Abstract
Post bariatric control of food intake is influenced by psychological and behavioral factors. We investigated dietary habits and food intake during COVID-19 quarantine among recently operated patients. Patients were assessed for total and per meal energy and macronutrient intake as well as frequency of food consumption per processing level. Patients were also classified according to adherence to nutritional recommendations from our outpatient clinic. Main results are indicative of inappropriate nutritional intake during COVID-19 quarantine in postoperative bariatric patients. We observed that many patients failed to meet the recommended protein intake (89.2%) along a relatively high intake of ultra-processed foods (~1/4 of the diet). Our data suggest the need for the implementation of strategies to extend nutritional care to at-risk patients during social distancing.
Keywords: COVID-19, Gastric bypass, Protein intake, Ultra-processed food, Nutritional status
It is well established that post-bariatric weight loss is mainly due to the dietary and gastrointestinal hormonal (such as satiety signals) adjustments imposed by surgery [1, 2]. However, postoperative control of food intake is also influenced by psychological and behavioral factors [3]. The outbreak of coronavirus disease 2019 (COVID-19) and social isolation measures taken worldwide to control the spread of the disease have dramatically changed the way of life among patients who underwent bariatric surgery, along with reduced access to face-to-face patient care [4]. In this scenario, adhering to postoperative dietary recommendations and maintaining appropriate control over dietary intake may be challenging. Considering these aspects, we investigated dietary habits and food intake during COVID-19 quarantine among patients who recently underwent bariatric surgery.
In this screening study, we enrolled patients who underwent bariatric surgery from the Metabolic and Bariatric Surgery Unit of the Clinical Hospital of the University of Sao Paulo, Brazil. Patients aged ≥ 18 years, with surgery elapsed time ≤ 12 months and without any COVID-19 symptoms, were considered eligible. Information about age, sex, surgery technique, postoperative time, and body mass index (BMI) were collected from medical records. Informed consent was obtained from all individual participants included in the study. Trained dietitians conducted three non-consecutive 24-h food recalls (two weekdays and one weekend day) over the phone to gather information on food consumption during quarantine. Using appropriate nutritional software (Dietbox software—online version), we analyzed energy (in kcal), protein, carbohydrates, and lipids (in grams and percentage of total energy intake—TEI) and fiber (grams) intake. Per meal (breakfast, lunch, dinner, and snacks) energy and macronutrients intake were also assessed. Food consumption was classified and calculated for processing level in accordance with the NOVA classification [5]. For this, we evaluated the frequency of food consumption (times/day) and energy contribution (%TEI) of each category (culinary ingredients; unprocessed or minimally processed foods; processed foods and ultra-processed foods). In addition, patients were categorically classified (“yes” or “no”) according to their adherence to nutritional recommendations provided during follow-up in our outpatient clinic for patients who underwent bariatric surgery for the following criteria: meal skipping habit; daily consumption of three structured meals (breakfast, lunch, and dinner); consumption of snacks; consumption of animal and vegetal protein source in main meals (breakfast, lunch, and dinner); and minimal protein intake of 60 g/day [6]. Data are presented as mean ± standard deviation or frequency (absolute and relative). Data on food consumption are presented as absolute values, %TEI and daily frequency of intake.
We evaluated 65 patients (56 (86.1%) women) aged 47.2 ± 11.4 years. Forty-seven (72.3%) underwent Roux-en-Y gastric bypass, whereas 18 (27.7%) underwent vertical gastrectomy. Average postoperative time and BMI were 7.4 ± 2.2 months and 35.5 ± 6.8 kg/m2, respectively. Twenty-one (32.3%) patients had the habit of skipping meals; thirty-four patients (52.3%) had daily consumption of three structured meals; and only twenty-three patients (38.5%) consumed animal protein in all main meals and thirty-seven (56.9%) consumed any source of protein (animal or vegetable) in all main meals (Table 1).
Table 1.
