Abstract
Background
With recent transformations in medical education, the integration of technology to improve medical students’ abilities has become feasible. Artificial intelligence (AI) has impacted several aspects of healthcare. However, few studies have focused on medical education. We performed an AI-assisted education study and confirmed that AI can accelerate trainees’ medical image learning.
Materials
We developed an AI-based medical image learning system to highlight hip fracture on a plain pelvic film. Thirty medical students were divided into a conventional (CL) group and an AI-assisted learning (AIL) group. In the CL group, the participants received a prelearning test and a postlearning test. In the AIL group, the participants received another test with AI-assisted education before the postlearning test. Then, we analyzed changes in diagnostic accuracy.
Results
The prelearning performance was comparable in both groups. In the CL group, postlearning accuracy (78.66 ± 14.53) was higher than prelearning accuracy (75.86 ± 11.36) with no significant difference (p = .264). The AIL group showed remarkable improvement. The WithAI score (88.87 ± 5.51) was significantly higher than the prelearning score (75.73 ± 10.58, p < 0.01). Moreover, the postlearning score (84.93 ± 14.53) was better than the prelearning score (p < 0.01). The increase in accuracy was significantly higher in the AIL group than in the CL group.
Conclusion
The study demonstrated the viability of AI for augmenting medical education. Integrating AI into medical education requires dynamic collaboration from research, clinical, and educational perspectives.
Keywords: Medical image education, Artificial intelligence, Deep learning, Fracture, Personalized education
Key points
A heatmap-producing radiography reading system can be utilized in medical education.
Artificial intelligence (AI) can offer low-level supervision for medical students to read hip fracture images.
AI proved viable for augmenting and being integrated into medical education.
Introduction
The history of medical education reform amply demonstrates that curricular change has been incremental, reactive, and mostly marginalized [1, 2]. With recent transformations in medical education, many efforts have been made to integrate technology and ethical aspects to efficiently improve the professionalism and clinical ability of medical students and trainees [3, 4]. Medical image learning is experience dependent, and trainees need to learn several kinds of images to achieve an expert level. This training requires acquiring skills to analyze and extract imaging features to identify patterns, generate a differential diagnosis that matches the patterns, and correlate the imaging features and differential with clinical findings to select the most likely diagnosis [5]. However, owing to time constraints, training opportunities might be compressed, and trainees may not be able to access as many images as their tutors or teachers. Individual variability is thought to substantially affect learning styles [6, 7]. Moreover, learning is dictated by the number and diversity of cases encountered, with varying practices and patient mixes. An artificial intelligence (AI)-assisted teaching platform can deliver personalized education and 24-h supervised tutoring that benefits both trainees and trainers. AI generated from deep neural network learning has been developed to help deliver precision medicine and health services [3, 8]. Researchers are increasingly embracing this modality to develop tools for diagnosis and prediction as well as to improve the effectiveness of healthcare. However, AI applications to medical education have been relatively underexplored. Although a tremendous amount of research has focused on AI in decision support [9–13], very little has focused on personalized medical education. Additionally, several AI methods use e-learning modules to identify the learning styles of individual students [6, 14, 15].
In this study, we developed a prospective blind image education system and confirmed that AI can support medical students and help them learn with confidence.
Materials and methods
Study population
This research involved the participation of students from the Medical School at Chang Gung University, Taiwan. We recruited undergraduate students in their fifth year of medical school training who had finished their basic medicine classes and entered the clinical rotation. All the participants volunteered to join this project. The study was designed as a single-center randomized controlled trial (RCT) conducted in the medical faculty of Chang Gung Memorial Hospital, Taiwan, between January 2020 and July 2020. At the beginning of the study, we introduced the study design, the method of image collection, the reading method of pelvis anteroposterior (AP) view radiographs (PXRs), and the characteristic features of hip fractures. The participants were enrolled in the study after an informed consent process.
