Abstract
Plants have evolved according to their environmental conditions and continuously interact with different biological entities. These interactions induce many positive and negative effects on plant metabolism. Many viruses also associate with various plant species and alter their metabolism. Further, virus–plant interaction also alters the expression of many plant hormones. To overcome the biotic stress imposed by the virus’s infestation, plants produce different kinds of secondary metabolites that play a significant role in plant defense against the viral infection. In this review, we briefly highlight the mechanism of virus infection, their influence on the plant secondary metabolites and phytohormone biosynthesis in response to the virus–plant interactions.
Keywords: Secondary metabolites, Phytohormones, Viruses, Biotic stress, Defense
Introduction
Plants have tremendously evolved owing to their sporadicity in continuum with the climatic conditions, where they are subjective to diverse and complex interactions involving multitudinous biotic and abiotic stress factors. Plant viruses are obligate and biotrophic pathogens, which are responsible for severe diseases in plants, resulting in major losses in many important crops. The development of symptoms is likely to be the result of a complex interplay between plant and virus in the context of cellular homeostasis. The viral infection affects the physiological and biochemical processes within the plant. These alterations often lead to the appearance of symptoms such as stunting, mottling or wrinkling of leaves, wilting, chlorotic or necrotic lesions, abscission of leaves and fruits, and the development of abnormal growth forms such as galls (enations) and phyllody.
During evolution, plants have developed some special mechanisms allowing them to live in stressful conditions, including a plethora of parasite infestation. These stresses are intensely complex which induce changes at the cellular, physiological, and genome levels, but to overcome this situation, the accumulation of secondary metabolites plays a major role (Atkinson and Urwin 2012; Srivastava et al. 2018). The secondary metabolites are small organic compounds present in their natural forms in plants. They are associated to develop the essential biological roles during biotic and abiotic stress, facilitating the defense signalling in plants. Besides secondary metabolites, viruses also impact significantly on the hormone biosynthesis pathways in plants. Plant hormones are small organic molecules and involved in growth, differentiation, defense, and cellular signalling. They are divided into nine major categories according to their function such as auxin, abscisic acid (ABA), brassinosteroids (BR), cytokinins (CK), gibberellin (GA), ethylene (ET), jasmonic acid (JA), salicylic acid (SA), and strigolactones (SL). The defense mechanism in plants is primarily regulated by hormones, i.e. ET, JA, and SA, which are also referred to as plant immunity hormones. Besides, the hormones ABA, GAs, auxins, CKs, BR, and Nitric oxide are also emerged as modulators of the plant immune signalling pathways (Pieterse et al. 2012). Hormonal cross-talk during viral pathogenesis in plants improves the defense mechanism and provides resistance; however, many plant viruses overcome this resistance and alter the hormone signalling (Jameson and Clarke 2002; Srivastava et al. 2014). This review will highlight the effects of virus–plant interaction on secondary metabolites production and phytohormones regulation. Through recent findings, the impact of virus infection has been elaborated on plant hormones and secondary metabolites production, which could be an essential improvement and function as a strategy to develop plants possessing an enhanced physiological response and medicinal utility.
Mechanism of viral pathogenesis in plants
The virus constantly infects the plants by escaping the defense mechanism of the host to complete their replication. The mechanism of virus pathogenesis and interaction in plants are reviewed recently by many researchers (Culver and Padmanabhan 2007; Calil and Fontes 2017; Wu et al. 2019; Islam et al. 2019; Hyodo and Okuno 2020). Previously, Culver and Padmanabhan 2007 suggest two models to enlighten virus-induced symptom and disease development. First, in a competitive disease model, viruses compete with the host plants for the available genetic and metabolic resources. This model demonstrates the plant–virus replication within the host and restricted host metabolic resources, which affect plant growth and development. Further, the continuous replication of viral particles disrupts the host processes and develops the symptoms of the disease by the interaction of virus and host constituents. The viruses control host transcription and translation processes for their resource utilization. The synthesis of viruses required a substantial amount of host resources, which is possible by the action of the shutdown of host genes (Thivierge et al. 2005). The competitive disease model also suggests the appearance of disease symptoms upon viral infection is not the issue of competition for host resources. However, the replication of viruses in hosts increasing rapidly and then fall, often within an hour which reveals the transient nature of host resources competition in viruses (Culver and Padmanabhan 2007). The other interaction disease mode presents a substitute description of the specific virus and host components. Albeit, it is more complex than simple resource competition, specific virus–host interactions induce disease, which deciphers the variations in the severity of the disease observed when differentiating the similar viruses on the same host or the same virus on different hosts. It is very well known that induction of disease relies on specific virus–host interaction (Culver and Padmanabhan 2007).
Plant viruses have expanded versatile approaches to destroy and exploit host defenses to confirm their effective infection. One emerging theme is that plant virus that not only avoids plant defense mechanisms but also integrates a host defense factor, which is typically involved in plant defense against pathogen infestation into a viral protein complex and thereby become a part of viral pathogenesis. For example, mutation strategy as a master plan for escaping host antiviral mechanisms, host innate immunity inhibition, suppression, and utilization of host RNA silencing, destabilize host autophagy-mediated and ubiquitination-mediated antiviral machinery (Machado et al. 2015; Vierstra 2009; Nicaise and Candresse 2017; Wu et al. 2010, 2019; Diaz-Pendon et al. 2007; Islam et al. 2019).
Plant secondary metabolites: insights and their biosynthetic pathways
Plants produce a large and diverse array of organic compounds known as secondary metabolites in response to pathogen attacks and environmental stress. Secondary metabolites have no direct functions in the growth and reproduction of plants even though they play an imperative role in the development of defense mechanisms against biotic and abiotic stresses (Dixit et al. 2020; Srivastava et al. 2018; Pandey et al. 2016). Further, a higher concentration of secondary metabolites also increases the resistance in plants, but sometimes it may produce an adverse effect by reducing the growth and reproduction in plants (Siemens et al. 2002). Plant secondary metabolites are majorly classified into three groups: terpenes/terpenoids, phenolic compounds (such as flavonoids and allied phenolic and polyphenolic compounds), and nitrogen-containing and sulfur-containing compounds (such as alkaloids and glucosinolates). Their classification is based on the chemical composition (containing nitrogen or sulphur or not), chemical structure (e.g., having rings, containing a sugar), the biosynthetic pathway (e.g., phenylpropanoid, which produces tannins) or their solubility (Kabera et al. 2014).
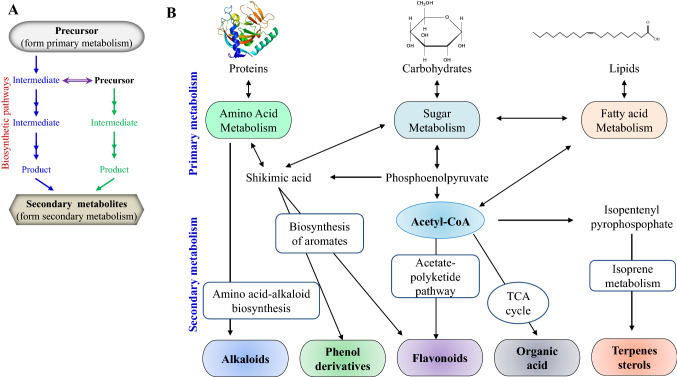
In the biosynthesis of plant secondary metabolites, the sequence of reactions involves through which the primary metabolites convert into the final molecule by the cell. The glycolysis of the carbohydrate and citric acid cycles provides energy for biosynthetic reactions. A high energy molecule, such as ATP, is formed by the oxidation of the glucose, fatty acid, and amino acids (catabolism of the primary compounds) (Lancini and Lorenzetti 1993). The secondary metabolite’s biosynthetic pathways initiate from different precursors of primary metabolism (Fig. 1a). These precursors are resulting from various primary metabolism pathways comprising carbohydrates (sugars), proteins (amino acids), and lipids (fatty acid). These precursors are used as a substrate by the enzyme, which could be involved in a particular biosynthetic pathway and convert precursor into the product for the next step. This product may be an intermediate molecule in that biosynthetic pathway and utilized as a precursor for an enzyme's next step. On the other hand, it could be the last product of the biosynthetic reaction pathway (Fig. 1) (Gutzeit and Ludwig-M 2014).
Fig. 1.
Biosynthetic pathways for secondary metabolites production. a An outline for the formation of secondary metabolite production from the conversion of precursor (substrate) into the product by biosynthetic enzymes. b Biosynthetic pathways and precursors form the major classes of secondary metabolites. Deriving pathways from primary metabolites amino acids, carbohydrates, and lipids (adapted and modified from Ferdes 2018). Shikimic acid is an intermediate for the amino acid metabolism. Additionally, acetyl-CoA is involved in the synthesis of terpenes, flavonoids, and organic acids which form a distinct class of metabolites
Interestingly, similar precursors can be utilized in a particular group of compounds and an array of diverse compounds for the metabolites' biosynthesis. Accumulating evidences suggest that the primary metabolism products are synthesized from various processes, including the glycolysis, the TCA (tricarboxylic acid) cycle, amino acids, pentose phosphate or the shikimate signalling pathways. These commonly act as precursors for the secondary metabolites synthesis (Gutzeit and Ludwig-M 2014; Kabera et al. 2014; Ncube and Van Staden 2015; Pott et al. 2019). Acetyl-CoA plays a key role in the formation of several secondary metabolites biosynthesis formed from glycolysis and fatty acid oxidation. Additionally, the acetyl-CoA is used for the synthesis of organic acids (secondary metabolites precursors) in the TCA cycle. the acetyl-CoA is also participated in different secondary metabolites such as terpenes and flavonoids synthesis (Gutzeit and Ludwig-M 2014; Kabera et al. 2014). The chief source of aromatic secondary metabolites (phenols, flavonoids, and some alkaloids) is aromatic amino acids. Shikimic acid plays an intermediate role in the metabolism of amino acid and acts as a precursor for aromatic secondary metabolites synthesis (Gutzeit and Ludwig-M 2014; Kabera et al. 2014).
Influence of virus infection on plant secondary metabolites production
Many plant viruses have a very broad host range. For instance, Tobacco mosaic virus (TMV) infects many plant species including tobacco, tomato, and other solanaceous crops; Cucumber mosaic virus (CMV) is capable of infecting over 1000 plant species, Citrus tristeza virus affects several citrus species, Tomato yellow leaf curl virus infects tomato and other crops in many countries, and Rice tungro virus causes a problem for rice production globally (Hamidun et al. 2014; Loebenstein et al. 1995; Scholthof 2004). Different biotic and abiotic elicitors regulate the production of the secondary metabolites in plants that have been reported. However, fewer studies are available on the influence of the viral infections on plant secondary metabolites. Interestingly, these accumulating studies suggest that plant viruses control the accumulation of various secondary metabolites at the industry level (Table 1).