Meal pattern behavior among post-bariatric patients (n = 65)
| Eating three structured meals daily (breakfast, lunch, and dinner) | 44 (67.7%) |
| Consuming five or more than five meals daily, including snacks | 34 (52.3%) |
| Consuming animal protein source in main meals | 25 (38.5%) |
| Consuming animal or vegetal protein source in main meals | 37 (56.9%) |
| Number of meals/day | 4.9 ± 1 (2–8) |
| According outpatients’ dietitian advices | |
The average energy intake was 824.5 ± 255.4 kcal/day. The frequencies of unprocessed or minimally processed foods, processed foods, and ultra-processed foods consumption were 10.1 ± 2.9, 2.2 ± 1.1, and 3.1 ± 1.5 times/day, respectively. Energy from ultra-processed and processed foods corresponded to 23.8 ± 12.3% and 11.1 ± 10.1% of TEI, respectively (Table 2). We observed that most of the consumed protein was during lunch, whereas carbohydrate was mostly present during snacks (Fig. 1). In comparison to guideline recommendations, we observed that most patients (fifty-eight patients (89.2%)) did not achieve the recommended daily protein intake (animal + vegetal source). A representative 24-h recall from one of the patients is showed in Table 3.
Table 2.
Post-bariatric patients’ food intake (n = 65)
| Daily nutrient consumption | |
| Energy (kcal/day) | 824.5 ± 255.4 (385–1776.7) |
| Protein (g/day) | 42.9 ± 13 (23.6–80.7) |
| Protein (%TEI) | 21.5 ± 5.8 (12–40.6) |
| Carbohydrate (g/day) | 112.6 ± 39.3 (34.6–245) |
| Carbohydrate (%TEI) | 55.1 ± 10.9 (26.4–82.9) |
| Sugar (%TEI) | 2.5 ± 4 (0–22.3) |
| Lipid (g/day) | 33.3 ± 16.7 (13.4–136.6) |
| Lipid (%TEI) | 36.2 ± 9.8 (18.1–69.2) |
| Fiber (g/day) | 8.7 ± 4 (2–19.6) |
| Energy density (kcal/g) | 1 ± 0.3 (0.5–1.9) |
| Food consumption per processing level | |
| Culinary ingredients (%TEI) | 10.1 ± 5 (0–23.7) |
| Unprocessed or minimally processed foods (frequency) | 10.1 ± 2.8 (5–19) |
| Unprocessed or minimally processed foods (%TEI) | 54.9 ± 14.6 (13.7–86.4) |
| Processed foods (frequency) | 2.2 ± 1.1 (1–6) |
| Processed foods (%TEI) | 11. ± 10.1 (0–43.8) |
| Ultra-processed foods (frequency) | 3.1 ± 1.5 (1–8) |
| Ultra-processed foods (%TEI) | 23.8 ± 12.3 (2.5–71.6) |
Data are shown as frequency (% of patients) or mean ± standard deviation (95% confidence interval). TEI total energy intake
Fig. 1.
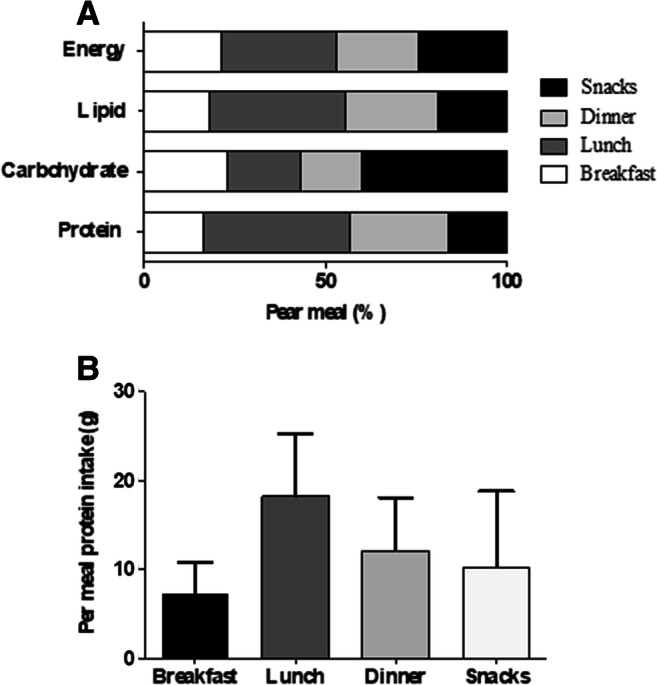
Panel a Per meal macronutrient contribution (relative to total energy intake) (breakfast, lunch, dinner, and snacks) (%). Panel b Per meal (breakfast, lunch, and dinner) protein intake (g)
Table 3.
