisplatin with an additional in vivo model, in which 4T-1 cancer cells were intravenously injected into BALB/C mice and the pulmonary metastatic growth was assessed; thus the ability for metastatic growth of circulating cancer cells would be assessed (Supplementary Fig. S7). In this model, NC13 showed no effects per se on the metastatic growth of cancer cells likely vehicle; but NC13 in combination with cisplatin significantly suppressed pulmonary metastasis compared to cisplatin monotherapy (Supplementary Fig. S7B,C). These phenomena were also true for other chemoadjuvants such as Rosi and metformin, which have no cytotoxic effects on cancer cells, but they confer synergistic effects on conventional chemotherapy23,35. Molecular mechanisms underlying the Rosi-induced synergistic effects on cancer cell death are still largely elusive. Among the various major components isolated from NC13 including picroside II, isovanilly catalpol, 6-O-veratroyl catalpol, and Vms, the most significant anti-metastatic effect of cisplatin treatment in vivo was found in combination with Vms, revealing that Vms could mostly account for the NC13-mediated chemoadjuvant effects in cisplatin.
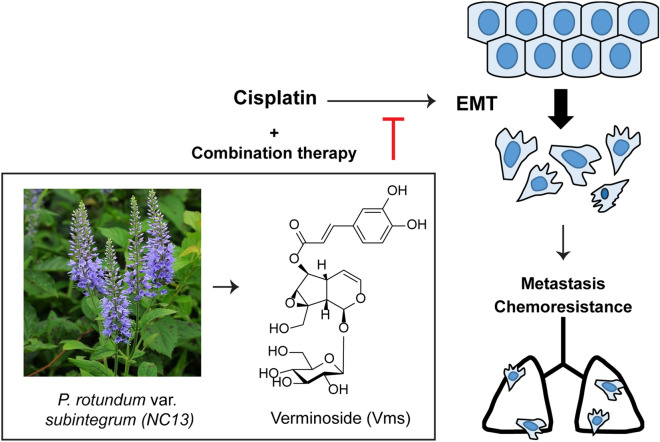
As tumor metastasis accounts for over 90% of mortalities in solid cancers, development of targeted therapeutic agents against cancer invasion or metastasis is important for drug discovery36. Several studies suggested the potential of anti-metastatic agents in the breast cancer preclinical models37–39, moreover, sacituzumab and govitecan-hziy for the patients of metastatic breast cancer are in phase III clinical trials40. Here, we suggested that Vms, isolated from NC13, gives a synergistic effect on cisplatin-based chemotherapy, particularly at the level of metastatic growth, and this is partly through the suppression of cisplatin-induced EMT processes (Fig. 7). Various applications of combination therapy have been developed to overcome the failure of conventional chemotherapy, and our study strongly suggested the efficacy of natural product NC13 and its isolated bioactive component, Vms as resources to be developed as chemoadjuvants and potentially as anti-metastatic agents in conventional chemotherapy; particularly, for metastatic breast cancer patients.
Figure 7.
Schematic view of chemoadjuvant effect of both NC13 and Vms in cisplatin treatment in metastatic breast cancer. Verminoside (Vms) isolated from Pseudolysimachion rotundum var. subintegrum (NC13) sensitizes cisplatin effects in breast cancer. Combined administration of cisplatin with either Vms or NC13 sensitizes cisplatin treatment, and further suppresses cancer cell migration through down-regulation of the EMT process leading to significant inhibition of pulmonary metastasis and chemoresistance.
Supplementary information
Acknowledgements
We thank UNIST-Olympus Bio-imaging Center and UNIST-Biological Sciences Department core facility for technical assistance, and UNIST Central Research Facilities for help with in vivo animal studies. This work was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI, HI14C1277), the Bio-Synergy Research Project of the Ministry of Science, ICT and Future Planning (NRF-2017M3A9C4065956), and the Basic Science Research Program (NRF-2018R1A2B6003878) through the National Research Foundation to J.P. This work was also supported by the Ministry of Education, Science and Technology (NRF-2018R1A5A1024340) through NRF, and by the KRIBB Research Initiative Program funded by the Ministry of Science and ICT (MSIT) of Republic of Korea.