Table 1.
Effects of the plant–virus interaction on the secondary metabolites
| Virus | Host | Secondary metabolites/metabolism | References |
|---|---|---|---|
| Cucumber mosaic virus | Passiflora edulis | Polyphenols and flavonoids | Lan et al. (2020) |
| Grapevine Leaf roll associated virus 3 | Vitis vinifera | Flavonols (myricetin, kaempferol and quercetin derivatives) and hydroxycinnamic acids (which include derivatives of caffeic acid) | Montero et al. (2016) |
| Grapevine red blotch-associated virus | Vitis vinifera |
Flavonoid and anthocyanin biosynthesiss (malvidin-3-O-glucoside, petunidin-3-O-glucoside, delphinidin-3-O-glucoside, pelargodin-3-O-glucoside, and cyanidin-3-O-glucoside) Phenylpropanoid metabolism Isoflavonoid metabolism |
Blanco-Ulate et al. (2017) |
|
Hop mosaic virus, Hop latent virus |
Humulus lupulus | α- and β-bitter acids, polyphenols, essential oils | Pethybridge et al. (2002); Jelínek et al. (2012) |
| Poppy mosaic virus | Papaver somniferum var. Sampada | Alkaloids-papaverine, narcotine, codeine, morphine, thebaine | Zaim et al. (2014a, b) |
| Prunus necrotic ringspot virus—PNRSV-A and PNRSV-I | Humulus lupulus | α- and β-bitter acids, polyphenols, essential oils | Pethybridge et al. (2002); Jelínek et al. (2012) |
| Saffron latent virus | Saffron | Crocetin, esters, picrocrocin, safranal, and kaempferols | Parizad et al. (2019) |
| Telosma mosaic virus | Passiflora edulis | Phenols | Chen et al. (2018) |
| Tobacco mosaic virus | Tobacco | Scopoletin and its glucoside scopolin | Chong et al. (2002); Costet et al. (2002) |
| Turnip crinkle virus, Cauliflower mosaic virus | Arabidopsis thaliana | Camalexin | Callaway et al. (1996); Dempsey et al. (1997) |
The virus infection to Arabidopsis thaliana induces camalexin formation, which plays a significant role in response to pathogen attack (Callaway et al. 1996; Dempsey et al. 1997). In Cauliflower mosaic virus (CaMV)-inoculated Arabidopsis ecotype Enkheim-2 seeding, level in camalexin accumulation increases after fourteen days of infection, but it is even not detected in Arabidopsis ecotype Col-0 seedling (Callaway et al. 1996). Infection with Turnip crinkle virus to Arabidopsis thaliana leaves induce 20-folds camalexin accumulation on the second day of infection (Dempsey et al. 1997). The antioxidants present in the Hop (Humulus lupulus L.) are significantly important for the brewing industry. Two calraviruses (Hop mosaic virus—HpMV and Hop latent virus—HpLV) and two serotypes of the ilavirus (Prunus necrotic ringspot virus—apple PNRSV-A and intermediate, PNRSV-I) affect on the contents of brewing essential Hop secondary metabolites α- and β-bitter acids, polyphenols, and essential oils production (Jelínek et al. 2012; Pethybridge et al. 2002). Another virus that is a threat to the wine industry is Grapevine red blotch-associated virus (GRBaV), which is a single-stranded circular DNA virus. GRBaV infections suppress the biosynthesis of the phenylpropanoid pathway and their derivatives in Vitis vinifera. GRBaV infection reduced the flavonoid and anthocyanin (malvidin-3-O-glucoside, petunidin-3-O-glucoside, delphinidin-3-O-glucoside, pelargodin-3-O-glucoside, and cyanidin-3-O-glucoside) biosynthesis (Blanco-Ulate et al. 2017). Another RNA virus Grapevine Leaf roll-associated virus 3 (GLRaV-3) enhances the production of flavonols (myricetin, kaempferol, and quercetin derivatives) and hydroxycinnamic acids (include derivatives of caffeic acid) in Vitis vinifera white cultivar Malvasía de Banyalbufar (Montero et al. 2016). Scopoletin and its glucoside scopolin are important secondary metabolites synthesized in plants as a defense mechanism against various environmental stresses. The transgenic tobacco containing the tobacco togt (tobacco salicylic acid—and pathogen-inducible UDP-Glc:glucosyltransferases) gene in antisense expression revealed that the reduction of scopoletin and the glucoside scopolin levels to infection with TMV, which has been associated with a decrease of resistance to TMV infection showing an indication of the antimicrobial function (Chong et al. 2002). It is also reported that scopoletin levels increased in high amounts until day 5 due to TMV infection in tobacco leaves (Costet et al. 2002).
Opium poppy (Papaver somniferum) is an important medicinal plant as the source of several pharmacologically secondary metabolites. RNA virus Poppy mosaic virus (PMV-P) consistently infected the poppy plants and thereby regulated the secondary metabolites according to the genotype-dependent (Zaim et al. 2014a, b). Due to PMV-P infection, five alkaloids such as papaverine, thebaine, narcotine, codeine, and morphine are accumulated in poppy genotype IM, except of thebaine levels which decreased in P. somniferum var Sampada (Opium poppy). However, the short duration of the PMV-P infection causes the improvement in the production of five alkaloids including thebaine in P. somniferum var. Shweta (Zaim et al. 2014a, b). The recent reports suggest that some virus infection modulates the levels of secondary metabolites in Passiflora edulis, which has high medicinal properties for various human health diseases (Lan et al. 2020; Chen et al. 2018). CMV-infected P. edulis fruits and leaves also show improvement of the total polyphenol (26–28%) and flavonoids (48–58%) content (Lan et al. 2020). A potyvirus Telosma mosaic virus infection cause a significantly increased in total phenol contents of P. edulis fruits, which gives resistance against viral infections (Chen et al. 2018). Another recent study on the saffron (Crocus sativus) affects the accumulation of secondary metabolites due to virus infection (Parizad et al. 2019). Saffron latent virus distinctly affects the spice quality of saffron by altering concentration and proportions of the crocetin, esters, picrocrocin, safranal, and kaempferols (Parizad et al. 2019). Altogether, these studies suggest that it is significant to comprehend several viruses pathogenesis in the modification of secondary metabolites in plants.
Virus–phytohormone interaction in plants
Plants have developed specified recognition and signalling systems for the rapid recognition of phytopathogen invasion and initiation of defense responses. The contact of the plant with pathogens or elicitors leads to the activation of metabolic fluxes, proteins phosphorylation/dephosphorylation and the production of other signalling molecules. This activation promotes hormone signalling, regulation of the defense-responsive genes, strengthening of the cell wall, phytoalexins accumulation and several other physiological and molecular processes. Phytohormones display several different roles in plant development and growth, and are progressively being acknowledged for their application in plant–virus interactions. The viruses affect the phytohormones level depending on the combination of virus–host interaction. Virus infection could be spread systemically in some hosts, including vegetable crops, medicinal plants, ornamentals, weeds, etc. while some virus movement is restricted to phloem in some plants such as Barley yellow dwarf virus in Barley. The large diversification in virus forms, replication and pathogenic consequences reveal diverse effects in the hormone metabolism of the different host plants. Furthermore, the virus interaction with plant changes the hormone concentration and it is difficult to measure the level of hormone concentration at this stage. Therefore, the immunlogical techniques [enzyme-linked immunosorbent assay (ELISA) and radioimmunoassay (RIA)] based on antigen–antibody (Ab–Ag) reactions merged with purification techniques such as high-profile liquid chromatography (HPLC) and mass spectrometry (MS) used to measure the level of hormones, which have enhanced the value of investigations to identify the role and function of plant hormones affected during the plant–virus interaction (Jameson and Clarke 2002; Sakamoto et al. 2018). Understanding the link between the phytohormones and the compounds involved in the induction of defense-related pathways is very imperative for the description of hormone–virus interactions. However, the hormone signalling in plants during viral pathogenesis gives more robust defense responses.
Impact of viral infection on phytohormone biosynthesis
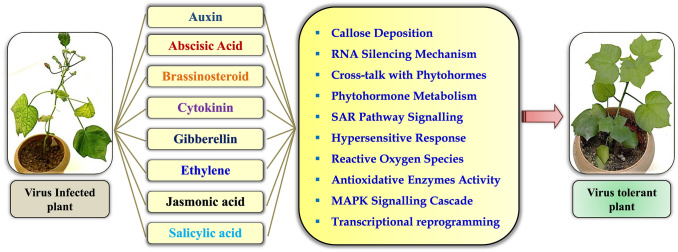
Understanding the molecular mechanisms of phytohormone biosynthesis–virus associations can facilitate new insights into virus tolerance or resistance, and can result in the improvement of novel approaches for breeding of tolerant/resistant varieties (Fig. 2). The viral infection has continuously manipulated phytohormonal regulation that results in modifications of the plant growth and development. Therefore, to understand the regulation of phytohormones during viral infections, it is important to enlighten the role of virus–hormone interaction in plants. Viruses can even manipulate plant hormone biosynthesis to deactivate defense or development signalling and reprogram the cellular conditions to improve their replication process and spread the infection.
Fig. 2.
Influence on phytohormone due to the plant–virus interaction. Virus infections modulate the plant hormone levels result in various mechanism of the plant defense strategies
Influence of virus–plant interaction on phytohormones
Abscisic acid
Abscisic acid (ABA) is a major plant hormone that has a remarkable impact on the plant's ability to endure biotic and abiotic stresses (Table 2). The virus infection prompts the ABA synthesis, which impedes the defense mechanism by disturbing other defensive hormonal pathways including SA, JA, or ET synthesis (Alazem and Lin 2017). Earlier reports indicate an alteration in the level of ABA increased in tobacco and banana plants infected by TMV and Banana bunchy top virus, respectively (Rajagopal 1977; Zhang et al. 1997). In some studies, accumulation of ABA level in virus-infected plants represents the compatible interactions in a host such as CMV in N. benthamiana, Bamboo mosaic virus (BaMV) in A. thaliana and N. benthamiana and TMV in N. tabacum (Alazem and Lin 2017; Alazem et al. 2014, 2017). Interestingly, few RNA viruses have been involved in drought tolerance in plants i.e., CMV, TMV, Lily symptomless virus and Tobacco rattle virus-infected host plants, including Nicotiana tabacum, Beta vulgaris, Lilium species and Oryza sativa. This induction of drought tolerance by viral infection also increases the concentration of osmoprotectants and antioxidants which increases the ABA content in plants (Chinestra et al. 2010; Xu et al. 2008). The expression of ABA biosynthetic and signalling genes (biosynthetic genes, OsNCED3 and OsABA8ox; ABA receptors, OsPYL1 and OsPYL5; ABA pathway negative modulators, OsABIL1 and OsABIL2) is reduced in Rice black‐streaked dwarf virus (RBSDV) infected rice plants, suggesting ABA pathway was significantly down‐regulated in response to viral infection and helped rice susceptibility to the virus infection (Xie et al. 2018). Furthermore, some reports enlightened the predominantly positive effects upon viral infection on ABA contents viz. Mal de Rio cuarto virus (MRCV) infection in wheat (Triticum aestivum) (de Haro et al. 2019), inoculation of Plum pox virus (PPV) on peach (Dehkordi et al. 2018), and BaMV infection in model plants N. benthamiana and Arabidopsis (Alazem et al. 2014, 2017). Several genes from ABA biosynthesis and signaling are stimulated promptly against Soybean mosaic virus (SMV) avirulent strain G5H in soybean cultivar L29 (Alazem et al. 2018). In the context of these studies, an additional report described the role of the ABA pathway in the development of resistance against BaMV via regulation of Argonaute (AGO), dicer like (DCL) proteins, and RNA-dependent RNA polymerase (RDR) genes in the antiviral RNA-silencing pathway (Alazem et al. 2019).