Representative 24-h recall and classification according to food processing level [5]
| Meal/food | Amount | Food processing level |
|---|---|---|
| Breakfast | ||
| Instant coffee | 1 cup | Unprocessed or minimally processed |
| Whole milk | 1 cup | Unprocessed or minimally processed |
| Sugar | 1 medium spoon | Culinary ingredient |
|
Bread Margarine |
2 slices 1 teaspoon |
Ultra-processed Ultra-processed |
| Lunch | ||
| White rice | 2 medium spoons | Unprocessed or minimally processed |
| Cooked beans | 3 medium spoons | Unprocessed or minimally processed |
| Cooked chicken leg | 1 medium piece | Unprocessed or minimally processed |
|
Lettuce and tomato salad Instant juice, orange flavored |
3 medium spoons 1 glass |
Unprocessed or minimally processed Ultra-processed |
| Afternoon snack | ||
| Tangerine | 1 unit | Unprocessed or minimally processed |
| Dinner | ||
| Cream cracker | 4 small units | Ultra-processed |
| Ginger tea | 1 cup | Unprocessed or minimally processed |
| Artificial sweetener | 3 drops | Ultra-processed |
Our results are indicative of inappropriate nutritional intake during COVID-19 quarantine in patients who underwent bariatric surgery. Despite current recommendations regarding the implementation (whenever possible) of telemedicine, online, or phone follow-up strategies during quarantine in order to provide health support, especially nutritional, to patients who are less willing or unable to travel [7], the emergency status of the pandemic impeded the implementation of remote follow-up in our institution. This is of clinical relevance, as inadequate eating practices and lifestyle arising from lack of nutritional guidance and counseling (as those experienced by our cohort due to constraints imposed by social isolation measures) may compromise the effectiveness of surgery on weight loss [8]. More than one-third of the patients had the habit of skipping main meals (breakfast, lunch, or dinner), which could possibly be related to an elevated consumption of snacks throughout the day. Considering the energy content of snacks (293.6 ± 196 kcal), their frequent consumption may lead to excessive calorie intake, which in turn could result in reduced weight loss or even weight recover in the postoperative period [9]. The rather low sugar intake observed in most of our patients (2.5% TEI) is not surprising. Previous studies have shown a decreased preference for sugar among patients undergoing bariatric surgery [10] as its highly osmotic feature increases the incidence of dumping syndrome [11]. Despite adequate dietary fiber intake being essential for gut function and laxation (bowel habits) [12], there is a lack of fiber intake recommendation in current guidelines [6, 13]. The number of available studies on post-bariatric dietary fiber intake is limited, but food intolerance to cereals, rice, fruits, and vegetables has been considered as a potential contributing factor to reduced postoperative fiber intake [14].