Author contributions
C.L. conceived the project, and performed experiments, analyzed data, and wrote the manuscript. H.W.R performed natural product extractions, analyzed data, and wrote the manuscript. S.K. and M.K. performed experiments and analyzed data. S.O. and K.A. provided critical comments and analyzed data. J.P. designed and performed experiments, analyzed data, wrote the manuscript, and all authors approved the manuscript. J.P. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Changhu Lee and Hyung Won Ryu.
Supplementary information
is available for this paper at 10.1038/s41598-020-77401-7.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J. Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Hassan M, Ansari J, Spooner D, Hussain S. Chemotherapy for breast cancer. Oncol. Rep. 2010;24:1121–1131. doi: 10.3892/or_00000963. [DOI] [PubMed] [Google Scholar]
- 4.Kerbel RS. A cancer therapy resistant to resistance. Nature. 1997;390:335. doi: 10.1038/36978. [DOI] [PubMed] [Google Scholar]
- 5.Wang X, Zhang H, Chen X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019;2:141–160. doi: 10.20517/cdr.2019.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dasari S, Tchounwou PB. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014;740:364–378. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dimery IW, Legha SS, Shirinian M, Hong WK. Fluorouracil, doxorubicin, cyclophosphamide, and cisplatin combination chemotherapy in advanced or recurrent salivary gland carcinoma. J. Clin. Oncol. 1990;8:1056–1062. doi: 10.1200/JCO.1990.8.6.1056. [DOI] [PubMed] [Google Scholar]
- 8.Valle J, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 9.Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: An evolving paradigm. Nat. Rev. Cancer. 2013;13:714–726. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 10.De Jongh F, et al. Weekly high-dose cisplatin is a feasible treatment option: Analysis on prognostic factors for toxicity in 400 patients. Br. J. Cancer. 2003;88:1199–1206. doi: 10.1038/sj.bjc.6600884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohnishi S, Takeda H. Herbal medicines for the treatment of cancer chemotherapy-induced side effects. Front. Pharmacol. 2015;6:14. doi: 10.3389/fphar.2015.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cragg GM, Newman DJ, Yang SS. Natural product extracts of plant and marine origin having antileukemia potential. The NCI experience. J. Nat. Prod. 2006;69:488–498. doi: 10.1021/np0581216. [DOI] [PubMed] [Google Scholar]
- 13.Itokawa, H., Wang, X. & Lee, K.-H. Anticancer agents from natural products. In Homoharringtonine and Related Compounds (ed. Cragg, G. M.). 65–94 (CRC Press, New York, 2005).
- 14.Dyshlovoy SA, Honecker F. Marine compounds and cancer: 2017 Updates. Mar. Drugs. 2018;16:41. doi: 10.3390/md16020041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li C-J, et al. Synergistic anticancer activity of triptolide combined with cisplatin enhances apoptosis in gastric cancer in vitro and in vivo. Cancer Lett. 2012;319:203–213. doi: 10.1016/j.canlet.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Ryu HW, et al. 3-Methoxy-catalposide inhibits inflammatory effects in lipopolysaccharide-stimulated RAW264. 7 macrophages. Cytokine. 2017;91:57–64. doi: 10.1016/j.cyto.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Choi J, et al. Picroside II attenuates airway inflammation by downregulating the transcription factor GATA3 and Th2-related cytokines in a mouse model of HDM-induced allergic asthma. PLoS ONE. 2016;11:e0167098. doi: 10.1371/journal.pone.0167098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song HH, et al. Piscroside C, a novel iridoid glycoside isolated from Pseudolysimachion rotundum var. subinegrum suppresses airway inflammation induced by cigarette smoke. J. Ethnopharmacol. 2015;170:20–27. doi: 10.1016/j.jep.2015.04.043. [DOI] [PubMed] [Google Scholar]
- 19.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 20.Park J, Scherer PE. Adipocyte-derived endotrophin promotes malignant tumor progression. J. Clin. Investig. 2012;122:4243–4256. doi: 10.1172/JCI63930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blanquicett C, Roman J, Hart CM. Thiazolidinediones as anti-cancer agents. Cancer Ther. 2008;6:25. [PMC free article] [PubMed] [Google Scholar]