Table 2.
Effects of the plant–virus interaction on the phytohormone biosynthesis
| Virus | Host | Phytohormone | References |
|---|---|---|---|
| Bamboo mosaic virus |
Arabidopsis thaliana Nicotiana benthamiana |
Abscisic acid | Alazem et al. (2014); Alazem and Lin (2017) |
| Banana bunchy top virus | Banana |
Gibberellic acid Abscisic acid Isopentenyladenine group contents |
Zhang et al. (1997a ) |
| Barley yellow dwarf virus | Barley | Gibberellin | Russell and Kimmins (1971) |
| Bean golden mosaic virus | Phaseolus vulgaris | Cytokinin | Fazio (1981) |
| Begomoviruses (cabbage leaf curl virus and Tomato golden mosaic virus), Curtoviruses (spinach curly top virus) and Potexviruses (white clover mosaic potexvirus) | Arabidopsis thaliana | Cytokinin-responsive genes | Baliji et al. (2010) |
| Cauliflower mosaic virus | Arabidopsis thaliana | Ethylene | Geri et al. (2004) |
| Crucifer-infecting tobacco mosaic virus | Arabidopsis thaliana | Salicylic acid | Islam et al. (2019) |
| Cucumber mosaic virus | Nicotiana benthamiana | Abscisic acid | Alazem et al. (2014); Alazem and Lin (2017) |
| Cucumber mosaic virus | Cucumber | Gibberellin | Clarke et al. (1999, 2002) |
| Cucumber mosaic virus | Nicotiana tabacum | Ethylene | Chaudhry et al. (1998) |
| Curly top virus | Tomato | Auxin transportation | Clarke et al. (2002) |
| Mal de Rio Cuarto virus | Maize | Auxin | Abdala et al. (1999) |
| Mal de Rio Cuarto virus | Oryza sativa | Auxin | de Haro et al. (2019) |
| Plum pox virus | Peach |
Gibberellin Abscisic acid Salicylic acid Jasmonic acid |
Dehkordi et al. (2018) |
| Potato leaf-roll virus | Solanum tuberosum | Auxin | Clarke et al. (1999, 2002) |
| Potato virus M | Solanum tuberosum | Jasmonates | Clarke et al. (2002); Ravnikar et al. (1990) |
| Potato virus Y | Solanum tuberosum | Cytokinin-9–glucosylation | Dermastia et al. (1995) |
| Potato virus Y | Solanum tuberosum | Jasmonates | Petrovič et al. (1997) |
| Potato virus Y | Solanum tuberosum | Salicylic acid | Baebler et al. (2014) |
| Potyvirus | Solanum tuberosum | Auxin synthesis | Muletarova et al. (1995) |
| Rice black streaked dwarf virus | Oryza sativa |
Auxin signalling pathway Aux/IAA |
Zhang et al. (2019) |
| Rice black streaked dwarf virus | Oryza sativa | Brassinosteroid | He et al. (2017) |
| Rice dwarf virus | Oryza sativa | Auxin signalling | Jin et al. (2016) |
| Rice dwarf virus | Oryza sativa |
Ethylene Gibberellin |
Zhao et al. (2017); Zhu et al. (2005) |
| Rice tungro virus | Oryza sativa |
Gibberellin Cytokinin |
Sridhar et al. (1978) |
| Tobacco Mosaic virus | Nicotiana tabacum var. Mammoth |
Indole-3-acetic Phenylacetic Abscisic acid |
Rajagopal (1977) |
| Tobacco mosaic virus |
Nicotiana tabacum Xanthi tobacco |
Abscisic acid Cytokinin |
Alazem et al. (2014); Alazem and Lin (2017); Balázs et al. (1977) |
| Tobacco mosaic virus | Tomato | Aux/IAA | Padmanabhan et al. (2008) |
| Tobacco mosaic virus | Nicotiana tabacum | Ethylene | Siefert et al. (1995) |
| Tobacco mosaic virus |
Nicotiana tabacum Nicotiana benthamiana |
Salicylic acid | War et al. (2011); Diao et al. (2019) |
| Tobacco mosaic virus | Nicotiana tabacum | Brassinolide | Nakashita et al. (2003) |
| Tobacco necrosis virus | Chenopodium amaranticolor | Cytokinin | Faccioli et al. (1984) |
| Tobacco ringspot virus | Vigna unguiculata | Cytokinin | Kuriger and Agrios (1977); Tavantzis et al. (1979) |
| Tobacco ringspot virus | Tobacco | Cytokinin | Kuriger and Agrios (1977); Tavantzis et al. (1979) |
| White clover mosaic virus | Phaseolus vulgaris | Jasmonic acid dihydrojasmonic | Clarke et al. (2000a) |
Even though the several functions of ABA in response to bacterial or fungal pathogens infestation have been comprehensively studied, the interaction between ABA and plant viruses is less well understood and the only reported studies have shown that ABA enhances resistance. During virus infection, ABA increased callose deposition and thereby, limited virus movement (Rezzonico et al. 1998). Callose deposition at plasmodesmata and the RNA silencing pathway are thought to be the main ABA-dependent antiviral mechanisms (Alazem and Lin 2017).
Cytokinins
The cytokinins (CKs) have significantly influenced agriculturally important processes including growth, nutrients responses, and response against biotic and abiotic stresses. Besides, CK has been involved in the cross-talk with other plant hormones to enhance the immunity of plants during viral pathogenesis. Thus, CKs play an essential and diverse role in the growth and development of plants by enhancement of resistance against the plant pathogenic viruses. Several reports have enlightened the effect of CKs in the development of resistance in plants against viral pathogens (Table 1). However, it is also revealed that the virus infections cause changes in the endogenous level of CK in the plants (Kieber and Schaller 2018; Alazem and Lin 2015; Jameson and Clarke 2002).
Accumulating reports suggest that virus infections significantly affect and modify the activity and quantity of the CK level, which could be either reduced or increased, subject to the virus infection. Tobacco ringspot virus declines the CK level (decline Zeatin and/or Zeatin Riboside) in the cowpea root exudates and tissue and tobacco leaves (Kuriger and Agrios 1977; Tavantzis et al. 1979). Thompson et al. 1983 suggests that Tomato spotted wilt virus infection results in a decline in the cytokinin level (Zeatin and/or Zeatin Riboside), which affect the water content of aerial parts of plants (Thompson et al. 1983; Jameson and Clarke 2002). Arabidopsis thaliana seedlings infected with CMV show change in root growth patterns, which are accompanied by a decrease in the cytokinin levels (trans-zeatin riboside and dihydrozeatin riboside), as a likely result of the regulation of genes involved in cytokinin biosynthesis (AtIPT, isopentenyl transferases gene) and cytokinin degradation (AtCKX, cytokinin oxidase gene) (Vitti et al. 2013). Potato leafroll virus (PLRV) and Potato virus Y (PVY) infections lead in the reduction to endogenous content of CK level (ZR) in potato. Furthermore, viruses like Tungro virus infecting susceptible rice and Bean golden mosaic virus-infected beans increase the cytokinin-like activity (Fazio 1981; Sridhar et al. 1978). But in Chenopodium amaranticolor, both systemically resistant leaves and Tobacco necrosis virus-infected leaves showed an escalation in the cytokinin-like activity (Zeatin and/or Zeatin Riboside, and isopentenyladenine/isopentenyladenosine) (Faccioli et al. 1984). The pathogenicity proteins (AL2/C2) of begomoviruses and curtoviruses have inhibited the action of adenosine kinase (ADK) that affects the expression of primary cytokinin-responsive genes in Arabidopsis during pathogen attack (Baliji et al. 2010). It is also suggested that up-regulation in the expression of endogenous cytokinin-responsive genes, which is accompanied by the increase in CK levels and an increase in the pool of bioactive CKs (by preventing their phosphorylation) in plants infected with geminivirus (Cabbage leaf curl virus and Tomato golden mosaic virus, and Spinach curly top virus). This strategy of viral infection provides a favorable condition for their replication and maintenance of the viral genome (Baliji et al. 2010). Additionally, it is reported that it affects the active forms of cytokinins upon viral infection leading to alterations in its metabolism (zeatin-O-glucoside and zeatin-O-glucoside riboside) in tobacco (Whenham 1989). Dermastia et al. (1995) reported the changes in cytokinin metabolism after the infection of Potato Virus Y (PVY) in potato plants (Dermastia et al. 1995). White clover mosaic virus-infected bean plants showed the alteration in cytokinin metabolism, showing the effect of the virus on the cytokinin biosynthesis, catabolism, and/or conjugation. These investigations help to understand that enhanced virus titer (due to virus replication) is associated with reduced activity of CK, which further confirms the involvement of CK in the host–virus interaction (Clarke et al. 1999).
Although, the alteration in the level of CK contents also helps to induce resistance to other viruses e.g. systemic infection on tobacco plant by CMV helps to induce resistance against TMV (Sziráki et al. 1980). The increase in endogenous level CK concentration in the noninfected leaves of Xanthi tobacco causes systematically control the TMV infection in the upper leaves, suggesting systemic acquired resistance (SAR) plays important role in controlling the challenged infection (Balázs et al. 1977). In another aspect, the virus infection also reduced the active CK and in reactive oxygen species (ROS) scavenging enzymes which reveal that the low level of ROS species helps to viral replication (Clarke et al. 2002). In addition, antisense RNA inhibition of S-adenosylhomocysteine hydrolase exhibits cytokinins accumulation in tobacco, which may be responsible for enhanced resistance to a broad spectrum of plant virus e.g. TMV, CMV, Potato virus X, and PVY (Masuta et al. 1995). The higher level of CKs in plants increased the expression of SA-responsive defense genes during viral pathogenesis. Interestingly, CKs are actively involved in the development of resistance against phytopathogens via increasing expression of pathogenessis-related proteins in Arabidopsis (Choi et al. 2010).