Protein is thought to play a central role in postoperative nutrition of bariatric patients. Current recommendations state that protein-rich foods (e.g., dairy products, eggs, fish, and lean meat) should be consumed in the main meals of the day [6]. We observed that many of our patients failed to meet both the recommended daily protein intake (89.2%) and the recommendation for frequent animal protein intake (38.5%). Inadequate protein intake has been previously reported in Brazilian patients undergoing bariatric surgery [15, 16]. Considering the relatively short postoperative time (7.4 ± 2.2 months), one may speculate that food intolerance may have hampered proper protein intake [17]; however, previous studies did not observe any association between food intolerance and protein consumption [15, 18]. Although speculative, the low protein ingestion may be related to our population’s low socioeconomic status [19]. These results reinforce the importance of monitoring protein intake after surgery. No recommendation is currently available for per-meal protein intake in postoperative bariatric surgery; however, there is evidence to suggest that a high per-meal protein intake (> 30 g/meal) is associated with better functional status in older obese adults engaged in a weight loss intervention [20]. In our patients, average per meal protein intake was ~ 17 g. Reductions in fat-free mass during energy restriction typically account for a significant amount of total weight loss. This is also the case in patients who underwent bariatric surgery, for whom the loss of fat-free mass may rise as high as 23% within the first months after surgery [21] and may significantly impact functional capacity, bone remodeling, and cardiometabolic parameters [22]. In this scenario, adequate protein provision may constitute an important nutritional support to mitigate energy restriction-induced muscle waste.
Moreover, no guideline is available for carbohydrate and lipid daily intakes during the postoperative period. Despite the last UpToDate Report [13] allude carbohydrate and lipid intakes of approximately 130 g/day and 20 to 35% TEI, respectively, these are not official recommendations; therefore, no adequacy assessment was performed.
Despite the high intake of unprocessed or minimally processed foods (predominantly in the form of natural foods), our patients showed a high consumption of ultra-processed foods (23.8 ± 12.3% of TEI). Because of its energy dense profile characterized by high fat (mainly saturated fat), sugar, and sodium levels, the ingestion of ultra-processed foods is strongly associated with obesity and cardiovascular disease [23]. Moreover, ultra-processed foods can activate brain regions associated with the reward system, affecting food choices and stimulating food consumption [24]. In line with this, previous studies have explored the caloric contribution as a function of food processing in patients who underwent bariatric surgery [25, 26]. Pinto et al. [25] observed that the caloric contribution of ultra-processed foods was 19.7% after 3 months of bariatric surgery, which is ~ 20% lower than that seen in the current study. The qualitative analysis of food intake has become more important recently, as good-quality diets have been considered a protective factor against weight recovery after surgery [23]. It is possible, however, that the longer shelf life and lower cost of ultra-processed foods [27] may have driven its increased consumption during quarantine. Longitudinal assessments are warranted in order to confirm whether the nutritional inadequacies observed in this study are causally associated with social isolation and lack of face-to-face health care.
In conclusion, the screening of dietary habits and food intake during COVID-19 quarantine of patients who underwent bariatric surgery showed low protein consumption and a relatively high intake of ultra-processed foods (~ 1/4 of the diet). Despite the inherent limitations of an observational study, thus hampering more in-depth investigation of the actual role of COVID-19 pandemic on eating habits, data herein allow us to suggest the need for the development and implementation of virtual strategies, such as app-based tools and telemedicine, in order to extend nutritional care to at-risk patients during social distancing.
Funding
This study was financially supported by the Sao Paulo Research Foundation (FAPESP) (grants #2015/26937-4, #2017/13552-2, #2019/18039-7, #2020/07860-9) and the National Council for Scientific and Technological Development (CNPq) (grant #402123/2020-4).