- 22.Park J, Morley TS, Scherer PE. Inhibition of endotrophin, a cleavage product of collagen VI, confers cisplatin sensitivity to tumours. EMBO Mol. Med. 2013;5:935–948. doi: 10.1002/emmm.201202006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Girnun GD, et al. Synergy between PPARγ ligands and platinum-based drugs in cancer. Cancer Cell. 2007;11:395–406. doi: 10.1016/j.ccr.2007.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang J, Li H, Ren G. Epithelial–mesenchymal transition and drug resistance in breast cancer. Int. J. Oncol. 2015;47:840–848. doi: 10.3892/ijo.2015.3084. [DOI] [PubMed] [Google Scholar]
- 25.Baribeau S, Chaudhry P, Parent S, Asselin É. Resveratrol inhibits cisplatin-induced epithelial-to-mesenchymal transition in ovarian cancer cell lines. PLoS ONE. 2014;9:e86987. doi: 10.1371/journal.pone.0086987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salaroglio IC, Mungo E, Gazzano E, Kopecka J, Riganti C. ERK is a pivotal player of chemo-immune-resistance in cancer. Int. J. Mol. Sci. 2019;20:2505. doi: 10.3390/ijms20102505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buonato JM, Lazzara MJ. ERK1/2 blockade prevents epithelial–mesenchymal transition in lung cancer cells and promotes their sensitivity to EGFR inhibition. Can. Res. 2014;74:309–319. doi: 10.1158/0008-5472.CAN-12-4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mayer LD, Janoff AS. Optimizing combination chemotherapy by controlling drug ratios. Mol. Interv. 2007;7:216. doi: 10.1124/mi.7.4.8. [DOI] [PubMed] [Google Scholar]
- 29.Vassilomanolakis M, et al. First-line chemotherapy with docetaxel and cisplatin in metastatic breast cancer. Breast. 2005;14:136–141. doi: 10.1016/j.breast.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 30.Zhang J, et al. Cisplatin and gemcitabine as the first line therapy in metastatic triple negative breast cancer. Int. J. Cancer. 2015;136:204–211. doi: 10.1002/ijc.28966. [DOI] [PubMed] [Google Scholar]
- 31.Song W, et al. Polypeptide-based combination of paclitaxel and cisplatin for enhanced chemotherapy efficacy and reduced side-effects. Acta Biomater. 2014;10:1392–1402. doi: 10.1016/j.actbio.2013.11.026. [DOI] [PubMed] [Google Scholar]
- 32.Chew HK. Adjuvant therapy for breast cancer: Who should get what? West. J. Med. 2001;174:284. doi: 10.1136/ewjm.174.4.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qi F, et al. Chinese herbal medicines as adjuvant treatment during chemoor radio-therapy for cancer. Biosci. Trends. 2010;4(6):297–307. [PubMed] [Google Scholar]
- 34.Aung TN, Qu Z, Kortschak RD, Adelson DL. Understanding the effectiveness of natural compound mixtures in cancer through their molecular mode of action. Int. J. Mol. Sci. 2017;18:656. doi: 10.3390/ijms18030656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peng M, et al. Combination of metformin with chemotherapeutic drugs via different molecular mechanisms. Cancer Treat. Rev. 2017;54:24–33. doi: 10.1016/j.ctrv.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 36.Gandalovičová A, et al. Migrastatics—anti-metastatic and anti-invasion drugs: Promises and challenges. Trends Cancer. 2017;3:391–406. doi: 10.1016/j.trecan.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang T, et al. Cucurbitacin E inhibits breast tumor metastasis by suppressing cell migration and invasion. Breast Cancer Res. Treat. 2012;135:445–458. doi: 10.1007/s10549-012-2175-5. [DOI] [PubMed] [Google Scholar]
- 38.Zhao W, et al. Candidate antimetastasis drugs suppress the metastatic capacity of breast cancer cells by reducing membrane fluidity. Can. Res. 2016;76:2037–2049. doi: 10.1158/0008-5472.CAN-15-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thaiparambil JT, et al. Withaferin A inhibits breast cancer invasion and metastasis at sub-cytotoxic doses by inducing vimentin disassembly and serine 56 phosphorylation. Int. J. Cancer. 2011;129:2744–2755. doi: 10.1002/ijc.25938. [DOI] [PubMed] [Google Scholar]
- 40.Bardia A, et al. Sacituzumab govitecan-hziy in refractory metastatic triple-negative breast cancer. N. Engl. J. Med. 2019;380:741–751. doi: 10.1056/NEJMoa1814213. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.