Auxins
Auxin is a potent plant growth and development hormone that directly sabotaged by viral components and it can stimulate the differential growth responses against gravity or light stimuli. But, virus–host interaction affects the activity and concentration of auxin in plants (Alazem and Lin 2015; Jameson and Clarke 2002) (Table 2).
The several studies suggest that the virus infection increased the auxin levels, for example, Abdala et al. (1999) reported that the Mal de Rio Cuarto virus (MRCV) infection of maize increases the higher levels of auxin, such as Indole 3-acetic acid (IAA), in comparison to healthy tissue (Abdala et al. 1999). In wheat (Triticum aestivum), the infection of MRCV has increased the expression of auxin level in leaves (de Haro et al. 2019). Some reports described that the replication protein of TMV is disrupted auxin signaling by interaction with Aux/IAA proteins and develop the disease symptoms in a plant (Collum and Culver 2016). Some studies reported that virus infections PLRV and Potato virus A (PVA) reduced both auxin activity and concentration (Muletarova et al. 1995; Pennazio and Roggero 1996; Li et al. 2013). Interestingly, peroxidase isoenzymes play a role as inhibitors and suppressors of auxin synthesis during PVA infection on potato (Muletarova et al. 1995). But, it is reported that the white clover mosaic potexvirus infection rapidly increases the peroxidase activity in Phaseolus vulgaris plants (Clarke et al. 2002).
The TMV replication protein disturbs auxin signaling through an interaction with specific members of Aux/IAA family TMV, which acts as a negative regulator for auxin signaling and thereby enhances the infection process in older mature tissues (Padmanabhan et al. 2008). In rice, the P2 protein (capsid protein) of Rice dwarf virus (RDV) disrupted the auxin signaling by their interaction with Aux/IAA proteins (Jin et al. 2016). These results signify that viral proteins association with Aux/IAA protein leads to cellular reprogramming to make a compatible condition for the virus pathogenesis. Recent reports also revealed that auxin signaling pathways in rice are affected by the infection of different viruses, such as RBSDV, Southern rice black-streaked dwarf virus (SRBSDV) and Rice stripe virus (RSV), in a distinct mode of action (Zhang et al. 2019, 2020). It reveals that higher expression of auxin signalling in rice helps to develop the resistance against RBSDV (Zhang et al. 2019). The P2 of RSV and the SP8 of SRBSDV associate with rice Auxin RF family member ARF17 to expedite virus infection. SRBSDV P8 disrupts the auxin pathway by repressing the transcriptional activation activity of OsARF17 and interfering with OsARF-OsARF dimerization. RSV P2 associate with OsARF17 through the DNA binding domain and inhibited the OsARF17 DNA binding ability to the promoter of auxin response genes (Zhang et al. 2020).
There is a gap in the metabolic processes that leads to the escalation of auxin in virus-infected plants. But, reports suggest that the development of resistance by the interference of viral proteins with auxin signaling. Arabidopsis mutant vid1 (virus-inducible dwarf) resemble the healthy without any infection. However, The Turnip vein clearing virus (TVCV) infection in Arabidopsis vid1 mutant causes a dwarfed phenotype, loss of apical dominance, and interfere with the intracellular transport system. Interestingly, Sheng et al (1998) show the reversal of TVCV-infected vid1 plant deformities by exogenous application of auxin (Sheng et al. 1998). Additionally, the early report also indicates that the Curly top virus infection in tomato reduces the transportation of auxin (Jameson and Clarke 2002).
Gibberellins
The gibberellins (GA) are plant growth regulators that control plant development by their effects on stem growth via cell division and elongation. Several previous studies suggest that the virus infection on plants changed the GA level and decreased their activity (Table 2) e.g. Barley yellow dwarf virus reduces the GA3-like activity in barley (Russell and Kimmins 1971), Rice tungro virus decreases the free and bound GA like substances in plants (Sridhar et al. 1978). RNA virus CMV infect cucumber seedlings, which also reduced the GA-like substances such as GA1- and GA3-like. In this context, the less GA-like activity was reported in infected leaves of Citrus sinensis by Mosaic virus that causes leaf yellowing and abscission in plants (Rao et al. 1977). However, it is also reported that increased levels of GA3 in single PVY-infected, and PLRV and PVY co-infected shoots in potato (Li et al. 2013).
However, the infection of viruses in plants also disrupts the biosynthesis of GA. The ent-kaurene oxidase is involved in the synthesis of GA and participated in the dwarfing of rice. The Rice dwarf virus (RDV) outer capsid protein P2 is interacted with ent-kaurene oxidase in rice (Oryza sativa) and disrupts its activity, which results in the level of endogenous GA1 has been shown to decrease (Zhu et al. 2005). Application of GA3 exogenously to rice plants infected with RDV has restored the normal growth phenotypes. The inoculation of Plum pox virus (PPV) in peach (Prunus persica) plants significantly elevated the level of GA3 and reduced GA4. Further, grafting of “Garrigues” almond onto the PPV-inoculated peach showed a reverse effect and reduced the level of GA3 (Dehkordi et al. 2018). In this context, GA has played a central role in the development of resistance in plants affected by the virus. The virus interaction in host plants also imbalance the GA regulation, which develop symptoms of the disease. More significantly, the qualitative difference in the metabolism of 3H-GA3 between healthy and infected cucumber plants by CMV has been shown (Ben-Tal and Marco 1980).
Du et al. (2014) also revealed that the Fny-2b protein of CMV prohibits miR159 function, which interacts with the GA-mediated MYB33 and MYB65 transcript, and thereby causes Arabidopsis developmental deficiencies with Fny-CMV infection (Du et al. 2014). Kriznik et al. 2017 described the role of microRNAs (miR167) and small interfering RNAs (siRNAs- phasiRNA931) in the silencing of endogenous RNA and play an important role in the development of plant immunity (Kriznik et al. 2017). These sRNAs-gibberellin regulatory circuit is identified before the viral multiplication (Potato virus Y tuber necrosis strain-PVYNTN) in Potato cv. Desiree. The two endogenous predicated siRNAs viz. miR167 and phasiRNA931 regulate the GA biosynthesis genes (GA20-oxidase and GA3-oxidase). However, elevated expression of phasiRNA931 decrease the level of GA3-oxidase transcripts. This regulation describes the link between sRNAs and gibberellin (GA) biosynthesis that represents the connection between the immune and signaling pathway of development (Kriznik et al. 2017).
Ethylene
Ethylene (ET) is a multifunctional plant hormone that plays an important role to control the growth and senescence of plants associated with other hormones. The change in the level of ethylene is directly or indirectly associated with the regulation of the plant’s life. The virus infection in plants alters the regulation of ethylene and promotes the changes in growth and senescence of plants. Many studies suggest that ethylene modulate host defense in both positive and negative ways (Table 2).
Ethylene production appears to be enhanced by the virus infection, which is associated with the expansion of necrotic or chlorotic lesions and abnormal growth, hypersensitive response (HR) induction in plants (Jameson and Clarke 2002). The ACC (1-aminocyclopropane-1-carboxylic acid) is the metabolic precursor of ethylene and in the rate-limiting step of ET synthesis, ACC synthase converts S-AdoMet (S-adenosylmethionine) to ACC. Knoester et al. (1995) reveal that the TMV infection in tobacco plants enhances the transcription of genes which are coding for ACC oxidase and ACC synthase (Knoester et al. 1995). Another virus CMV infected tobacco leaves shows an increase in ethylene production, which is accompanied by a rise in ACC level and ACC synthase and ACC oxidase activities in systemically-CMV-infected leaves (Chaudhry et al. 1998). Another study proposed that Turnip mosaic virus may modulate ethylene responses to increase susceptibility to viral infection in Arabidopsis (Casteel et al. 2015).
However, the stimulation of a key component as S-adenosyl-l-methionine synthetase (SAMS) in the ethylene synthesis pathway by Rice dwarf virus (RDV) helps to trigger ethylene production in plants and developing a susceptibility to RDV (Zhao et al. 2017). Some research findings elaborated that ethylene production can induce the pathogen-related (PR) proteins in virus-infected plants. In this context, Ohtsubo et al. (1999) suggest that due to enhanced ACC level facilitate the high expression of defense-induced pathogenesis genes (PR-1 and proteinase inhibitor II genes) (Ohtsubo et al. 1999). The P6 protein of CaMV is involved in the interaction with the ethylene pathway in Arabidopsis transgenic plants. The interference of P6 protein with ethylene signaling promotes the development of symptoms and is involved in the RNAi suppression during the pathogenesis (Geri et al. 2004).
Jasmonic acid
Jasmonates (JAs) including jasmonic acid (JA) is lipid-derived stress hormones and synthesized from α-linolenic acid (α-LeA/18:3) via the octadecanoid pathway. They regulate the adaptation in plants to biotic, abiotic stresses and play an important function such as primary root growth, reproductive development, and leaf senescence. Albeit, they control the plant defenses against various pathogen infection. Furthermore, the interaction between plant and viruses affects the production of JA (Table 2), e.g. Potato virus M infected potato has an enhanced JA concentration in meristematic tissue (Ravnikar et al. 1990), while the enhanced level of JA reported in roots and reduced in shoots of PVY-infected potato plantlets in comparison to healthy potato plantlets (Petrovič et al. 1997). The Phaseolus vulgaris infected by White clover mosaic virus (WClMV) increased the level of JA and dihydrojasmonic acid, which reveals that the wound site (damaged cellular membranes) produces the linolenic acid that synthesizes the JA after virus infection (Clarke et al. 2000b).
Endogenous JA is shown to accumulate in plant–virus incompatible interactions (Dhondt et al. 2000; Kovač et al. 2009), for example, tobacco leaves respond hypersensitively to TMV due to increase of JA and 12-oxophytodienoic thereby phospholipase A2 (PLA2) activity increase and ultimately provide a hypersensitive response to viral pathogens. A distinct increase of JA level is noted in PVY virus-inoculated leaves of the resistant cultivar in comparison of the susceptible cultivar of potato (Kovač et al. 2009). Other reports suggest invade the host defense mechanism by inducing miR319 by Rice ragged stunt virus RRSV infection in rice suppresses JA-mediated defense to enable viral pathogenesis (Zhang et al. 2016). A study demonstrated that the silencing of MAPKs, such as wound-induced protein kinase (WIPK) and SA-induced protein kinase (SIPK) results in the reduction of JA level and enhanced local resistance to TMV in tobacco (Kobayashi et al. 2010). The interaction of the C2 protein of geminivirus with the catalytic subunit of the COP9 signalosome compromised the activity of SCF ubiquitin ligase and altered its activity to regulate JA. The targeting of SCF ubiquitination by C2 providing a mechanism to a virus in the modulation of resistance in the host. The accumulation of a higher level of JA is not favorable all the time in viral pathogenesis. In this context, a study highlights that the JA mediated defense system in plant adversely affect it after the establishment of matured viral infection such as geminivirus (Lozano-Durán et al. 2011).