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Approval and Consent to Participate
This study has been approved by the Hospital’s Ethical Committee. All patients gave their written consent for participation in the study.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sarwer DB, Wadden TA, Moore RH, Baker AW, Gibbons LM, Raper SE, Williams NN. Preoperative eating behavior, postoperative dietary adherence, and weight loss after gastric bypass surgery. Surg Obes Relat Dis. 2008;4(5):640–646. doi: 10.1016/j.soard.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ionut V, Burch M, Youdim A, Bergman RN. Gastrointestinal hormones and bariatric surgery-induced weight loss. Obesity. 2013;21(6):1093–1103. doi: 10.1002/oby.20364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Madsbad S, Dirksen C, Holst JJ. Mechanisms of changes in glucose metabolism and bodyweight after bariatric surgery. Lancet Diabetes Endocrinol. 2014;2(2):152–164. doi: 10.1016/S2213-8587(13)70218-3. [DOI] [PubMed] [Google Scholar]
- 4.Sockalingam S. The impact of coronavirus disease 2019 on bariatric surgery: redefining psychosocial care. Obesity (Silver Spring) 2020;28(6):1010–1012. doi: 10.1002/oby.22836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monteiro CA, Cannon G, Levy R, Moubarac JC, Jaime P, Martins AP, et al. NOVA. The star shines bright. World Nutr. 2016;7(1–3):28–38. [Google Scholar]
- 6.Mechanick JI, Youdim A, Jones DB, Garvey WT, Hurley DL, McMahon MM, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient—2013 update: cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery. Obesity (Silver Spring) 2013;21(Suppl 1):S1–27. doi: 10.1002/oby.20461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang W, Wang C, Shikora S, Kow L. Recommendations for metabolic and bariatric surgery during the COVID-19 pandemic from IFSO. Obes Surg. 2020;30(6):2071–2073. doi: 10.1007/s11695-020-04578-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masood A, Alsheddi L, Alfayadh L, et al. Dietary and lifestyle factors serve as predictors of successful weight loss maintenance postbariatric surgery. J Obes. 2019;7295978 [DOI] [PMC free article] [PubMed]
- 9.Brolin RE, Robertson LB, Kenler HA, Cody RP. Weight loss and dietary intake after vertical banded gastroplasty and Y-en-Roux gastric bypass. Ann Surg. 1994;220(6):782–790. doi: 10.1097/00000658-199412000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Vuuren MA, Strodl E, White KM, Lockie PD. Taste, enjoyment, and desire of flavors change after sleeve gastrectomy—short term results. Obes Surg. 2017;27(6):1466–1473. doi: 10.1007/s11695-016-2497-1. [DOI] [PubMed] [Google Scholar]
- 11.Fujioka K. Follow-up of nutritional and metabolic problems after bariatric surgery. Diabetes Care. 2005;28:481–484. doi: 10.2337/diacare.28.2.481. [DOI] [PubMed] [Google Scholar]
- 12.Handzlik-Orlik G, Holecki M, Orlik B, Wylezol M, Dulawa J. Nutrition management of the post-bariatric surgery patient. Nutr Clin Prac. 2015;30(3):383–392. doi: 10.1177/0884533614564995. [DOI] [PubMed] [Google Scholar]
- 13.Mechanick JI, Apovian C, Brethauer S, Garvey WT, Joffe AM, Kim J, et al. Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures – 2019 update: cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, The Obesity Society, American Society for Metabolic and Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists. Obesity. 2020;28:1–58. doi: 10.1002/oby.22719. [DOI] [Google Scholar]
- 14.Grosse CS, Cope VC. Dietary fibre intake and bowel habits after bariatric surgery: structured literature review. Obes Surg. 2019;29:2247–2254. doi: 10.1007/s11695-019-03837-0. [DOI] [PubMed] [Google Scholar]