The AI education system—HipGuide
We collected 3605 PXRs, which are commonly used to diagnose hip fractures, from the patients recorded in the trauma registry of January 2008 to December 2016 to train a hip fracture detection AI system, “HipGuide,” using a deep learning algorithm of DenseNet-121. All the images were reviewed by a trauma specialist with clinical information including injury mechanism, advanced images, surgical reports, and final diagnosis in the medical record. The development dataset contains 1975 (54.8%) images with hip fracture (including femoral neck fracture and trochanteric fracture) and 1630 (45.2%) images without hip fracture. The technical part of the system development is detailed in our previous work [16]. After the model trained, we collected an independent test set using 50 hip fracture and 50 normal PXR from 2017 for validation. When tested on new test images, the algorithm generates a probability of hip fracture, and an overlay heatmap representing the area contributes to this decision using a grad-CAM algorithm. The AI system achieved 91% accuracy and 98% sensitivity in the independent test set, and the heatmap identified 95.9% of the fracture sites. The model and parameters were adjusted to point out the fracture area more precisely to make the algorithm proper for education. The algorithm determined negative images will not present any heatmap to prevent confusion. The predicted fracture sites will be highlighted in positive images to guide the participants.
Experimental protocol and randomization
The participants received a test composed of 100 randomized images from the 2017 PXR dataset, and the accuracy of the results was defined by the prelearning score. Furthermore, we randomized the students with the simple method of flipping a coin. They were divided into two groups: the AI-assisted learning (AIL) group and the conventional (CL) group. In the CL group, the students received a postlearning test composed of another 100 PXR images 2 weeks after the first test, and the performance was defined as the postlearning test score. In the AIL group, the students took one additional test composed of 100 AI-augmented PXRs with AI-introduced heatmap images (as shown in Fig. 1) 1 week after the prelearning test, and the performance was defined as the WithAI score. One week later, the AIL students took another test composed of 100 more PXR images without AI augmentation, and the performance was defined as the postlearning score. The study flow is shown in Fig. 2. To evaluate the improvement in this learning process, we defined the gained score as the postlearning score minus the prelearning score.
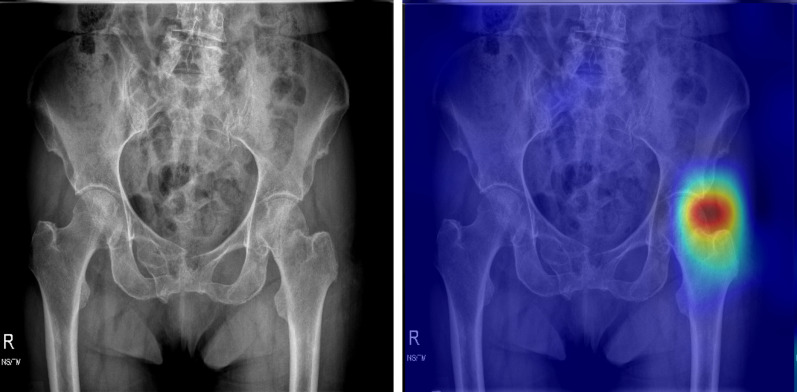
Fig. 1.
The demonstration of AI-augmented PXR with AI-introduced heatmap images. The augmented image includes the original plain pelvic film and one highlighted image that indicates the possible fracture area
Fig. 2.
Diagram describing the randomized method of our study
Main outcome measures
The primary outcome of the study was the difference between the study groups in overall gained score. In addition, the gained score for the AIL group was calculated and analyzed to determine which group of students achieved the most improvement after AI-assisted training.
Statistical analysis and software
The Shapiro–Wilk test was used to test whether the distribution was normal. Student’s t-test was used to compare the quantitative variables. Subsequently, the paired t-test was used to compare the accuracy scores to the corresponding baseline scores in each group. One-way analysis of variance (ANOVA) was used to compare the differences in improvement between the pre- and postlearning scores in the two groups. The Pearson correlation was used to compare the correlation between gained accuracy and prelearning accuracy. Statistical analyses were performed with SPSS v 20.0 for Macintosh (SPSS, Inc., Chicago, IL, USA). A p value < 0.05 was considered statistically significant.