Salicylic acid
Salicylic acid (SA) is a phytohormone synthesized from trans-cinnamic acid via benzoic acid and it plays an important role in the induction of plant defense during biotic and abiotic stresses (Agarwal et al. 2020). SA is continuously involved in different processes of resistance during viral pathogenesis such as SAR, R gene-mediated resistance, and basal defense mechanisms. Although, activation of SA biosynthesis and signaling promotes the PR (pathogenesis-related) proteins, callose deposition, induction of hypersensitive response, and accumulation of ROS in plants (Collum and Culver 2016). The reduction of endogenous SA leads to the defective response of defense mechanisms and increasing the susceptibility in plants during viral infection.
The viral infection increased the concentration of salicylic acid (glucose conjugate and methyl ester) in plants e.g. the benzoic acid 2-hydroxylase catalyzes the biosynthesis of salicylic acid from benzoic acid and is expressed constitutively in tobacco plant but inoculation of TMV enhanced the production of SA which denotes de novo biosynthesis of SA during viral infection (León et al. 1995; Jameson and Clarke 2002).
However, ATAF2 is a NAC domain transcription factor and involved to regulate the basal defense in a host. The degradation of ATAF2 by TMV results in the suppression of defense response mediated by SA (Wang et al. 2009; Wang and Culver 2012). The infection of PVY in transgenic NahG (salicylate hydroxylase) potato (Solanum tuberosum L.) plants leads to lacking SA accumulation and develop the symptom of the disease (Baebler et al. 2014). The CaMV P6 protein is needed for the translation of 35S RNA as well as acts as an RNA silencing suppressor. This protein in the Arabidopsis plant indirectly disrupts the SA signaling. The crucifer strain of TMV (TMV-Cg) coat protein (CgCP) has suppressed the SA signaling in crucifer by stabilization of DELLA proteins. The DELLA proteins act as negative regulators of GA signaling but repressed the SA defense responses by modulation of the antagonistic cross-talk between SA and JA pathways (Rodriguez et al. 2014). The TMV infection in N. benthamiana has induced the expression of SA marker gene NbPR1a and significantly increases the level of SA (Diao et al. 2019).
However, the cumulative action of hormones such as cytokinin, JA, and SA show interfere with the replication of white clover mosaic virus (WClMV) at the sub-genomic dsRNA level in the inoculated tissue reported by Clarke et al. (2000a). Hormone treatment on Phaseolus vulgaris plants show partial inhibition of WClMV in infected primary leaves. This inhibition revealed that a decrease in viral mRNA and viral coat protein accumulation. The salicylic acid treatment causes increased phenylalanine ammonia-lyase, NPR1, PR1, and HSP70 gene expression, which contribute to pathogen resistance in plants (Gális et al. 2004).
Brassinosteroids
Brassinosteroids (BRs) are identified as the sixth class of steroidal hormones produced by plants in the regulation of development and growth as well as a response to challenge the environmental condition for proper maintenance of the plant homeostasis. The BRs genes are induced during the viral pathogenesis by TMV (Alazem and Lin 2015). The development of disease resistance in tobacco and rice plants is affected by BRs (Nakashita et al. 2003). Nakashita et al. (2003) revealed that brassinolide (BL)-induced the resistance in tobacco against TMV, which could be due to N gene‐mediated resistance (Nakashita et al. 2003). BL-pretreated Arabidopsis plants show an increase in the resistance against the CMV infection, confirming the role of BRs in plant innate immunity. BR-induced CMV tolerance is associated with and defense-induced genes expression and the antioxidant system by enhancing antioxidative enzymes’ activities, such as superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), and ascorbate peroxidase (APX) (Zhang et al. 2015). Tobacco rattle virus-based VIGS method indicates that the BR signaling pathway, MAPK cascades, and NADPH oxidase play significant roles in BR-mediated TMV defense in tobacco (Deng et al. 2016a). Another study proposed that the local treatment of lower leaves to BRs efficiently prompted systemic virus resistance, with a reduction in TMV infection in the upper untreated leaves of N. benthamiana, which is complemented by accumulations of ROS such as H2O2 and Nitric oxide (Deng et al. 2016b). BR-induced inhibition of TMV replication is due to the sifting of H2O2 or NO in upper leaves, which could activate the immunity system, and thus result in the enhancement of virus resistance (Deng et al. 2016b).
However, BR is responsible to increase the susceptibility in rice plants against RBSDV by JA-mediated suppression of BR (He et al. 2017). The interaction of Sweet potato leaf curl virus (SPLCV-JS) C4 protein with brassinosteroid-insensitive 2 (AtBIN2) in the plasma membrane of N. bethamiana result re-localization of AtBIN2-interacting proteins viz. AtBES1/AtBZR1. The relocalization of AtBIN2-interacting proteins altered the expression of BR responsive genes which activating the BR-signaling pathway (Bi et al. 2017).
Conclusion
Several plants including both medicinal, ornamental, weeds, and cultivated crops are simultaneously exposed to diverse pathogens including viruses. Viruses are an obligate parasite and require the living tissue of an organism for their replication. The host–virus interaction results in the development of various strategies to strengthen the plant defense mechanism. However, the plant viruses encounter the plant defense mechanism by modulating the functions of different host factors. Besides, the virus plays an important role to trigger the plant secondary compounds (i.e. alkaloids, phenol, flavonoids, and terpenes, etc.) and alter the biosynthesis of plant hormones (e.g., abscisic acid, cytokinin, auxin, gibberellin, jasmonic acid, ethylene, salicylic acid, and brassinosteroids). Additionally, this review emphasizes on the regulation of hormone biosynthesis by virus–phytohormone interaction that triggers the signaling pathways. Besides, in recent years, the technical advancement in mass spectrometry and high-throughput metabolite profiling helps to discover the roles of secondary metabolites in pathogen defense mechanisms.
Acknowledgements
JM duly acknowledge Academy of Scientific and Innovative Research (AcSIR), Ghaziabad, Uttar Pradesh, India.
Author contributions
JM and RS drafted and wrote the manuscript, and involved in drawing the figures and tables in this review article. PKT reviewed and evaluated the manuscript. PCV reviewed and overall guided the manuscript.
Funding
This work was supported by the SERB, New Delhi, Government of India [Project No. GAP-3401]. Institute Manuscript Number is CSIR-NBRI_ MS/2020/06/25.
Compliance with ethical standards
Conflict of interest
The authors declared no conflict of interest with respect to the authorship, and publication of this review article.
References
- Abdala G, Milrad S, Vigliocco A, Lorenzo E, Pharis R, Wanner G. Hyperauxinity in diseased leaves affected by Mal de Rio Cuarto Virus (MRCV) Biocell. 1999;23(1):13–18. [Google Scholar]
- Agarwal N, Srivastava R, Verma A, Rai KM, Singh B, Verma PC. Unravelling cotton nonexpressor of pathogenesis-related 1(NPR1)-like genes family: evolutionary analysis and putative role in fiber development and defense pathway. Plants. 2020;9(8):999. doi: 10.3390/plants9080999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alazem M, Lin NS. Roles of plant hormones in the regulation of host–virus interactions. Mol Plant Pathol. 2015;16(5):529–540. doi: 10.1111/mpp.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alazem M, Lin NS. Antiviral roles of abscisic acid in plants. Front Plant Sci. 2017;8:1760. doi: 10.3389/fpls.2017.01760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alazem M, Lin KY, Lin NS. The abscisic acid pathway has multifaceted effects on the accumulation of Bamboo mosaic virus. Mol Plant Microbe Interact. 2014;27(2):177–189. doi: 10.1094/MPMI-08-13-0216-R. [DOI] [PubMed] [Google Scholar]
- Alazem M, He MH, Moffett P, Lin NS. Abscisic acid induces resistance against Bamboo mosaic virus through argonaute2 and 3. Plant Physiol. 2017;174(1):339–355. doi: 10.1104/pp.16.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alazem M, Tseng K-C, Chang W-C, Seo J-K, Kim K-H. Elements involved in the Rsv3-mediated extreme resistance against an avirulent strain of soybean mosaic virus. Viruses. 2018;10(11):581. doi: 10.3390/v10110581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alazem M, Kim KH, Lin NS. Effects of abscisic acid and salicylic acid on gene expression in the antiviral RNA silencing pathway in Arabidopsis. Int J Mol Sci. 2019 doi: 10.3390/ijms20102538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson NJ, Urwin PE. The interaction of plant biotic and abiotic stresses: from genes to the field. J Exp Bot. 2012;63(10):3523–3543. doi: 10.1093/jxb/ers100. [DOI] [PubMed] [Google Scholar]
- Baebler Š, Witek K, Petek M, Stare K, Tušek-Žnidarič M, Pompe-Novak M, Renaut J, Szajko K, Strzelczyk-Żyta D, Marczewski W, Morgiewicz K, Gruden K, Hennig J. Salicylic acid is an indispensable component of the Ny-1 resistance-gene-mediated response against Potato virus Y infection in potato. J Exp Bot. 2014;65(4):1095–1109. doi: 10.1093/jxb/ert447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balázs E, Sziráki I, Király Z. The rôle of cytokinins in the systemic acquired resistance of tobacco hypersensitive to tobacco mosaic virus. Physiol Plant Pathol. 1977;11(1):29–IN23. doi: 10.1016/S0048-4059(77)80003-9. [DOI] [Google Scholar]