- 15.Nicoletti CF, Oliveira BAP, Barbin R, Marchini JS, Salgado Junior W, Nonino CB. Red meat intolerance in patients submitted to gastric bypass: a 4-year follow-up study. Surg Obes Relat Dis. 2015;11(4):842–846. doi: 10.1016/j.soard.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Menegati GC, de Oliveira LC, Santo ALA, Cohen L, Mattos F, Mendonça LMC, et al. Nutritional status, body composition, and bone health in women after bariatric surgery at a University Hospital in Rio de Janeiro. Obes Surg. 2016;26:1517–1524. doi: 10.1007/s11695-015-1910-5. [DOI] [PubMed] [Google Scholar]
- 17.Giusti V, Theytaz F, Di Vetta V, Clarisse M, Suter M, Tappy L. Energy and macronutrient intake after gastric bypass for morbid obesity: a 3-y observational study focused on protein consumption. Am J Clin Nutr. 2016;103:18–24. doi: 10.3945/ajcn.115.111732. [DOI] [PubMed] [Google Scholar]
- 18.Godoy CMA, Cunha BAQ, Furtado MC, Godoy EP, Souza LBR, Oliveira AG. Relationship of food intolerance 2 years after roux-en-y gastric bypass surgery for obesity with masticatory efficiency and protein consumption. Obes Surg. 2020;30(8):3093–3098. doi: 10.1007/s11695-020-04669-z. [DOI] [PubMed] [Google Scholar]
- 19.Soares FL, Souza LB, Corradi-Perini C, Cruz MRR, Nunes MGJ, Branco-Filho AJ. Food quality in the late postoperative period of bariatric surgery: an evaluation using the bariatric food pyramid. Obes Surg. 2014;24(9):1481–1486. doi: 10.1007/s11695-014-1198-x. [DOI] [PubMed] [Google Scholar]
- 20.Starr KNP, Pieper CF, Orenduff MC, McDonald SR, McClure RB, Zhou M, et al. Improved function with enhanced protein intake per meal: a pilot study of weight reduction in frail, obese older adults. J Gerontol A Biol Sci Med Sci. 2016;71(10):1369–1375. doi: 10.1093/gerona/glv210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nuijten MAH, Monpellier VM, Eijsvogels TMH, Janssen IMC, Hazebroek EJ, Hopman MTE. Rate and determinants of excessive fat-free mass loss after bariatric surgery. Obes Surg. 2020;30(8):3119–3126. doi: 10.1007/s11695-020-04654-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murai IH, Roschel H, Dantas WS, Gil S, Merege-Filho C, de Cleva R, de Sá-Pinto AL, Lima F, Santo MA, Benatti FB, Kirwan JP, Pereira RM, Gualano B. Exercise mitigates bone loss in women with severe obesity after roux-en-y gastric bypass: a randomized controlled trial. J Clin Endocrinol Metab. 2019;104(10):4639–4650. doi: 10.1210/jc.2019-00074. [DOI] [PubMed] [Google Scholar]
- 23.Juul F, Martinez-Steele E, Parekh N, Monteiro CA, Chang VW. Ultra-processed food consumption and excess weight among US adults. Br J Nutr. 2018;120:90–100. doi: 10.1017/S0007114518001046. [DOI] [PubMed] [Google Scholar]
- 24.Small D. Processed foods and food reward. Science. 2019;363(6425):346–347. doi: 10.1126/science.aav0556. [DOI] [PubMed] [Google Scholar]
- 25.Pinto SL, Silva DCG, Bressan J. Absolute and relative changes in ultra-processed food consumption and dietary antioxidants in severely obese adults 3 months after Roux-en-Y gastric bypass. Obes Surg. 2019;29(6):1810–1815. doi: 10.1007/s11695-019-03749-z. [DOI] [PubMed] [Google Scholar]
- 26.Farias G, Silva RMO, Silva PPP, Vilela RM, Bettini SC, Dâmaso AR, et al. Impact of dietary patterns according to NOVA food groups: 2 y after Roux-en-Y gastric bypass surgery. Nutrition. 2020;74:110746. doi: 10.1016/j.nut.2020.110746. [DOI] [PubMed] [Google Scholar]
- 27.Moubarac JC, Martins APB, Claro RM, Levy RB, Cannon G, Monteiro CA. Consumption of ultra-processed foods and likely impact on human health. Evidence from Canada. Public Health Nutr. 2013;16(12):2240–2248. doi: 10.1017/S1368980012005009. [DOI] [PMC free article] [PubMed] [Google Scholar]