Results
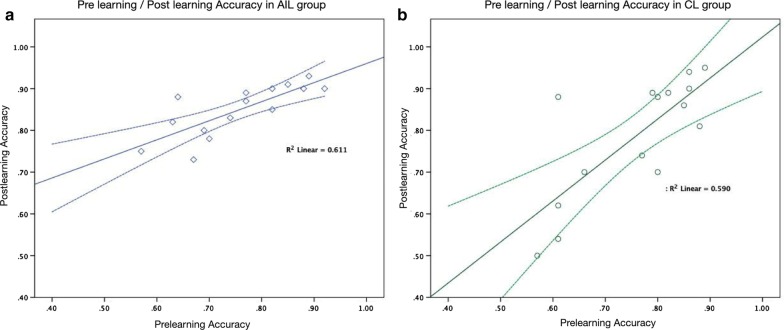
This study enrolled 34 medical students within the range of 22–32 years old who were randomly divided into the AIL and CL groups. Three of the students dropped out because they could not adhere to the evaluation schedule, and one could not complete the course. Therefore, a total of 30 students completed this study. The participants’ age, gender distribution, prelearning accuracy, sensitivity, and specificity were comparable in the two groups, as shown in Table 1. In the CL group, the postlearning score (78.66 ± 14.53) was higher than the prelearning score (75.86 ± 11.36), but there was no significant difference (p = 0.264). In the AIL group, all the participating students showed remarkable improvement with AI support. The WithAI score (88.87 ± 5.51) was significantly higher than the prelearning score (75.73 ± 10.58, p < 0.01). Moreover, the postlearning score (84.93 ± 14.53) was also better than the prelearning score (p < 0.01), as shown in Table 2 and Fig. 3. Figure 4 demonstrates the shift of the scores of both groups. In the AIL group, the prelearning/postlearning plot shows considerable improvement after AIL, as shown in Fig. 4a; however, the learning effect in the CL group was less significant, as shown in Fig. 4b.
Table 1.
Comparison between AI-assisted learning and conventional groups
| AI-assisted learning group | Conventional group | p value | |
|---|---|---|---|
| Case number | 15 | 15 | |
| Age (mean + SD) | 24.00 ± 1.60 | 24.67 ± 3.13 | 0.471 |
| Gender | |||
| Male | 9 | 10 | 0.740 |
| Female | 6 | 5 | |
| Pre-learning accuracy | 75.73 ± 10.58 | 75.86 ± 11.36 | 0.974 |
| Pre-learning sensitivity | 77.33 ± 18.15 | 72.53 ± 15.37 | 0.441 |
| Pre-learning specificity | 74.13 ± 17.42 | 79.20 ± 15.47 | 0.407 |
| With AI accuracy | 88.87 ± 5.51 | – | |
| With AI sensitivity | 94.93 ± 5.23 | – | |
| With AI specificity | 82.80 ± 10.50 | – | |
| Post-learning accuracy | 84.93 ± 14.53 | 78.66 ± 14.53 | 0.141 |
| Post-learning sensitivity | 86.40 ± 6.42 | 75.47 ± 22.10 | 0.084 |
| Post-learning specificity | 83.47 ± 14.03 | 81.87 ± 14.27 | 0.759 |
Table 2.
Comparison of the gain scores of diagnostic performances between AI-assisted learning and conventional groups
| AI-assisted learning group | Conventional group | p value | |
|---|---|---|---|
| Gained accuracy | 9.2 ± 6.9 | 2.8 ± 9.3 | 0.042 |
| Gained sensitivity | 9.1 ± 1.7 | 2.9 ± 1.4 | 0.299 |
| Gained specificity | 9.3 ± 1.8 | 2.6 ± 1.1 | 0.237 |
Bold indicates p < 0.05 which was considered statistical significance
Fig. 3.
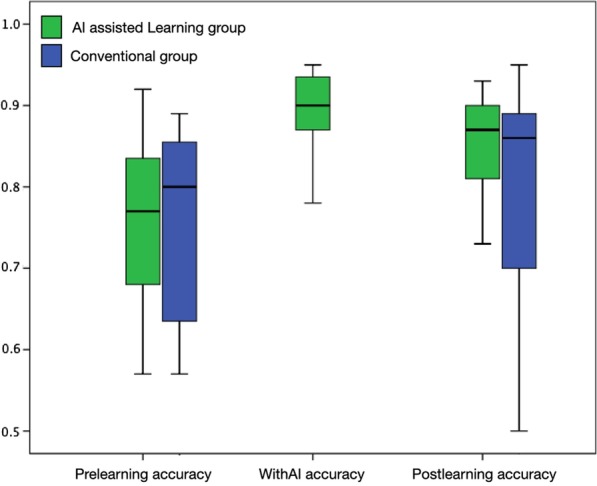
The box-plot graph of the diagnostic accuracy of the two groups
Fig. 4.
The prelearning/postlearning accuracy plot of the AI-assisted learning group and the conventional group
In comparing the AIL and CL groups, we identified a significantly higher gained accuracy in the AIL group (9.2 ± 6.9) than in the CL group (2.8 ± 9.3), p = 0.042, as shown in Table 3. As shown in Fig. 5, most of the AIL students obtained better scores and were located above the line of improvement. Just one participant’s score was below the line. However, in the CL group, five students (33%) did not improve and even had worse results after the learning.