- Baliji S, Lacatus G, Sunter G. The interaction between geminivirus pathogenicity proteins and adenosine kinase leads to increased expression of primary cytokinin-responsive genes. Virology. 2010;402(2):238–247. doi: 10.1016/j.virol.2010.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Tal Y, Marco S. Qualitative changes in cucumber gibberellins following cucumber mosaic virus infection. Physiol Plant Pathol. 1980;16(3):327–336. doi: 10.1016/S0048-4059(80)80004-X. [DOI] [Google Scholar]
- Bi H, Fan W, Zhang P. C4 protein of sweet potato leaf curl virus regulates brassinosteroid signaling pathway through interaction with AtBIN2 and affects male fertility in Arabidopsis. Front Plant Sci. 2017;8:1689–1689. doi: 10.3389/fpls.2017.01689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Ulate B, Hopfer H, Figueroa-Balderas R, Ye Z, Rivero RM, Albacete A, Pérez-Alfocea F, Koyama R, Anderson MM, Smith RJ, Ebeler SE, Cantu D. Red blotch disease alters grape berry development and metabolism by interfering with the transcriptional and hormonal regulation of ripening. J Exp Bot. 2017;68(5):1225–1238. doi: 10.1093/jxb/erw506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calil IP, Fontes EPB. Plant immunity against viruses: antiviral immune receptors in focus. Ann Bot. 2017;119(5):711–723. doi: 10.1093/aob/mcw200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway A, Liu W, Andrianov V, Stenzler L, Zhao J, Wettlaufer S, Jayakumar P, Howell SH. Characterization of cauliflower mosaic virus (CaMV) resistance in virus-resistant ecotypes of Arabidopsis. Mol Plant Microbe Interact. 1996;9(9):810–818. doi: 10.1094/mpmi-9-0810. [DOI] [PubMed] [Google Scholar]
- Casteel CL, De Alwis M, Bak A, Dong H, Whitham SA, Jander G. Disruption of ethylene responses by turnip mosaic virus mediates suppression of plant defense against the green peach aphid vector. Plant Physiol. 2015;169(1):209–218. doi: 10.1104/pp.15.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry Z, Yoshioka T, Satoh S, Hase S, Ehara Y. Stimulated ethylene production in tobacco (Nicotiana tabacum L. cv. Ky 57) leaves infected systemically with cucumber mosaic virus yellow strain. Plant Sci. 1998;131(2):123–130. doi: 10.1016/S0168-9452(97)00257-4. [DOI] [Google Scholar]
- Chen S, Yu N, Yang S, Zhong B, Lan H. Identification of Telosma mosaic virus infection in Passiflora edulis and its impact on phytochemical contents. Virol J. 2018;15(1):168. doi: 10.1186/s12985-018-1084-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinestra SC, Facchinetti C, Curvetto NR, Marinangeli PA. Detection and frequency of lily viruses in Argentina. Plant Dis. 2010;94(10):1188–1194. doi: 10.1094/pdis-07-09-0419. [DOI] [PubMed] [Google Scholar]
- Choi J, Huh SU, Kojima M, Sakakibara H, Paek KH, Hwang I. The cytokinin-activated transcription factor ARR2 promotes plant immunity via TGA3/NPR1-dependent salicylic acid signaling in Arabidopsis. Dev Cell. 2010;19(2):284–295. doi: 10.1016/j.devcel.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Chong J, Baltz R, Schmitt C, Beffa R, Fritig B, Saindrenan P. Downregulation of a pathogen-responsive tobacco UDP-Glc:phenylpropanoid glucosyltransferase reduces scopoletin glucoside accumulation, enhances oxidative stress, and weakens virus resistance. Plant Cell. 2002;14(5):1093–1107. doi: 10.1105/tpc.010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke SF, McKenzie MJ, Burritt DJ, Guy PL, Jameson PE. Influence of white clover mosaic potexvirus infection on the endogenous cytokinin content of bean. Plant Physiol. 1999;120(2):547–552. doi: 10.1104/pp.120.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke SF, Burritt DJ, Jameson PE, Guy PL. Effects of plant hormones on white clover mosaic potexvirus double stranded RNA. Plant Pathol. 2000;49(4):428–434. doi: 10.1046/j.1365-3059.2000.00476.x. [DOI] [Google Scholar]
- Clarke SF, Guy PL, Jameson PE, Schmierer D, Burritt DJ. Influence of white clover mosaic potexvirus infection on the endogenous levels of jasmonic acid and related compounds in Phaseolus vulgaris L. seedlings. J Plant Physiol. 2000;156(4):433–437. doi: 10.1016/S0176-1617(00)80155-8. [DOI] [Google Scholar]
- Clarke SF, Guy PL, Burritt DJ, Jameson PE. Changes in the activities of antioxidant enzymes in response to virus infection and hormone treatment. Physiol Plant. 2002;114(2):157–164. doi: 10.1034/j.1399-3054.2002.1140201.x. [DOI] [PubMed] [Google Scholar]
- Collum TD, Culver JN. The impact of phytohormones on virus infection and disease. Curr Opin Virol. 2016;17:25–31. doi: 10.1016/j.coviro.2015.11.003. [DOI] [PubMed] [Google Scholar]
- Costet L, Fritig B, Kauffmann S. Scopoletin expression in elicitor-treated and tobacco mosaic virus-infected tobacco plants. Physiol Plant. 2002;115(2):228–235. doi: 10.1034/j.1399-3054.2002.1150208.x. [DOI] [PubMed] [Google Scholar]
- Culver JN, Padmanabhan MS. Virus-induced disease: altering host physiology one interaction at a time. Annu Rev Phytopathol. 2007;45(1):221–243. doi: 10.1146/annurev.phyto.45.062806.094422. [DOI] [PubMed] [Google Scholar]
- de Haro LA, Arellano SM, Novák O, Feil R, Dumón AD, Mattio MF, Tarkowská D, Llauger G, Strnad M, Lunn JE, Pearce S, Figueroa CM, del Vas M. Mal de Río Cuarto virus infection causes hormone imbalance and sugar accumulation in wheat leaves. BMC Plant Biol. 2019;19(1):112. doi: 10.1186/s12870-019-1709-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehkordi AN, Rubio M, Babaeian N, Albacete A, Martínez-Gómez P. Phytohormone signaling of the resistance to Plum pox virus (PPV, sharka disease) induced by almond (Prunus dulcis (Miller) Webb) grafting to peach (P. persica L. Batsch) Viruses. 2018;10(5):238. doi: 10.3390/v10050238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey DA, Pathirana MS, Wobbe KK, Klessig DF. Identification of an Arabidopsis locus required for resistance to turnip crinkle virus. Plant J. 1997;11(2):301–311. doi: 10.1046/j.1365-313x.1997.11020301.x. [DOI] [PubMed] [Google Scholar]
- Deng XG, Zhu T, Peng XJ, Xi DH, Guo H, Yin Y, Zhang DW, Lin HH. Role of brassinosteroid signaling in modulating Tobacco mosaic virus resistance in Nicotiana benthamiana. Sci Rep. 2016;6:20579. doi: 10.1038/srep20579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng XG, Zhu T, Zou LJ, Han XY, Zhou X, Xi DH, Zhang DW, Lin HH. Orchestration of hydrogen peroxide and nitric oxide in brassinosteroid-mediated systemic virus resistance in Nicotiana benthamiana. Plant J. 2016;85(4):478–493. doi: 10.1111/tpj.13120. [DOI] [PubMed] [Google Scholar]
- Dermastia M, Ravnikar M, Kovač M. Increased cytokinin-9-glucosylation in roots of susceptible Solanum tuberosum cultivar infected by potato virus Y. Mol Plant Microbe Interact. 1995;8(2):327–330. doi: 10.1094/MPMI-8-0327. [DOI] [Google Scholar]
- Dhondt S, Geoffroy P, Stelmach BA, Legrand M, Heitz T. Soluble phospholipase A2 activity is induced before oxylipin accumulation in tobacco mosaic virus-infected tobacco leaves and is contributed by patatin-like enzymes. Plant J. 2000;23(4):431–440. doi: 10.1046/j.1365-313x.2000.00802.x. [DOI] [PubMed] [Google Scholar]
- Diao P, Zhang Q, Sun H, Ma W, Cao A, Yu R, Wang J, Niu Y, Wuriyanghan H. miR403a and SA are involved in NbAGO2 mediated antiviral defenses against TMV infection in Nicotiana benthamiana. Genes (Basel) 2019 doi: 10.3390/genes10070526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Pendon JA, Li F, Li WX, Ding SW. Suppression of antiviral silencing by cucumber mosaic virus 2b protein in Arabidopsis is associated with drastically reduced accumulation of three classes of viral small interfering RNAs. Plant Cell. 2007;19(6):2053–2063. doi: 10.1105/tpc.106.047449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit G, Srivastava A, Rai KM, Dubey RS, Srivastava R, Verma PC. Distinct defensive activity of phenolics and phenylpropanoid pathway genes in different cotton varieties toward chewing pests. Plant Signal Behav. 2020;15(5):1747689. doi: 10.1080/15592324.2020.1747689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Z, Chen A, Chen W, Westwood JH, Baulcombe DC, Carr JP. Using a viral vector to reveal the role of MicroRNA159 in disease symptom induction by a severe strain of Cucumber mosaic virus. Plant Physiol. 2014;164(3):1378–1388. doi: 10.1104/pp.113.232090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faccioli G, Rubies-Autonell C, Albertini R. Role of cytokinins in the acquired resistance of Chenopodium amaranticolor towards an infection of Tobacco necrosis virus. Phytopathol Mediterr. 1984;23(1):15–22. [Google Scholar]
- Fazio Gd. Cytokinin levels in healthy and bean golden mosaic virus (BGMV) infected bean plants (Phaseolus vulgaris) Rev Brasil Botanica. 1981;4(2):57–61. [Google Scholar]
- Ferdes M (2018) Antimicrobial compounds from plants. In: Budimir A (ed) Fighting Antimicrobial Resistance. IAPC Publishing, Zagreb, Croatia, pp 243-271. 10.5599/obp.15.15
- Gális I, Smith JL, Jameson PE. Salicylic acid-, but not cytokinin-induced, resistance to WClMV is associated with increased expression of SA-dependent resistance genes in Phaseolus vulgaris. J Plant Physiol. 2004;161(4):459–466. doi: 10.1078/0176-1617-01255. [DOI] [PubMed] [Google Scholar]
- Geri C, Love AJ, Cecchini E, Barrett SJ, Laird J, Covey SN, Milner JJ. Arabidopsis mutants that suppress the phenotype induced by transgene-mediated expression of cauliflower mosaic virus (CaMV) gene VI are less susceptible to CaMV-infection and show reduced ethylene sensitivity. Plant Mol Biol. 2004;56(1):111–124. doi: 10.1007/s11103-004-2649-x. [DOI] [PubMed] [Google Scholar]