Table 3.
The learning efficiency and post-learning performance of AIL and CL groups
| Pre-learning | With AI | Post-learning | |
|---|---|---|---|
| AIL group | |||
| Accuracy | 75.73 ± 10.58 | 88.87 ± 5.51* | 84.93 ± 14.53⍏ |
| *Pre-learning versus with AI: p < 0.01 | ⍏Pre-learning versus post-learning: p < 0.01 | ||
| CL group | |||
| Accuracy | 75.86 ± 11.36 | 78.66 ± 14.53⍏ | |
| ⍏Pre-learning versus post-learning: p = 0.264 |
Bold indicates p < 0.05 which was considered statistical significance
Fig. 5.
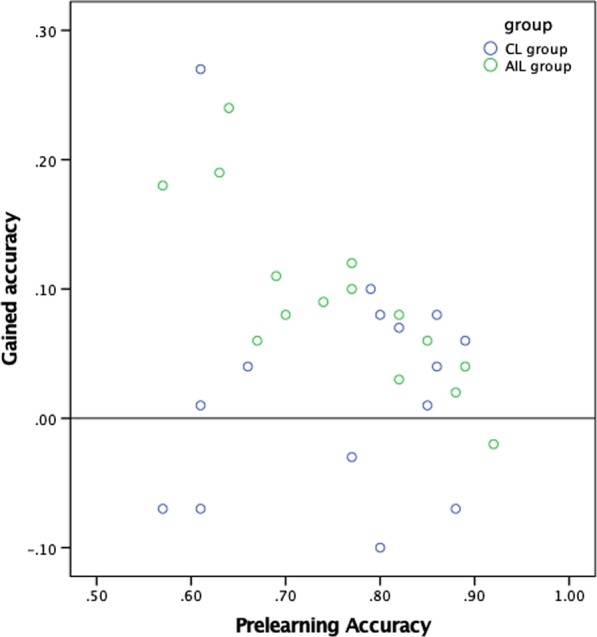
The comparison of gained accuracy with prelearning accuracy in the two groups
In the AIL group, the gained accuracy improvement was greater in the participants who had lower prelearning accuracy than in those who had higher prelearning accuracy. The strong inverse correlation is statistically significant, and the Pearson correlation coefficient (Pearson's r) is − 0.83 (p < 0.01). However, in the CL group, the gained accuracy showed no correlation with prelearning accuracy (Pearson's r: − 0.02, p = 0.94), as presented in Table 4.
Table 4.
The correlation between gained accuracy with pre-learning accuracy in AI-assisted learning group and conventional learning group
| Pre-learning accuracy | ||
|---|---|---|
| r | p value | |
| AIL group | ||
| Gained accuracy | − 0.83 | < .01 |
| CL group | ||
| Gained accuracy | − 0.02 | .94 |
Bold indicates p < 0.05 which was considered statistical significance
Discussion
In this study, we presented AI-augmented medical education that can help medical students efficiently learn to detect fracture of the hip; the gained accuracy score improved with a significant difference between the two groups. This is the first study to integrate AI into medical image education. In the AIL group, the WithAI score was significantly higher than the prelearning score. The AIL students who had been coached by the HipGuide system, even without AI-augmented image support, understood the key features of fracture and had learned how to identify the correct diagnosis. The postlearning score in the AIL group was significantly better than the prelearning score. Thus, the AI-based education system demonstrated its utility in improving trainee performance through quality measures that should be integral to the improvement in medical education, especially with the utilization of AI [7, 17, 18]. Furthermore, we found that the HipGuide system particularly helped novice students who had lower prelearning scores. The gained scores showed a strongly inverse correlation with the prelearning scores, which indicates that HipGuide helped novice students more than it helped their experienced classmates.
In the past, medical image training has relied on the traditional apprenticeship model. Because this model depends on trainee relationships, and because there is limited time available to review preliminary reports with a staff radiologist, gains in knowledge and skills can vary among trainees [19–21]. Hence, this apprenticeship education model is characterized by ever-increasing workload demands on both attending physicians and trainees and can be improved by a better understanding of relations between humans and tools. [8, 22, 23]. In conventional image teaching, students are expected to first learn diagnostic characteristics from educators and practice afterward. AI in medical education is still in the development stage [2, 7, 24–26]. AI can empower flipped classroom-like teaching [27, 28] and complete the existing bottom-up platforms used to teach radiology [29]. It has been argued that case-based learning should be implemented because it is more effective than traditional top-down approaches and is preferred by radiology educators and students [30, 31]. In the hip fracture detection task, the medical students had fundamental knowledge of anatomy, pathology, and the principle of imaging. The heatmap generated by AI provided an opportunity for the students to connect this domain knowledge and generate a new understanding of radiographic interpretation skills. Thus, they could improve their diagnostic accuracy with limited learning cases. Visualization techniques such as grad-CAM provide a rationale for humans to understand the black box mechanism [32]. Students can utilize AI to supplement unsupervised learning during personal time. AI can be used for low-level supervision, while attending and staff physicians can continue to provide high-level supervision [14, 33]. This change would allow human educators to tailor training methods and lesson content to their students’ strengths and weaknesses, promoting bidirectional information exchange and saving training time. Thus, AI supports unique education platforms that balance a standardized curriculum with inherently individualized learning.
A well-designed self-learning platform with AI augmentation will be an adequate model for medical education in the next generation. Kolachalama and Garg proposed the integration of machine learning-related content in medical education. They recommended integrating real-world clinical examples into machine learning courses and applying practical guidelines to choose the best tools [34, 35]. Because of a current lack of tutors’ direct access to appropriate AI education, AI-assisted teaching methods are rarely embedded in undergraduate and graduate medical education training. Creating a user-friendly automated platform to help both trainers and trainees is essential for developing an AI educational system [36]. In this study, we presented a straightforward pathway to support AI-based technology that can also help medical students or novice doctors learn and obtain experience quickly. This study offered a method to improve medical students’ learning of medical images with instinctive AI diagnostic support.
Limitations
There have been significant breakthroughs in AI in medical diagnostics in recent years. Our study provides evidence supporting the proposal that AI can help medical education. There are still some limitations. There have been few, if any, direct comparisons between conventional and AI-augmented medical image education. To determine and verify the utility of AI in precision education, trainee performance must be assessed through reliable and valid measures [7]. Second, the sample size was relatively small (N = 30), which might affect the power of the study, although an analysis of 560 studies indicated that this number is within the normal range of experimental groups [37]. Third, because the educator curates and collects the data for AI algorithm development, selection bias might exist due to the data distribution. Fourth, given our training capacity, we decided to recruit thirty-six participants for this study and opened it to residents on a first-come, first-served basis. There might be selection bias because we assume that those who chose to participate had high motivation and interest in learning novel technology. Further, when we proceeded with this study, all the medical students also had access to other approaches for learning medical image diagnosis. Because all the medical students may not have had equal learning opportunities, there may have been unpreventable bias. Finally, owing to the limitations of the technology, we can offer only the heatmap rather than using an arrowhead to point directly to a lesion, which is less user-friendly. However, despite the limitations of this study, we demonstrated that AI can shorten the learning curve in learning to read PXRs.
Conclusion
In this study, we demonstrate that AI is viable for augmenting medical education and can shorten the learning curve in learning to read PXRs. With AI assistance, students can learn to efficiently read medical images. In addition, AI-based training can elevate diagnostic accuracy even further for students whose performance is initially poor. The next generation of students may learn how AI technologies are used in practice, and similar tools will also be used to enrich their education.
Acknowledgements
The authors thank CMRPGJ1121, CMRPG 3J631, and CIRPG3H0021 for supporting the study and the development of the system.
Abbreviations
- AI
Artificial intelligence
- AIL
AI-assisted learning
- ANOVA
Analysis of variance
- CL
Conventional learning
- PACS
Picture archiving and communication system
- PXRs
Pelvis AP view radiographs
Authors’ contributions
C-HL, C-TC, M-JH, C-HC are responsible for the main manuscript writing and project design. C-CC is responsible for the main statistical analysis and figure creation of the study. C-YF, Y-TW, C-PH, C-CC, and C-HH are responsible for the data acquisition of the study.
Funding
The supporting funding of this project is from the Chang Gung Memorial Hospital Research project CMRPGJ1121, CMRPG 3J631, and CIRPG3H0021.
Availability of data and materials
The authors declare the data that support the findings of this study are available on request from the corresponding author C.T. Cheng.
Ethics approval and consent to participate
The study was approved by the IRB Committee of the Chang Gung Memorial Hospital (IRB: 201900382B0). Informed consent was done after well discussing the participants.