- Gutzeit HO, Ludwig-M J. Plant natural products: synthesis, biological functions and practical applications. Weinheim: John Wiley & Sons; 2014. [Google Scholar]
- Hamidun B, Dusik L, Bunawan SN, Amin NM. Rice tungro disease: from identification to disease control. World Appl Sci J. 2014;31:1221–1226. [Google Scholar]
- He Y, Zhang H, Sun Z, Li J, Hong G, Zhu Q, Zhou X, MacFarlane S, Yan F, Chen J. Jasmonic acid-mediated defense suppresses brassinosteroid-mediated susceptibility to Rice black streaked dwarf virus infection in rice. New Phytol. 2017;214(1):388–399. doi: 10.1111/nph.14376. [DOI] [PubMed] [Google Scholar]
- Hyodo K, Okuno T. Hijacking of host cellular components as proviral factors by plant-infecting viruses. In: Carr JP, Roossinck MJ, editors. Advances in virus research. Cambridge: Academic Press; 2020. pp. 37–86. [DOI] [PubMed] [Google Scholar]
- Islam W, Naveed H, Zaynab M, Huang Z, Chen HYH. Plant defense against virus diseases; growth hormones in highlights. Plant Signal Behav. 2019;14(6):1596719. doi: 10.1080/15592324.2019.1596719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson PE, Clarke SF. Hormone–virus interactions in plants. Crit Rev Plant Sci. 2002;21(3):205–228. doi: 10.1080/0735-260291044241. [DOI] [Google Scholar]
- Jelínek L, Dolečková M, Karabin M, Hudcova T, Kotlikova B, Dostalek P. Influence of growing area, plant age, and virus infection on the contents of hop secondary metabolites. Czech J Food Sci. 2012;30(6):541–547. doi: 10.17221/50/2012-CJFS. [DOI] [Google Scholar]
- Jin L, Qin Q, Wang Y, Pu Y, Liu L, Wen X, Ji S, Wu J, Wei C, Ding B, Li Y. Rice dwarf virus P2 protein hijacks auxin signaling by directly targeting the rice OsIAA10 protein, enhancing viral infection and disease development. PLoS Pathog. 2016;12(9):e1005847. doi: 10.1371/journal.ppat.1005847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabera JN, Semana E, Mussa AR, He X. Plant secondary metabolites: biosynthesis, classification, function and pharmacological properties. J Pharm Pharmacol. 2014;2:377–392. [Google Scholar]
- Kieber JJ, Schaller GE. Cytokinin signaling in plant development. Development. 2018 doi: 10.1242/dev.149344. [DOI] [PubMed] [Google Scholar]
- Knoester M, Bol JF, van Loon LC, Linthorst HJ. Virus-induced gene expression for enzymes of ethylene biosynthesis in hypersensitively reacting tobacco. Mol Plant Microbe Interact. 1995;8(1):177–180. doi: 10.1094/mpmi-8-0177. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Seo S, Hirai K, Yamamoto-Katou A, Katou S, Seto H, Meshi T, Mitsuhara I, Ohashi Y. Silencing of WIPK and SIPK mitogen-activated protein kinases reduces tobacco mosaic virus accumulation but permits systemic viral movement in tobacco possessing the N resistance gene. Mol Plant Microbe Interact. 2010;23(8):1032–1041. doi: 10.1094/mpmi-23-8-1032. [DOI] [PubMed] [Google Scholar]
- Kovač M, Müller A, Jarh DM, Milavec M, Düchting P, Ravnikar M. Multiple hormone analysis indicates involvement of jasmonate signalling in the early defence of potato to potato virus Y NTN. Biol Plant. 2009;53(1):195–199. doi: 10.1007/s10535-009-0034-y. [DOI] [Google Scholar]
- Kriznik M, Petek M, Dobnik D, Ramsak Z, Baebler S, Pollmann S, Kreuze JF, Zel J, Gruden K. Salicylic acid perturbs sRNA-gibberellin regulatory network in immune response of potato to potato virus Y infection. Front Plant Sci. 2017;8:2192. doi: 10.3389/fpls.2017.02192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriger W, Agrios G. Cytokinin levels and kinetin–virus interactions in tobacco ringspot virus infected cowpea plants. Phytopathology. 1977;67:604–609. doi: 10.1094/Phyto-67-604. [DOI] [Google Scholar]
- Lan H, Lai B, Zhao P, Dong X, Wei W, Ye Y, Wu Z. Cucumber mosaic virus infection modulated the phytochemical contents of Passiflora edulis. Microb Pathog. 2020;138:103828. doi: 10.1016/j.micpath.2019.103828. [DOI] [PubMed] [Google Scholar]
- Lancini G, Lorenzetti R (1993) Biosynthesis of secondary metabolites. In: Biotechnology of antibiotics and other bioactive microbial metabolites. Springer US, Boston, pp 95–132. doi:10.1007/978-1-4757-9522-6_4
- León J, Shulaev V, Yalpani N, Lawton MA, Raskin I. Benzoic acid 2-hydroxylase, a soluble oxygenase from tobacco, catalyzes salicylic acid biosynthesis. Proc Natl Acad Sci USA. 1995;92(22):10413–10417. doi: 10.1073/pnas.92.22.10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J-W, Wang B, Song X-M, Wang R-R, Chen L, Zhang H, Zhang Z-B, Wang Q-C. Potato leafroll virus (PLRV) and potato virus Y (PVY) influence vegetative growth, physiological metabolism, and microtuber production of in vitro-grown shoots of potato Solanum tuberosum L. Plant Cell Tissue Organ Cult PCTOC. 2013;114(3):313–324. doi: 10.1007/s11240-013-0327-x. [DOI] [Google Scholar]
- Loebenstein G, Lawson RH, Brunt AA. Virus and virus-like diseases of bulb and flower crops. Bet Dagan: Wiley; 1995. [Google Scholar]
- Lozano-Durán R, Rosas-Díaz T, Gusmaroli G, Luna AP, Taconnat L, Deng XW, Bejarano ER. Geminiviruses subvert ubiquitination by altering CSN-mediated derubylation of SCF E3 ligase complexes and inhibit jasmonate signaling in Arabidopsis thaliana. Plant Cell. 2011;23(3):1014–1032. doi: 10.1105/tpc.110.080267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado JP, Brustolini OJ, Mendes GC, Santos AA, Fontes EP. NIK1, a host factor specialized in antiviral defense or a novel general regulator of plant immunity? BioEssays News Rev Mol Cell Dev Biol. 2015;37(11):1236–1242. doi: 10.1002/bies.201500066. [DOI] [PubMed] [Google Scholar]
- Masuta C, Tanaka H, Uehara K, Kuwata S, Koiwai A, Noma M. Broad resistance to plant viruses in transgenic plants conferred by antisense inhibition of a host gene essential in S-adenosylmethionine-dependent transmethylation reactions. Proc Natl Acad Sci USA. 1995;92(13):6117–6121. doi: 10.1073/pnas.92.13.6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero R, Pérez-Bueno ML, Barón M, Florez-Sarasa I, Tohge T, Fernie AR, Ouad Hel A, Flexas J, Bota J. Alterations in primary and secondary metabolism in Vitis vinifera 'Malvasía de Banyalbufar' upon infection with Grapevine leafroll-associated virus 3. Physiol Plant. 2016;157(4):442–452. doi: 10.1111/ppl.12440. [DOI] [PubMed] [Google Scholar]
- Muletarova S, Stoikova D, Ivanov K. Changes in the isoenzyme spectrum of peroxidase in potato and tobacco plants inoculated with potato virus A. Rastenievudni Nauki. 1995;32:118–120. [Google Scholar]
- Nakashita H, Yasuda M, Nitta T, Asami T, Fujioka S, Arai Y, Sekimata K, Takatsuto S, Yamaguchi I, Yoshida S. Brassinosteroid functions in a broad range of disease resistance in tobacco and rice. Plant J. 2003;33(5):887–898. doi: 10.1046/j.1365-313x.2003.01675.x. [DOI] [PubMed] [Google Scholar]
- Ncube B, Van Staden J. Tilting plant metabolism for improved metabolite biosynthesis and enhanced human benefit. Molecules. 2015;20(7):12698–12731. doi: 10.3390/molecules200712698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicaise V, Candresse T. Plum pox virus capsid protein suppresses plant pathogen-associated molecular pattern (PAMP)-triggered immunity. Mol Plant Pathol. 2017;18(6):878–886. doi: 10.1111/mpp.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsubo N, Mitsuhara I, Koga M, Seo S, Ohashi Y. Ethylene promotes the necrotic lesion formation and basic PR gene expression in TMV-infected tobacco. Plant Cell Physiol. 1999;40(8):808–817. doi: 10.1093/oxfordjournals.pcp.a029609. [DOI] [Google Scholar]
- Padmanabhan MS, Kramer SR, Wang X, Culver JN. Tobacco mosaic virus replicase-auxin/indole acetic acid protein interactions: reprogramming the auxin response pathway to enhance virus infection. J Virol. 2008;82(5):2477–2485. doi: 10.1128/JVI.01865-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey V, Srivastava R, Akhtar N, Mishra J, Mishra P, Verma PC. Expression of Withania somnifera steroidal glucosyltransferase gene enhances withanolide content in hairy roots. Plant Mol Biol Rep. 2016;34(3):681–689. doi: 10.1007/s11105-015-0955-x. [DOI] [Google Scholar]
- Parizad S, Dizadji A, Habibi MK, Winter S, Kalantari S, Movi S, Lorenzo Tendero C, Alonso GL, Moratalla-Lopez N. The effects of geographical origin and virus infection on the saffron (Crocus sativus L.) quality. Food Chem. 2019;295:387–394. doi: 10.1016/j.foodchem.2019.05.116. [DOI] [PubMed] [Google Scholar]
- Pennazio S, Roggero P. Plant hormones and plant virus diseases. The auxins. New Microbiol. 1996;19(4):369–378. [PubMed] [Google Scholar]
- Pethybridge SJ, Wilson CR, Hay FS, Leggett GW, Sherriff LJ. Effect of viruses on agronomic and brewing characteristics of four hop (Humulus lupulus) cultivars in Australia. Ann Appl Biol. 2002;140(1):97–105. doi: 10.1111/j.1744-7348.2002.tb00161.x. [DOI] [Google Scholar]
- Petrovič N, Miersch O, Ravnikar M, Kovač M. Potato virus YNTNalters the distribution and concentration of endogenous jasmonic acid in potato plants grownin vitro. Physiol Mol Plant Pathol. 1997;50(4):237–244. doi: 10.1006/pmpp.1997.0079. [DOI] [Google Scholar]
- Pieterse CM, Van der Does D, Zamioudis C, Leon-Reyes A, Van Wees SC. Hormonal modulation of plant immunity. Annu Rev Cell Dev Biol. 2012;28:489–521. doi: 10.1146/annurev-cellbio-092910-154055. [DOI] [PubMed] [Google Scholar]
- Pott DM, Osorio S, Vallarino JG. From central to specialized metabolism: an overview of some secondary compounds derived from the primary metabolism for their role in conferring nutritional and organoleptic characteristics to fruit. Front Plant Sci. 2019;10:835. doi: 10.3389/fpls.2019.00835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal R. Effect of tobacco mosaic virus infection on the endogenous levels of indoleacetic, phenylacetic and abscisic acids of tobacco leaves in various stages of development. J Plant Physiol. 1977;83(5):403–409. doi: 10.1016/S0044-328X(77)80046-9. [DOI] [Google Scholar]