Consent for publication
Not applicable.
Competing interests
The authors have no conflicts of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chi-Tung Cheng and Chih-Chi Chen contributed equally to this work
References
- 1.Skochelak SE. A decade of reports calling for change in medical education: what do they say? Acad Med. 2010;85:S26–33. doi: 10.1097/ACM.0b013e3181f1323f. [DOI] [PubMed] [Google Scholar]
- 2.Wartman SA, Combs CD. Reimagining medical education in the age of AI. AMA J Ethics. 2019;21:E146–152. doi: 10.1001/amajethics.2019.146. [DOI] [PubMed] [Google Scholar]
- 3.Pietrzak B, Ward A, Cheung MK, et al. Education for the future. Science. 2018;360:1409–1412. doi: 10.1126/science.aau3877. [DOI] [PubMed] [Google Scholar]
- 4.Briz-Ponce L, Juanes-Méndez JA, García-Peñalvo FJ, Pereira A. Effects of mobile learning in medical education: a counterfactual evaluation. J Med Syst. 2016;40:136. doi: 10.1007/s10916-016-0487-4. [DOI] [PubMed] [Google Scholar]
- 5.Reeder MM. Reeder and Felson’s Gamuts in radiology: comprehensive lists of roentgen differential diagnosis. New York: Springer; 2013. [Google Scholar]
- 6.Hart SA. Precision education initiative: moving towards personalized education. Mind Brain Educ. 2016;10:209–211. doi: 10.1111/mbe.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duong MT, Rauschecker AM, Rudie JD, et al. Artificial intelligence for precision education in radiology. Br J Radiol. 2019;92:20190389. doi: 10.1259/bjr.20190389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slanetz PJ, Reede D, Ruchman RB, et al. Strengthening the radiology learning environment. J Am Coll Radiol. 2018;15:1016–1018. doi: 10.1016/j.jacr.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 9.Kohli M, Prevedello LM, Filice RW, Geis JR. Implementing machine learning in radiology practice and research. AJR Am J Roentgenol. 2017;208:754–760. doi: 10.2214/AJR.16.17224. [DOI] [PubMed] [Google Scholar]
- 10.Choy G, Khalilzadeh O, Michalski M, et al. Current applications and future impact of machine learning in radiology. Radiology. 2018;288:318–328. doi: 10.1148/radiol.2018171820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kermany DS, Goldbaum M, Cai W, et al. Identifying medical diagnoses and treatable diseases by image-based deep learning. Cell. 2018;172:1122.e9–1131.e9. doi: 10.1016/j.cell.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 12.Stone JR, Wilde EA, Taylor BA, et al. Supervised learning technique for the automated identification of white matter hyperintensities in traumatic brain injury. Brain Inj. 2016;30:1458–1468. doi: 10.1080/02699052.2016.1222080. [DOI] [PubMed] [Google Scholar]
- 13.Olczak J, Fahlberg N, Maki A, et al. Artificial intelligence for analyzing orthopedic trauma radiographs: deep learning algorithms—are they on par with humans for diagnosing fractures? Acta Orthop. 2017;88:581–586. doi: 10.1080/17453674.2017.1344459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernard J, Chang T-W, Popescu E, Graf S. Learning style Identifier: Improving the precision of learning style identification through computational intelligence algorithms. Expert Syst Appl. 2017;75:94–108. doi: 10.1016/j.eswa.2017.01.021. [DOI] [Google Scholar]
- 15.Truong HM. Integrating learning styles and adaptive e-learning system: current developments, problems and opportunities. Comput Human Behav. 2016;55:1185–1193. doi: 10.1016/j.chb.2015.02.014. [DOI] [Google Scholar]
- 16.Cheng C-T, Ho T-Y, Lee T-Y, et al. Application of a deep learning algorithm for detection and visualization of hip fractures on plain pelvic radiographs. Eur Radiol. 2019;29:5469–5477. doi: 10.1007/s00330-019-06167-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarwar A, Boland G, Monks A, Kruskal JB. Metrics for radiologists in the era of value-based health care delivery. Radiographics. 2015;35:866–876. doi: 10.1148/rg.2015140221. [DOI] [PubMed] [Google Scholar]