- Rao M, Narasimham B, Reddy G, Murthy V. Effect of mosaic virus infection on the endogenous gibberellin levels of sathgudi leaves (Citrus sinensis Osbeck) Indian J Hortic. 1977;34(2):196–198. [Google Scholar]
- Ravnikar M, Gogala N, Miersch O, Bruckner C. The correlation between plant growth regulator jasmonic acid and PVM in the potato. Potato Res. 1990;33:144. [Google Scholar]
- Rezzonico E, Flury N, Meins F, Jr, Beffa R. Transcriptional down-regulation by abscisic acid of pathogenesis-related beta-1,3-glucanase genes in tobacco cell cultures. Plant Physiol. 1998;117(2):585–592. doi: 10.1104/pp.117.2.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez MC, Conti G, Zavallo D, Manacorda CA, Asurmendi S. TMV-Cg coat protein stabilizes DELLA proteins and in turn negatively modulates salicylic acid-mediated defense pathway during Arabidopsisthaliana viral infection. BMC Plant Biol. 2014;14:210. doi: 10.1186/s12870-014-0210-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell SL, Kimmins WC. Growth regulators and the effect of barley yellow dwarf virus on barley Hordeum vulgare L. Ann Bot. 1971;35(5):1037–1043. doi: 10.1093/oxfordjournals.aob.a084539. [DOI] [Google Scholar]
- Sakamoto S, Putalun W, Vimolmangkang S, Phoolcharoen W, Shoyama Y, Tanaka H, Morimoto S. Enzyme-linked immunosorbent assay for the quantitative/qualitative analysis of plant secondary metabolites. Journal of Natural Medicines. 2018;72(1):32–42. doi: 10.1007/s11418-017-1144-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholthof K-BG. Tobacco mosaic virus: a model system for plant biology. Annu Rev Phytopathol. 2004;42:13–34. doi: 10.1146/annurev.phyto.42.040803.140322. [DOI] [PubMed] [Google Scholar]
- Sheng J, Lartey R, Ghoshroy S, Citovsky V. An Arabidopsis thaliana mutant with virus-inducible phenotype. Virology. 1998;249(1):119–128. doi: 10.1006/viro.1998.9238. [DOI] [PubMed] [Google Scholar]
- Siefert F, Kwiatkowski J, Sarkar S, Grossmann K. Changes in endogenous cyanide and 1-aminocyclopropane-1-carboxylic acid levels during the hypersensitive response of tobacco mosaic virus-infected tobacco leaves. Plant Growth Regul. 1995;17(2):109–113. doi: 10.1007/BF00024169. [DOI] [Google Scholar]
- Siemens DH, Garner SH, Mitchell-Olds T, Callaway RM. Cost of defense in the context of plant competition: Brassica rapa may grow and defend. Ecology. 2002;83(2):505–517. doi: 10.2307/2680031. [DOI] [Google Scholar]
- Sridhar R, Mohanty SK, Anjaneyulu A. Physiology of rice tungro virus disease: increased cytokinin activity in tungro-infected rice cultivars. Physiol Plant. 1978;43(4):363–366. doi: 10.1111/j.1399-3054.1978.tb01595.x. [DOI] [Google Scholar]
- Srivastava R, Srivastava R, Singh UM (2014) Understanding the patterns of gene expression during climate change. In Climate Change Effect on Crop Productivity; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, pp 279–328. ISBN 978-1-4822-2920-2
- Srivastava R, Rai KM, Srivastava R. Plant biosynthetic engineering through transcription regulation: an insight into molecular mechanisms during environmental stress. In: Varjani SJ, Parameswaran B, Kumar S, Khare SK, editors. Biosynthetic technology and environmental challenges. Singapore: Springer Singapore; 2018. pp. 51–72. [Google Scholar]
- Sziráki I, Balázs E, Király Z. Rôle of different stresses in inducing systemic acquired resistance to TMV and increasing cytokinin level in tobacco. Physiol Plant Pathol. 1980;16(2):277–284. doi: 10.1016/0048-4059(80)90042-9. [DOI] [Google Scholar]
- Tavantzis SM, Smith SH, Witham FH. The influence of kinetin on tobacco ringspot virus infectivity and the effect of virus infection on the cytokinin activity in intact leaves of Nicotiana glutinosa L. Physiol Plant Pathol. 1979;14(2):227–233. doi: 10.1016/0048-4059(79)90010-9. [DOI] [Google Scholar]
- Thivierge K, Nicaise V, Dufresne PJ, Cotton S, Laliberte JF, Le Gall O, Fortin MG. Plant virus RNAs. Coordinated recruitment of conserved host functions by (+) ssRNA viruses during early infection events. Plant Physiol. 2005;138(4):1822–1827. doi: 10.1104/pp.105.064105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson G, Martin M, Van Staden J. Relationship between tomato spotted wilt virus infection and cytokinin content of tomato. Phytophylactica. 1983;15(2):63–66. [Google Scholar]
- Vierstra RD. The ubiquitin-26S proteasome system at the nexus of plant biology. Nat Rev Mol Cell Biol. 2009;10(6):385–397. doi: 10.1038/nrm2688. [DOI] [PubMed] [Google Scholar]
- Vitti A, Nuzzaci M, Scopa A, Tataranni G, Remans T, Vangronsveld J, Sofo A. Auxin and cytokinin metabolism and root morphological modifications in Arabidopsis thaliana seedlings infected with Cucumber mosaic virus (CMV) or exposed to cadmium. Int J Mol Sci. 2013;14(4):6889–6902. doi: 10.3390/ijms14046889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Culver JN. DNA binding specificity of ATAF2, a NAC domain transcription factor targeted for degradation by Tobacco mosaic virus. BMC Plant Biol. 2012;12:157. doi: 10.1186/1471-2229-12-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Goregaoker SP, Culver JN. Interaction of the Tobacco mosaic virus replicase protein with a NAC domain transcription factor is associated with the suppression of systemic host defenses. J Virol. 2009;83(19):9720–9730. doi: 10.1128/jvi.00941-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- War AR, Paulraj MG, War MY, Ignacimuthu S. Role of salicylic acid in induction of plant defense system in chickpea (Cicer arietinum L.) Plant Signal Behav. 2011;6(11):1787–1792. doi: 10.4161/psb.6.11.17685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whenham RJ. Effect of systemic tobacco mosaic virus infection on endogenous cytokinin concentration in tobacco (Nicotiana tabacum L.) leaves: consequences for the control of resistance and symptom development. Physiol Mol Plant Pathol. 1989;35(1):85–95. doi: 10.1016/0885-5765(89)90009-X. [DOI] [Google Scholar]
- Wu Q, Wang X, Ding SW. Viral suppressors of RNA-based viral immunity: host targets. Cell Host Microbe. 2010;8(1):12–15. doi: 10.1016/j.chom.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Valli A, García JA, Zhou X, Cheng X. The tug-of-war between plants and viruses: great progress and many remaining questions. Viruses. 2019;11(3):203. doi: 10.3390/v11030203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie K, Li L, Zhang H, Wang R, Tan X, He Y, Hong G, Li J, Ming F, Yao X, Yan F, Sun Z, Chen J. Abscisic acid negatively modulates plant defence against rice black-streaked dwarf virus infection by suppressing the jasmonate pathway and regulating reactive oxygen species levels in rice. Plant Cell Environ. 2018;41(10):2504–2514. doi: 10.1111/pce.13372. [DOI] [PubMed] [Google Scholar]
- Xu P, Chen F, Mannas JP, Feldman T, Sumner LW, Roossinck MJ. Virus infection improves drought tolerance. New Phytol. 2008;180(4):911–921. doi: 10.1111/j.1469-8137.2008.02627.x. [DOI] [PubMed] [Google Scholar]
- Zaim M, Lal R, Verma R, Pandey R. Studies on effect of poppy mosaic virus infection on poppy produce and some secondary metabolites. Acta Horticult. 2014;1036:151–155. doi: 10.17660/ActaHortic.2014.1036.16. [DOI] [Google Scholar]
- Zaim M, Verma RK, Pandey R, Lal RK. Genotype-dependent response of an RNA Virus Infection on Selected pharmaceutically important alkaloids in Papaver somniferum. J Herbs Spices Med Plants. 2014;20(2):124–131. doi: 10.1080/10496475.2013.840817. [DOI] [Google Scholar]
- Zhang H, Zhu Liu XH. Effect Of banana bunchy top virus (BBTV) on endogenous hormones of banana plant. Acta Phytopathol Sin. 1997;27:79–83. [Google Scholar]
- Zhang DW, Deng XG, Fu FQ, Lin HH. Induction of plant virus defense response by brassinosteroids and brassinosteroid signaling in Arabidopsis thaliana. Planta. 2015;241(4):875–885. doi: 10.1007/s00425-014-2218-8. [DOI] [PubMed] [Google Scholar]
- Zhang C, Ding Z, Wu K, Yang L, Li Y, Yang Z, Shi S, Liu X, Zhao S, Yang Z, Wang Y, Zheng L, Wei J, Du Z, Zhang A, Miao H, Li Y, Wu Z, Wu J. Suppression of jasmonic acid-mediated defense by viral-inducible microRNA319 facilitates virus infection in rice. Mol Plant. 2016;9(9):1302–1314. doi: 10.1016/j.molp.2016.06.014. [DOI] [PubMed] [Google Scholar]
- Zhang H, Tan X, Li L, He Y, Hong G, Li J, Lin L, Cheng Y, Yan F, Chen J, Sun Z. Suppression of auxin signalling promotes rice susceptibility to rice black streaked dwarf virus infection. Mol Plant Pathol. 2019;20(8):1093–1104. doi: 10.1111/mpp.12814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Li L, He Y, Qin Q, Chen C, Wei Z, Tan X, Xie K, Zhang R, Hong G, Li J, Li J, Yan C, Yan F, Li Y, Chen J, Sun Z. Distinct modes of manipulation of rice auxin response factor OsARF17 by different plant RNA viruses for infection. Proc Natl Acad Sci USA. 2020;117(16):9112–9121. doi: 10.1073/pnas.1918254117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Hong W, Wu J, Wang Y, Ji S, Zhu S, Wei C, Zhang J, Li Y. A viral protein promotes host SAMS1 activity and ethylene production for the benefit of virus infection. Elife. 2017 doi: 10.7554/eLife.27529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Gao F, Cao X, Chen M, Ye G, Wei C, Li Y. The rice dwarf virus P2 protein interacts with ent-kaurene oxidases in vivo, leading to reduced biosynthesis of gibberellins and rice dwarf symptoms. Plant Physiol. 2005;139(4):1935–1945. doi: 10.1104/pp.105.072306. [DOI] [PMC free article] [PubMed] [Google Scholar]