- 18.Walker EA, Petscavage-Thomas JM, Fotos JS, Bruno MA. Quality metrics currently used in academic radiology departments: results of the QUALMET survey. Br J Radiol. 2017;90:20160827. doi: 10.1259/bjr.20160827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wildenberg JC, Chen P-H, Scanlon MH, Cook TS. Attending radiologist variability and its effect on radiology resident discrepancy rates. Acad Radiol. 2017;24:694–699. doi: 10.1016/j.acra.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Chen P-H, Loehfelm TW, Kamer AP, et al. Toward data-driven radiology education-early experience building multi-institutional academic trainee interpretation log database (MATILDA) J Digit Imaging. 2016;29:638–644. doi: 10.1007/s10278-016-9872-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cook TS, Hernandez J, Scanlon M, et al. Why isn’t there more high-fidelity simulation training in diagnostic radiology? Results of a survey of academic radiologists. Acad Radiol. 2016;23:870–876. doi: 10.1016/j.acra.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 22.Liao C-H, Ooyang C-H, Chen C-C, et al. Video coaching improving contemporary technical and nontechnical ability in laparoscopic education. J Surg Educ. 2020;77:652–660. doi: 10.1016/j.jsurg.2019.11.012. [DOI] [PubMed] [Google Scholar]
- 23.Lubowitz JH, Provencher MT, Brand JC, Rossi MJ. The apprenticeship model for surgical training is inferior. Arthroscopy. 2015;31:1847–1848. doi: 10.1016/j.arthro.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 24.Sapci AH, Sapci HA. Artificial intelligence education and tools for medical and health informatics students: systematic review. JMIR Med Educ. 2020;6:e19285. doi: 10.2196/19285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan KS, Zary N. Applications and challenges of implementing artificial intelligence in medical education: integrative review. JMIR Med Educ. 2019;5:e13930. doi: 10.2196/13930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park SH, Do K-H, Kim S, et al. What should medical students know about artificial intelligence in medicine? J Educ Eval Health Prof. 2019;16:18. doi: 10.3352/jeehp.2019.16.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Belfi LM, Bartolotta RJ, Giambrone AE, et al. “Flipping” the introductory clerkship in radiology: impact on medical student performance and perceptions. Acad Radiol. 2015;22:794–801. doi: 10.1016/j.acra.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Flanders AE. What is the future of electronic learning in radiology? Radiographics. 2007;27:559–561. doi: 10.1148/rg.272065192. [DOI] [PubMed] [Google Scholar]
- 29.Maleck M, Fischer MR, Kammer B, et al. Do computers teach better? A media comparison study for case-based teaching in radiology. Radiographics. 2001;21:1025–1032. doi: 10.1148/radiographics.21.4.g01jl091025. [DOI] [PubMed] [Google Scholar]
- 30.Terashita T, Tamura N, Kisa K, et al. Problem-based learning for radiological technologists: a comparison of student attitudes toward plain radiography. BMC Med Educ. 2016;16:236. doi: 10.1186/s12909-016-0753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Welter P, Deserno TM, Fischer B, et al. Towards case-based medical learning in radiological decision making using content-based image retrieval. BMC Med Inform Decis Mak. 2011;11:68. doi: 10.1186/1472-6947-11-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samek W, Müller K-R (2019) Towards explainable artificial intelligence. Explainable AI: interpreting, explaining and visualizing deep learning, pp 5–22
- 33.Lam CZ, Nguyen HN, Ferguson EC. Radiology resident’ satisfaction with their training and education in the United States: effect of program directors, teaching faculty, and other factors on program success. AJR Am J Roentgenol. 2016;206:907–916. doi: 10.2214/AJR.15.15020. [DOI] [PubMed] [Google Scholar]
- 34.Kolachalama VB, Garg PS. Machine learning and medical education. NPJ Digit Med. 2018;1:54. doi: 10.1038/s41746-018-0061-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beam AL, Kohane IS. Translating artificial intelligence into clinical care. JAMA. 2016;316:2368–2369. doi: 10.1001/jama.2016.17217. [DOI] [PubMed] [Google Scholar]
- 36.van der Niet AG, Bleakley A. Where medical education meets artificial intelligence: “does technology care?”. Med Educ. 2020 doi: 10.1111/medu.14131. [DOI] [PubMed] [Google Scholar]
- 37.Mason M. Sample size and saturation in PhD studies using qualitative interviews. Forum Qual Soc Res. 2010 doi: 10.17169/fqs-11.3.1428. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declare the data that support the findings of this study are available on request from the corresponding author C.T. Cheng.